Abstract
Many biochemical, physiological and behavioural processes show circadian rhythms which are generated by an internal time-keeping mechanism referred to as the biological clock. According to rapidly developing models, the core oscillator driving this clockis composed of an autoregulatory transcription–(post) translation-based feedback loop involving a set of ‘clock’ genes1,6. Molecular clocks do not oscillate with an exact 24-hour rhythmicity but are entrained to solar day/night rhythms by light. The mammalian proteins Cry1 and Cry2, which are members of the family of plant blue-light receptors (cryptochromes) and photolyases, have been proposed as candidate light receptors for photoentrainment of the biological clock7,8,9,10. Here we show that mice lacking the Cry1 or Cry2 protein display accelerated and delayed free-running periodicity of locomotor activity, respectively. Strikingly, in the absence of both proteins, an instantaneous and complete loss of free-running rhythmicity is observed. This suggests that, in addition to a possible photoreceptor and antagonistic clock-adjusting function, both proteins are essential for the maintenance of circadian rhythmicity.
Similar content being viewed by others
Main
We were interested in identifying mammalian homologues of the DNA-repair enzyme photolyase, a protein that undoes ultraviolet-induced DNA damage in a single-step process (photoreactivation) requiring light energy captured by blue-light-collecting chromophores11,12. In this search, we and others have cloned two genes with strong homology to class I photolyases of lower species7,8,9,10. In addition to the photolyase core domain, the gene products appeared to contain a carboxy-terminal extension also found in plant blue-light receptors (cryptochromes), for which the mammalian photolyase-like genes were designated cry1 and cry2. Plant Cry proteins mediate light-dependent processes such as phototropism, growth and flowering13,15. Since placental mammals as well as endogenous or recombinant mammalian Cry proteins lack clearly detectable photoreactivating activity, the mammalian Cry proteins may act as photoreceptors rather than photolyases7,9,10. The biological ‘master’ clock in the suprachiasmatic nucleus (SCN) of the brain controls many physiological processes, from body temperature to the sleep–wake cycle. A major question in mammalian chronobiology is how the clock is entrained to solar time, thereby keeping an organism in an exact 24-h rhythm. The absence of photoentrainment in eye-less rodents indicates that the light receptors feeding into the SCN circadian system must reside in the eye16,17, but the process does not seem to depend on retinal photoreceptor cells and their visual pigments, as Retinal-degenerate (Rd) mice show a normal circadian response to light18,18. Since mammalian cry genes are specifically expressed in the ganglion and inner nuclear layer of the retina, the Cry1 and Cry2 proteins are possible candidates for circadian photoreceptors19.
To explore the biological function of mammalian Cry1 and Cry2, we have generated cry1 and cry2 mutant mice through gene targeting in embryonic stem cells (Fig. 1). Analysis of the transcriptional status of the targeted cry1 and cry2 genes, using the reverse transcription-long-range polymerase chain reaction, revealed no detectable transcripts in the corresponding knockout animals, thus demonstrating that we have created null-mutant mice. Targeted cry1 and cry2 alleles both segregate at expected mendelian ratios, indicating that the absence of either Cry1 or Cry2 does not interfere with embryonic development. Moreover, cry1 and cry2 mutant mice are completely healthy and show no overt phenotype (the oldest animals are now 14 and 7 months, respectively). We analysed the possible role for Cry proteins in the biological clock by measuring the circadian wheel-running behaviour of cry -knockout mice under normal light/dark (LD) cycles and in constant darkness (dark/dark; DD). We made two unexpected observations. First, compared with wild-type mice which, when subjected to DD conditioning, have a free-running rhythm close to 24 hours (τ = 23.77 ± 0.07 h (n = 14)), the internal clock of cry1 mutants runs significantly faster (τ = 22.51 ± 0.06 h (n = 9); P < 0.00001) (Fig. 2a, b). In contrast, cry2 mutants exhibit a clear increase in period length (τ = 24.63 ± 0.06 h (n = 5); P < 0.00001) (Fig. 2c). Heterozygous animals showed wheel-running patterns comparable to wild-type mice, and there were no clear sex- or age-related differences (data not shown). These findings suggest that Cry1 and Cry2 antagonistically modulate the period length of the clock. Second, under LD conditions, both mutants show a circadian periodicity of 24 h (Fig. 2a–c), suggesting that a deficiency in either cry1 or cry2 does not produce a detectable loss of light entrainment of locomotor activity. However, as cry1 mice still contain a functional Cry2 protein which may (partly) take over the function of Cry1 (and vice versa), functional redundancy may blur the phenotypic outcome. Thus, it was of interest to examine double-mutant mice.
