Abstract
Gene therapy has been proposed as a potential treatment for Wiskott–Aldrich syndrome (WAS), a severe primary immune deficiency characterized by multiple hematopoietic-specific cellular defects. In order to develop an optimal lentiviral gene transfer cassette for this application, we compared the performance of several internal promoters in a variety of cell lineages from human WAS patients. Vectors using endogenous promoters derived from short (0.5 kb) or long (1.6 kb) 5′ flanking sequences of the WAS gene, expressed the transgene in T, B, dendritic cells as well as CD34+ progenitor cells, but functioned poorly in non-hematopoietic cells. Defects of T-cell proliferation and interleukin-2 production, and the cytoskeletal anomalies in WAS dendritic cells were also corrected. The levels of reconstitution were comparable to those obtained following transduction with similar lentiviral vectors incorporating constitutive PGK-1, EF1-alpha promoters or the spleen focus forming virus gammaretroviral LTR. Thus, native regulatory sequences target the expression of the therapeutic WAS transgene to the hematopoietic system, as is naturally the case for WAS, and are effective for correction of multiple cellular defects. These vectors may have significant advantages for clinical application in terms of natural gene regulation, and reduction in the potential for adverse mutagenic events.
Similar content being viewed by others
Introduction
The WAS gene is mutated in Wiskott–Aldrich syndrome (WAS) and in X-linked thrombocytopenia. These diseases are characterized by platelet defects with variable immune dysfunction, as a result of abnormal signaling and impaired cytoskeletal regulation in hematopoieitic cells.1 The WAS protein (WASp) is expressed exclusively in hematopoietic cells, where it functions as a regulator of actin cytoskeleton reorganization, by linking various types of signals to Arp2/3-mediated actin polymerization.2, 3 In T cells, WASp is an essential component of the signal transduction cascade initiated by T-cell receptor engagement, contributing to the establishment of immunological synapse and T-cell activation.4, 5 In highly motile cells such as dendritic cells (DC), WASp is essential for motility and for the formation of podosomes that are actin-rich highly dynamic cytoskeletal structures.6, 7
Several groups have demonstrated that gene transfer, via Moloney-derived gammaretroviral vectors (MLV-RV) or via HIV-1-derived lentiviral vectors (HIV-LV), effectively restores protein expression and corrects the defects in cytoskeletal organization, cellular activation and inflammation in various in vitro or in vivo models of WAS5, 6, 8, 9, 10, 11, 12, 13, 14, 15 These studies provide a strong rationale to develop clinical gene therapy protocols for this disease. The advanced generations of HIV-LV are self-inactivating (SIN) constructs that regulate the expression of the transgene through an internal promoter. This safety feature provides the opportunity to use cellular promoters that may provide a physiological regulation of transgene expression. Normally, WASp is expressed in all leukocyte lineages and found only in the hematopoietic system. Two recent studies have demonstrated in the context of SIN LV that either a 1.6 kb fragment or a shorter 0.5 kb fragment corresponding to the WAS 5′ flanking regions, function well in T cells and are otherwise weakly active in non-hematopoietic cells.12, 14 Further studies were therefore warranted to evaluate the activity of these two WAS promoters in an equivalent vector backbone, in multiple lineages of hematopoietic cells and in comparison with previously tested constitutive internal promoters. For example, the ubiquitously-active PGK-1 promoter is known to induce WASp expression in vitro in T cells derived from WAS patients12 and in vivo in WASp knockout mice after engraftment of transduced hematopoietic progenitor cells.13, 15 Other internal promoters have also been successfully used in HIV-LV to express genes in hematopoietic cells, including a short intron-less EF1-alpha promoter16 and the spleen focus forming virus (SFFV) viral promoter,17 which, in particular, is capable of expressing WASp in human DC.18 Our results showed that all lentiviral WASp expression systems worked efficiently in multiple hematopoietic lineages. Although transcriptionally weaker, the WAS promoters restored sufficient levels of WASp to correct the defects in patients' T cells and DC. The transduction of patient's CD34+ progenitor cells also led to a restoration of WASp expression without measurable toxicity.
Results
Generation of WAS-LV utilizing various internal promoter elements
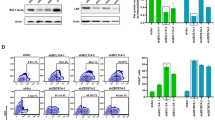
To optimize a WAS gene expression cassette, we compared the performance of several internal promoters driving the expression of human WAS cDNA in the context of an identical lentiviral backbone. The pRRL SINcPPT/CTS vector is an advanced generation SIN and Tat-independent HIV-LV system, which produces high titers and has successfully been used to transduce various types of cells.19 Using this backbone, several human WAS cDNA expression cassettes were constructed, varying the internal promoter to include the human short intron-less EF1-alpha promoter, the SFFV LTR promoter or WAS promoter fragments consisting of 1.6 or 0.5 kb of the 5′ flanking sequences of the WAS gene from the start of transcription (Figure 1). The corresponding LV (W0.5, W1.6, PGK, EF1, SFFV) were pseudotyped with VSV-G and were produced to equivalent titers, being on average 6.9±5 × 109 infectious genome (i.g.)/ml and 7.2±6.4 × 104 ng p24/ml (n=17 total, at least two separate batches produced per vector). Each vector was functional, efficiently inducing WASp in a high proportion of WAS B-lymphoblastic cell lines (B-LCL) (60–80% of normal levels) (Figure 2a). As expected from prior studies,12, 14 the W1.6 and W0.5 LV induced very little WAS transgene expression in non-hematopoietic cells. Less than 20% of HCT116 (epithelial carcinoma), HT1080 (fibrosarcoma) or 293T cells (embryonic kidney) expressed WASp following transduction with high concentrations of the W0.5 and W1.6 vectors (data not shown). In contrast, high frequencies of WASp-expressing cells were induced following transduction with the PGK, EF1 and SFFV vectors utilizing ubiquitously active promoters. Altogether these data validate the different vectors and confirm the hematopoietic-restriction of WAS promoters.
Comparison of efficacy of the five LV in WAS B cells. Transgene expression following transduction with various concentrations of vector was compared in WAS B-LCL derived from three different patients (04W, 08W and 010W). (a) A total of five experiments (lines 04W and 08W B-LCL being tested twice) were performed to correlate the number of vector copies per cell to: normalized WAS mRNA levels measured by Q-RT-PCR and expressed as the percentage of WAS mRNA of B-LCLs of normal individuals which are given an arbitrary value of 100% (left graph); normalized levels of WASp measured by Western blot analysis and densitometry, expressing results as a ratio of WASp over the β-actin signal which is normalized to the ratios obtained with normal B-LCLs, which are given an arbitrary value of 100% (middle graph) or percentage of WASp+ cells as detected by intracellular staining and FACS analysis (right graph). (b) A total of four experiments were performed with the three different B-LCL lines (line 04W tested twice) to measure mRNA (left graph), WASp by Western blot (middle graph) and percentage of WASp+ cells by FACS (right graph) as described above and following transduction with increasing concentrations of the W0.5, W1.6 and PGK vectors. (c) FACS analysis illustrating the transduction of the WAS B-LCL line 08W with increasing concentrations of the LV W1.6. Controls include a normal B-LCL line shown in the upper left panel and small insets showing the staining of 08W and normal cells with an irrelevant immunoglobulin (Ig) control.
