Abstract
The Human Papillomavirus (HPV) E6 protein is one of three oncoproteins encoded by the virus. It has long been recognized as a potent oncogene and is intimately associated with the events that result in the malignant conversion of virally infected cells. In order to understand the mechanisms by which E6 contributes to the development of human malignancy many laboratories have focused their attention on identifying the cellular proteins with which E6 interacts. In this review we discuss these interactions in the light of their respective contributions to the malignant progression of HPV transformed cells.
Similar content being viewed by others
Introduction
Human Papillomaviruses (HPVs) infect keratinocytes in the basal layers of stratified epithelia at a variety of anatomical sites and their replicative cycle is intimately linked with the differentiation of the infected cell. Mucosal HPVs are the best characterized, and include the high-risk types, such as HPV-16 and HPV-18, which cause lesions that can progress to cervical carcinoma. In contrast, the low-risk types, such as HPV-6 and HPV-11, induce benign genital warts and are very rarely associated with malignancies (zur Hausen and Schneider, 1987). Less is known about the cutaneous HPV types but, once again, a subset of these types, including HPV-5 and HPV-8, is linked with the development of human cancers, particularly squamous cell carcinoma (SCC) at sun exposed sites in immunocompromised individuals (for review see Benton and Arends, 1996).
Due to their limited coding capacity, HPVs have to use the cellular DNA synthesis machinery in order to replicate their genomes. However, while low-risk HPVs begin replication in cells that are still proliferating, the replicative phase of high-risk HPV infection is confined to more differentiated cells that have already exited the cell cycle and are non-permissive for DNA synthesis (Doorbar et al., 1997). In order to overcome this problem, the high-risk HPV E7 protein targets a number of cell cycle regulatory proteins, including the ‘pocket protein’ family of pRb, p107 and p130, thereby upregulating genes required for G1/S transition and DNA synthesis (see Münger et al., this issue). However, the host cell's normal response to this unscheduled induction of proliferation would be to trigger apoptosis and/or growth arrest. To overcome these obstacles, the high-risk E6 protein targets a variety of cellular proteins involved in regulating these defence mechanisms, as well as those involved in terminal differentiation and antiviral defence. Under normal circumstances, viral replication would then continue, resulting in production and release of infectious virions. On rare occasions, however, the viral life cycle is interrupted and processes are initiated that lead to immortalization and ultimately to full transformation of the cell.
In this review we shall address the functions of E6 which appear most relevant for cell transformation, highlighting those aspects which appear to be conserved across many different viral oncoproteins, as well as those reflecting unique aspects of E6 function. Prior to this we will review the studies which define the high-risk HPV E6 as a potent viral oncoprotein.
Transformation by HPV E6
The HPV E6 proteins are small polypeptides of approximately 150 amino acids and contain two zinc-finger motifs (Cole and Danos, 1987; Barbosa et al., 1989), whose integrity is essential for E6 function (Kanda et al., 1991; Sherman and Schlegel, 1996). The first indirect evidence that E6 was a viral oncoprotein came from studies on cervical tumours and derived cell lines, where E6 was found to be retained and expressed many years after the initial transforming events (Schwarz et al., 1985; Androphy et al., 1987; Banks et al., 1987). Subsequently E6 was found to possess intrinsic transforming activity in a variety of different assay systems. Although E6 has weak transforming activity in established rodent cells (Sedman et al., 1991), high-risk but not low-risk E6 proteins can efficiently cooperate with an activated ras oncogene in the transformation of primary rodent cells (Storey and Banks, 1993; Pim et al., 1994; Liu et al., 1994). In addition, E6 has also been found to immortalize primary human mammary epithelial cells at late passage (Band et al., 1991; Wazer et al., 1995), although this activity is also exhibited by the low-risk HPV E6 proteins (Band et al., 1993).
Perhaps the most relevant system for evaluating the transforming potential of the HPV oncoproteins is immortalization of primary human keratinocytes, which are the natural host cells of the virus in vivo. Numerous studies have shown that high-risk E6 and E7 are together sufficient to induce immortalization of primary human keratinocytes (Münger et al., 1989; Hawley-Nelson et al., 1989), while the low-risk HPV proteins are completely inactive in this assay (Woodworth et al., 1989; Pecoraro et al., 1989). It is also interesting to note that these keratinocytes, although immortalized, will not form tumours in nude mice. Only following expression of activated oncogenes (DiPaolo et al., 1989) or after extended passage in tissue culture (Dürst et al., 1989; Hurlin et al., 1991) do these cells become fully transformed. This nicely resembles the process of HPV induced tumorigenicity in vivo, where there are long periods between the initial immortalization events and the ultimate progression to cervical cancer, thereby highlighting the multistep nature of the disease progression.
More recently, efficient models of HPV-induced carcinogenesis have been obtained through the generation of transgenic mice. E6 and E7 together can induce various types of tumours, depending on the particular tissue in which they are expressed (Arbeit et al., 1993; Griep et al., 1993; Comerford et al., 1995): when targeted to the basal cells of the squamous epithelium, under control of the human keratin 14 promoter (K14), the transgenic mice developed progressive squamous epithelial neoplasia (Arbeit et al., 1994). Individual expression of each oncogene induced epithelial hyperplasia and skin tumours (Herber et al., 1996; Song et al., 1999), however E7 was found to primarily cause benign, highly differentiated tumours, whereas those promoted by E6 were mostly malignant. This suggests that the two oncoproteins play different roles in the process of carcinogenesis, and also supports the notion that they act cooperatively to induce transformation. Further investigation was performed on E6- and E7-transgenic mice, following treatment with specific carcinogens known to affect distinct stages of tumour formation. Interestingly, E7 was found to primarily cause tumour promotion, whereas E6 contributed weakly to the early stages, acting more strongly during tumour progression, accelerating the malignant conversion of benign tumours (Song et al., 2000): this finding was particularly important, since it suggests that E6 may be responsible for the malignant progression of HPV induced tumours in vivo.
Interactions between HPV E6 and p53
Analysis of the cellular targets of the HPV E6 proteins has provided a wealth of information on how E6 contributes to malignant transformation. The first cellular target of E6 to be identified, and probably still the most important, is p53.
The p53 tumour suppressor represents a major constraint to viral replication, since, once activated by the unscheduled induction of DNA replication, it can promote cell cycle arrest or apoptosis of the infected cell (El-Deiry et al., 1993; Harper et al., 1993; Lowe et al., 1994; Wu and Levine, 1994). To overcome this obstacle, several viruses encode proteins that functionally inactivate p53. SV40 LT prevents transactivation of p53 target genes through association with its DNA binding domain (Ruppert and Stillmann, 1993), Ad E1B-55K abolishes the same function by binding to the transactivating domain of p53 (Lin et al., 1994), yet in association with E4orf6 it can also lead to p53 degradation (Steegenga et al., 1998, Querido et al., 2001; Täuber and Dobner, this issue), while the HBV X protein sequesters p53 in the cytoplasm (Elmore et al., 1997). A major strategy employed by the high-risk HPV E6 proteins to abrogate p53's oncosuppressive functions is to induce its degradation through the ubiquitin-proteasome pathway (Scheffner et al., 1990). As a consequence, p53 levels are extremely low in cervical tumour cells (Matlashewski et al., 1986), and p53-induced growth arrest and apoptosis in response to DNA damage are abolished (Kessis et al., 1993; Foster et al., 1994). Under normal growth conditions in the absence of HPV, p53 is also turned over by the ubiquitin proteasome pathway, and this is mediated by the ubiquitin ligase Mdm2 (Honda et al., 1997). However under stress conditions, e.g. the expression of viral oncogenes, this degradative pathway is inhibited and p53 is both stabilized and activated (Ashcroft and Vousden, 1999, for review). Recent experiments have indeed shown that in HPV-positive cancer cells the Mdm2 pathway is completely inactive, while p53 degradation depends entirely on E6 (Hengstermann et al., 2001). This indicates that E6 can target p53 for degradation under conditions when this would be normally inhibited, e.g. after DNA damage, thereby allowing the accumulation of genomic mutations in the infected keratinocytes, and thus contributing towards malignant progression. E6 reactivates degradation of p53 by recruiting E6AP, a ubiquitin ligase. E6AP belongs to the HECT-domain family of ubiquitin ligases, whose large and divergent N-terminal domains mediate substrate recognition, while ubiquitination of bound substrates is catalysed by the conserved C-terminal HECT domain (Schwarz et al., 1998). High-risk HPV E6 binds to E6AP within its N-terminal substrate recognition domain (Huibregtse et al., 1993a), and formation of a stable E6–E6AP complex precedes association with p53, thereby redirecting the substrate specificity of E6AP towards p53 (Huibregtse et al., 1993b). Indeed, approaches aimed at blocking E6AP activity, either by the use of antisense oligonucleotides (Beer-Romero et al., 1997) or dominant negative mutants (Talis et al., 1998), increased the levels of p53 in HPV-positive, but not in HPV-negative cells, confirming that E6AP plays an essential role in E6 directed degradation of p53 in vivo, but has no effect on p53 levels in cells lacking E6.