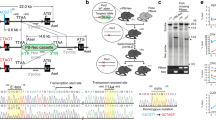
a, Physical map of the wild-type cry1 locus, the targeting construct and the disrupted cry1 locus. Exons are indicated by black filled boxes. Note that the use of PCR-derived genomic DNA does not allow proper exon numbering. The probe used for screening homologous recombinants and genotyping mice, localized external to the construct, is represented by a grey box. Primers used for RNA analysis by RT-long-range PCR are depicted as black arrowheads. b, Southern blot analysis of tail DNA of cry1 +/+, +/− and −/− mice, obtained from heterozygote intercrosses. In Nco I-digested DNA, the cry1 probe hybridizes to a 9-kilobase (kb) fragment of the wild-type cry1 locus and a 4-kb fragment of the targeted locus. c, Expression of the wild-type and targeted cry1 locus. The left panel shows ethidium-bromide-stained RT-long-range PCR products obtained from cry1 +/+, +/− and −/− mouse embryonic fibroblasts using primer set C1f/C1r. The absence of the 1,440-bp cry1 -specific PCR product in −/− animals is confirmed by Southern blot analysis using the complete cry1 cDNA as a probe (right panel). d, Physical map of the wild-type cry2 locus, the targeting construct and the disrupted cry2 locus. The use of symbols is explained in a. e, Southern blot analysis of tail DNA of cry2 +/+, +/− and −/− mice, obtained from heterozygote intercrosses. The cry2 probe hybridizes to 7- and 3.5-kb Eco RI fragments of the wild-type and disrupted cry2 locus, respectively. f, Expression of the wild-type and targeted cry2 locus. Left panel, ethidium-bromide-stained RT-long-range PCR products obtained from kidney RNA using primer set C2f/C2r; right panel, the corresponding Southern blot probed with cry2 cDNA and used to discriminate between specific and background fragments.
Representative examples are shown of voluntary wheel-running records of wild-type (n = 14), cry1 −/− (n = 9), cry2 −/− (n = 5), cry1 +/−cry2 −/− (n = 4), cry1 −/−cry2 +/− (n = 4) and cry1 −/−cry2 −/− (n = 8) mutant mice under LD (12:12 h) and DD conditions. Daily activity patterns are double plotted (for example, day 1+ 2, day 2+ 3, day 3+ 4) on consecutive lines. Periods of darkness are indicated by a grey background. Free-running periods (τ + s.e.m.) were calculated as described in the Methods section.
Like cry -single-knockout mice, double-mutant animals are viable and show no gross phenotypic abnormalities (by 5 months old). Unexpectedly, these mice (n = 8) still display an essentially 24-h circadian rhythm under LD conditions (Fig. 2f, upper part). This suggests that, despite the absence of both Cry proteins, the biological clock—as reflected by locomotor activity–may still receive a light input. However, when double-mutant mice are shifted to a DD regime, they show a striking instantaneous and complete circadian arrhythmicity (Fig. 2f). This indicates that there is no internal circadian clock running with any significant momentum, although we do not exclude the possibility that there is still an ultradian component. Note also that the instantaneous arrhythmicity in double-mutant mice differs from any clock mutant analysed thus far. This includes the only mouse ‘clock’ mutant (clock) described to date, which shows a more gradual loss of periodicity20,21. Interestingly, mice (n = 4) with only one functional cry2 allele out of the four cry gene copies initially display a free-running rhythm even shorter than cry1 -knockout mice, which gradually progresses into arrhythmicity (Fig. 2e). Apparently, these mice possess a clock, but one dose of cry2 can only keep it running for a limited number of cycles in the absence of light. On transfer into LD again these mice regain their original clock, showing that the gradual loss of rhythmicity in DD is reversible (data not shown). This demonstrates a direct involvement of Cry2 in maintaining the clock. On the other hand, in the presence of one allele of cry1, mice (n = 4) show rhythmic activity but the periodicity is intermediate to that of cry1 and cry2 single-mutant mice (Fig. 2d). Mice heterozygous for both cry1 and cry2 show wild-type DD wheel-running patterns (data not shown). Thus, our results demonstrate that the Cry proteins are involved in maintaining period length and circadian rhythmicity, and that a critical balance between Cry1 and Cry2 is required for proper clock functioning.