Activity of WAS-LV utilizing various internal promoter elements in B-LCL
The various promoter systems induced WAS gene expression in WAS B-LCL that were derived from three different patients (04W, 08W and 010W) (Table 1). In all cases, WAS mRNA and protein levels were copy number-dependent (Figure 2a and b). The WAS promoters appeared to be transcriptionally weaker than the other promoters since lower levels of mRNA were produced following transduction with the W0.5 or W1.6 vectors compared to the PGK, EF1 or SFFV vectors, for equivalent transgene copies integrated per cell (Figure 2a). The levels of protein expression achieved seemed to be comparable from one vector to another as measured by Western blot analysis or by the percentage of WASp+ cells. For clarity, Figure 2b compares various concentrations of W0.5, W1.6 and PGK vectors. In spite of a high concentration of vector and multiplicity of infection (MOI), none of the conditions tested was able to induce protein overexpression and, in particular, there appears to be a saturation of protein expression following transduction with the two highest concentrations of PGK vector. This is illustrated also by a representative fluorescence-activated cell sorter (FACS) analysis of the 08W B-LCL line transduced with the W1.6 vector athough similar results were obtained with all vectors (Figure 2c). As can be seen, the upregulation of WASp is vector dose-dependent up to a plateau beyond which there is no further increase. This could suggest that WASp may not easily be overexpressed. At optimal levels, the proportion of cells stained and the intensity of the stain is comparable to that of a normal B-LCL line confirming that transduction with the LV provides physiological levels of gene expression. Altogether, all vectors tested functioned adequately in B cells over a wide range of concentration.
Activity of WAS-LV utilizing various internal promoter elements in T cells
A T cell line was established from patient WAS1 (Table 1). These cells that express no detectable levels of WASp by Western blot analysis were transduced with low (8 × 106 i.g./ml; MOI 20) or high (4 × 107 i.g./ml; MOI 100) concentrations of the five different LV. This resulted in variable numbers of copies integrated per cell ranging from 0.4 (W 1.6) to 0.7 (SFFV) copies per cell at MOI 20 and from 1.1 (W 1.6) to 2.5 (SFFV) copies per cell at MOI 100. Western blot analysis (Figure 3a) showed that each vector induced WASp expression in a dose-dependent manner. Vectors PGK, EF1 or SFFV induced higher levels of WASp than W0.5 and W1.6. The incomplete restoration of WASp expression in the T-cell cultures compared to healthy control cells was related to limited percentages of transduced cells (Figure 3b). Intracytoplasmic WASp staining and FACS analysis showed that at the MOI of 20, the initial percentage of WASp+ cells was low and ranged from 9% (W0.5) to 26% (PGK), therefore consistent with the low and variable range of protein levels detected by Western blot. Following transduction at the MOI of 100, the initial percentage of WASp+ cells was higher for all vectors and ranged from 16% (W1.6) to 45% (SFFV), correlating also with the Western blot analysis. These results are consistent with the measures of integrated vector per cell and suggest that the differences of WASp expression levels observed after transduction with the different vectors were due in part to different transduction efficiencies. Transduced WAS T-cell populations were cultured for several weeks to check that WASp expression was sustained over time. Except for the WAS T cells transduced with the W0.5 vector at the MOI of 20 for which WASp expression was not detectable, all transduced WAS T-cell populations expressed the transgene during the 10 weeks of culture. Remarkably, the fraction of WASp+ cells increased over time in all transduced conditions (Figure 3b). At week 10 of culture, the proportion of WASp+ cells ranged from 33 to 68% for the T cells transduced at the MOI of 20, and from 35 to 72% for the T cells transduced at the MOI of 100. These results provide additional evidence for a selective in vitro growth advantage of transduced WAS patients' T cells over their untransduced counterparts, as previously reported.12, 14
Transgene expression in transduced T lymphocytes derived from WAS1 patient. Results from a single experiment. (a) Evidence of WASp expression by Western blot analysis following transduction with low (8 × 106 i.g./ml; MOI 20) or high (4 × 107 i.g./ml; MOI 100) concentrations of the different vectors. Unt.=Untransduced WAS1T cells. ND=T cells from a normal donor. Staining of one portion of the blot with Ponceau Red (indicated as loading control) shows comparable protein loading in each lane. (b) The percentage of WASp+ cells was measured by intracellular FACS staining at different times following transduction of WAS1T cells with the different concentrations of the different vectors.
To further compare the activity of the short or long WAS promoter sequences in T cells, we tested the corresponding LV in a model of WASp-deficiency created in Jurkat T cells by RNA interference. We have previously described a LV (W8) encoding a small hairpin RNA that targets the 3′UTR of the WAS gene and stably inhibits 85–90% of WASp in Jurkat T cells.18 This system allows WASp protein to be re-expressed when cells are transduced with a WASp-encoding LV such as SFFV, which does not encode the region targeted by the shRNA.18 To compare the activity of the 0.5 and 1.6 kb promoter sequences in the context of otherwise identical 3′ and 5′ sequences of the WAS cDNA, we constructed two LV gene transfer cassettes expressing the longer cDNA (1585 nucleotides) either under control of the WAS 1.6 or 0.5 kb promoter to produce respectively the W1.6ex12 and W0.5ex12 vectors, which we used only in this set of experiments. Transduction of Jurkat-W8 cells with increasing concentrations of these two vectors, induced equivalent re-expression of WASp, and in both cases restored the levels found in parental Jurkat cells (reaching 120% of parental levels with the highest concentration of vector) (Figure 4). The main point here is that the short or long WAS promoter seem to function equivalently in this T-cell model. Besides, these results provide additional evidence of efficacy of the WAS promoters in the human T-cell lineage.12, 14
Re-expression of equivalent levels of WASp in Jurkat-W8 cells with the short or long WAS promoter. Results from a single experiment. Following transduction of Jurkat-W8 cells with increasing concentrations of the W0.5ex12 or W1.6ex12 vectors, WASp was measured by Western blotting, using β-actin as loading control. Results were quantified by densitometry analysis and expressed as fold increase in WASp/β-actin ratios relative to basal levels in Jurkat-W8 cells. Normal levels indicate the levels obtained in parental WASp+ Jurkat cells.