The efficiency in mediating degradation of p53 varies among different E6 proteins, depending on their ability to interact with both p53 and E6AP. Both high- and low-risk mucosal HPV E6 proteins are able to bind the p53 C-terminus, however such interactions do not induce degradation. Binding to the core region of p53 is much greater for high-risk E6 proteins and is enhanced by the presence of E6AP, and it is this interaction that allows efficient degradation of p53 (Li and Coffino, 1996). In addition, HPV-16 E6 binds E6AP more strongly, and concomitantly degrades p53 more effectively, than HPV-18 E6. HPV-11 E6 has minimal levels of binding to E6AP (Huibregtse et al., 1993b), and degrades p53 in vivo only weakly (Storey et al., 1998). Interestingly, the E6 proteins of both high- and low-risk cutaneous HPV types do not associate with either E6AP or p53 and are incapable of affecting p53 stability (Elbel et al., 1997), therefore the mechanism by which these viruses evade the constraints that p53 places on viral replication remain to be determined.
Although targeting p53 for degradation is the major route by which E6 overcomes its effects, several reports indicate that E6 makes use of additional pathways to abrogate p53's growth suppressive activities. Both low- and high-risk HPV E6 proteins are capable of abolishing p53-mediated transcriptional repression in vivo (Lechner et al., 1992) and this is likely to occur through binding to the p53 C-terminus (Li and Coffino, 1996). Moreover, the capacity of the high-risk E6 proteins to abrogate transactivation of p53 target genes does not only depend on p53 destabilization, since E6 mutants defective for degradation retain the ability to abrogate transcriptional activation by p53 in vivo (Pim et al., 1994). Several mechanisms can be invoked to explain this property of E6. First, E6 can interfere with binding of p53 to its DNA recognition site (Lechner and Laimins, 1994; Thomas et al., 1995). In addition, repression of p53-responsive promoters could be mediated through the interaction of high-risk HPV-16 E6 with the transcriptional coactivators p300/CBP (Patel et al., 1999; Zimmermann et al., 1999), similarly to what has been reported for Ad E1A (Somasundaram and El-Deiry, 1997). Finally, cytoplasmic sequestration of p53 is another common strategy adopted by different viral proteins, such as Ad E1B 55K (König et al., 1999), HBV protein X (Elmore et al., 1997) and HPV E6. It has been reported that in HPV-positive cancer cells, the nuclear localization of p53 in response to DNA damage is blocked even if proteasome degradation is inhibited (Mantovani and Banks, 1999). Cytoplasmic retention may be due to masking p53's nuclear localization signal by E6 binding to the p53 C-terminus, or to enhanced nuclear export of p53. The latter mechanism is supported by the observation that inhibition of nuclear export in HPV-positive tumour cells by the drug leptomycin B results in accumulation of p53, indicating that E6-mediated degradation of p53 is, at least in part, dependent on nuclear export (Freedman and Levine, 1998). Although these data strongly suggest that it occurs in cytoplasmic proteasomes, colocalization of the two proteins in the cytoplasm is not alone sufficient to trigger degradation. In neuroblastoma cells p53 is constitutively cytoplasmic and appears to be resistant to proteolysis induced by either Mdm2 or E6 (Isaacs et al., 1999), implying the requirement for nucleocytoplasmic shuttling to allow degradation. Whether this process is mediated directly by E6, its cellular partner E6AP, or another protein, however remains to be determined, since E6, E6AP and p53 all contain putative nuclear export signals.
It is quite clear, however, that during viral infection and in HPV-induced cervical lesions not all p53 is degraded, as several studies have reported detectable levels of p53 in HPV-infected cells (Mantovani and Banks, 1999; Cooper et al., 1993; Lie et al., 1999). A possible viral pathway for regulating E6 activity with respect to p53 relies on a series of polypeptides termed E6*, which are expressed by the high-risk HPV types through alternative splicing of E6 mRNA (Schneider-Gädicke et al., 1988). Interestingly, HPV-18 E6*I was found to interact with both the full-length E6 and E6AP, thereby blocking degradation of p53 (Pim et al., 1997): this might allow a fine-tuning of the activity of E6 with respect to p53 during viral infection. Interestingly, while p53 has been shown to specifically inhibit HPV amplificational DNA replication in vivo, it did not affect episomal maintenance, which occurs in synchrony with the cell cycle (Lepik et al., 1998). In order to elicit a productive infection, viral DNA amplification needs to be controlled and it is plausible that the activity of E6* could ensure the presence of a limited amount of p53 at the replication sites, where it could both prevent overreplication of the viral genome and, possibly, assist DNA synthesis by means of its proofreading capacity. Indeed, HPV recruits DNA polymerase α for DNA replication, and the 3′–5′ exonuclease activity of p53 could enhance the replicative fidelity of this enzyme (for review, Albrechtsen et al., 1999). It is interesting to note that p53 has been found in the replication centres of Herpes Simplex Virus (Wilcock and Lane, 1991), Cytomegalovirus (Fortunato and Spector, 1998) and Adenovirus (König et al., 1999), and recent studies reported an interaction between the HPV ori-complex binding protein E2 and p53, further suggesting a potential positive role for p53 in viral replication (Massimi et al., 1999).
It is quite clear that the E6–p53 interaction represents one of the key events in E6 induced malignancy: continuous degradation of p53 can lead to the accumulation of genetic mutations in the infected cell. Indeed, loss of p53 leads to early tumour development (Donehower et al., 1992), and enhances the malignant progression of chemically induced skin cancers in mice (Kemp et al., 1993), which is consistent with the observation that E6 accelerates the malignant conversion of tumours promoted by E7 (Song et al., 2000). In addition, E6 has been shown to induce gross chromosomal alterations in cell culture, including translocations and aneuploidy (Reznikoff et al., 1994; White et al., 1994). These chromosomal changes are also common in malignant cells that have lost p53 function, indicating that E6 can induce genomic instability through inactivation of p53.
p53-independent activities of E6
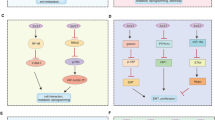
Although p53 is a vital aspect of E6 function, analysis of E6 mutants has shown that activities other than targeting p53 are required for its full transforming potential (Pim et al., 1994; Nakagawa et al., 1995; Liu et al., 1999). Moreover, the oncogenic potential of cutaneous HPVs mainly relies on their E6 proteins which, however, do not interact with p53 (Elbel et al., 1997). It is now clear that, in common with many viral oncoproteins, E6 is multifunctional and, in line with the above observations, numerous cellular targets have been identified, some of which are listed in Table 1 and Figure 1. There is now active debate as to which of these other activities of E6 are relevant for the development of malignancy.
