Abstract
Myeloid sarcoma (MS) is a rare neoplasm whose knowledge is largely based on case reports and/or technically dated contributions. Ninety-two MSs in adulthood with clinical data available were evaluated both morphologically and immunohistochemically. Seventy-four cases were also studied by fluorescent in situ hybridization on tissue sections and/or conventional karyotyping on bone marrow or peripheral blood. Histologically, 50% of the tumors were of the blastic type, 43.5% either monoblastic or myelomonocytic and 6.5% corresponded to different histotypes. CD68/KP1 was the most commonly expressed marker (100%), followed by myeloperoxidase (83.6%), CD117 (80.4%), CD99 (54.3%), CD68/PG-M1 (51%), CD34 (43.4%), terminal-deoxy-nucleotidyl-transferase (31.5%), CD56 (13%), CD61/linker for activation of T cells (2.2%), CD30 (2.2%) and CD4 (1.1%). Foci of plasmacytoid monocyte differentiation were observed in intestinal cases carrying inv16. Chromosomal aberrations were detected in about 54% of cases: monosomy 7(10.8%), trisomy 8(10.4%) and mixed lineage leukemia-splitting (8.5%) were the commonest abnormalities, whereas t(8;21) was rare (2.2%). The behavior was dramatic irrespective of presentation, age, sex, phenotype and cytogenetics. Most if not all, long survivors received bone-marrow transplantation. The present report expands the spectrum of our knowledge showing that MS has frequent monoblastic/myelomonocytic differentiation, displays distinctive phenotypic profile, carries chromosomal aberrations other than t(8;21), and requires supra-maximal therapy.
Similar content being viewed by others
Introduction
Myeloid sarcoma (MS) (ICD-O code 9930/3) is a rare condition that is characterized by the occurrence of one or more tumor masses, consisting of immature myeloid cells presenting at an extra-medullary site.1 The latter has been reported more often to correspond to the skin, bone or lymph node, although the process can affect almost every site of the body.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 It may develop de novo or concurrently with acute myeloid leukemia (AML), myeloproliferative disorder (MPD) or myelodysplastic syndrome (MDS).1, 2, 6, 11, 13, 14, 15, 16, 17, 18, 19, 20 Interestingly, MS may be the first evidence of AML or precede it by months or years.1, 13 Finally, it can represent the initial manifestation of relapse in a previously treated AML in remission.1, 21, 22
The recent World Health Organization (WHO) classification1 has proposed the subdivision of MS into several histotypes, whose exact incidence has not been established yet. At conventional light microscopy, the picture can be misdiagnosed as lymphoblastic, Burkitt's or diffuse large B-cell lymphoma (DLBCL) or even as a non-hematopoietic tumor.1, 6, 13 As this implies a wrong treatment, the application of immunophenotyping is mandatory.1, 2, 3, 23, 24, 25, 26, 27, 28 No concrete attempts have so far been made to determine the prevalence of conventional and more recent markers on large series of cases,1, 3, 26, 27 as well as to draw clear-cut criteria for the differentiation of MS from the plasmacytoid dendritic cell precursor tumor (PMPT)29, 30 (also termed ‘natural killer blastic lymphoma’).1
MS has been described in association with a variety of chromosomal abnormalities18, 31, 32, 33, 34, 35, 36, 37 as well as with mixed lineage leukemia (MLL) rearrangements.38, 39 In particular, t(8;21)(q22;q22) is regarded as a recurrent aberration in MS.1 However, review of the literature highlights that such translocation has been detected in tumors more often occurring in childhood and/or at the orbital level.31, 32, 33
On prognostic grounds, the knowledge of an underlying MPD, MDS or AML is felt to be a negative prognostic factor.1 By contrast, de novo MS has been reported to be sensitive to radiotherapy and/or chemotherapy (CHT) with possible prolonged survival.1, 4, 5, 12, 40, 41 However, recent studies suggest that either highly specific drugs42 or aggressive schedules should also be applied to the latter, if one aims to achieve stable remission and cure.5, 14, 15 So far, no definitive conclusion has been drawn concerning MS optimal treatment.
In this study, we investigated a large series of MS in adult patients by an updated approach, including fluorescent in situ hybridization (FISH). The aims were to assess: (1) the frequency of genetic lesions and (2) the possible correlations among molecular findings, phenotype, clinical features and outcome.
Materials and methods
Tissue samples and clinical information
All cases were retrieved from the files of Italian Pathologic Anatomy Services. In particular, 55 had been collected at the Hematopathology Unit of Bologna University between 1990 and 2004: of these, five had been provided by a Dermatology-oriented Institution and three had been the object of brief reports.12, 13, 14 In all instances, formalin-fixed, paraffin-embedded tissue blocks were available, as were the following information: age and sex of the patients, sites of MS presentation, original morphologic diagnoses and presence of previous, concomitant or following AML, MPD, MDS or non-hematopoietic tumor. Follow-up data were available in 67 patients. Among these, 47 had undergone CHT according to the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) guidelines43, 44, 45 (70.1%), six allogeneic bone-marrow transplantation (AlloBMT) (9.0%), five surgery (7.4%), four autologous BMT (AutoBMT) (6.0%), three – with chronic myeloid leukemia (CML) in blast crisis – imatinib–mesylate (4.5%) and one radiotherapy (1.5%). One patient (1.5%) had received no treatment, as he died immediately after the diagnosis. Informed consent was obtained from all patients or their relatives and each institutional ethical committee approved the tissue collection.
Histology and immunohistochemistry
Sections were cut from the paraffin blocks and either routinely stained (hematoxylin–eosin, Giemsa, periodic acid-Schiff and Gomori) or used for immunohistochemistry. The latter was performed by applying antibodies against: CD1a, CD3, CD4, CD30, CD34, CD56, CD61, CD68/KP1, CD68/PG-M1, CD79a, CD99, CD117, terminal-deoxy-nucleotidyl-transferase (TdT), glycophorin A, glycophorin C, myeloperoxidase (MPO), Factor VIII-related antigen (FVIIIRAg), linker for activation of T cells, cutaneous lymphocyte antigen (CLA/HECA452), protein S-100, tryptase, B-cell-specific activator protein, Ki-67 and keratins. Details on the antibodies and immunohistochemical techniques46 are available on the Supplementary Information file 1. The percentage of neoplastic-positive cells was assessed by four experienced pathologists. Each marker was regarded as positive when it was expressed by at least 20% of the neoplastic cells.
