Abstract
Spinal cord transection at a thoracic level activates fictive ejaculation (FE) in the male rat. It has earlier been demonstrated that fictive motor patterns may be activated by pharmacological means and that the noradrenergic system seems to be particularly efficient in triggering locomotor fictive patterns in spinal animals. In the present study, the hypothesis was tested that the spinal noradrenergic system participates in the activation of the spinal generator for ejaculation (SGE). To this aim, the effect of the adrenergic agents, methoxamine, prazosin, clonidine, and yohimbine, upon FE was evaluated in spinal male rats using electromyographic techniques. The results obtained show that ejaculatory rhythmic patterns, accompanied by the expulsion of urethral contents and phasic penile movements, can be elicited by the intravenous (i.v.) injection of methoxamine or yohimbine. These drug-induced motor sequences appear superimposed to the intrinsic ejaculatory spinal rhythm. By contrast, i.v. injection of prazosin or clonidine blocked the expression of the spontaneous ejaculatory rhythmic pattern without inducing any other genital response. These data suggest that an increased noradrenergic tone, either by blockade of presynaptic α2-adrenoceptors or by stimulation of postsynaptic α1-adrenoceptors, results in the activation of the SGE. Present findings provide the evidence that the SGE might be importantly influenced by the noradrenergic system, which exerts a facilitatory control on the expression of the genital motor pattern of ejaculation.
Similar content being viewed by others
Introduction
There is abundant evidence that locomotor activity is largely produced by spinal networks. Central pattern generators (CPG) are neural networks that generate the basic pattern of motor outputs in the absence of peripheral sensory feedback.1, 2, 3 The hallmark for the identification of a CPG within the central nervous system (CNS) is the production of recognisable and reproducible patterns of rhythmic output.3 Although the term CPG refers both to rhythmic and to single automatic movements, the most detailed analysis of its operation has been conducted for rhythmic movements.3 A recent study has provided physiological evidence that a spinal CPG is involved in the control of the genital motor pattern of ejaculation (GMPE).4 Data of this report show that in anaesthetised spinal male rats, the spinal cord is capable of expressing a pattern of rhythmic activity recorded in genital muscles that closely resembles the ejaculatory motor pattern registered in anaesthetised, spinally-intact, and in copulating animals.4, 5 Spinally-transected, anaesthetised male rats express the rhythmic motor pattern of ejaculation accompanied by complex pelvic activity that includes phasic and strong penile erections, as well as penile movements followed by the potent expulsion of urethral contents. Altogether this genital motor activity was termed fictive ejaculation (FE).4 An understanding of the spinal mechanisms involved in the generation of the rhythmic muscular activity underlying this ejaculatory motor pattern can be relevant to clarify the neural basis of ejaculation.
Different pharmacological agents are capable of inducing fictive rhythmic activity that closely resembles locomotor patterns registered in intact animals,6 among them the noradrenergic agents seem to be the most efficient in triggering locomotor fictive patterns in spinal animals.7, 8, 9 Thus, activation of α2-adrenoceptors with the agonist clonidine elicits dramatic responses on fictive locomotion that turn a virtually paralysed state into full-blown movement patterns.6 This effect is blocked by the α2-adrenoceptor antagonist yohimbine.8 Under the same conditions, activation of α1-adrenoceptors by the agonist methoxamine also elicits locomotor patterns, although more modest than those obtained after activation of α2-adrenoceptors.6
Several studies have demonstrated that specific components of male sexual behaviour are differentially modulated by the central noradrenergic system.10, 11, 12, 13, 14, 15, 16, 17 The role played by this neurotransmitter in the control of copulation has been primarily studied through pharmacological activation of α-adrenoceptors.11 In general, these studies suggest that an increased noradrenergic tone, elicited either by blockade of presynaptic α2-autoreceptors or stimulation of postsynaptic α1-adrenoceptors results in a facilitation of copulatory activity that includes a reduction of the ejaculation latency.10 Conversely, stimulation of α2- or blockade of α1-adrenoceptors results in diminished sexual activity.11 However, when examining the effect of α-adrenoceptor activation specifically on penile reflexes in animals with movement restriction (ex-copula), the opposite effect is observed. Thus, systemic injection of yohimbine results in a dose-dependent reduction in the proportion of males displaying erection and ejaculation, while administration of clonidine facilitates the ex-copula penile reflexes.14 Activation of α1-adrenoceptors by methoxamine lacks of an effect on these reflexes, whereas high doses of this agonist decreases the number of erections, cups and flips per test and increases the incidence of seminal emission.14 Despite the conflicting results obtained on in-copula and ex-copula responses in male rats, it can be concluded that α-adrenoceptors influence ejaculatory function. It is important to note that the majority of the data on the consequences of stimulating the different α-adrenoceptor subtypes on male rat ejaculatory function are centred in the behavioural aspects of ejaculation. If ejaculation in behaving animals is significantly affected by noradrenergic agents acting at α-adrenoceptors and the NA system has constantly been implicated in the control of CPGs at a spinal level, it is likely that the spinal circuitry that constitutes the spinal generator for ejaculation (SGE) is also modulated by noradrenaline acting at these receptor subtypes.