Functional restoration obtained after transduction of WAS T lymphocytes
As shown in Figure 5, WAS patient's T cells display profound defects in their ability to proliferate and to produce interleukin-2 in response to TCR stimulation. To determine the efficacy of the various LV gene expression cassettes to correct these parameters, we measured the response of WAS T cells to anti-CD3 mAbs after transduction and after a period of culture of 8 weeks. Based on data in Figure 4b, this period of time favors the expansion of the fraction of WASp-expressing T cells in the culture. WAS T cells transduced with any of the five different LV tested increased their response to anti-CD3 antibody stimulation with a dose-dependent proliferation and IL-2 production (Figure 5). The data were roughly segregated along two groups, according to vector dose. The group corresponding to MOI 20 (dotted lines) provided intermediate levels of restoration of proliferation and IL-2 whereas the group corresponding to MOI 100 (plain lines) displayed high levels of activity, close to that observed in normal T cells. Therefore, at sufficiently high levels of transduction, the LV tested can similarly correct in vitro the TCR unresponsiveness of WAS patient's T cells, regardless of promoter usage.
Restoration of functional responses in WAS T lymphocytes. Results from a single experiment. WAS1 T cells were transduced with the various LV as in Figure 4. After 8 weeks, the anti-CD3-mediated proliferative response was measured by 3H-Thymidine incorporation, and IL-2 production in the medium was measured by ELISA.
Comparison of WASp expression and functional restoration obtained in dendritic cells
The lack of WASp induces defects not only in lymphocytes but also in antigen-presenting cells, which are likely to contribute to the immune dysfunction of WAS. To evaluate the efficacy of the WAS gene expression vectors in DC, we transduced bone marrow (BM)-derived immature DC cultures obtained from patients 04W and 07W with 1 × 108 i.g./ml of W0.5, W1.6 or PGK LV. Equivalent levels of WAS mRNA were induced by each of the LV, being approximately 100-fold higher than in untransduced cells, as determined in one experiment (data not shown). The lack of WASp is known to cause marked cytoskeletal anomalies in DC.20 Normally, these cells elaborate podosomes that form upon cell-substrate contact and consist of a ring of approximately 0.5 μm diameter containing vinculin and actin.21 Podosomes are absent in DC or macrophages of WAS patients.22 Only 2±2% of immature DC prepared from either the 04W or 07W patient could produce recognizable podosomes upon adherence onto fibronectin, whereas normal control cultures contained approximately 50–60% of cells with podosomes. Figure 6A shows representative results obtained after transduction of DC from patient 04W in which we found respectively 16±1, 16±1 and 18±1% cells with podosomes after transduction with the W0.5, W1.6 and PGK LV, respectively. All three LV tested were capable of inducing DC that assembled 20 or more podosomes per cell, indicating that such cells probably contained near normal amounts of WASp. Figure 6B illustrates the cellular structures and shows that classic clusters of podosomes were obtained after transduction of WAS DC, resembling those of DC from normal individuals.21 Similarly to T cells, the overall numbers of functionally corrected DC were lower than those obtained in DC from normal individuals as a result of incomplete transduction of the whole-cell population (data not shown). Probably, our transduction protocol for DC is not optimal.23 Overall, the results show that all of the vectors tested, including those with WAS promoters, induced expression and equal functional correction in this cell lineage.
Restoration of podosome formation in immature DC following transduction with LV. (A) One representative experiment out of two. Cells from patient 04W were transduced with the indicated LV and podosomes were enumerated after adherence to fibronectin. Cells were separated into two categories depending on the number of podosomes present per cell (between 1 and 20 per cell or greater than 20 per cell). Percentages above bars indicate the total percentage of podosome-positive cells for each vector with average±s.d. counts from duplicate slides. (B) Representative photographs of the two experiments illustrating the presence of podosomes in DC from a normal individual (a), their absence in DC from a WAS patient (b) and the restoration of these structures following transduction of these WAS DC with W1.6 (c) or PGK (d) vectors. Cell nuclei are recognized by DAPI stain (blue). Podosomes correspond to a specific co-localisation of vinculin (green) and actin (red) as magnified in the insets of panels a, c and d. WAS DC cells express vinculin and actin but fail to co-assemble these proteins into organized structures (b, inset).
Transduction of WAS patient CD34+ cells
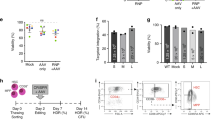
It is important to test the vectors on CD34+ hematopoietic progenitor cells as these cells would be targeted for gene transfer in a clinical application. Since transduction conditions of WAS CD34+ cells have not yet been reported, we first designed two transduction protocols for BM CD34+ cells from WAS4 patient (one or two exposures to the vector). Importantly, both the proportion of BM CD34+ cells by FACS (2.1% of total nucleated CD45+ cells) and their recovery after processing were within normal range. Indeed, after density gradient and immunomagnetic purification 26 × 106 mononuclear cells (MNC) and 0.2 × 106 CD34+ cells per ml of BM were recovered, respectively. These cells were first transduced with an LV encoding GFP under control of the PGK promoter. The 1-exposure and 2-exposure transduction protocols led to 74 and 89% GFP+ cells, respectively (data not shown), which indicates that the cells can be transduced without difficulty. Transduction with W1.6 induced the expression of WASp as shown by Western blot analysis in Figure 7a. The 2-exposure protocol that resulted in higher vector copies (4.8 copies per cell) was estimated to provide approximately 80% of the WASp found in normal BM CD34+ cells compared to the 1-exposure method (1.1 copies per cell) providing approximately 50% of normal protein levels. Transduction with W1.6 LV had no detrimental effect on CD34+ cell growth or survival (data not shown). The hematopoietic differentiation of transduced WAS CD34+ cells was normal in clonogenic assays as measured by the numbers of erythroid, myeloid or mixed colonies produced (Table 2). The appearance and size of colony-forming unit (CFU) colonies derived from WAS CD34+ cells untransduced or transduced with W1.6 was found to be normal (Supplementary data S1). A second sample of CD34+ cells was obtained from patient WAS5. These cells were transduced with the 1-exposure protocol using a slightly higher concentration of LV (5 × 107 i.g/ml MOI=400). The W0.5 and W1.6 vectors were compared and found to provide equivalent levels of transduction with 3.1 and 3.7 copies per cell, respectively. WASp expression in individual cells was measured by FACS (Figure 7b) and about 30% of the cells treated with W0.5 or W1.6 was positive. While this may seem partial, these percentages are similar to those of normal umbilical cord blood (UCB) CD34+-derived cells, thus indicating that the vectors induced near normal levels of WASp expression. Western blot analysis confirmed high levels of restored WASp following transduction with either vector, with an estimate of about 70% of the levels of UCB control cells (Figure 7c). Altogether, these data show that gene transfer into BM progenitor cells from WAS patients can be achieved efficiently. The W0.5 and W1.6 vectors functioned comparably. Both induced practically normal levels of WASp with the integration of relatively few (3) copies of vector per cell.