FISH studies
Technical details are available on the Supplementary Information file 2.
Probes
The following commercially available probes (Abbott Laboratories Inc., Abbot Park, IL, USA) were used for FISH studies: AML1/ETO (dual color, dual fusion); CBF-Beta (dual color, break apart rearrangement); MLL (dual color, break apart rearrangement); EGR1/D5S23, D5S721 (dual color); D7S486/CEP7 (dual color); CEP4; CEP8; CEP11; CEP16 (spectrum green and orange). Locus-specific probes were routinely used also to detect aneuploidies: AML1/ETO probe for chromosomes 8 and 21, the MLL probe for chromosome 11, the CBF-Beta probe for chromosome 16. If aneuplody was suspected, a confirmatory hybridization test was carried out with centromeric probes (CEP 8, CEP 11 and CEP 16). DNA sequences targeted by the above-mentioned probes are available on http://www.vysis.com. The probe mixtures were prepared following the manufacturer's instructions.
Normal control study and criteria for analysis
A normal value study was performed in 25 control samples, 12 lymphomas and 13 reactive lymph nodes known by karyotype analysis to be negative for the anomalies tested. Lymphatic tissue was chosen as control as it has the same embryological origin of MSs and also a similar high-density cell pattern.
The following scoring criteria47, 48 were adopted to diminish the impact of nuclei either truncated or with sub-optimal hybridization: (1) nuclei with an inconsistent or misleading FISH pattern were ignored, (2) only those areas with ⩾90% of nuclei showing at least one hybridization signal were evaluated and (3) the analysis was repeated twice, blindly and in different sessions. For further details, see the Supplementary Information file 2.
In addition, in 28 patients with synchronous or metachronous AML, MDS or MPD, karyotypic data on the marrow or peripheral blood were available: of these, 14 underwent FISH on tissue sections.
Statistical analysis
As to what FISH results are concerned, the normal distribution of all variables was ascertained and the t-test was evaluated with both the pooled method (for equal variances)49 and the Satterthwaite method (for unequal variances).50 The cutoff values for each probe was calculated in the control samples as the mean+3 s.d.
For clinical analysis, all data were evaluated with the Stat view 5.0 software package (SAS Institute Inc, Cary, NC, USA). Differences in marker expression were demonstrated by the use of χ2 analyses. Survival curves were plotted according to the Kaplan-Meier method.51 Overall survival (OS) was calculated from the date of diagnosis until death or date of the last contact for living patients. The univariate association between individual clinical features and OS was determined with the log-rank52 or Wilcoxon's test, whichever appropriate. Factors independently associated with OS were identified in multivariate analysis by the Cox proportional hazards regression model.53 The limit of significance for all analyses was defined as a P<0.05; two-sided tests were used in all calculations.
Results
Clinical data
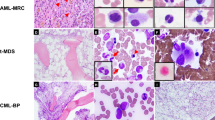
The male-to-female ratio was 1.42:1. The median age was 55.8 years (range 16–87). The commonest sites of MS presentation were the skin (28.2%), lymph node (16.3%), testis (6.5%), intestine (6.5%), bone (3.25%) and central nervous system (CNS) (3.25%) (Figure 1a–d). The complete list of organs involved is shown in the Supplementary Information file 3.
(a) An example of MS occurring at the testis level. Note the residual seminiferous tubules (arrowed) (H&E, × 200); (b) MS grows with the context of an adenomatous polyp of the intestine: please, note residual dysplastic glands arrowed (anti-MPO antibody, immunohistochemistry: APAAP technique, Gill's hematoxylin counterstaining (APAAP-IHC+GHC); × 250); (c) typical indian-file growth pattern in a skin biopsy (H&E, × 250); (d) infiltration of the lymph node paracortex; a residual follicle is arrowed (H&E, × 100); (e) example of blastic population (H&E, × 400); (f) differentiated MS of the kidney. Note the tendency to segmentation of the neoplastic cells and a residual tubule (arrowed) (H&E, × 400); inset: macroscopic detail of the tumoural growth in the same case showing the typical green color; (g) tumor with features of megakaryocytic differentiation, as shown by the FVIIIRAg staining (APAAP-IHC+GHC; × 250); the inset highlights the MPO-positive component (idem, × 400); (h) MS of the myelo-monocytic type: the growth consists of highly proliferating and variably sized cells, showing a greyish cytoplasm at Giemsa (× 400); (i) monoblastic MS: the neoplastic elements of large size display indented, kidney-shaped nuclei (H&E; × 400); (j) MS of the intestine: staining for MPO. Note the central unstained area (encircled) that corresponds to a focus of plasmacytoid monocyte differentiation; inset: staining for CLA/HECA452 of the above mentioned focus; the surrounding cells (on the left) are negative (APAAP-IHC+GHC; × 100; inset: × 250); (k) in the same case, CD117 results: once again the encircled focus is negative (APAAP-IHC+GHC; × 100); (l) in the same tumor, CD68/PGM1 positivity is limited to the plasmacytoid monocyte nodule (encircled) (APAAP-IHC+GHC; × 100).
Twenty-five patients (27%) presented as de novo MS, as assessed by the results of contemporary bone-marrow examination. In two of them, the tumor was followed by AML (M2 and M4, respectively) some months later.
Thirty-two subjects (35%) had simultaneous AML (M1, N=3; M2, N=5; M4, N=8; M5, N=10), MPD (idiopathic myelofibrosis (IM), N=1) or MDS (refractory anemia with excess of blasts (RAEB)-1, N=3; RAEB-2, N=1; 5q− syndrome, N=1).