The main purpose of the present study was to establish the possible participation of α-adrenoceptors in the functioning of the SGE. To that aim, we evaluated the effect of the intravenous (i.v.) injection of agonists and antagonists to the different α-adrenoceptor subtypes on the expression of the rhythmic activity featuring the GMPE in spinally-transected rats.
Methods
Animals
Sexually experienced male Wistar rats (300–350 g body weight) were used. Animals were housed in groups (four rats per cage), with free access to food and water and under an inverted light: dark cycle 1212, at 22°C. The Local Committee of Ethics on Animal Experimentation approved all experimental procedures, which followed the regulations established in the Mexican official norm for the use and care of laboratory animals ‘NOM-062-ZOO-1999’.
Drugs
Yohimbine, clonidine, methoxamine, and prazosin were all purchased from Sigma Chemical Co. (St Louis, USA), dissolved in physiological saline solution and injected in a final volume of 0.4 ml/rat. All adrenergic agents were i.v. applied 15–20 min after spinalisation. Doses of yohimbine and clonidine were selected on the basis of previous data.18 Methoxamine and prazosin doses were chosen based on a pilot study.
General surgical procedures
All animals were urethane-anaesthetised (0.7 g/kg i.p.), and the bulbospongiosus genital muscles were identified by a surgical incision on the perineum. Two platinum wires (Grass) were inserted into the muscles to record electromyographic (EMG) activity, which was registered on a polygraph (Grass M7). For a better visualisation of the genital activity associated to the rhythmic motor pattern, an additional surgery was performed to expose the bulbar portion of the penis and its anatomical connections with the striated bulbospongiosus muscles. The right femoral vein was cannulated for drug administration. At the end of the surgery the spinal cord was blunt transected at T6 level.
Groups
Animals were divided into six groups. The first group (n=3) was used to record the GMPE and served as control. Groups 2–5 (n=3, each) were employed to analyse the effect of the following α-adrenoceptor drugs: yohimbine (1 μg/animal)), clonidine (3 μg/animal), methoxamine (1 μg/animal) and prazosin (1 μg/animal), respectively, on FE. Finally, animals in group 6 (n=3) were used to elicit FE by sensory means, that is, mechanical stimulation of the urethra, for comparison purposes.
Activation of the rhythmic genital motor pattern of ejaculation
Immediately after spinal cord transection, spontaneously expressed GMPE appeared and could be recorded in the genital muscles. Animals in groups 2–5 were used to register pharmacologically-activated GMPEs. In these groups, after spinalisation, two to three consecutive GMPEs spontaneously expressed were recorded during at least a 10 min-interval to establish the capacity of the spinal apparatus to produce the genital muscular rhythmic pattern. Thereafter, one of the selected adrenergic agents was i.v. injected immediately after the expression of a spontaneous GMPE and the response obtained under their influence, registered. After drug injection, three additional motor patterns, if present, were allowed to be expressed before the next drug injection. When no response was obtained, a 1-min recording period was left between injections. To obtain a representative number of pharmacologically-induced GMPEs and to determine whether consecutive individual injections were all able to induce FE without eliciting habituation to the drugs, five consecutive i.v. injections were administered and their respective responses recorded. For animals in group 1, the recording of spontaneously expressed GMPEs was continued beyond the initial 10 min interval until at least five responses were completed (around 30 min). Finally, for animals in group 6 GMPEs were sensorially activated by mechanically stimulating the urethra as previously described.4 In brief, after spinalisation, GMPEs were repeatedly evoked, at 3-min intervals, by the injection of saline solution (200 μl/min) through a PE-50 catheter (0.965 mm OD) inserted into the pelvic urethra via a bladder incision.
Data analysis
The parameters recorded for each motor train were the number and frequency of EMG bursts. Values were expressed as mean±s.e.m. Mean values were calculated for each animal and quantitative comparisons between groups were calculated from those means. Statistical comparisons were conducted by means of a Kruskal–Wallis ANOVA followed by the Dunnett t-test. The Sigma Stat program (version 2.03) was used for all statistical analyses.