Induction of WASp in CD34+ cells. (a) Following transduction of WAS4 patient CD34+ cells with the W1.6 vector, cells were cultured in the presence of the transduction cytokines for about 10 days and levels of WASp were measured in this expanded cell population by Western blot analysis using GAPDH levels as loading control. The expression of WASp was quantified by densitometry analysis relative to GAPDH and relative to a normal donor. Unt.=Untransduced CD34+ cells from WAS4 or normal donor, ND=CD34+ cells from a normal BM donor, Control=WASp levels in a control T cell line. (b) Following transduction of WAS5 patient CD34+ cells with the W0.5 or W1.6 vector, cells were expanded by culture in the presence of cytokines as indicated above. Expression of WASp in the cell population was analyzed by flow cytometry. Staining controls included WAS and normal B-LCL, untransduced cells and normal UCB CD34+-derived cells cultured in the same conditions. Results from a single experiment. (c) The same cells that were used in (b) were also analyzed by Western blotting using simultaneoud immunoblotting of WASp and actin. The expression of WASp was quantified by densitometry analysis relative to actin and relative to the levels found in the UCB-derived cells.
Lack of hematopoietic toxicity of WAS gene transfer in CD34+ cells
To assess the risk of toxicity associated with the potential overexpression of WASp, we transduced CD34+ cells obtained from normal UCB or BM (Table 3) with the various WASp-encoding LV using relatively high concentrations of vector particles (2 to 10 × 107 i.g./ml), which introduced between 0.5 to two copies of viral genome per cell (data not shown). FACS analysis of the control culture treated with the GFP-encoding LV showed that 35–45% of the cells were transduced (n=3 experiments). Growth and hematopoietic colony formation were not significantly impaired by transduction with any of the control or WASp-encoding LV including those with strong promoter activity (Table 3). One experiment was performed to examine long-term culture-initiating cells (LTC-IC) activity following transduction of BM CD34+ cells, as this activity originates from a primitive fraction of progenitor cells. Results of this experiment showed no effect of WAS gene transfer since cells treated with the W0.5, PGK vectors or untransduced produced respectively 33, 24 and 41 colonies. Thus, hematopoietic activity does not seem to be compromised after transduction of WASp+ cells with WASp-encoding vectors but it remains to be demonstrated whether the corrected cells are preferentially differentiated towards one lineage over the others.
Discussion
In order to develop a safe and effective LV gene transfer cassette for gene therapy of WAS, we assessed the potential of several internal promoters to regulate expression of a human WAS cDNA transgene in multiple hematopoietic cell lineages and induce correction of the cellular anomalies.
We used the pRRL cassette which had already demonstrated its efficacy to express a human WAS cDNA under control of the PGK-1 promoter or of the native 1.6 kb WAS gene promoter sequence, both in vivo in murine hematopoietic progenitor cells13, 15 and in WAS T lymphocytes in vitro.12 Here, we extend these results by showing that several other internal promoters can be used in the pRRL backbone to express the human WAS cDNA in lymphoid and myeloid cells. Some of the promoters appeared to be transcriptionally stronger, as observed in B cells where higher levels of mRNA were induced from the EF1-alpha, SFFV or PGK-1 compared to WAS promoters. Yet, at equivalent levels of transduction, promoter usage made little difference in total amounts of protein produced by the cells or in the frequency of cells capable of expressing the protein after transduction of T or B lymphocytes. In addition, equivalent levels of functional correction were obtained in T cells and DC obtained from WAS patients. There may be several reasons to explain why differences between the promoters were observed at the mRNA level but not at the protein or at the functional level. One reason may be that Q-reverse transcription-polymerase chain reaction (Q-RT-PCR) is a more discriminative technique than Western blot or functional testing. Alternatively, the lower transcriptional strength of the WAS promoters could be partially compensated by a relative stability and accumulation of WASp in the cells after transduction. Regardless of the mechanism, we find that globally, the LV gene expression system tested here is efficient with any one of the internal promoter used. Transduction can lead to a reconstitution of protein levels that can be estimated to be between 50 and 80% of normal levels. In T lymphocytes, one round of transduction can achieve near complete functional restoration of IL-2 production.
A main point of our study is that it validates the efficacy of gene expression cassettes utilizing native WAS promoter sequences in vitro in multiple lineages of patients cells including hematopoietic progenitor cells. Two putative promoter regions have previously been identified for WAS in the human genome.24, 25 The strongest is a proximal promoter that starts immediately upstream of the transcription start site. Progressive 5′ deletions from an initial 1.6 kb fragment of the WAS 5′ flanking region have identified shorter fragments that are also active in CAT reporter assays, including a 0.5 kb fragment and a minimal sequence of approximately 150 bp conferring hematopoietic restriction and activity (Supplementary Figure S2). In the present study, we tested the 0.5 or 1.6 kb 5′ flanking regions of WAS, since these two fragments have been previously tested into LV expression systems and validated in human T cells.12, 14 Possibly, shorter fragments could also be used as internal promoters and this could be addressed in a separate study. Our present results extend prior findings by presenting evidence that both WAS promoters function not only in human T lymphocytes but also in human B cells, dendritic cells and CD34+ cells. This confirms earlier studies, notably studies using GFP as a reporter system to show that the WAS 0.5 kb promoter is active in all types of human myeloid cells,14 and data obtained in vivo in mice showing that the 1.6 kb WAS promoter functions in T and B lymphocytes as well as in platelets and myeloid cells.15 The WAS proximal promoter is TATA-less and contains several Ets sites that may be responsible for regulating its expression in hematopoietic cells25 although a detailed understanding of this restriction is not available yet. Indeed, we find here that the WAS promoters are poorly active in non-hematopoietic cells in agreement with prior results.12, 14 This feature is adequate to restrict transgene expression to the compartment of cells targeted in the context of the gene therapy of WAS. In addition, this could be useful for other applications requiring the restriction of transgene expression to the hematopoietic system. In the assays performed, we were not able to detect differences in the activity of the short or long WAS promoter sequences in terms of levels of transgene expression and functional correction. An in silico analysis of these two sequences using the GenBank, JASPAR and TFBS resources26, 27 showed that they both contain putative Ets, PU.1, c-Rel, Rel-A, Spi-1, Spi-B, Yin-Yang and MZF1-4 binding motifs. The Ets, PU.1 and Rel sites were previously reported and proposed to contribute to the regulation of WAS expression in hematopoietic cells.25 The longer WAS promoter also contains additional putative MZF1-4 and GATA-3 sequences, which may play an important role, albeit entirely speculative at this point, in the regulation of WAS expression in T cells or myeloid cells (Supplementary Figure S2). Additional studies may therefore be needed to distinguish if the short or long WAS promoters lead to functionally distinct outcomes in models of gene therapy of WAS.