As the Cry proteins were expected to function as circadian photoreceptors, it was surprising to see an apparent effect of light on the running-wheel behaviour of totally Cry-deficient animals (Fig. 2f, LD part). Therefore, we analysed the behaviour of wild-type and double-mutant mice under changing light conditions. Photoentrained wild-type mice (n = 4), when shifted from the normal LD (12:12 h) regime to a different LD regime (6:18 h), maintain a virtually normal period of activity that gradually shifts towards the new ‘lights-off’ set-point according to the free-running rhythmicity (Fig. 3a). In sharp contrast, cry double-mutant mice (n = 4) abruptly adapt to the new situation by starting wheel running as soon as the light is off and by expanding their period of fragmented activity to 18 h (Fig. 3b). When given daily random blocks of 8 h of light, wild-type mice (n = 5) try to adjust their biological clock, whereas double-mutant cry mice (n = 5) maintain arrhythmic wheel-running behaviour that is only interrupted by light (Fig. 3d–f). Moreover, the running activity of wild-type mice seems also to be transiently and completely suppressed by light (see Fig. 3d, arrow). These observations suggest that light has a dominant influence on the behaviour of (nocturnal) mice, preventing them from using the running wheel when the light is on. Thus, our findings support the idea that under a normal light regime (LD 12:12 h) a dominant effect of light on the running behaviour masks the defective biological clock in totally cry -deficient mice, apparent from their behaviour in DD (see also Fig. 3c). We are currently generating rod-less cry mutant mice to study in detail the photoreceptor function of the cry proteins in the absence of the visual light-perceptive system.
Wild-type (n = 4) and cry double-mutant mice (n = 4) were maintained under normal LD (12:12 h) and subsequently exposed to a short day (LD, 6:18 h) light regime (a, b). In addition, animals were maintained under continuous darkness (DD) and shifted to a normal LD (12:12 h) protocol (c, representative example of 6 cry1 −/−cry2 −/− mice) or a light regime where they encountered random daily light blocks of 8 h (d–f, representative examples of 5 wild-type and 5 cry1 −/−cry2 −/− mice). The arrowhead in d illustrates the suppressing effect of light on wheel-running behaviour of a wild-type mouse. Periods of darkness are indicated by a grey background.
How do Cry1 and Cry2 fit into the rapidly advancing model of the molecular mechanism of the clock? Recently, behavioural analysis of cry2 -knockout mice has revealed altered photoresponses but persistent circadian rhythmicity in DD22, which is consistent with our findings for both cry mutants. This is consistent with the idea that the Cry proteins are circadian photoreceptors. However, our observations with cry double-mutants and with mice carrying one active cry2 allele disclose a second important function of Cry proteins in mammals, namely, involvement in maintaining rhythmicity in constant darkness. This indicates that the Cry proteins not only function in the light-input pathway but are also clock components. As such, expression of the mammalian cry genes resembles that of the clock genes (see ref. 1 and refs therein, and refs 23, 24) in that they are not only expressed in the retina and SCN but also in all other organs and tissues analysed8,10,18. Moreover, the cry1 gene is rhythmically expressed in the mouse SCN even in the absence of Cry222. Using mutant and overexpressing flies, the recently discovered drosophila cry gene product has also been shown to act as a photoreceptor25,26. In cry mutant flies the rhythmically expressed clock genes per and tim show flat expression levels, except in a subset of lateral neurons. We do not exclude the possibility that, analogous to the mammalian situation, Drosophila also contains a second cry gene, which may explain the persisting rhythmic behaviour of the mutant flies.
Future research will focus on the precise roles of the mammalian cry genes in the master ‘clock’ in the SCN and the autonomous clock in every cell. In particular, it will be important to know how these proteins are involved in regulating the expression of known clock genes, or in light-dependent stabilization of (clock) proteins. Finally, one interesting aspect is the subcellular localization of the Cry proteins. Previously, we have shown that, in mouse liver cells and cultured human fibroblasts, Cry2 resides in both the nucleus and mitochondria, whereas all detectable Cry1 appears to be in mitochondria10. These findings suggest that mitochondria may play an important role in controlling the biological clock. This may not be without precedent, as inhibitors of mitochondrial function can induce a phase-shift in the circadian rhythm of Neurospora27,28. Future research must focus on the intriguing connection between this organelle and the biological clock.
Methods
Generation of cry1 and cry2 targeting constructs. Isogenic mouse genomic DNA was obtained by amplification of Ola129-derived E14 ES cell DNA (Takara, LA-PCR; E14 line was provided by A. Berns, Netherlands Cancer Institute) using primer sets designed on the basis of the mouse cry1 and cry2 complementary DNA sequence (GenBank accession nos 000777 and 003433, respectively10). The construction of the Neo -selectable cry1 and cry2 targeting vectors (see Fig. 1a, d) used to delete coding sequences corresponding to base pairs (bp) 730–1,479 and 397–810 of the respective cDNA sequences and isolation of genomic probes will be described in detail elsewhere. Note that the use of PCR-derived genomic DNA does not allow appropriate exon numbering.