The high-risk HPV E6 protein. Schematic diagram of E6 showing the two zinc fingers, together with the regions involved in interactions with cellular proteins that are targeted by the oncoproteins of other viruses. Also shown is the C-terminal PDZ binding motif, ETQV, and the overlapping site of PKA phosphorylation is arrowed
De-regulation of transcription and DNA replication by HPV E6
The E6 proteins of both high- and low-risk HPV types have long been known to modulate transcription from many cellular and viral promoters (Sedman et al., 1991; Desaintes et al., 1992; Etscheid et al., 1994; Veldman et al., 2001). It is only recently, however, that clues to the mechanisms by which this may occur have come, with the demonstration that E6 interacts with p300/CBP (Patel et al., 1999; Zimmermann et al., 1999). The p300/CBP transcriptional co-activators play important roles in activating a great number of genes involved in the regulation of cell cyle, differentiation and immune response. Indeed, many viral oncoproteins including SV40 LT, Py LT, EBNA2, Ad E1A, and HTLV-1 Tax have been shown to require the interaction with p300 for optimal transforming activity (Goodman and Smolik, 2000, for review), highlighting its central importance in regulating cellular homeostasis. HPV-16 E6 was shown to directly bind three regions of p300/CBP, namely C/H1, C/H3 and the carboxy terminus, while low-risk HPV-6 E6 was found to only weakly bind to the C/H1 region (Patel et al., 1999). Moreover, HPV-16 E6 was also shown to inhibit the intrinsic transcriptional activity of p300/CBP on both p53- and NF-κB-responsive promoter elements. Obviously, p300/CBP affects expression of many different cellular promoters, including those regulating differentiation (Bannister and Kouzarides, 1995; Goodman and Smolik, 2000), and at present it is not clear which are the true targets for E6 mediated inhibition via the p300/CBP interaction. However, possible E6 inhibition of NF-κB-responsive promoters is particularly intriguing, since dysregulation of the NF-κB pathway can result in hyperproliferation of the stratum spinosum, the epithelial layer in which HPV DNA amplification occurs (Hu et al., 1999). NF-κB is activated upon viral infection and promotes transcription of a number of genes involved in the local immune response such as class I MHC, interleukins and GM–CSF, some of which are synthesized directly by the keratinocytes (for review see Baldwin, 1996), therefore inhibition of NF-κB target genes may help the virus to escape immune recognition. Moreover, NF-κB activation by viral infection stimulates the IFN-β promoter (Thanos and Maniatis, 1995), and thus E6 inhibition of NF-κB could help overcome the interferon-mediated antiviral response. Interestingly, HPV-16 E6 has also been found to bind the interferon regulatory factor 3 (IRF-3) (Ronco et al., 1998), which is an important transactivator of interferons and binds to the regulatory elements of the IFN-β promoter (Wathelet et al., 1998). As a consequence of this interaction, E6 was also found to inhibit IFN-β induction (Ronco et al., 1998). Interestingly, a recent screen of cDNA microarrays demonstrated that transfection of HPV-16 E6 in differentiating cervical keratinocytes also downregulates the expression of interferon-responsive genes (Nees et al., 2001). Since viral infection stimulates the assembly and activation of a large transcription factor complex that includes IRF-3 and CBP/p300, which in turn activates α/β interferon-responsive genes (Weaver et al., 1998), it is likely that the combined targeting of all these components by E6 will contribute to the disruption of the cellular antiviral response.
Finally, it should also be borne in mind that the E6/p300 interaction, and its possible contribution towards survival of the transformed cell, could be a by-product of the regulation of viral gene expression. Thus, the HPV major transcriptional activator, E2, also interacts with p300/CBP (Lee et al., 2000a; Marcello et al., 2000). This appears to involve a cellular protein, AMF-1/Gps2, which in turn enhances p300 activity but which, intriguingly, is also a target for E6 mediated degradation (Peng et al., 2000; Degenhardt and Silverstein, 2001). Therefore the interaction between E6 and p300 may represent a means of downregulating E2 transcriptional activity, and thereby controlling the levels of E6 expression by a feed-back mechanism.
An additional link with tumour progression and HPV E6 activity has come from studies showing deregulation of the cellular DNA replication machinery. Normal somatic cells terminate their replicative life span through a pathway leading to cellular senescence, which is triggered in response to critically shortened telomere DNA. Telomerase activity is absent from most normal somatic cells and neoplastic cells must first overcome the senescence checkpoint mechanisms, and subsequently activate telomerase to propagate indefinitely. In senescent cells the levels of p16ink are elevated, and its inactivation has been shown to abrogate a late step during senescence of human epithelial cells (Kiyono et al., 1998). Interestingly, the immortalization of human uroepithelial cells by HPV-16 E6 correlated with undetectable levels of p16ink due to gene inactivation. In addition, activation of telomerase is detected in cervical carcinomas and in a subset of high grade cervical lesions associated with high-risk HPV (Snijders et al., 1998). Telomerase can be induced by HPV-16 E6 in primary epithelial cells through a p53-independent mechanism (Klingelhutz et al., 1996; Veldman et al., 2001) via E6-mediated transcriptional activation of the gene encoding the telomerase catalytic subunit, hTERT, and the minimal promoter region involved in induction by E6 was found to require an intact E box (Veldman et al., 2001; Gewin and Galloway, 2001). Interestingly, this activation of the hTERT promoter was found to be dependent on the ability of E6 to interact with E6AP, suggesting that the activity of the E6-E6AP complex may target a regulator of the hTERT promoter (Gewin and Galloway, 2001).
E6 proteins of both benign and oncogenic HPV types have also been found to interact with hMcm7, a component of the DNA replication licensing complex, suggesting that this interaction might be required for viral genome replication (Kukimoto et al., 1998; Kühne and Banks, 1998). However, hMcm7 is a substrate for E6AP-dependent ubiquitination, and HPV-18 E6 was able to enhance its proteasome degradation in vivo (Kühne and Banks, 1998): this might also be expected to cause p53-independent chromosomal abnormalities in HPV-positive cells.
Mitogenic activities of E6
It has been shown that expression of oncogenic E6 can affect the early stages of carcinogenesis in transgenic mice, although to a much lesser extent than E7 (Song et al., 2000), and this probably relies on the ability of E6 to induce cellular hyperproliferation and epidermal hyperplasia (Song et al., 1999). Interestingly, this appears to be largely independent of p53, since similar levels of proliferation are induced by E6 in p53-null mice (Song et al., 1999). At present, little is known about the pathways by which E6 could stimulate proliferation but a recent study identified E6TP1 in a two-hybrid screen. This protein shows high homology with a family of GTPase activating proteins (GAPs) that are negative regulators of Rap (Gao et al., 1999). Therefore E6TP1 might be involved in the inhibition of Rap-mediated mitogenic signalling. Moreover, its gene has been mapped to a putative tumour suppressor locus on chromosome 14 (Menon et al., 1997). High-risk and, to a lesser extent, low-risk HPV E6 proteins can bind E6TP1, while only the oncogenic E6s can promote its degradation. Interestingly, E6AP was reported to participate in the interaction, and it might therefore be responsible for ubiquitination of E6TP1 (Gao et al., 1999). There is a strong correlation between the ability of HPV-16 E6 mutants to degrade E6TP1 and to immortalize human mammary epithelial cells (Gao et al., 2001), however more data concerning the GAP function of E6TP1 are necessary to understand its role in E6-induced malignancy.
Inhibition of apoptosis
Oncogenic HPV E6 proteins counteract the induction of apoptosis by activated p53 by inducing its degradation. However, there is increasing evidence that E6 can also inhibit p53-independent apoptotic pathways, promoted by different stimuli. Indeed, in transgenic mice expressing HPV-16 E6 in the ocular lens, E6 was found to prevent apoptosis both in wt and in p53-null animals (Pan and Griep, 1995), and it has also been reported to inhibit drug-induced apoptosis in cells lacking p53 (Steller et al., 1996). Among the more recently discovered cellular targets of E6, there are several proapoptotic factors. One of them is Bak, whose high levels of expression in the upper epithelial layers (Krajewski et al., 1996) appear to represent a common obstacle for a broad range of HPV types that replicate in differentiating keratinocytes, since the E6 proteins of both high and low-risk mucosal HPVs, plus those of high-risk cutaneous types, have been shown to inhibit Bak-induced apoptosis (Thomas and Banks, 1998, 1999; Jackson and Storey, 2000). HPV-18 E6 stimulates the ubiquitin-dependent degradation of Bak catalysed by E6AP, probably by accelerating a normal cellular process, since Bak appears to be a substrate of E6AP in the absence of E6 (Thomas and Banks, 1998). Degradation of Bak by HPV-11 E6 is less effective, and this correlates with a weaker anti-apoptotic activity of the low-risk mucosal HPV types (Thomas and Banks, 1999). Recently, a link was demonstrated between cutaneous HPV infection and the induction of HPV-associated skin cancer by UV radiation. The cutaneous E6 proteins were shown to abrogate both p53-dependent and independent apoptosis in response to UV-induced DNA damage. p53 protein levels were unaffected in cells expressing cutaneous E6s, and its transcriptional activity with respect to p21 also did not change (Jackson and Storey, 2000). The mechanism used by these E6 types to inhibit p53-dependent apoptosis is not known, although one possibility is that they mediate the specific inhibition of p53 target genes directly involved in apoptosis, such as the PIG genes induced by DNA damage (Polyak et al., 1997). The cutaneous HPV E6 proteins also abrogate Bak function by promoting its ubiquitin-mediated degradation and Bak protein is undetectable in HPV-positive skin cancers, in contrast to HPV negative cancers which express it (Jackson et al., 2000). Interestingly, Ad E1B 19K also inhibits Bak induced apoptosis (Farrow et al., 1995; White, this issue), demonstrating a high degree of conservation of function among these viruses.