For the first time, we show that WAS patient's CD34+ cells can be efficiently transduced with the VSV-g-pseudotyped pRRL LV system and that physiological levels of transgene can be achieved with WAS promoters. In particular, the present data validate the ability to restore WASp expression in patients' CD34+ cells while preserving their in vitro differentiation potential, two important requisites for clinical use. The potential for hematopoietic toxicity induced by WASp-encoding vectors seems limited. Transduction of normal hematopoietic progenitor cells expressing WASp with the various vectors did not affect their short- or long-term hematopoietic differentiation potential into myeloid and erythroid lineages. A toxicity-limiting factor could be the inability to over-express high levels of WASp as observed in several experiments within the range of concentrations and MOI tested. Altogether, these results concur with successful prior gene therapy studies in mice.10, 13, 15, 28 to suggest the feasibility and possible efficacy of ex vivo gene therapy of WAS through transduction of hematopoietic progenitor cells.
Several types of viral vectors can be used to express the WAS transgene.5, 6, 8, 9, 10, 11, 12, 13, 14 For several reasons, HIV-LV are good candidates for ex vivo gene transfer into hematopoietic stem cells. Contrary to MLV-RV, HIV-LV integrate into non-dividing hematopoietic stem/progenitor cells under conditions of limited preactivation with cytokines which are more likely to preserve long-term multilineage hematopoietic reconstitution and the sustained expression of transgene.29, 30 Contrary to MLV-RV, which are biased for promoter-proximal integration, HIV-LV appear to integrate throughout transcriptional units31 and this may reduce the risk of insertional mutagenesis following transduction of hematopoietic stem/progenitor cells, although this is not demonstrated. The risk of insertional mutagenesis is not easily addressed, particularly in human cells as there are no experimental methods and insufficient clinical data. However, the tumor-prone Cdkn2a−/− mouse model appears to be a sensitive assay and it provided direct experimental evidence that SIN HIV-LV have low genotoxicity even upon integration of about six copies of vector per cell.32 In contrast, LTR-active MLV-RV were found to carry a significant risk of genotoxicity. The SIN design is therefore probably a key element that reduces this risk by providing the opportunity to use cellular promoters that have less long range enhancer activity than viral LTRs. Furthermore, by reducing the possibility of expression in non-hematopoietic cells, WAS promoters should reduce the risk of toxicity associated with ectopic or illicit expression of a cytoskeletal regulator. For these reasons and because the present study provides evidence of the efficacy of both the W 0.5 and W 1.6 vectors in various types of patients' cells including, B, T and DC and CD34+ cells, vectors with WAS promoters appear to be good candidate vectors to pursue for the development of a gene therapy protocol for WAS.
Materials and methods
Constructions
The different WAS cDNA-encoding gene transfer cassettes are described in Figure 1. All include human WAS coding sequences that start at the ATG in position 35 of the reference sequence GenBank Accession Number NM_000377. The pRRL SIN cPPT/cTS W 1.6-hWASp-WPRE plasmid, described previously as pRRLW1.6pWW12 and corresponding to vector W1.6, contains the 1508 nucleotides of the entire coding sequence. All other plasmids contain 1585 nucleotides of WAS cDNA as they further extend 3′ of the stop codon by 77 nucleotides. These plasmids, pRRL SIN cPPT/cTS W0.5-hWASp-WPRE (vector W0.5), pRRL SIN cPPT/cTS EF1a-WASp-WPRE (vector EF1) and pRRL SIN cPPT/cTS SFFV-WASp-WPRE (vector SFFV) were produced from the pRRL SIN cPPT/cTS PGK-WASp-WPRE plasmid (which was described as HIV-PGK-WASP13 and corresponding to vector PGK) in which the PGK-1 promoter was replaced by ligating the 7.7 kb fragment obtained after EcoRV and BamHI digestion with, respectively, a 0.5 kb sequence of the WAS proximal promoter obtained as a ClaI–BamHI fragment of the pHR'sincpptWpWW vector,14 or the short intronless EF1-alpha promoter obtained as a XhoI–BamHI fragment of the pRRL sin cPPT EF1-GFP-WPRE vector,16 or the SFFV LTR obtained as a ClaI–BamHI fragment of the pHR'sin18cppSWW vector.14 The pRRL SIN cPPT/cTS W1.6-hWASp-ex12 WPRE plasmid (vector W1.6ex12) was constructed by exchanging the BspEI–SalI fragment with the pRRL SIN cPPT/cTS W0.5ex12-hWASp-WPRE plasmid, the latter differing from the pRRL SIN cPPT/cTS W0.5-hWASp-WPRE plasmid by the removal of a short stretch of nucleotides (corresponding to a cloning site) inserted 5′ of translation initiation. The two gene transfer cassettes of W1.6ex12 and W0.5ex12 are therefore identical except in length of promoter.
Generation and titration of lentiviruses
VSV-G-pseudotyped LV were produced by transient quadritransfection of 293T cells as previously described.13 Titers (i.g./ml) were determined by quantitative PCR on genomic DNA of serially-infected HCT116 cells by quantifying proviral sequences in the Psi region normalized to the human albumin gene (ALB), using primers described in Table 4 and based on standard curves generated from the pRRLcpptPGKGFP-WPRE-Alb plasmid containing the appropriate sequences in cis from the vectors and ALB gene.
PCR primers and probes used in the study
All sequences are indicated in Table 4.
Derivation, culture and transduction of cells from WAS patients
Blood and BM from WAS patients and normal donors were obtained according to approved research protocols after informed consent, following institutional and national ethical guidelines.
B cell lines
Epstein–Barr virus (EBV)-transformed B-LCL were derived from peripheral blood (PB) MNC of WAS patients (Table 2) after infection with B95-8 cell supernatant in the presence of phytohemagglutinin (PHA) and cyclosporine, using standard procedures. Cells were grown at 37°C, 5% CO2 in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), penicillin–streptomycin and L-glutamine (R10 medium). B-LCL were transduced at the concentration of 2 × 105 cells/0.2 ml per well in the presence of polybrene (6 μg/ml) for 6 h with various concentrations of vectors ranging from 0.5 × 107 i.g./ml with a MOI (multiplicity of infection) 5 to 1 × 108 i.g./ml with MOI 100. After transduction, cells were washed and incubated for 7–10 days, adding fresh medium every other day.
T cell lines
Untransformed T cell lines were derived as described previously from the peripheral blood mononuclear cells (PBMC) of a WAS patient previously referred to as WAS1.12 Briefly, PBMC were stimulated by a feeder cell mixture comprising irradiated allogenic PBMC (1 × 106/ml), irradiated EBV-transformed JY cells (1 × 105/ml), PHA (1 μg/ml), IL-2 (100 IU/ml) and IL-7 (10 ng/ml). These allospecific T cells were cultured in IMDM medium, 10% FCS, penicillin/streptomycin, at 37°C, 5% CO2 and were stimulated every other week with the feeder cell mixture. Lentiviral transduction of T cells from the WAS patient was performed as previously described.12 Briefly, T cells were prestimulated at 1 × 106 cells/ml for 48 h with immobilized anti-CD3 mAb (1 μg/ml), soluble anti-CD28 mAb (1 μg/ml), IL-2 (100 U/ml) and IL-7 (10 ng/ml). Transduction was performed by overnight incubation of prestimulated T cells (2 × 105 cells in a volume of 0.5 ml) with vectors at the concentration 8 × 106 i.g./ml (MOI 20) and 4 × 107 i.g./ml (MOI 100) in the presence of polybrene (8 μg/ml).