Disruption of cry1 and cry2 in mouse ES cells. ES cell line E14 was maintained on gelatine-coated dishes in 60% BRL conditioned DMEM/40% fresh DMEM medium, supplemented with 15% fetal calf serum, 0.1 mM non-essential amino acids, 2 mM glutamine, 50 µg ml−1 penicillin and streptomycin, 1,000 U ml−1 leukaemia inhibitory factor (all components purchased from Gibco) and 0.1 mM 2-mercaptoethanol. Linearized targeting vector DNA (15 µg) was transfected into E14 cells (1 × 107 cells in 400 µl PBS) by electroporation for 10 ms at 1,200 µF and 117 V, using a Progenetor II Gene Pulser (Hoeffer). Electroporated cells were reseeded onto 10-cm dishes and subjected to neomycin selection by addition of 200 µg ml−1 G418 (Geneticin, Gibco) the following day. In the case of cry2, counterselection against randomly integrated DNA was obtained by including 0.2 µM fialuridine in the selection medium. After nine days colonies were randomly picked and expanded in 24-well dishes. Duplicate dishes were used for cryopreservation and genotyping (Southern blot analysis), respectively. Both experiments produced targeting frequencies of 5%.
Generation of cry1 - and cry2 -knockout mice. Gene-targeted ES cells, checked for proper chromosome composition by karyotyping, were injected into C57BL/6 blastocysts by standard procedures29. Chimaeric male mice were mated with C57BL/6 females and transmission of E14-derived germ cells was identified by an agouti coat colour in the offspring. Heterozygous male and female mice were interbred to generate cry1 and cry2 and cry1/cry2 (double) knockout mice.
DNA and RNA analysis. Genotyping of ES cell, mouse embryonic fibroblast (MEF) or tail DNA was performed by Southern blot analysis. For cry1, Nco I-digested DNA was probed with a 560-bp Nco I–Xba I fragment. For cry2, Eco RI-digested DNA was probed with a 519-bp PCR fragment flanking the 5′ border of the construct. Both probes were located outside the targeting construct. RNA was examined by RT-long-range PCR (Takara) analysis using primer sets C1F (5′-CGCATTTCACATACACTGTATGACCTGGACAA-3′)/C1R (5′-TTACTGCTCAGCTGCTGGGACTTTG-3′) and C2F (5′-GTGCACTGGTTCCGGAAGGG-3′)/C2R (5′-AGCACTGCAGGACAGCCACA-3′) for cry1 and cry2, respectively. Randomly primed cDNA was synthesized from RNA isolated with a Rneasy kit (Qiagen). The specificity of the PCR products was confirmed by Southern blot analysis using complete cDNA probes.
Monitoring of locomotor-activity rhythm. Mice of either sex and of different ages (8-20 weeks) were used. They were individually housed in a light-proof chamber in cages (30 × 45 cm) equipped with a running wheel (11 cm in diameter) and a magnetic sensor system to detect wheel rotations30. Animals were maintained in a cycle of 12 h light (150 lux) and 12 h complete darkness (LD) or in continuous complete darkness (DD) in constant ambient temperature (21 ± 0.5 °C) with water and food available ad libitum. Voluntary wheel running (wheel turns per unit of time) was continuously recorded by an online IBM computer using a modified version of the Rodent Activity Meter program30. Activity records were plotted as actograms and the period of locomotor activity was determined from the slope of an eye-fitted straight line through the daily activity onset. The reproducibility of this method was demonstrated by the minimal difference in period estimations (<5 min) when performed by three independent investigators. Unpaired Student's t -tests were used to make statistical comparisons between the different genotypes.
References
Whitmore, D., Sassone-Corsi, P. & Foulkes, N. S. PASting together the mammalian clock. Curr. Opin. Neurobiol. 8, 635–641 (1998).
Wilsbacher, L. D. & Takahashi, J. S. Circadian rhythms: molecular basis of the clock. Curr. Opin. Genet. Dev. 8, 595–602 (1998).
Reppert, S. M. Aclockwork explosion! Neuron 21, 1–4 (1998).
Dunlap, J. An end in the beginning. Science 280, 548–549 (1998).
Sassone-Corsi, P. Molecular clocks: mastering time by gene regulation. Nature 392, 871–874 (1998).