The c-Myc transcription factor is normally degraded through the proteasome pathway, and it was shown that high-risk, but not low-risk HPV E6, accelerates degradation of c-Myc by recruiting E6AP (Gross-Mesilaty et al., 1998). It may seem unusual that E6 targets a protein which would normally stimulate cell proliferation, but this finding can be also viewed in the light of c-Myc proapoptotic activity. TGFβ is a potent growth inhibitor for many cell types, including most epithelial cells (Lyons and Moses, 1990), and its effects are mediated at least in part by suppression of c-myc transcription (Pietenpol et al., 1990a). It has been demonstrated that HPV-16 E7 and other viral proteins binding pRb, such as SV40 LT and Adenovirus E1A, are able to block TGFβ-mediated downregulation of c-Myc, in order to sustain proliferation of the infected keratinocytes (Pietenpol et al., 1990b; Münger et al., this issue). However, deregulated expression of c-Myc in differentiating cells induces apoptotic cell death (Askew et al., 1991). It is therefore possible that degradation of c-Myc by E6 contributes towards maintaining an equilibrium between E7-promoted proliferation and cell survival.
Interference with epithelial organization and differentiation
High-risk HPV types have evolved to replicate in the differentiated layers of the squamous epithelium, in an environment that is non-permissive for DNA replication. A characteristic of the high-risk E6 proteins is their ability to inhibit terminal differentiation of epithelial cells, which normally leads to keratinization and cell death. HPV-16 E6 impairs cell differentiation in the ocular lens of transgenic mice (Pan and Griep, 1994) and causes expansion of the undifferentiated compartment of the epithelia in K14-E6 transgenic mice and, interestingly, this activity of E6 appears to be p53-independent (Song et al., 1999). Consistent with this, HPV-16 E6 was also found to increase the resistance of human keratinocytes to serum and calcium induced differentiation through p53-independent pathways (Sherman et al., 1997; Sherman and Schlegel, 1996), although little is known about the molecular mechanisms by which E6 does this. However, HPV-16 E6 was reported to interact with E6BP/ERC-55 (Chen et al., 1995) which is a putative calcium binding protein localized in the endoplasmic reticulum (Weis et al., 1994). E6BP was found to form a complex with both E6 and E6AP in vivo, however direct binding to E6AP and E6 targeted degradation were not shown (Chen et al., 1995). Epithelial differentiation is responsive to Ca2+ mediated signalling, and it might be speculated that targeting E6BP contributes to E6's impairment of terminal differentiation. Ca2+ signalling also has a role in blocking apoptosis, and it is interesting that the antiapoptotic Ad E1B 19K protein also interacts with a putative calcium binding protein localized in the nuclear envelope and ER (Boyd et al., 1994). Hence, the E6–E6BP interaction might similarly be involved in the p53-independent inhibition of apoptosis in HPV infected cells.
Cellular adhesion to the extracellular matrix affects many different cellular processes including cell morphology, proliferation and migration. The restriction of cell proliferation to matrix–interacting cells serves to prevent dysplasia, and the circumvention of anchorage dependence plays an important role in tumorigenesis (Sastry and Horwitz, 1996). Cell–matrix adhesion is mediated by specialized structures called focal adhesions, which contain integrins, vinculin, focal adhesion kinase and paxillin (Sastry and Burridge, 2000). Paxillin is involved in mediating signalling from the plasma membrane to focal adhesions and to the actin cytoskeleton, and its activity is regulated by tyrosine phosphorylation in response to various stimuli, including integrin crosslinking and treatment with growth factors (Turner, 2000). HPV-16 E6 has also been shown to bind paxillin and this interaction correlates with E6 transforming activity, although it does not lead to paxillin degradation (Tong and Howley, 1997). However, since most of the biological effects of this interaction have been determined for the more highly abundant BPV-1 E6 protein, its relevance for high-risk HPV E6 proteins remains to be determined.
Interactions with PDZ proteins: interference with cell–cell adhesion, polarity and proliferation control
A striking feature of all E6 proteins derived from the high-risk HPV types is the presence of a highly conserved C-terminal domain (see Figure 1), which is not involved in p53 binding and degradation (Crook et al., 1991; Pim et al., 1994), but which nonetheless contributes to E6 transforming activity, since its deletion impairs E6's ability to transform rodent cells (Kiyono et al., 1997) and immortalize keratinocytes (C Meyers, personal communication). This region contains a PDZ-binding motif (XT/SXV), a short stretch of amino acids which mediates the specific interaction with proteins containing PDZ domains (Doyle et al., 1996; Songyang et al., 1997). These are 80–90 amino-acid long motifs, present on a variety of proteins involved in clustering ion channels, signalling enzymes, and adhesion molecules to specific structures at the membrane-cytoskeleton interface of polarized cells (reviewed in Kim, 1997). The first PDZ-protein shown to be a target for high-risk E6 was hDlg (Kiyono et al., 1997; Lee et al., 1997). This is the human homologue of the Drosophila tumour suppressor Dlg, required for formation of adherens junctions and for regulation of cell adhesion, apicobasal polarity and proliferation in epithelial tissues. Indeed, loss of Dlg function causes aberrant morphology and invasive growth of epithelial cells, resulting in embryonic lethality (Woods et al., 1996; Bilder et al., 2000). A recent study also described a Dlg truncation mutant that resulted in impaired morphogenesis and perinatal death during murine development (Caruana and Bernstein, 2001). Human hDlg colocalizes with E-cadherin at adherens junctions of epithelial cells, (Reuver and Garner, 1998; Ide et al., 1999) and interacts through different domains (see Figure 2) with several proteins, including Shaker-type K+ channels (Kim et al., 1995), cytoskeletal protein 4.1 (Lue et al., 1994; Marfatia et al., 1996), and the APC tumour suppressor protein (Matsumine et al., 1996), which is mutated in the majority of colon cancers (Kinzler and Vogelstein, 1996). Indeed, complex formation between hDlg and APC was reported to block cell cycle progression (Ishidate et al., 2000). HPV E6 can target hDlg for ubiquitin mediated degradation (Gardiol et al., 1999), probably by enhancing a physiological process, since hDlg appears to be ubiquitinated and degraded by the proteasome in cells even in the absence of E6 (Gardiol et al., 1999; Mantovani et al., 2001, in press). This interaction between E6 and hDlg might be necessary at a defined point during the viral life cycle, in order to disrupt cell junctions and to abolish cell polarity, thereby altering the normal maturation and inducing proliferation of the infected keratinocytes. However, the consequences for the metastatic potential of tumours harbouring high-risk HPVs are clear. While HPV-18 E6 has a canonical PDZ-binding motif (ETQV), HPV-16 E6 has only a sub-optimal consensus site (ETQL). Consequently, HPV-18 E6 binds hDlg with higher affinity than HPV-16 E6 (Pim et al., 2000) and can degrade it more efficiently (Gardiol et al., 1999; Pim et al., 2000). Interestingly, this also correlates with the different malignant potential of the viruses, since it has been reported that cervical tumours associated with HPV-18 are more invasive and recurrent than those caused by HPV-16 (Burnett et al., 1992; Kurman et al., 1988; Zhang et al., 1995); this is in marked contrast with the reported lower efficiency with which HPV-18 E6 degrades p53 (Scheffner et al., 1990). Interestingly, low-risk E6 proteins lack PDZ-binding motifs, and indeed they can neither bind hDlg (Kiyono et al., 1997; Lee et al., 1997) nor induce its degradation (Gardiol et al., 1999; Pim et al., 2000), however they acquire this ability if provided with a C-terminal PDZ binding domain derived from a high-risk E6 protein (Pim et al., 2000). In contrast, cutaneous HPV E6 proteins are unable to degrade hDlg even when the interaction is allowed by adding a PDZ-binding motif to their C-termini (Pim et al., submitted). This suggests that the mucosal HPV E6 proteins, whether derived from high or low-risk HPV types, can interact similarly with the cellular proteolytic machinery, whereas the high-risk cutaneous HPV E6 proteins appear to significantly differ in this aspect.