The remaining 35 individuals (38%) had a previous history of AML (M1, N=1; M2, N=5; M3, N=2; M4, N=1; M5, N=3), MPD (polycythemia vera (PV), N=2; essential thrombocytemia, N=1; IM, N=3; CML, N=7), mastocytosis (N=1) or MDS (RA, N=7; RAEB-1, N=1; RAEB-2, N=1). Notably, among these patients the ones with AML and RAEB had been treated according to GIMEMA protocols that included intercalating agents,43, 44, 45 whereas those with MPD, RA and mastocytosis had received hydroxyurea, supportive therapy and α-interferon, respectively.
In two patients (one with de novo disease and the other with associated M5), a simultaneous non-Hodgkin's lymphoma was detected (i.e. follicular lymphoma and mycosis fungoides). In another one, a peripheral T-cell lymphoma, unspecified had been diagnosed 10 years before and repeatedly treated with radiotherapy and CHT: the latter included antracyclines.
In six cases, a previous history of non-hematopoietic tumor was recorded: embryonal carcinoma of the testis (N=1), prostate carcinoma (N=1), endometrial carcinoma (N=1), breast carcinoma (N=1), intestinal carcinoma with liver metastases (N=1) and association of prostate and larynx carcinoma (N=1). All these patients had undergone CHT, including the administration of intercalating agents, and had developed some years later either AML (M2, N=1; M4, N=1; M5, N=2) or MDS (RA, N=1; RAEB-1, N=1). Interestingly, in these patients AML was always simultaneous to MS, whereas MDS preceded MS.
Finally, a de novo MS was associated with a colonic adenoma (Figure 1b).
Ten out of 25 de novo cases (40%) had been submitted in consultation with a misdiagnosis of DLBCL (N=5), small lymphocytic lymphoma (N=1), peripheral T-cell lymphoma, unspecified (N=1), T-cell precursor (lymphoblastic) lymphoma/leukemia (N=1), anaplastic lymphoma kinase (ALK)-negative anaplastic large-cell lymphoma (N=1) and myeloid metaplasia (N=1). Immunohistochemistry was instrumental to correctly interpret these cases. Notably, three of the patients considered to suffer from DLBCL had been unsuccessfully treated for a malignant lymphoma, until the diagnosis of MS was made.
Histology
When occurring at an extra-nodal site, MS was usually characterized by a diffuse or Indian-file growth pattern, depending on the degree of stromal reaction evoked (Figure 1c). In the lymph node, the neoplastic population either diffused through sinuses or infiltrated the paracortex, entrapping some residual follicles (Figure 1d).
According to the WHO criteria, corroborated by immunophenotyping, the 92 cases were subdivided into: undifferentiated (N=3), blastic (N=46), differentiated (N=1), trilinear (N=1), with megakaryocytic differentiation (N=1), monoblastic (N=20) and myelomonocytic (N=20) (Figure 1e–i). Interestingly, no association was found between the histotype and anatomic site involved with the exception of the monoblastic forms, 50% of them occurring in the skin.
Immunohistochemistry
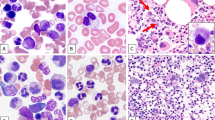
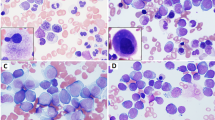
The main immunohistochemical results are summarized in Table 1 and illustrated in Figures 1 and 2. They merit some brief comments.
(a, b) in a myelo-monocytic MS, the opposite patterns observed with the anti-MPO and CD68PG-M1 antibodies (APAAP-IHC+GHC; × 300); (c) diffuse CD99 positivity (APAAP-IHC+GHC; × 400); (d–f) range of results observed at CD56 determination. Note the internal controls (arrowed) represented by ganglion cells in d and a nerve in f (APAAP-IHC+GHC; × 300); (g) dot-like keratin expression in a MS of the left testis followed an embryonic carcinoma of the contro-lateral organ (APAAP-IHC+GHC; × 600); (h) MS cells are negative at the search for BSAP that is expressed by a residual follicle (arrowed) (APAAP-IHC+GHC; × 150); (i) interphase FISH analysis with the MLL dual color: break apart rearrangement probe in a sample with MLL gene rearrangement; arrows: the MLL gene appears splitted (one red and one green signal lay apart in the nuclei); arrow-heads: the MLL gene is not rearranged (red and green spots co-localize into two fusion red/green signal); the rectangle limits a perfectly on-focus area; (j) interphase FISH analysis with the AML1/ETO dual color: dual fusion translocation probe in a sample showing AML1/ETO gene fusion; arrow: AML1/ETO gene fusion (one red/green fusion signal, two red and two green spots, this is a less common pattern of AML1/ETO genes fusion); arrow-head: a normal cell (two green and two red spots); (k) interphase FISH analysis with the MLL dual color: break apart rearrangement probe in a sample with trisomy 11 (three red/green fusion spots); the rectangle limits a perfectly on-focus area; (l) interphase FISH analysis with the chromosome 4 centromeric (Alfa-Satellite). Spectrum green probe: three trisomic and one disomic cells are shown; arrow: not evaluable cell (NE).
The Ki-67/MIB1 score was always high, ranging from 50 to 95%.
CD56 turned out to be variably positive in 12 cases (Table 1) (Figure 2d–f). Interestingly, in none of these cases there was reactivity for CLA/HECA452 and in only one CD4 was detected. By contrast, in four examples of blastic MS (two in the intestine, one in the rectum and one in the lymph node, respectively) foci of plasmacytoid monocytes were observed as shown by the immunostaining results (CLA/HECA452+, CD68/PG−M1+, CD56−, MPO−, CD117−, CD34−, TdT−) that contrasted with those of the surrounding population (CLA/HECA452−, CD68PG−M1−, CD56−, MPO+, CD117+, TdT+) (Figure 1j–l). Notably, at FISH analysis two of such cases (i.e., the intestinal ones) were characterized by the presence of the same chromosomal aberration (inv 16) in both the blastic and plasmacytoid monocyte components. In addition, inv 16 was detected at conventional karyotyping in the rectal case, which did not undergo FISH because of paraffin block exhaustion.