Schibler, U. New cogwheels in the clockworks. Nature 393, 620–621 (1998).
Todo, T. et al. Similarity among the Drosophila (6-4)photolyase, a human photolyase homolog, and the DNA photolyase-blue-light photoreceptor family. Science 272, 109–112 (1996).
van der Spek, P. J. et al. Cloning, tissue expression, and mapping of a human photolyase homolog with similarity to plant blue-light receptors. Genomics 37, 177–182 (1996).
Hsu, D. et al. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry 35, 13871–13877 (1996).
Kobayashi, K. et al. Characterization of photolyase/blue-light receptor homologs in mouse and human cells. Nucleic Acids Res. 26, 5086–5092 (1998).
Sancar, A. Structure and function of DNA photolyase. Biochemistry 33, 2–9 (1994).
Yasui, A. & Eker, A. P. M. in DNA Damage and Repair, Vol. 2: Repair in Higher Eukaryotes(eds Nickoloff, J. A. & Hoekstra, M. F.) 9–31 (Humana, Totowa, NJ, 1998).
Ahmad, M., Jarillo, J. A. & Cashmore, A. R. Chimeric proteins between cry1 and cry2 Arabidopsis blue light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell 10, 197–207 (1998).
Guo, H., Yang, H., Mockler, T. C. & Lin, C. Regulation of flowering time by Arabidopsis photoreceptors. Science 279, 1360–1363 (1998).
Suárez-López, P. & Coupland, G. Plants see the blue-light. Science 279, 1323–1324 (1998).
Nelson, R. J. & Zucker, I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp. Biochem. Physiol. A 69, 145–148 (1981).
Foster, R. G. Shedding light on the biological clock. Neuron 20, 829–832 (1998).
Foster, R. G. et al. Circadian photoreception in the retinally degenerate mouse (rd/rd). J. Comp. Physiol. A 619, 39–50 (1991).
Miyamoto, Y. & Sancar, A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc. Natl Acad. Sci. USA 95, 6097–6102 (1998).
King, D. P. et al. Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 (1997).
Antoch, M. P. et al. Functional identification of the mouse circadian clock gene by transgenic BAC rescue. Cell 89, 655–667 (1997).
Tresher, R. J. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282, 1490–1494 (1998).
Sangoram, A. M. et al. Mammalian circadian autoregulatory loop: A Timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron 21, 1101–1113 (1998).
Zylka, M. J. et al. Molecular analysis of mammalian timeless. Neuron 21, 1115–1122 (1998).
Emery, P., So, W. V., Kaneko, M., Hall, J. C. & Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679 (1998).
Stanewsky, R. et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95, 681–692 (1998).
Brody, S. Circadian rhythms in Neurospora crassa: the role of mitochondria. Chronobiol. Int. 9, 222–230 (1992).
Millar, A. J. Circadian rhythms: PASsing time. Current Biology 7, R474–R476 (1997).
Bradley, A. in Teratocarcinomas and Embryonic Stem Cells. A Practical Approach(ed. Joyner, A. L.) 113–151 (IRL, Oxford, 1987).
Horton, R. M. Simple, inexpensive computerized rodent activity meters. Biotechniques 19, 594–597 (1995).
Acknowledgements
We thank J. Miyazaki for help with construction of targeting vectors, and Y.Tsukahara and S. Okano for discussions on circadian rhythms. M. Kuit is acknowledged for photographic work. This work was supported in part by grants from the Dutch Cancer Society, the Association for International Cancer Research, Human Frontier Science, The Louis Jeantet Foundation and the Ministry of Education, Science, Sports and Culture of Japan, and by a Spinoza premium of the Dutch Scientific Organization NWO.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Horst, G., Muijtjens, M., Kobayashi, K. et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999). https://doi.org/10.1038/19323
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/19323
This article is cited by
-
Microfluidic models of the neurovascular unit: a translational view
Fluids and Barriers of the CNS (2023)
-
Astrocytic insulin receptor controls circadian behavior via dopamine signaling in a sexually dimorphic manner
Nature Communications (2023)
-
Artificial induction of circadian rhythm by combining exogenous BMAL1 expression and polycomb repressive complex 2 inhibition in human induced pluripotent stem cells
Cellular and Molecular Life Sciences (2023)
-
Genome-Wide Identification and Analysis of the Cryptochrome/Photolyase Family in the Brown Alga Saccharina japonica
Journal of Applied Phycology (2023)
-
Circadian disruption alters gut barrier integrity via a ß-catenin-MMP-related pathway
Molecular and Cellular Biochemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.