The hDlg protein. Schematic diagram showing the location of hDlg at the membrane-cytoskeleton interface of epithelial cells at regions of cell–cell contact. hDlg contains 3 PDZ domains, an SH3 signalling domain and a Guanylate Kinase (GUK) homology domain. Here it co-localizes with E-cadherin and binds the APC tumour suppressor, which in turn regulates β-catenin. The interaction between hDlg and APC is via PDZ domain 2 and this is the same domain targeted by the high risk HPV E6 proteins. Also shown is the interaction with protein 4.1, which connects hDlg to the actin cytoskeleton
An increasing number of PDZ domain-containing proteins involved in the organization of epithelial architecture have now been reported to be targeted by E6 through their PDZ domains. Among them is hScrib, a protein expressed at epithelial tight junctions, which has recently been shown to be a substrate for ubiquitination by the E6-E6AP complex in vitro, and for proteasome degradation mediated by high-risk E6 in vivo (Nakagawa and Huibregtse, 2000). hScrib is the human homologue of Drosophila tumour suppressor Scrib, which cooperates with Dlg and Lgl to control both formation of cell junctions and inhibition of epithelial cell growth, possibly through controlling the localization of growth factor receptors and signalling molecules (Bilder and Perrimon, 2000; Bilder et al., 2000). Interestingly, expression of oncogenic HPV E6 in mammalian cells was reported to disrupt the integrity of epithelial tight junctions, while E6 mutated in the PDZ-binding motif did not (Nakagawa and Huibregtse, 2000). This further suggests that combined degradation of different PDZ-proteins by E6 might be responsible for the loss of epithelial cell adhesion and polarity of HPV-positive cancer cells. E6 proteins from high-risk, but not low-risk HPV types were also shown to bind another tight junction protein, MAGI-1 (Dobrosotskaya et al., 1997; Ide et al., 1999), through the first of its five PDZ domains (Thomas et al., 2001), and to reduce its steady-state levels and half-life (Glaunsinger et al., 2000). MAGI-1 forms a complex with β-catenin (Dobrosotskaya and James, 2000), the expression of which is deregulated in many human cancers (reviewed in Polakis, 1999). Interestingly, the closely related proteins MAGI-2 and MAGI-3 are involved in the regulation of the PTEN tumour suppressor, a component of the Akt kinase signalling pathway that promotes cell survival and proliferation (Marte and Downward, 1997): MAGI proteins are required to enhance PTEN's ability to suppress Akt activation, probably through assembling a multiprotein complex at the cell membrane (Wu et al., 2000a,b). Therefore it is plausible that MAGI-2/3 degradation, promoted by high-risk HPV E6 (M Thomas, personal communication), might also affect Akt signalling and thereby inhibit apoptosis independently of targeting p53, as well as representing an alternative mitogenic activity of E6 (see above).
HPV-18 E6 has also been shown to bind MUPP1, a large multi-PDZ scaffold protein with a putative role in signal transduction (Ullmer et al., 1998). Indeed, MUPP1 interacts selectively through its 10th PDZ domain with the cytoplasmic portion of the 5-HT2c serotonin receptor (Ullmer et al., 1998; Becamel et al., 2001), and with the unphosphorylated c-Kit tyrosine kinase receptor (Mancini et al., 2000). HPV-18 E6 was shown to reduce MUPP1 half-life in vitro and in vivo (Lee et al., 2000b), and there is evidence that this also occurs through proteasome mediated degradation (F Mantovani, personal observations). Destruction of MUPP1 by E6 could thereby interfere with the assembly of signalling complexes at the epithelial cell membranes.
It has been frequently reported that different oncogenic viruses inactivate the same cellular targets to overcome common obstacles to their replication: thus, there are viral oncoproteins other than HPV E6 which bind PDZ-proteins, and this contributes to their transforming potential. hDlg has been shown to interact, through its PDZ domains, with both Ad 9 E4-ORF1 (Lee et al., 1997; Täuber and Dobner, this issue) and HTLV-1 Tax, and these interactions interfere with the binding of APC, thereby perturbing cell growth control (Lee et al., 1997; Suzuki et al., 1999). MUPP1 and MAGI-1 are also bound by the Ad 9 E4-ORF1, which abolishes their activities by sequestrating them into cytoplasmic bodies (Lee et al., 2000b; Glaunsinger et al., 2000). However, ubiquitin mediated degradation of PDZ-proteins is, to date, an exclusive property of the high-risk HPV E6 proteins. It has recently been shown that E6 can bridge the interaction between hScrib and the E6AP ubiquitin ligase, which normally would not recognize hScrib (Nakagawa and Huibregtse, 2000). This is reminiscent of p53 degradation, yet a clear role for E6AP in hScrib degradation in vivo has not been confirmed. In contrast, proteasome degradation of hDlg appears to be independent of E6AP (Pim et al., 2000), as deduced from the basis of E6's ability to degrade hDlg in extracts lacking E6AP, and from the fact that E6 mutants defective for E6AP binding and p53 degradation can still degrade hDlg. Similarly, low-risk E6 proteins can also target hDlg for degradation, with an efficiency similar to that of the high-risk E6 proteins, when they are provided with a PDZ-binding motif (Pim et al., 2000). However, these low risk E6 proteins show only minimal levels of interaction with E6AP (Huibregtse et al., 1991, 1993b).
E6 binding motifs: a matter of specificity
It is clear from the above discussion that E6 is a multifunctional protein which efficiently interferes with diverse cellular pathways. However, it is logical to ask how such a small and low abundance viral protein could evolve to interact with so many different cellular proteins, both with respect to the specificity of the interactions and to the relatively high abundance of the partners. The analysis of E6 targets has identified conserved E6 binding motifs, thereby characterizing clusters of proteins which are bound by E6 through similar domains. The PDZ domain, for instance, defines a family of proteins with common functions, which are targeted by high-risk E6 proteins through their C-terminal PDZ-binding motifs. E6 interaction with proteins containing multiple PDZ domains is, however, highly specific: thus, despite their high overall homology, only single PDZ domains on hDlg and MAGI-1 (Kiyono et al., 1997; Thomas et al., 2001) are recognized by the E6 protein. Another conserved binding site, the L2G box which comprises a α-helical motif, has been found on a number of cellular targets of E6, including E6AP, hMcm7, E6BP and paxillin. The binding specificity of E6AP is mediated by this motif, present on both enzyme and substrate, and this in turn is used by E6 to interact with both (Chen et al., 1998; Elston et al., 1998; Kühne and Banks, 1998). Not surprisingly, E6 has also been reported to induce self-ubiquitination of E6AP (Kao et al., 2000), nonetheless, there are proteins which interact with E6 through an L2G box without being targeted for degradation, such as E6BP (Chen et al., 1995) and paxillin (Tong and Howley, 1997).
The amount of the E6 protein produced during a viral infection is very low, and the problem of targeting cellular proteins which are much more abundant within the cell is efficiently solved by inducing their ubiquitin-mediated degradation. Moreover, it seems plausible that the virus does not require the complete destruction of its targets, rather, even a transient decrease of their local concentration within the cell is likely to perturb the physiological conditions in favour of viral replication. This also implies that E6 does not need to interact with all of its putative targets at the same time, since probably only a limited subset will be available at defined stages during cell differentiation and in specific compartments within the cell. It is therefore possible that the intracellular localization of E6 might change, either as a consequence of differentiation or as a result of exogenous stimuli. Indeed, the PDZ-binding specificity of high-risk E6 has also been shown to be regulated by a cellular pathway: the E6/hDlg interaction is inhibited by PKA phosphorylation of the conserved threonine residue within the PDZ binding domain on E6, and this was also shown to inhibit E6-mediated degradation of hDlg. Indeed, high levels of hDlg could be restored in HPV-18 positive cervical cancer cells by induction of PKA activity (Kühne et al., 2000). Interestingly, PKA regulation of PDZ domain binding was also reported to control the interaction between the K+ channel Kir2.3 and the PDZ protein PSD-95 (Cohen et al., 1996), and this would suggest that the high-risk E6 proteins might have acquired the PDZ binding domain, with its PKA-associated regulation, from the cellular genome. Its strict conservation among all high-risk E6 proteins is intriguing, and implies a requirement for the virus to finely balance its effects upon hDlg and other PDZ domain-containing proteins. Recent studies have also shown that E6 is phosphorylated by PKN (Gao et al., 2000) and, although the site of phosphorylation has not been mapped, it will nonetheless be extremely interesting to evaluate the effects of this phosphorylation upon E6's other activities.
E6 as a therapeutic target
Since E6 is invariably expressed in cervical lesions and would appear to be responsible for their malignant progression, this protein represents an attractive candidate for developing therapeutic strategies against cervical cancer. Many approaches have been tested to block the expression of E6 in HPV-positive cervical cancer cells, e.g. by selectively inhibiting viral transcription (Goodwin and DiMaio, 2000), or by using antisense constructs (Hamada et al., 1996; von Knebel Doeberitz et al., 1992) or ribozymes (Alvarez-Salas et al., 1998) directed against the polycistronic E6/E7 mRNA. All these approaches cause growth suppression and concomitant reduction of tumorigenicity in vivo, implying the feasibility of reactivating functional tumour suppressor pathways in HPV-positive cells. It can be reasoned that blocking the activity of E6, while retaining that of E7 as a proapoptotic stimulus, might provide a higher therapeutic potential. Since this would be difficult to obtain by targeting the viral mRNAs, since the E6 and E7 genes are expressed together as polycistronic transcripts, this could be done by using peptide aptamers to block the E6 protein in vivo. Indeed, administration of such peptides was found to induce apoptosis of HPV-positive cancer cells (Butz et al., 2000). Thus blocking the E6-mediated degradation of p53 is a major therapeutic goal, since there is strong evidence that the p53-responsive pathways are fully functional in cervical tumour cell lines (Butz et al., 1995, 1999), and reactivating p53 would then bring about growth arrest and/or apoptosis of the HPV transformed cells. However this is unlikely to be universally applicable, since inhibition of E6 induced degradation does not always lead to increased p53 levels. In several cervical cancer cell lines p53 can be stabilized only after additional genotoxic insult, indicating a lack of intrinsic activation of p53 despite the presence of the viral oncogenes (Mantovani and Banks, 1999). A note of caution should also be added, since targeting the p53/E6AP/E6 complex could interfere with normal E6AP functions, and mutations of E6AP have been implicated in a serious developmental disorder, the Angelman Syndrome (Kishino et al., 1997; Matsuura et al., 1997).