A dot-like positivity for cytokeratins was detected (Figure 2g) in a MS of the blastic type arising in the left testis of a patient, who had undergone CHT 10 years before for an embryonal carcinoma of the contro-lateral testis, followed 7 years later by RAEB-1.
In one further case, foci of tryptase-positive cells were found. The patient had no previous history of mastocytosis, but else suffered from RA.
Finally, three MSs (one blastic and two monoblastic) were associated with a minor lymphoblastic component (B in two instances and T in the remaining one) (details on the phenotype of these cases are available on the Supplementary Information file 4).
FISH analysis
Seven samples of sarcoma out of 56 (12.5%), were not evaluable by FISH analysis, because of the lack of hybridization signals with all the probe sets. Thirty-two could be fully analyzed (57.2%), whereas in the remaining 17 (30.3%) the success rate of FISH analysis varied within the different probe used (Table 2). In the control samples, only two out of 25 analyzed cases were not fully evaluable (8%), whereas in the remaining 23, the full probe set could be tested. A patient sample was considered as positive for a given aberration, if the percentage of cells showing the aberrant pattern was above the cutoff value for the probe examined.
FISH showed clonal abnormalities in 25 out of 49 (54.3%) sarcomas fully or partially analyzed. Of note, in two cases two different aberrations were observed: 20q− associated with CBF-Beta deletion and trisomy 4 in conjunction with 5q−, respectively (Figure 2i–l). Table 2 summarizes the results obtained with the different probes, as well as the main clinical characteristic of positive cases.
Cytogenetics
In 28 patients, cytogenetic analysis had been performed on the bone marrow or peripheral blood owing to synchronous or metachronous AML, MDS or MPD. Thirteen patients (46.4%) showed a normal karyotype, whereas the remaining 15 (53.6%) carried a series of aberrations as shown in Table 3.
Comparison between FISH and cytogenetics
In 14 patients, the results of full or partial FISH analysis could be compared with those of conventional karyotyping. In 10 cases, there was full concordance between the two approaches, whereas in the remaining four no correspondence was recorded (Table 3). In particular, in one of the discordant cases, MS (with uterine and breast involvement) preceded AML-M4 of about 1 year: the former was characterized by trisomy 8 at FISH, whereas the latter showed a rare translocation [(1;9)(q11;q34)] in the absence of +8 at cytogenetics on the peripheral blood (Table 3). Such discrepancy might be due either to +8 loss during leukemic evolution or to the fact that AML represented a secondary event unrelated to MS. In the remaining three discordant patients, monosomy 7 was detected by FISH but not by karyotyping. One of them with testicular MS had been treated 3 years before for RAEB-1, possibly secondary to poly-CHT administered for an embryonal carcinoma of the controlateral testis. Cytogenetics carried out at the time of MDS showed a complex karyotype, including 5q− and monosomy 16 in a subclone (Table 3): MS–FISH provided unreliable results for the former and failed to identify the latter. Interestingly, the CBFB probe used for FISH was a locus-specific sequence that showed a diploid asset: this finding might be related to a nonreciprocal translocation missed by karyotype analysis. The second MS case with monosomy 7 had simultaneous AML-M5 with normal karyotype on the peripheral blood. Finally, the third patient had developed testicular MS 1 year after treatment for CML blastic crisis. In these three cases, monosomy 7 might represent a chromosomal abnormality (subclonal?) acquired during the course of the disease and/or following CHT and favoring MS formation.
Correlations between chromosomal aberrations and tumor characteristics
In general, no statistically significant correlation was found between the recorded cytogenetic abnormalities and age, sex, histotype, de novo presentation, concomitant or previous history of AML, MPD, MDS, malignant lymphoma or nonhematological neoplasm, previous administration of antracyclins or intercalating agents, clinical course or response to therapy. Only, patients with inv16, t(8;21), t(15;17), identified by FISH and/or karyotype, appeared to be usually younger and carried de novo AML or MS.
CD56 positivity was detected in one case with AML1/ETO fusion, but was also recorded in five and four cases with no detectable aberrations and aberration other than t(8;21), respectively. MLL rearrangement was always associated with bone-marrow involvement and still recorded in a 80-year-old patient. In one of the two cases with 5q monosomy, a bone-marrow biopsy performed 1 year before MS was consistent with the 5q− syndrome, whereas in the other there was de novo CNS involvement. Finally, the three patients with inversion 16 had intestinal MS with plasmacytoid monocyte clusters.
Type of therapy and follow-up
At a median follow-up of 150 months, 60 patients died of disease (89.5%), whereas seven are still alive and in complete remission (CR) (10.5%). Figure 3 shows the OS curve of all 67 patients. Among those in CR, one had a history of de novo tumor and six had MS associated with AML (M1, N=1; M3, N=2; M5, N=1), RAEB-1 (N=1) or PV (N=1). There were no significant differences in terms of survivor incidence between the group with de novo tumor and the one with concomitant or previous hematological disorder (1/15 and 6/52). Interestingly, all survivors achieved CR following the first line of therapy. By contrast, only eight out of 60 patients who deceased, obtained CR following the initial therapeutic approach. Six out of seven survivors underwent AlloBMT, the remaining one having received several courses of conventional CHT. These six patients are alive and in CR from 6 months to 8 years after the diagnosis (mean: 52.5 months). Notably, the patients who died of disease within the group of transplanted patients (two following AutoBMT used as initial therapy and two who received AlloBMT as salvage therapy) experienced prolonged survival (from 8 months to 6 years: mean: 41 months). By contrast, the mean survival times of the patients who underwent CHT, imatinib-mesylate, surgery and radiotherapy were as follows: 7.1 months, 5.6 months, 36 days and 1 week, respectively. The OS curves of the patients who, did and did not undergo Auto/AlloBMT (survival rates at 48 months: 76% vs 0%; P=0.0000) are shown in Figure 3.