Jurkat-W8 cells
This cell line was produced as described.18
Dendritic cells
Immature DC were obtained from an adherent cell fraction of BM. Briefly, BM-MNC obtained by density centrifugation were plated onto tissue culture plastic flasks for 2 h at 37°C in R10 medium. Non-adherent cells were washed off and the remaining plastic-adherent cells were cultured at 37°C in R10 medium supplemented with human recombinant granulocyte–monocyte colony-stimulating factor (GM-CSF) (50 ng/ml) and human IL-4 (20 ng/ml) (R&D Systems, Minneapolis, MN, USA). The following day, 1 × 106 cells per well and per ml were transduced with 1 × 108 i.g./ml of vector (MOI=100) in the presence of polybrene (4 μg/ml), for 3 h. Fresh medium with GM-CSF and IL-4 was added and renewed every other day for 7 days.
BM progenitor cells
CD34+ cells were purified from BM-MNC of healthy individual donors using immunomagnetic beads according to the manufacturer's instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany). Purified CD34+ cells were preactivated with the cytokines thrombopoietin (TPO) (100 ng/ml), stem cell factor (SCF) (300 ng/ml), flt-3-ligand (300 ng/ml) and IL-3 (60 ng/ml) in XVIVO medium (Cambrex, Belgium) for a day. In the set of experiments with WAS4 patient's CD34+ cells, the cells were then exposed onto retronectin-coated plates, to the W1.6 LV at 2 × 107 i.g./ml (or control PGK-GFP vector at 1 × 108 TU/ml) either in a single hit (12 h-long exposure to LV and 36 h total time of culture) or in a double hit (12 h each interspaced by a recovery of 12 h and for a total time of culture of 60 h) using a MOI=100 for each infection. In the set of experiments with WAS5 patient's CD34+ cells, a single-hit protocol using the W1.6 or W0.5 LV at 5 × 107 i.g./ml and MOI=400 with polybrene (6 μg/ml) was used. After the transduction, cells were washed and tested in various assays: CFU in culture (CFU-C), LTC-IC assays or were expanded for about 10 days in the cytokine-containing medium used for the transduction.
Cord blood progenitor cells
CD34+ cells were obtained from UCB-MNC by immunomagnetic selection (Miltenyi Biotec). For transduction, 5 × 104 cells in 0.2 ml were preactivated by overnight incubation in medium supplemented with SCF (50 ng/ml) (R&D Systems), Flt-3 ligand (25 ng/ml) (Abcys SA, Paris, France), TPO (25 ng/ml) (kindly provided by Kirin Brewery, Gunma, Japan), IL-6 (10 ng/ml) (R&D Systems). Preactivated cells were then transduced with LV at 1 × 108 i.g./ml (MOI 100) for 3–6 h in the presence of polybrene (6 μg/ml). After the transduction, cells were washed and CFU-C assays were performed.
PCR analysis of transduction efficiency
The number of vector integrated per cell was measured by quantitative PCR detection of WPRE or Psi sequences normalized to ALB, as described for titrations, using genomic DNA extracted from transduced cells with the ‘Wizard genomic DNA purification kit’ (Promega Corporation, Madison, WI, USA). WAS mRNA expression was measured by quantitative RT-PCR normalized to expression of the transcription factor hTFIID. Total RNA was extracted with the SV total RNA isolation system (Promega) and 1 μg was reverse transcribed into cDNA with the Super-Script II RT (Gibco BRL, Paisley, UK) using random hexamer primers as described elsewhere.33 Amplification reactions (17 μl) contained 8 μl of sample cDNA or genomic DNA and 9 μl of TaqMan buffer (TaqMan Universal PCR Master Mix, No AmpErase UNG, Applied Biosystems, Foster City, CA, USA), 0.2 μ M primers (forward and reverse), 0.1 μ M TaqMan probe (5′ reporter dye (6-carboxyfluorescein) and a downstream, 3′ quencher dye (6-carboxytetramethyl rhodamine)) and consisted of 40 cycles at 95°C (15 s) then 60°C (1 min) on an ABI PRISM 7700 sequence detector (Perkin-Elmer, Applied Biosystems). Standard amplification curves were obtained by serial dilutions of a known cDNA sample. All PCR measures were performed at least in duplicate. Data were edited using the Primer Express software. Results were expressed as the ratio of WAS mRNA to TFIID, which is highly stable in normal B-LCL (S Charrier, data not shown). The WAS/TFIID mRNA ratio obtained after transduction of patients' cells was normalized to that of a normal B-LCL control (arbitrary 100% value) versus the non-transduced cells (arbitrary 0% value).
Detection of WASp by intracellular immunofluorescence staining
Cells were suspended in phosphate-buffered saline (PBS) buffer containing 0.2% bovine serum albumin (BSA) and 0.02% NaN3 were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences Pharmingen, San Diego, CA, USA). Nonspecific binding was prevented by a 10 min blocking step with gamma-immune human normal immunoglobulin (IgG) (1 mg/ml, Sigma-Aldrich, St Louis, MO, USA) prior to incubating the cells for 30 min at 4°C with PE-conjugated anti-WASp (B9, clone Santa Cruz Biotechnology, Santa Cruz, CA, USA) or with PE-IgG2a control antibodies diluted in saponin buffer (Caltag, Burlingame, CA, USA). T cells were fixed and permeabilized as above, but nonspecific binding was blocked with 5% normal rabbit serum and cells were incubated with 5 μg/ml of purified anti-WASp monoclonal antibody (mAb) 5A5 (kind gift from Dr S Tsuchyia, Tohoku University, Sendai, Japan) for 20 min at 4°C. After washing, 5 μg/ml of PE-conjugated goat anti-mouse IgG Abs (Southern Biotechnology Associates, Birmingham, AL, USA) was added for 15 min at 4°C. Stained cells were analyzed using a FACScan with CELLQuest software (BD Biosciences).
Western blot analysis
For Western blot analysis in Figures 2, 4 and 7c, cells were washed and lysed in buffer containing 50 mM Tris-HCl pH 7.5, 200 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 10% glycerol, 1 mM ethylene diaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonylfluoride (PMSF, Sigma) and a cocktail of protease inhibitors (Roche Diagnostics, Basel, Switzerland) consisting of leupeptin, pepstatin and aprotinin (10 μg/ml each). Lysates were boiled in the presence of 5 × Laemmli buffer. Protein concentration was measured by colorimetry (BioRad Laboratories, Hercules, CA, USA) and 30 μg total protein was deposited per lane, separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE), transferred onto nitrocellulose membranes (Hybond ECL, Amersham Biosciences, Piscataway, NJ, USA), blotted with a combination of mouse mAbs D1 specific for human WASp (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) and AC-15 specific for human β-actin (Sigma-Aldrich, Saint Louis, MO, USA), detected with peroxidase-conjugated AffinityPure goat anti-mouse IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA), revealed by chemiluminescence (ECL Western blotting detection kit, Amersham, Piscataway, NJ, USA) and Kodak Biomax film (Sigma-Aldrich).