The interactions of E6 with PDZ-proteins are also excellent therapeutic targets, because of their correlation with the malignant progression of HPV-associated disease. Moreover, the binding motif on E6 is small and exposed, and the structures of a number of PDZ domains have been solved (Doyle et al., 1996; Tochio et al., 2000). In addition, the interaction between E6 and its PDZ domain-containing substrates is highly specific, with E6 binding only single PDZ domains on both hDlg and MAGI-1. Therefore it might be feasible to design chemotherapeutic agents, capable of specifically inhibiting the interaction between E6 and this class of targets.
Concluding remarks
During HPV infection E6 plays multiple roles, interfering with several cellular pathways in order to create a favourable environment for viral replication, and neutralizing the cellular surveillance controls that are turned on as the infected cell is unnaturally forced to restart DNA replication. Thus, high-risk E6 blocks apoptosis by targeting p53, Bak and Myc proteins for degradation, however this leaves the cell unprotected from the detrimental effects of DNA mutations. This is confirmed by biochemical and epidemiological studies reporting a synergy between HPV oncogenes and chemical carcinogens in the development of malignancy (Song et al., 2000; Daling et al., 1992). Moreover, E6 inhibits terminal differentiation and senescence, thereby stimulating cell immortalization. Finally, by targeting a class of PDZ domain containing proteins, E6 can also affect cell contact and polarity and, when the pathways regulating these interactions are impaired, either due to mutation or exogenous stimuli, this final loss of control in the immortalized cell can finally lead to an invasive and metastatic phenotype (see Figure 3).
Contribution of HPV E6 to different stages of tumour progression. The role of E6 in tumour promotion is weak compared with that of E7 which actively stimulates cell proliferation. In contrast E6 promotes malignant progression: degradation of p53 overcomes growth arrest and/or apoptosis allowing accumulation of DNA damage, and induction of telomerase contributes towards immortalization. Finally, targeting PDZ-containing proteins through the C-terminal XT/SXV sequence of E6 causes loss of cell polarity and contact, strongly contributing to the malignant phenotype
It should be clear, however, that the above picture is far from the ‘physiological’ outcome of an HPV infection, representing instead the most unfortunate result of a progressive and multifactorial process that only occurs on some occasions. Nonetheless, cervical cancer is an extremely serious disease, representing the third major cause of cancer-related death in women worldwide (Parkin et al., 1999), and the study of the E6 cellular targets has important implications for the development of effective therapeutic strategies.
References
Albrechtsen N, Dornreiter I, Grosse F, Kim E, Wiesmuller, Deppert W . 1999 Oncogene 18: 7706–7717
Alvarez-Salas L, Cullinan A, Siwkowski A, Hampel A, DiPaolo J . 1998 Proc. Natl. Acad. Sci. USA 95: 1189–1194
Androphy EJ, Hubbert NL, Schiller JT, Lowy DR . 1987 EMBO J. 6: 992
Arbeit J, Münger K, Howley P, Hanahan D . 1993 Am. J. Pathol. 142: 1187–1197
Arbeit J, Münger K, Howley P, Hanahan D . 1994 J. Virol. 68: 4358–4368
Ashcroft M, Vousden K . 1999 Oncogene 18: 7637–7643
Askew D, Ashmun R, Simmons B, Cleveland J . 1991 Oncogene 6: 1915–1922
Baldwin A . 1996 Annu. Rev. Immunol. 14: 649–683
Band V, DeCaprio J, Delmolino L, Kulesa V, Sager R . 1991 J. Virol. 65: 6671–6676
Band V, Dalal S, Delmolino L, Androphy E . 1993 EMBO J. 12: 1847–1852
Banks L, Spence P, Androphy E, Hubbert N, Matlashewski G, Murray A, Crawford L . 1987 J. Gen. Virol. 68: 1351–1359
Bannister A, Kouzarides T . 1995 EMBO J. 14: 4758–4762
Barbosa M, Lowy D, Schiller J . 1989 J. Virol. 63: 1404–1407
Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C . 2001 J. Biol. Chem. 276: 12974–12982
Beer-Romero P, Glass S, Rolfe M . 1997 Oncogene 14: 595–602
Benton C, Arends MJ . 1996 Papillomavirus Reviews: Current Research on Papillomaviruses. C. Lacey (ed) Leeds Medical Information pp. 271–279
Bilder D, Perrimon N . 2000 Nature 403: 676–680
Bilder D, Li M, Perrimon N . 2000 Science 289: 113–116
Boyd J, Malstrom S, Subramaniam T, Venkatesh L, Schaeper U, Elangovan B, D'Sa-Eipper C, Chinnadurai G . 1994 Cell 79: 341–351
Burnett A, Barnes W, Johnson J, Grendys E, Willett G, Barter J, Doniger J . 1992 Gynecol. Oncol. 47: 343–347
Butz K, Denk C, Ullmann A, Scheffner M, Hoppe-Seyler F . 2000 Proc. Natl. Acad. Sci. USA 97: 6693–6697
Butz K, Shahabedin L, Geisen C, Spitkovsky D, Ullmann A, Hoppe-Seyler F . 1995 Oncogene 10: 927–936
Butz K, Whitaker N, Denk C, Ullmann A, Geisen C, Hoppe-Seyler F . 1999 Oncogene 18: 2381–2386
Caruana G, Bernstein A . 2001 Mol. Cell Biol. 21: 1475–1483
Chen J, Hong Y, Rustamzadeh E, Baleja J, Androphy E . 1998 J. Biol. Chem. 73: 13537–13544
Chen J, Reid C, Band V, Androphy E . 1995 Science 269: 529–531
Cohen N, Brenman J, Snyder S, Bredt D . 1996 Neuron 17: 759–767
Comerford SA, Maika SD, Laimins LA, Messing A, Elsasser HP, Hammer RE . 1995 Oncogene 10: 587–597
Cole ST, Danos O . 1987 J. Mol. Biol. 193: 599–608
Cooper K, Herrington CS, Evans MF, Gatter KC, McGee JO . 1993 J. Pathol. 171: 27–34
Crook T, Tidy J, Vousden K . 1991 Cell 67: 547–556
Daling J, Sherman K, Hislop T, Maden G, Mandelson M, Beckmann A, Weiss N . 1992 Am. J. Epidemiol. 135: 180–189
Degenhardt Y, Silverstein S . 2001 J. Virol. 75: 151–160
Desaintes C, Hallez P, Van Alphen P, Burney A . 1992 J. Virol. 66: 325–333
DiPaolo J, Woodworth C, Popescu MC, Notario V, Doniger J . 1989 Oncogene 4: 395–399
Dobrosotskaya I, James G . 2000 Biochem. Biophys. Res. Commun. 270: 903–909
Dobrosotskaya I, Guy R, James G . 1997 J. Biol. Chem. 272: 31589–31597
Donehower L, Harvey M, Slagle B, McArthur M, Montgomery C, Butel J, Bradley A . 1992 Nature 356: 215–221
Doorbar J, Foo C, Coleman N, Medcalf L, Hartley O, Prospero T, Napthine S, Sterling J, Winter G, Griffin H . 1997 Virology 238: 40–52
Doyle D, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R . 1996 Cell 85: 1067–1076
Dürst M, Gallahan D, Jay G, Rhim J . 1989 Virology 173: 767–771
Elbel M, Carl S, Spaderna S, Iftner T . 1997 Virology 239: 132–149