The clinical behavior and response to therapy were not influenced by any of the following factors: age, sex, anatomic site(s) involved, clinical presentation, previous clinical history, histotype, phenotype and cytogenetic findings.
Discussion
To the best of our knowledge, this is the largest series of MSs so far collected in the literature and comprehensively studied both at the clinical and pathobiological levels: it offers several hints for the better knowledge and management of the tumor.
The present report does not allow definitive conclusions on MS epidemiology, being based on a nationally gathered series. Interestingly, our case-mix ensued from a random collection among Pathologic Anatomy Services and only five samples were provided by a Dermatology-oriented Institution. Thus, the lack of pediatric cases and the high prevalence of skin locations seem to represent fortuitous events. With these limitations in mind, at least in Italy, MS seems to mostly affect adult males. Almost any site of the body can be involved, with a certain predilection for the skin (see Supplementary Information file 3). In 86% of our cases, MS presented as a single mass and in 27% as de novo disease. Importantly, 10/25 de novo tumors had originally been misdiagnosed and three unsuccessfully treated as DLBCL. In none of these cases, an adequate immunohistochemical study had been performed. This strengthens the relevance of phenotyping for MS diagnosis.23, 24, 25, 28
At the morphological level, our series represents the first opportunity to assess the exact incidence of the histotypes quoted in the WHO classification.1 The blastic variant was indeed the commonest form, followed by the monoblastic and myelomonocytic ones that turned out to be much more frequent than thought previously.1
Our immunohistochemical data confirm the relevance of some key markers (such as MPO, CD117 and CD68PG-M1) both for the diagnosis and subclassification of MS.23, 24, 25, 26, 27, 28 In addition, they shed some light on the real incidence of CD99, CD56 and CD30 that can cause diagnostic pitfalls.29, 54, 55, 56 Whereas the former was actually detected in more than 50% of the cases in line with its not infrequent expression in hematopoietic tumors,54 the latter two markers were seldom recorded. CD30 was expressed in only two cases, one of which, however, had been misdiagnosed at another center as CD45-positive, ALK-negative anaplastic large-cell lymphoma of the null type. On the one hand, this confirms that CD30 can be rarely expressed by myeloid tumor, in the other it underlines the need to include MPO among the markers that should be applied to ALK-negative CD30-positive blastic neoplasms of the immune system. Variable CD56-positivity was encountered in 12 tumors, but did not represent a diagnostic problem. In fact, the lack of CD4 and CLA/HECA452 expression allowed the easy differentiation of MS from PMPT.29, 30, 57, 58 Conversely to what reported by others,59, 60 in our hands CD56 was not associated with monocytic derivation or t(8;21). Two further findings merit a brief comment. In one case, the expression of hematopoietic markers was associated with that of cytokeratins. Such event that has already been described in AML cells, either spontaneously or under experimental conditions,61, 62 was recorded in a patient with testicular MS treated 10 years before for an embryonic carcinoma of the contra-lateral organ. This raises the question as to whether the observed cytokeratin positivity should be regarded as an aberrant phenotypic expression of a myeloid neoplasm or a histogenetic link with the previous germ-cell tumor. Finally, four patients presenting with MS sarcoma of the intestine (N=3) and lymph node (N=1) showed foci of plasmacytoid monocyte differentiation within an obvious myeloid population. Interestingly, the two cellular components that showed opposite phenotype (see Figure 1j–l) apparently belonged to the same clone as suggested by the detection of the same chromosomal aberration (inversion 16) in the two intestinal cases that underwent cytogenetic studies. This finding confirms the possible detection of tumor-forming accumulations of plasmacytoid monocytes in the course of myeloid disorders and strengthen the hypothesis that they actually represent foci of differentiation of the underlying disorder.57, 58 In addition, it expands the spectrum of our knowledge by showing such occurrence in MS and the association with a translocation (inv 16) other than the monosomy 7 detected by Vermi et al.57 Last, but not least, the recorded CD56 negativity in our series does confirm the existence of a subset of plasmacytoid monocytes lacking such marker.
FISH analysis revealed a spectrum of abnormalities that expands our knowledge concerning MS.63 In particular, the recorded incidence of chromosomal aberrations (54.3%) is in line with what reported in adulthood AML (40–60%).64, 65 However, taking into consideration the fact that FISH reveals only part of the defects seen at conventional karyotyping as well as the heterogeneity of abnormalities encountered in our series, such finding might suggest that the load of chromosomal aberrations is higher in MS than in AML. This would not be surprising as most examples of MS occur in patients with a simultaneous or pre-existing myeloid disorder and might thus correspond to clonal evolution due to accumulation of chromosomal damages. Bearing in mind the above mentioned epidemiologic limitations and our case-mix arrangement, the differences between our results and those of previous studies31, 32, 33, 66 merit some comments. For instance, the incidence of t(8;21) turned out to be lower in our hands than in those of others.31, 32, 33, 66 Interestingly, the association between t(8;21) and MS was incidentally found in the course of retrospective studies on large cohorts of pediatric patients, who clinically presented with AML.31, 33, 66 In this setting, a significant proportion of cases carrying such translocation had simultaneous MS, more often at the orbital level.31, 32 Our collective corresponded instead to adult patients, included both de novo and AML/MPD/MDS-associated or secondary cases, and contained only one orbital tumor. In our hands, other aberrations showed higher incidence than t(8;21): MLL rearrangement (four cases and one more by karyotype on marrow blood), monosomy 7 (five cases,) and trisomy 8 (five cases). MLL rearrangement was reported by others38, 39, 67 to be possibly associated with MS and its prevalence thought to be equal to or greater than that of t(8;21).38 Interestingly, 3/5 patients with monosomy 7 had a previous history of MPD and MDS, respectively, and another one showed testicular involvement. This is in keeping with the concept that such aberration may be related to secondary leukemias and testicular tumors (8.2% in the Mitelman catalog).63 The detection of trisomy 8 in five cases is of some relevance: in fact, along with the occurrence of t(8;21) in one case (one additional case being doubtful: see Table 2), the above mentioned four MLL rearrangements and one recorded trisomy 11, it supports the recent observation of Deeb et al.,36 who found by CGH a preferential clustering of aberrations at chromosomes 8 and 11 in seven patients with MS. Noteworthy is the association of inv 16 with both the intestinal location and focal plasmacytoid monocyte differentiation of the tumor. Although the former finding has been described in rare AML cases,68 the latter is totally new and merits attention in future studies to assess its exact incidence and relevance. The occurrence of monosomy 16/16q− in 2/44 evaluable cases is of interest: in fact, this cryptic aberration – observed in conjunction with del 20q in one instance – might be underestimated as recently suggested by Casas et al.,65 who found it in 5/127 AML cases by GCH. Finally, monosomy 5/del 5q was detected in two patients: in one of them was associated with trisomy 4 and preceded by MDS of the 5q− type. Although our FISH study significantly expands the array of known chromosomal aberrations occurring in MS, it does not allow drawing definitive conclusions as to their prognostic impact and absolute incidence. In fact, all patients with positive cytogenetics died of disease irrespectively of the fact that they were older or younger than 60 years or had de novo MS or not. In addition, scoring of FISH results in paraffin sections (with special reference to the detection of monosomies and deletions) may be hampered by artefacts, like truncated nuclei, overlay of adjacent nuclei and diminished hybridization efficiency, although it is accomplished in most cases and made more practical by methods that minimize these biases.47, 48 In our series, the comparison between FISH and cytogenetic studies provided similar results in 10/14 cases studied with both techniques. This prompts to conclude that FISH is certainly a powerful tool when only fixed-tissue is available: however, collection of fresh samples – that allow conventional karyotyping – should be encouraged if one aims to assess the full range of chromosomal aberrations in MS.