For Western blot analysis in Figures 3a and 7a, lysates were prepared from 1 × 106 PBS-washed cells in 20 μl lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1% NP-40, 2 mM EDTA) supplemented with 100 μg/ml PMSF and protease inhibitors (Roche Diagnostics). Total protein concentration was determined with the BCA protein assay (Pierce, Rockford, IL, USA). Samples were separated by SDS-PAGE, transferred onto nitrocellulose membranes, stained with Ponceau Red or with the G3PDH mAb specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Chemicon, Temecula, CA, USA) to control loading and transfer. Membranes were incubated with 5A5 ascites diluted 1:2000 in 5% milk in Tris-buffered saline withTween-20 (0.05%) followed by incubation with 1:2000 secondary HRP-coupled antibodies (Dako A/S, Denmark) and detected with ECL. The quantification of WASp to β-actin (or to GAPDH) levels was carried out by densitometry analysis using ImageJ software (NIH, Bethesda, MD, USA).
T cell proliferation and IL-2 production assay
For the proliferation assay, resting T cells (9–14 days poststimulation with feeder cell mixture) were harvested, centrifuged and incubated over-night at 2 × 106 per ml in fresh medium without cytokine. They were plated at 1 × 105 cells per well in 96-well flat-bottom plates precoated with the indicated dose of anti-CD3 mAbs. After 72 h, cells were labeled with 3H-thymidine for 18 h and thymidine incorporation was measured in triplicates by liquid scintillation counting. IL-2 production was measured in the supernatants of cells that had been stimulated for 24 h with the indicated concentrations of immobilized anti-CD3 mAbs using capture enzyme-linked immunosorbent assay (ELISA) (BD Biosciences Pharmingen), peroxidase-conjugated streptavidin (Roche Diagnostics) and tetramethylbenzidine (Fluka Chemie, Switzerland) as substrate.
Detection of podosomes in dendritic cells
Preparations of immature dendritic cells were deposited onto glass coverslips coated with human fibronectin (coated at 100 ng/ml for 4 at 4°C and washed with PBS) and cells were incubated for 2 h at 37°C to allow adherence and formation of podosomes. Cells were then fixed in 2% paraformaldehyde for 10 min, washed with PBS and permeabilized using Triton X-100 (0.2%) for 4 min. Podosomes were detected with antibodies to vinculin (1:400, Sigma-Aldrich) revealed by secondary goat anti-mouse antibodies conjugated to Alexa 488 (1:1000, Molecular Probes, Invitrogen, Carlsbad, CA, USA) and with filamentous actin staining by tetramethylrhodamine-phalloidin (1:1000). Nuclei were labeled with DAPI in the Fluoromount G anti-fading mounting medium. Cells were examined by confocal microscopy using the Leica TCS SP2 AOBS laser scanning microscope. Imaging was performed with a × 40 oil-immersion objective using the laser wavelengths 488 nm (argon), 543 nm (HeNe) excitation and 405 nm (diode laser). All images represent a single section through the focal plane. Images were processed with Leica TCS Start and Adobe Photoshop software. Percentage of cells displaying podosomes were counted by enumerating at least 100 cells using duplicate measures per sample.
CFU-C and LTC-IC assays
CFU-C assays were performed by plating 1000 transduced or untransduced cells per milliliter in methocult medium (H4434) (Stem Cell Technologies, Vancouver, CA, USA). After 2 weeks, CFU-E, BFU-E, CFU-GM and CFU-mix were counted. For the LTC-IC assay, 6 × 105 CD34+ cells were plated on irradiated fetal BM stroma in Myelocult medium (Stem Cell Technologies) as previously described.34 After 5 weeks of culture, CD34+ cells were plated in CFU-C assays and colonies counted after 2 weeks.
Promoter sequence analysis
Annotation of the two human WAS promoter sequences (HWAS 1.6 and HWAS 0.5) was performed by combining several approaches. Three public data sources were used. Genbank (release 145.0) was downloaded from the National Center for Biotechnology Information (NCBI, ftp://ftp.ncbi.nih.gov/) and the GoldenPath annotations (build hg16) were downloaded from the University of California, Santa Cruz (UCSC, ftp://hgdownload.cse.ucsc.edu/goldenPath/hg16/database). JASPAR, the transcription factor binding profile database of the Karolinska institute (http://jaspar.cgb.ki.se), was consulted on-line. The WAS promoter known consensus binding sequences were obtained from Genbank (Accession Y16094.1) by using in-house keyword searching software.
References
Ochs HD, Thrasher AJ . The Wiskott–Aldrich Syndrome. J Allergy Clin Immunol 2006; 117: 725–738.
Badour K, Zhang J, Siminovitch KA . The Wiskott–Aldrich syndrome protein: forging the link between actin and cell activation. Immunol Rev 2003; 192: 98–112.
Burns S, Cory GO, Vainchenker W, Thrasher AJ . Mechanisms of WASp-mediated hematologic and immunologic disease. Blood 2004; 104: 3454–3462.
Sasahara Y, Rachid R, Byrne MJ, de la Fuente MA, Abraham RT, Ramesh N et al. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell 2002; 10: 1269–1281.
Dupre L, Aiuti A, Trifari S, Martino S, Saracco P, Bordignon C et al. Wiskott–Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity 2002; 17: 157–166.
Jones GE, Zicha D, Dunn GA, Blundell M, Thrasher A . Restoration of podosomes and chemotaxis in Wiskott–Aldrich syndrome macrophages following induced expression of WASp. Int J Biochem Cell Biol 2002; 34: 806–815.
Calle Y, Jones GE, Jagger C, Fuller K, Blundell MP, Chow J et al. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood 2004; 103: 3552–3561.
Candotti F, Facchetti F, Blanzuoli L, Stewart DM, Nelson DL, Blaese RM . Retrovirus-mediated WASP gene transfer corrects defective actin polymerization in B cell lines from Wiskott–Aldrich syndrome patients carrying ‘null’ mutations. Gene Therapy 1999; 6: 1170–1174.
Wada T, Jagadeesh GJ, Nelson DL, Candotti F . Retrovirus-mediated WASP gene transfer corrects Wiskott–Aldrich syndrome T-cell dysfunction. Hum Gene Ther 2002; 13: 1039–1046.