El-Deiry W, Tokino T, Velculescu V, Levy D, Parsons R, Trent J, Lin D, Mercer W, Kinzler K, Vogelstein B . 1993 Cell 75: 817–825
Elmore LW, Hancock AR, Chang SF, Wang XW, Chang S, Callahan CP, Geller DA, Will H, Harris CC . 1997 Proc. Natl. Acad. Sci. USA 94: 14707–14712
Elston R, Napthine S, Doorbar J . 1998 J. Gen. Virol. 79: 371–374
Etscheid BG, Foster SA, Galloway DA . 1994 Virology 205: 583–585
Farrow S, White J, Martinou I, Raven T, Pun K, Grinham C, Martinou J, Brown C . 1995 Nature 374: 731–733
Fortunato EA, Spector DH . 1998 J. Virol. 72: 2033–2039
Foster S, Demers W, Etscheid B, Galloway D . 1994 J. Virol. 68: 5698–5705
Freedman D, Levine AJ . 1998 Mol. Cell Biol. 18: 7288–7293
Gao Q, Kumar A, Srinivasan S, Singh L, Mukai H, Ono Y, Wazer D, Band V . 2000 J. Biol. Chem. 275: 14824–14830
Gao Q, Singh L, Kumar A, Srinivasan S, Wazer D, Band V . 2001 J. Virol. 75: 4459–4466
Gao Q, Srinivasan S, Boyer S, Wazer D, Band V . 1999 Mol. Cell Biol. 19: 733–744
Gardiol D, Kühne C, Glausinger B, Lee S, Javier R, Banks L . 1999 Oncogene 18: 5487–5496
Gewin L, Galloway DA . 2001 J. Virol. 75: 7198–7201
Glaunsinger B, Lee S, Thomas M, Banks L, Javier R . 2000 Oncogene 19: 5270–5280
Goodman R, Smolik S . 2000 Genes Dev 14: 1553–1577
Goodwin E, DiMaio D . 2000 Proc. Natl. Acad. Sci. USA 97: 12513–12518
Griep AE, Herber R, Jeon S, Lohse JK, Dubielzig RR, Lambert PF . 1993 J. Virol. 67: 1373–1384
Gross-Mesilaty S, Reinstein E, Bercovich B, Tobias K, Schwartz A, Kahana C, Ciechanover A . 1998 Proc. Natl. Acad. Sci. USA 95: 8058–8063
Hamada K, Sakaue M, Alemany R, Zhang W, Horio Y, Roth J, Mitchell M . 1996 Gynecol. Oncol. 63: 219–227
Harper J, Adami G, Wei N, Keyomarsi K, Elledge S . 1993 Cell 75: 805–816
Hawley-Nelson P, Vousden K, Hubbert N, Lowy D, Schiller J . 1989 EMBO J. 8: 3905–3910
Hengstermann A, Linares L, Ciechanover A, Whitaker N, Scheffner M . 2001 Proc. Natl. Acad. Sci. USA 98: 1218–1223
Herber R, Liem H, Pitot H, Lambert P . 1996 J. Virol. 70: 1873–1881
Honda R, Tanaka H, Yasuda H . 1997 FEBS Lett. 420: 25–27
Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M . 1999 Science 284: 316–320
Huibregtse J, Scheffner M, Howley P . 1991 EMBO J. 10: 4129–4135
Huibregtse J, Scheffner M, Howley P . 1993a Mol. Cell. Biol. 13: 775–784
Huibregtse J, Scheffner M, Howley P . 1993b Mol. Cell. Biol. 13: 4918–4927
Hurlin P, Kaur P, Smith P, Perez-Reyes N, Blanton R, McDougall J . 1991 Proc. Natl. Acad. Sci. USA 88: 570–574
Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, Mizoguchi A, Ishimori H, Tokino T, Nakamura Y, Takai Y . 1999 Oncogene 18: 7810–7815
Isaacs JS, Barrett JC, Weissman BE . 1999 Mol. Carcinog. 24: 70–77
Ishidate T, Matsumine A, Toyoshima K, Akiyama T . 2000 Oncogene 19: 365–372
Jackson S, Storey A . 2000 Oncogene 19: 592–598
Jackson S, Harwood C, Thomas M, Banks L, Storey A . 2000 Genes Dev. 14: 3065–3073
Kanda T, Watanabe S, Zanma S, Sato H, Furuno A, Yoshiike K . 1991 Virology 185: 536–543
Kao W, Beaudenon S, Talis A, Huibregtse J, Howley P . 2000 J. Virol. 74: 6408–6417
Kemp C, Donehower L, Bradley A, Balmain A . 1993 Cell 74: 813–822
Kessis T, Slebos R, Nelson W, Kastan M, Plunkett B, Han S, Lorincz A, Hedrick L, Cho K . 1993 Proc. Natl. Acad. Sci. USA 90: 3988–3992
Kim E, Niethammer M, Rothschild A, Nung Yan Y, Sheng M . 1995 Nature 378: 85–88
Kim S . 1997 Curr. Opin. Cell. Biol. 9: 853–859
Kinzler K, Vogelstein B . 1996 Cell 87: 159–170
Kishino T, Lalande M, Wagstaff J . 1997 Nat. Genet. 15: 70–73
Kiyono T, Foster S, Koop J, McDougall J, Galloway D, Klingelhutz A . 1998 Nature 396: 84–88
Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M . 1997 Proc. Natl. Acad. Sci. USA 94: 11612–11616
Klingelhutz A, Foster S, McDougall J . 1996 Nature 380: 79–82
König C, Roth J, Dobbelstein M . 1999 J. Virol. 73: 2253–2262
Krajewski S, Krajewska M, Reed J . 1996 Cancer Res. 56: 2849–2855
Kühne C, Banks L . 1998 J. Biol. Chem. 273: 34302–34309
Kühne C, Gardiol D, Guarnaccia C, Amenitsch H, Banks L . 2000 Oncogene 19: 5884–5891
Kukimoto I, Aihara S, Yoshiike K, Kanda T . 1998 Biochem. Biophys. Res. Commun. 249: 258–262
Kurman R, Schiffman M, Lancaster W, Reid R, Jenson A, Temple G, Lorincz A . 1988 Am. J. Obstet. Gynecol. 159: 293–296
Lechner M, Laimins L . 1994 J. Virol. 68: 4262–4273
Lechner M, Mack D, Finicle A, Crook T, Vousden K, Laimins L . 1992 EMBO J. 11: 3045–3052
Lee D, Lee B, Kim J, Kim W, Choe J . 2000a J. Biol. Chem. 275: 7045–7051
Lee S, Glausinger B, Mantovani F, Banks L, Javier R . 2000b J. Virol. 74: 9680–9693
Lee S, Weiss R, Javier R . 1997 Proc. Natl. Acad. Sci. USA 94: 6670–6675
Lepik D, Ilves I, Kristjuhan A, Maimets T, Ustav M . 1998 J. Virol. 72: 6822–6831
Li X, Coffino P . 1996 J. Virol. 70: 4509–4516
Lie AK, Skarsvag S, Skomedal H, Haugen OA, Holm R . 1999 Int. J. Gynecol. Pathol. 18: 5–11
Lin J, Chen J, Elenbaas B, Levine AJ . 1994 Genes Dev. 8: 1235–1246
Liu Y, Chen J, Gao Q, Dalal S, Hong Y, Mansur C, Band V, Androphy E . 1999 J. Virol. 73: 7297–7307
Liu Z, Ghai J, Ostrow RS, McGlennen RC, Faras AJ . 1994 Virology 201: 388–396
Lowe S, Jacks T, Houseman D, Ruley E . 1994 Proc. Natl. Acad. Sci. USA 91: 2026–2030
Lue R, Marfatia S, Branton D, Chishti A . 1994 Proc. Natl. Acad. Sci. USA 91: 9818–9822
Lyons R, Moses H . 1990 Eur. J. Biochem. 187: 467–473
Mancini A, Koch A, Stefan M, Niemann H, Tamura T . 2000 FEBS Lett. 482: 54–58
Mantovani F, Banks L . 1999 Oncogene 18: 3309–3315
Mantovani F Massimi P, Banks L . 2001 J. Cell Sci. in press
Marcello A, Massimi P, Banks L, Giacca M . 2000 J. Virol. 74: 9090–9098
Marfatia S, Morais Cabral J, Lin L, Hough C, Bryant P, Stolz L, Chishti A . 1996 J. Cell. Biol. 135: 753–766
Marte B, Downward J . 1997 Trends Biochem. Sci. 22: 355–358
Massimi P, Pim D, Bertoli C, Bouvard V, Banks L . 1999 Oncogene 18: 7748–7754
Matlashewski G, Banks L, Pim D, Crawford L . 1986 Eur. J. Biochem. 154: 665–672
Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg G, Kawahara T, Tobayashi S, Okada M, Toyoshima K, Akyama T . 1996 Science 272: 1020–1023
Matsuura T, Sutcliffe J, Fang P, Galjaard R, Jiang Y, Benton C, Rommens J, Beaudet A . 1997 Nat. Genet. 15: 74–77
Menon AG, Rutter JL, von Sattel JP, Synder H, Murdoch C, Blumenfeld A, Martuza RL, von Deimling A, Gusella JF, Houseal TW . 1997 Oncogene 14: 611–616
Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R . 1989 J. Virol. 63: 4417–4421
Nakagawa S, Huibregtse J . 2000 Mol. Cell Biol. 20: 8244–8253