Finally, our study provides some interesting information on the clinical behavior and therapeutic response of MS. Conversely to what reported in the past,1 no difference was found between the patients with de novo disease and those with tumors related to AML, MDS or MPD. This finding maintained its significance also when the age of the patients (below or higher than 60 years) and chromosomal abnormalities were evaluated (data not shown). Notably, the subjects treated with Auto/AlloBMT corresponded to long survivors, whereas those who received conventional therapies most often rapidly died of disease (OS at 48 months: 76 vs 0%; see Figure 3). Although not based on a randomized clinical trial, our study provides robust support to the recent concept – based on anecdotal observations5, 15, 41 – that patients with MS should undergo high-dose therapies as front-line approach. The latter seems to represent the only chance to achieve CR and to possibly cure the disease.
References
Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). Pathology and Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2001, pp 104–105.
Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer 1981; 48: 1426–1437.
Menasce LP, Banerjee SS, Beckett E, Harris M . Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology 1999; 34: 391–398.
Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia 2003; 17: 1100–1103.
Breccia M, Mandelli F, Petti MC, D'Andrea M, Pescarmona E, Pileri SA et al. Clinico-pathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institution. Leuk Res 2004; 28: 1165–1169.
Ferry JA, Srigley JR, Young RH . Granulocytic sarcoma of the testis: a report of two cases of a neoplasm prone to misinterpretation. Mod Pathol 1997; 10: 320–325.
Oliva E, Ferry JA, Young RH, Prat J, Srigley JR, Scully RE . Granulocytic sarcoma of the female genital tract: a clinicopathologic study of 11 cases. Am J Surg Pathol 1997; 21: 1156–1165.
Corpechot C, Lemann M, Brocheriou I, Mariette X, Bonnet J, Daniel MT et al. Granulocytic sarcoma of the jejunum: a rare cause of small bowel obstruction. Am J Gastroenterol 1998; 93: 2586–2588.
McCluggage WG, Boyd HK, Jones FG, Mayne EE, Bharucha H . Mediastinal granulocytic sarcoma: a report of two cases. Arch Pathol Lab Med 1998; 122: 545–547.
Gorczyca W, Weisberger J, Seiter K . Colonic adenomas with extramedullary myeloid tumor (granulocytic sarcoma). Leuk Lymphoma 1999; 34: 621–624.
Au WY, Shek TW, Ma SK, Leung G, Ooi GC, Liang R et al. Myeloblastoma (chloroma) in leukemia: case 2. Meningeal granulocytic sarcoma (chloroma) in essential thrombocythemia. J Clin Oncol 2000; 18: 3996–3997.
Breccia M, Petti MC, Fraternali-Orcioni G, Monarca B, Latagliata R, D'Elia GM et al. Granulocytic sarcoma with breast and skin presentation: a report of a case successfully treated by local radiation and systemic chemotherapy. Acta Haematol 2000; 104: 34–37.
Ascani S, Piccaluga PP, Pileri SA . Granulocytic sarcoma of main biliary ducts. Br J Haematol 2003; 121: 534.
Breccia M, D'Andrea M, Mengarelli A, Morano SG, D'Elia GM, Alimena G . Granulocytic sarcoma of the pancreas successfully treated with intensive chemotherapy and stem cell transplantation. Eur J Haematol 2003; 70: 190–192.
Imamura T, Matsuo S, Yoshihara T, Chiyonobu T, Mori K, Ishida H et al. Granulocytic sarcoma presenting with severe adenopathy (cervical lymph nodes, tonsils, and adenoids) in a child with juvenile myelomonocytic leukemia and successful treatment with allogeneic bone marrow transplantation. Int J Hematol 2004; 80: 186–189.
Elenitoba-Johnson K, Hodges GF, King TC, Wu CD, Medeiros LJ . Extramedullary myeloid cell tumors arising in the setting of chronic myelomonocytic leukemia. A report of two cases. Arch Pathol Lab Med 1996; 120: 62–67.
Hancock JC, Prchal JT, Bennett JM, Listinsky CM . Trilineage extramedullary myeloid cell tumor in myelodysplastic syndrome. Arch Pathol Lab Med 1997; 121: 520–523.
Kasahara S, Tsurumi H, Hara T, Goto H, Moriwaki H . Idiopathic myelofibrosis developing isolated granulocytic sarcoma with der (1;7)(q10; p10) after splenectomy and finally transforming to acute myelogenous leukemia. Leuk Lymphoma 2000; 39: 427–433.