Klein C, Nguyen D, Liu CH, Mizoguchi A, Bhan AK, Miki H et al. Gene therapy for Wiskott–Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood 2003; 101: 2159–2166.
Strom TS, Gabbard W, Kelly PF, Cunningham JM, Nienhuis AW . Functional correction of T cells derived from patients with the Wiskott–Aldrich syndrome (WAS) by transduction with an oncoretroviral vector encoding the WAS protein. Gene Therapy 2003; 10: 803–809.
Dupre L, Trifari S, Follenzi A, Marangoni F, Lain de Lera T, Bernad A et al. Lentiviral vector-mediated gene transfer in T cells from Wiskott–Aldrich syndrome patients leads to functional correction. Mol Ther 2004; 10: 903–915.
Charrier S, Stockholm D, Seye K, Opolon P, Taveau M, Gross DA et al. A lentiviral vector encoding the human Wiskott–Aldrich syndrome protein corrects immune and cytoskeletal defects in WASP knockout mice. Gene Therapy 2005; 12: 597–606.
Martin F, Toscano MG, Blundell M, Frecha C, Srivastava GK, Santamaria M et al. Lentiviral vectors transcriptionally targeted to hematopoietic cells by WASP gene proximal promoter sequences. Gene Therapy 2005; 12: 715–723.
Dupre L, Marangoni F, Scaramuzza S, Trifari S, Hernandez RJ, Aiuti A et al. Efficacy of gene therapy for Wiskott–Aldrich syndrome using a WAS Promoter/cDNA-containing lentiviral vector and nonlethal irradiation. Hum Gene Ther 2006; 17: 303–313.
Salmon P, Kindler V, Ducrey O, Chapuis B, Zubler RH, Trono D . High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood 2000; 96: 3392–3398.
Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 2002; 13: 803–813.
Olivier A, Jeanson-Leh L, Bouma G, Compagno D, Blondeau J, Seye K et al. A partial down-regulation of WASP is sufficient to inhibit podosome formation in dendritic cells. Mol Ther 2006; 13: 729–737.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Thrasher AJ . WASp in immune-system organization and function. Nat Rev Immunol 2002; 2: 635–646.
Calle Y, Chou HC, Thrasher AJ, Jones GE . Wiskott–Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J Pathol 2004; 204: 460–469.
Linder S, Wintergerst U, Bender-Gotze C, Schwarz K, Pannicke U, Aepfelbacher M . Macrophages of patients with X-linked thrombocytopenia display an attenuated Wiskott–Aldrich syndrome phenotype. Immunol Cell Biol 2003; 81: 130–136.
Breckpot K, Corthals J, Heirman C, Bonehill A, Michiels A, Tuyaerts S et al. Activation of monocytes via the CD14 receptor leads to the enhanced lentiviral transduction of immature dendritic cells. Hum Gene Ther 2004; 15: 562–573.
Hagemann TL, Kwan SP . The identification and characterization of two promoters and the complete genomic sequence for the Wiskott–Aldrich syndrome gene. Biochem Biophys Res Commun 1999; 256: 104–109.
Petrella A, Doti I, Agosti V, Giarrusso PC, Vitale D, Bond HM et al. A 5′ regulatory sequence containing two Ets motifs controls the expression of the Wiskott–Aldrich syndrome protein (WASP) gene in human hematopoietic cells. Blood 1998; 91: 4554–4560.
Sandelin A, Alkema W, Engstrom P, Wasserman WW, Lenhard B . JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res 2004; 32: D91–D94.
Lenhard B, Wasserman WW . TFBS: computational framework for transcription factor binding site analysis. Bioinformatics 2002; 18: 1135–1136.
Strom TS, Turner SJ, Andreansky S, Liu H, Doherty PC, Srivastava DK et al. Defects in T-cell-mediated immunity to influenza virus in murine Wiskott–Aldrich syndrome are corrected by oncoretroviral vector-mediated gene transfer into repopulating hematopoietic cells. Blood 2003; 102: 3108–3116.
Uchida N, Sutton RE, Friera AM, He D, Reitsma MJ, Chang WC et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA 1998; 95: 11939–11944.
Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC . Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther 2005; 11: 932–940.
De Palma M, Montini E, de Sio FR, Benedicenti F, Gentile A, Medico E et al. Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells. Blood 2005; 105: 2307–2315.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 2006; 24: 687–696.
Towers GJ, Stockholm D, Labrousse-Najburg V, Carlier F, Danos O, Pages JC . One step screening of retroviral producer clones by real time quantitative PCR. J Gene Med 1999; 1: 352–359.
Aiuti A, Friedrich C, Sieff CA, Gutierrez-Ramos JC . Identification of distinct elements of the stromal microenvironment that control human hematopoietic stem/progenitor cell growth and differentiation. Exp Hematol 1998; 26: 143–157.
Acknowledgements
We thank the patients and their families. We are grateful to A Fischer, C Picard, B Varet (Hôpital Necker, Paris), R Favier (Hôpital Trousseau, Paris) and S Martino (Department of Pediatrics, Turin University) for providing WAS patient samples. We are thankful to Genethon collaborators, in particular to G Griffith, S Martin, S Bucher, S Potier, A Jollet, K Seye and J Blondeau for help with vector construction, production and transduction experiments, to T Larmonier for establishment and maintenance of patient B-LCL lines. Special thanks to D Stockholm for help with image acquisition and analysis. We are grateful to S Tsuchyia (Tohoku University, Sendai, Japan) for providing the 5A5 anti-WASP mAbs and to L Naldini (Tiget, Milan, Italy) for providing the pRRL vector system. This work was supported by equipment funds from ‘Génopole Recherche’, Evry, France, by grants from the AFM (French Muscular Dystrophy Association, Evry, France) and Italian Telethon (to MGR, LD), from the EU CONSERT integrated project (FP6-Project No. 005242), the Wellcome Trust (AJT) and EU STREP EURO-POLICY PID (FP6 Project No. SP23-CT-2005-006411, MPB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt).
Supplementary information
Rights and permissions
About this article
Cite this article
Charrier, S., Dupré, L., Scaramuzza, S. et al. Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Ther 14, 415–428 (2007). https://doi.org/10.1038/sj.gt.3302863
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302863
Keywords
This article is cited by
-
Genetic abrogation of immune checkpoints in antigen-specific cytotoxic T-lymphocyte as a potential alternative to blockade immunotherapy
Scientific Reports (2018)
-
Biological and functional characterization of bone marrow-derived mesenchymal stromal cells from patients affected by primary immunodeficiency
Scientific Reports (2017)
-
Optimization of methods for the genetic modification of human T cells
Immunology & Cell Biology (2015)
-
Generation of a lentiviral vector producer cell clone for human Wiskott-Aldrich syndrome gene therapy
Molecular Therapy - Methods & Clinical Development (2015)
-
Lentiviral vectors for the treatment of primary immunodeficiencies
Journal of Inherited Metabolic Disease (2014)