Nakagawa S, Watanabe S, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T . 1995 Virology 212: 535–542
Nees M, Geoghean JM, Hyman T, Frank S, Miller L, Woodworth CD . 2001 J. Virol. 75: 4283–4296
Pan H, Griep A . 1994 Genes Dev. 8: 1285–1299
Pan H, Griep A . 1995 Genes Dev. 9: 2157–2169
Parkin D, Pisani P, Ferlay J . 1999 Int. J. Cancer 80: 827–841
Patel D, Huang S, Baglia L, McCance . 1999 EMBO J. 18: 5061–5072
Pecoraro G, Morgan D, Defendi V . 1989 Proc. Natl. Acad. Sci. USA 86: 563–567
Peng Y, Breiding D, Sverdrup F, Richard J, Androphy E . 2000 J. Virol. 74: 5872–5879
Pietenpol J, Holt J, Stein R, Moses R . 1990a Proc. Natl. Acad. Sci. USA 87: 3758–3762
Pietenpol J, Stein R, Moran E, Yaciuk P, Schlegel R, Lyons R, Pittelkow M, Münger K, Howley P, Moses H . 1990b Cell 61: 777–785
Pim D, Storey A, Thomas M, Massimi P, Banks L . 1994 Oncogene 9: 1869–1876
Pim D, Massimi P, Banks L . 1997 Oncogene 15: 257–264
Pim D, Thomas M, Javier R, Gardiol D, Banks L . 2000 Oncogene 19: 719–725
Polakis P . 1999 Curr. Opin. Genet. Dev. 9: 15–21
Polyak K, Xia Y, Zweler J, Kinzler K, Vogelstein B . 1997 Nature 389: 300–305
Querido E, Morisson M, Chu-Pham-Dang H, Thirlwell S, Boivin D, Branton P . 2001 J. Virol. 75: 699–709
Reuver SM, Garner CC . 1998 J. Cell. Sci. 111: 1071–1080
Reznikoff C, Belair C, Savelieva E, Zhai Y, Pfeifer K, Yeager T, Thompson K, DeVries S, Bindley C, Newton M . 1994 Genes Dev. 8: 2227–2240
Ronco L, Karpova A, Vidal M, Howley P . 1998 Genes Dev. 12: 2061–2072
Ruppert J, Stillmann B . 1993 Mol. Cell Biol. 13: 3811–3820
Sastry S, Burridge K . 2000 Exp. Cell. Res. 261: 25–36
Sastry S, Horwitz A . 1996 Dev. Biol. 180: 455–467
Scheffner M, Werness B, Huibregtse J, Levine A, Howley P . 1990 Cell 63: 1129–1136
Schneider-Gädicke A, Kaul S, Schwarz E, Gausepohl H, Frank R, Bastert G . 1988 Cancer Res. 48: 2969–2974
Schwarz E, Freese U, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H . 1985 Nature 314
Schwarz S, Rosa J, Scheffner M . 1998 J. Biol. Chem. 273: 12148–12154
Sedman SA, Barbosa MS, Vass WC, Hubbert NL, Haas JA, Lowy DR, Schiller JT . 1991 J. Virol. 65: 4860–4866
Sherman L, Schlegel R . 1996 J. Virol. 70: 3269–3279
Sherman L, Jackman A, Itzhaki H, Stoppler M, Koval D, Schlegel R . 1997 Virology 237: 296–306
Snijders P, van Duin M, Walboomers J, Steenbergen R, Risse E, Helmerhorst T, Verheijen R, Meijer C . 1998 Cancer Res. 58: 3812–3818
Somasundaram K, El-Deiry WS . 1997 Oncogene 14: 1047–1057
Song S, Pitot H, Lambert P . 1999 J. Virol. 73: 5887–5893
Song S, Liem A, Miller J, Lambert P . 2000 Virology 267: 141–150
Songyang Z, Fanning A, Fu C, Xu J, Marfatia S, Chishti A, Crompton A, Chan A, Anderson J, Cantley L . 1997 Science 275: 73–77
Steegenga W, Riteco N, Jochemsen AG, Fallaux F, Bos J . 1998 Oncogene 16: 349–357
Steller M, Zou Z, Schiller J, Baserga R . 1996 Cancer Res. 56: 5087–5091
Storey A, Banks L . 1993 Oncogene 8: 919–924
Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, Breuer J, Leigh I, Matlashewski G, Banks L . 1998 Nature 393: 229–234
Suzuki T, Ohsugi Y, Uchida-Toita M, Akiyama T, Yoshida M . 1999 Oncogene 18: 5967–5972
Talis A, Huibregtse J, Howley P . 1998 J. Biol. Chem. 273: 6439–6445
Thanos D, Maniatis T . 1995 Cell 83: 1091–1100
Thomas M, Banks L . 1998 Oncogene 17: 2943–2954
Thomas M, Banks L . 1999 J. Gen. Virol. 80: 1513–1517
Thomas M, Massimi P, Jenkins J, Banks L . 1995 Oncogene 10: 261–268
Thomas M, Glaunsinger B, Pim D, Javier R, Banks L . 2001 Oncogene 20: 5431–5439
Tochio H, Huang F, Li M, Bredt D, Zhang M . 2000 J. Mol. Biol. 295: 225–237
Tong X, Howley P . 1997 Proc. Natl. Acad. Sci. USA 94: 4412–4417
Turner CE . 2000 J. Cell. Sci. 113: 4139–4140
Ullmer C, Schmuck K, Figge A, Lubbert H . 1998 FEBS Lett 424: 63–68
von Knebel Doeberitz M, Rittmuller C, zur Hausen H, Dürst M . 1992 Int. J. Cancer 51: 831–834
Veldman T, Horikawa I, Barret J, Schlegel R . 2001 J. Virol. 75: 4467–4472
Wathelet M, Lin C, Parekh B, Ronco L, Howley P, Maniatis T . 1998 Mol. Cell 1: 507–518
Wazer DL, Liu XL, Chu Q, Gao Q, Band V . 1995 Proc. Natl. Acad. Sci. USA 92: 3687–3691
Weaver B, Prasanna Kumar K, Reich N . 1998 Mol. Cell Biol. 18: 1359–1386
Weis K, Griffiths G, Lamond A . 1994 J. Biol. Chem. 269: 19142–19150
White A, Livanos E, Tlsty T . 1994 Genes Dev. 8: 666–677
Wilcock D, Lane DP . 1991 Nature 349: 429–431
Woods D, Hough C, Peel D, Callaini G, Bryant PJ . 1996 J. Cell Biol. 134: 1469–1482
Woodworth C, Doniger J, DiPaolo J . 1989 J. Virol. 63: 159–164
Wu X, Levine A . 1994 Proc. Natl. Acad. Sci. USA 91: 3602–3606
Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye M, Yuan X, Wood J, Ross C, Sawyers C, Whang Y . 2000a Proc. Natl. Acad. Sci. USA 97: 4233–4238
Wu Y, Dowbenko D, Spencer S, Laura R, Lee J, Gu Q, Lasky L . 2000b J. Biol. Chem. 275: 21477–21485
Zhang J, Rose B, Thompson C, Jarrett C, Russell P, Houghton R, Cossart Y . 1995 Gynecol. Oncol. 57: 170–177
Zimmermann H, Degenkolbe R, Bernard H, O'Connor M . 1999 J. Virol. 73: 6209–6219
zur Hausen H, Schneider A . 1987 The Papillomaviruses. Salzman NP, Howley PM (eds). Plenum Publishing Corp.: New York pp. 245–263
Acknowledgements
We are grateful to C Meyers, D Pim and M Thomas for allowing us to cite their unpublished work. We are also grateful to M Thomas for valuable comments on the manuscript. L Banks gratefully acknowledges research support provided by the Associazione Italiana per la Ricerca sul Cancro.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mantovani, F., Banks, L. The Human Papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20, 7874–7887 (2001). https://doi.org/10.1038/sj.onc.1204869
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204869
Keywords
This article is cited by
-
HPV-associated oropharyngeal cancer: in search of surrogate biomarkers for early lesions
Oncogene (2024)
-
Clinical, morphologic and molecular heterogeneity of HPV-associated oropharyngeal cancer
Oncogene (2023)
-
Mutational profiles of head and neck squamous cell carcinomas based upon human papillomavirus status in the Veterans Affairs National Precision Oncology Program
Journal of Cancer Research and Clinical Oncology (2023)
-
Development of immunodiagnostic tools for in situ investigation of Ovis aries papillomavirus 3 (OaPV3)
Veterinary Research Communications (2023)
-
In silico design of a multi-epitope vaccine against HPV16/18
BMC Bioinformatics (2022)