Cankaya H, Ugras S, Dilek I . Head and neck granulocytic sarcoma with acute myeloid leukemia: three rare cases. Ear Nose Throat J 2001; 80: 224–226, 228–229.
Suzer T, Colakoglu N, Cirak B, Keskin A, Coskun E, Tahta K . Intracerebellar granulocytic sarcoma complicating acute myelogenous leukemia: a case report and review of the literature. J Clin Neurosci 2004; 11: 914–917.
Szomor A, Baranyai F, Tornoczky T, Losonczy H . Penile chloroma in a patient with secondary acute myeloid leukemia. Eur J Haematol 2002; 68: 322.
Maeng H, Cheong JW, Lee ST, Yang WI, Hahn JS, Ko YW et al. Isolated extramedullary relapse of acute myelogenous leukemia as a uterine granulocytic sarcoma in an allogeneic hematopoietic stem cell transplantation recipient. Yonsei Med J 2004; 45: 330–333.
Quintanilla-Martinez L, Zukerberg LR, Ferry JA, Harris NL . Extramedullary tumors of lymphoid or myeloid blasts. The role of immunohistology in diagnosis and classification. Am J Clin Pathol 1995; 104: 431–443.
Roth MJ, Medeiros LJ, Elenitoba-Johnson K, Kuchnio M, Jaffe ES, Stetler-Stevenson M . Extramedullary myeloid cell tumors. An immunohistochemical study of 29 cases using routinely fixed and processed paraffin-embedded tissue sections. Arch Pathol Lab Med 1995; 119: 790–798.
Chang CC, Eshoa C, Kampalath B, Shidham VB, Perkins S . Immunophenotypic profile of myeloid cells in granulocytic sarcoma by immunohistochemistry. Correlation with blast differentiation in bone marrow. Am J Clin Pathol 2000; 114: 807–811.
Chen J, Yanuck III RR, Abbondanzo SL, Chu WS, Aguilera NS . c-Kit (CD117) reactivity in extramedullary myeloid tumor/granulocytic sarcoma. Arch Pathol Lab Med 2001; 125: 1448–1452.
Miettinen M, Lasota J . KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 2005; 13: 205–220.
Traweek ST, Arber DA, Rappaport H, Brynes RK . Extramedullary myeloid cell tumors. An immunohistochemical and morphologic study of 28 cases. Am J Surg Pathol 1993; 17: 1011–1019.
Kurata H, Okukubo M, Fukuda E, Ichihashi M, Ueda M . Myeloid markers should be undertaken in cases of CD56 positivity to exclude granulocytic sarcoma. Br J Dermatol 2002; 147: 609–611.
Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol 2005; 123: 662–675.
Schwyzer R, Sherman GG, Cohn RJ, Poole JE, Willem P . Granulocytic sarcoma in children with acute myeloblastic leukemia and t(8;21). Med Pediatr Oncol 1998; 31: 144–149.
Bonig H, Gobel U, Nurnberger W . Bilateral exopthalmus due to retro-orbital chloromas in a boy with t(8;21)- positive acute myeloblastic acute leukemia. Pediatr Hematol Oncol 2002; 19: 597–600.
Rubnitz JE, Raimondi SC, Halbert AR, Tong X, Srivastava DK, Razzouk BI et al. Characteristics and outcome of t(8;21)-positive childhood acute myeloid leukemia: a single institution's experience. Leukemia 2002; 16: 2072–2077.
Fiegl M, Rieger C, Braess J, Haferlach T, Schnittger S, Schoch C et al. Isolated epidural chloroma with translocation t(15; 17) successfully treated with chemotherapy and all-trans-retinoic acid. Br J Haematol 2003; 122: 688–689.
Cornfield DB, Sun G, Ahmed B . Granulocytic sarcoma associated with a der(7;12)(q10;q10). Cancer Genet Cytogenet 2005; 156: 89–91.
Deeb G, Baer MR, Gaile DP, Sait SN, Barcos M, Wetzler M et al. Genomic profiling of myeloid sarcoma by array comparative genomic hybridization. Genes Chromosomes Cancer 2005; 44: 373–383.
Nicci C, Ottaviani E, Luatti S, Grafone T, Tonelli M, Motta MR et al. Molecular and cytogenetic characterization of a new case of t(5;17)(q35;q21) variant acute promyelocytic leukemia. Leukemia 2005; 19: 470–472.
Park KU, Lee DS, Lee HS, Kim CJ, Cho HI . Granulocytic sarcoma in MLL-positive infant acute myelogenous leukemia: fluorescence in situ hybridization study of childhood acute myelogenous leukemia for detecting MLL rearrangement. Am J Pathol 2001; 159: 2011–2016.
Douet-Guilbert N, Morel F, Le Bris MJ, Sassolas B, Giroux JD, De Braekeleer M . Rearrangement of MLL in a patient with congenital acute monoblastic leukemia and granulocytic sarcoma associated with a t(1;11)(p36;q23) translocation. Leuk Lymphoma 2005; 46: 143–146.
Pulsoni A, Falcucci P, Anghel G, Ribersani M, Petrucci MT, Pescarmona E et al. Isolated granulocytic sarcoma of the skin in an elderly patient: good response to treatment with local radiotherapy and low-dose methotrexate. J Eur Acad Dermatol Venereol 2000; 14: 216–218.
Finnegan D, Jones F, McMullin M . Acute myeloid leukemia with concurrent myeloid sarcoma treated with autologous bone marrow transplantation: two illustrative cases and a literature review. Hematol Oncol 2005; 23: 133–135.
Piccaluga PP, Martinelli G, Rondoni M, Malagola M, Gaitani S, Isidori A et al. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymphoma 2004; 45: 1791–1795.
Carella AM, Carlier P, Pungolino E, Resegotti L, Liso V, Stasi R et al. Idarubicin in combination with intermediate-dose cytarabine and VP-16 in the treatment of refractory or rapidly relapsed patients with acute myeloid leukemia. The GIMEMA Cooperative Group. Leukemia 1993; 7: 196–199.
Avvisati G, Lo Coco F, Diverio D, Falda M, Ferrara F, Lazzarino M et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) pilot study. Blood 1996; 88: 1390–1398.
Hoffman R BEJ, Shattil S, Furie B, Cohen HJ, Silberstein LE, McGlave P . Hematology Basic Principles and Practice, 4th edn, Elsevier Churchill Livingstone: London, 2005, pp 1099–1120.
Pileri SA, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sabattini E et al. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol 1997; 183: 116–123.
Haralambieva E, Kleiverda K, Mason DY, Schuuring E, Kluin PM . Detection of three common translocation breakpoints in non-Hodgkin's lymphomas by fluorescence in situ hybridization on routine paraffin-embedded tissue sections. J Pathol 2002; 198: 163–170.
Cook JR . Paraffin section interphase fluorescence in situ hybridization in the diagnosis and classification of non-hodgkin lymphomas. Diagn Mol Pathol 2004; 13: 197–206.
Weir BS, Hill WG . Estimating F-statistics. Annu Rev Genet 2002; 36: 721–750.
Muir WM . Estimation of response to selection and utilization of control populations for additional information and accuracy. Biometrics 1986; 42: 381–391.
Kaplan EL, Meier P . Non-parametric estimation from incomplete observation. JAMA 1958; 58: 457–461.
Mantel N . Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170.
Cox DR . Regression models and life-tables. J Stat Soc 1982; 34: 187–220.
Zhang PJ, Barcos M, Stewart CC, Block AW, Sait S, Brooks JJ . Immunoreactivity of MIC2 (CD99) in acute myelogenous leukemia and related diseases. Mod Pathol 2000; 13: 452–458.
Chen VM, McIlroy K, Loui JP, Fay K, Ward C . Extramedullary presentation of acute leukaemia: a case of myeloid/natural killer cell precursor leukaemia. Pathology 2003; 35: 325–329.
Fickers M, Theunissen P . Granulocytic sarcoma with expression of CD30. J Clin Pathol 1996; 49: 762–763.
Vermi W, Facchetti F, Rosati S, Vergoni F, Rossi E, Festa S et al. Nodal and extranodal tumor-forming accumulation of plasmacytoid monocytes/interferon-producing cells associated with myeloid disorders. Am J Surg Pathol 2004; 28: 585–595.
Facchetti F, Vermi W, Santoro A, Vergoni F, Chilosi M, Doglioni C . Neoplasms derived from plasmacytoid monocytes/interferon-producing cells: variability of CD56 and granzyme B expression. Am J Surg Pathol 2003; 27: 1489–1492; author reply 1492–1493.
Byrd JC, Weiss RB . Recurrent granulocytic sarcoma. An unusual variation of acute myelogenous leukemia associated with 8;21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer 1994; 73: 2107–2112.
Psiachou-Leonard E, Paterakis G, Stefanaki K, Mikraki-Christou V, Haidas S . Cerebellar granulocytic sarcoma in an infant with CD56+ acute monoblastic leukemia. Leuk Res 2001; 25: 1019–1021.
Jarvinen M, Andersson LC, Virtanen I . K562 erythroleukemia cells express cytokeratins 8, 18, and 19 and epithelial membrane antigen that disappear after induced differentiation. J Cell Physiol 1990; 143: 310–320.
Turner JJ, Milliken S . A case of keratin-positive acute myeloid leukemia: a possible role for cytokeratin 19 as a specific epithelial marker. Pathology 2000; 32: 98–101.
Mitelman F, Heim S . Quantitative acute leukemia cytogenetics. Genes Chromosomes Cancer 1992; 5: 57–66.
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–266.
Casas S, Aventin A, Fuentes F, Vallespi T, Granada I, Carrio A et al. Genetic diagnosis by comparative genomic hybridization in adult de novo acute myelocytic leukemia. Cancer Genet Cytogenet 2004; 153: 16–25.
Dusenbery KE, Howells WB, Arthur DC, Alonzo T, Lee JW, Kobrinsky N et al. Extramedullary leukemia in children with newly diagnosed acute myeloid leukemia: a report from the Children's Cancer Group. J Pediatr Hematol Oncol 2003; 25: 760–768.
Xavier SG, Fagundes EM, Hassan R, Bacchi C, Conchon M, Tabak DG et al. Granulocytic sarcoma of the small intestine with CBFbeta/MYH11 fusion gene: report of an aleukaemic case and review of the literature. Leuk Res 2003; 27: 1063–1066.
Russell SJ, Giles FJ, Thompson DS, Scanlon DJ, Walker H, Richards JD . Granulocytic sarcoma of the small intestine preceding acute myelomonocytic leukemia with abnormal eosinophils and inv(16). Cancer Genet Cytogenet 1988; 35: 231–235.
Acknowledgements
This work was supported by Italian Association for Cancer Research (AIRC, Milan), Italian Ministry of University and Research (MIUR, Rome), Fondazione Cassa di Risparmio in Bologna, Fondazione della Banca del Monte e Ravenna (Bologna) and BolognAIL (Bologna).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Rights and permissions
About this article
Cite this article
Pileri, S., Ascani, S., Cox, M. et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 21, 340–350 (2007). https://doi.org/10.1038/sj.leu.2404491
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404491
Keywords
This article is cited by
-
Myeloid sarcoma and pathological fracture: a case report and review of literature
International Journal of Hematology (2023)
-
Top Ten Lymphoproliferative Lesions Not to Miss When Evaluating Oral Ulcer Biopsies
Head and Neck Pathology (2023)
-
Myeloid sarcoma: more and less than a distinct entity
Annals of Hematology (2023)
-
Gene Mutations and Targeted Therapies of Myeloid Sarcoma
Current Treatment Options in Oncology (2023)
-
Clinical characteristics, treatment, and prognosis of 118 cases of myeloid sarcoma
Scientific Reports (2022)