Results
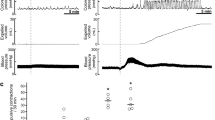
In the present series of experiments the EMG responses induced by α-adrenergic compounds appeared immediately after drug injection, superimposed to the intrinsic rhythm of the SGE. Thus, after spinal cord transection the GMPE was expressed exhibiting a specific rhythm (at 3-min intervals), the intrinsic one. The i.v. injection of methoxamine or yohimbine induced the immediate expression of a motor train thereby disrupting the initial pacing of the response. Thereafter, motor pattern expression regained its rhythm (every 3 min) that, however, did no longer follow the initial pacing, but established a new one starting from the drug-induced response (see Figure 1). It is important to mention that each motor sequence was accompanied by the expulsion of urethral contents and phasic penile movements and erections. The GMPE induced by methoxamine or yohimbine always consisted of a first motor train followed by after-discharge activity (Figure 2). After the first drug-induced GMPE, the following motor sequences recorded between injections lacked of an after-discharge response (see Figure 1). After-discharge activity appeared again during the first motor response that followed every injection. Methoxamine- or yohimbine-treated animals did not exhibit facilitation or habituation of the GMPE when the selected drugs were repeatedly administered; instead a single ejaculatory motor sequence was registered in response to each consecutive dose.
Activational effects of the noradrenergic agents, methoxamine and yohimbine, on the SGE in the spinal male rat. (a) Consecutive spontaneously-expressed genital motor patterns registered after spinal cord transaction, (b) consecutive rhythmic motor responses initiated by the i.v.injection of 1 μg of methoxamine or (c) yohimbine. Notice that drug administration immediately induced a motor response superimposed to the intrinsic response rhythm of the SGE. Arrows indicate drug delivery. Calibration bar 50 mV, 10 s.
Modulatory effects of noradrenergic agents upon FE. (a) Spontaneously expressed FE consisting of a unique motor train. Modulated ejaculatory motor pattern in response to (b) urethral stimulation, (c) i.v. injection of methoxamine or (d) of yohimbine. Notice that modulated patterns always consist of a complete ejaculatory motor response that includes a first train followed by after-discharge activity. Calibration bar 50 mV, 10 s. Arrows indicate mechanical (b) or pharmacological (c and d) stimulus application.
Statistical analysis of the parameters of the GMPE was carried out by comparing ejaculatory motor trains of control animals with those registered in response to methoxamine or yohimbine. This comparison revealed that there were no statistically significant differences in the features of the GMPE spontaneously expressed and the drug-induced ones, neither in the number of discharges nor in its frequency (Kruskal–Wallis one-way ANOVA for number of discharges, P=0.12, ns; for frequency of discharge P=0.11, ns). Figure 2 shows EMG sample tracings of control, spontaneously expressed (A) and mechanically evoked (B) GMPE and those elicited by 1 μg of methoxamine (C) or yohimbine (D).
On the other side, the i.v. injection of prazosin or clonidine blocked the expression of the basal rhythmic GMPE that appears following spinal transection, without eliciting additional motor activity. Thus, administration of prazosin (1 μg) or clonidine (3 μg) exerted an inhibitory effect both, on the spontaneously expressed GMPE and the penile phasic erections and movements. There were no motor responses between the administration of individual doses of prazosin or clonidine, even although a period of 1 min was allowed between injections (drug-induced motor responses appeared immediately after an injection).
In order to establish if there were differences in the features of the drug-induced and sensorially-elicited fictive GMPE, a comparison was conducted between the methoxamine- and yohimbine-induced GMPE with those mechanically elicited.
As it can be seen in Table 1, yohimbine elicited a GMPE with a statistically significantly lower number and decreased frequency of discharge as compared with the parameters obtained after urethral stimulation, but similar to the one spontaneously expressed (see above). By contrast, the methoxamine-induced GMPE did not differ from the spontaneously expressed response or the sensorially-elicited one. Although the two experimental conditions, that is, sensorial elicitation and pharmacological induction, are not strictly comparable, it appears interesting that the responses obtained by pharmacological agents that increase the noradrenergic tone and sensorial stimulation are similar in its modulatory effect. In both, the pharmacologically- and the sensory-elicited GMPE, each individual ejaculatory motor discharge registered in the bulbospongiosus genital muscle coincided with individual expulsive ejaculatory movements. Similarly, individual motor discharges of the genital bulbospongiosus muscle always coincided with phasic penile erections and with individual penile movements.
Discussion
The existence of a CPG for ejaculation in male rats has been postulated by several authors.19, 20 We have recently provided the physiological evidence that a spinal pattern generator is involved in the control of the GMPE.4 The present study investigated the possibility that noradrenergic agents, which significantly affect ejaculation in behaving animals and coordinate the initiation of several CPG-controlled fictive motor patterns, could also influence the SGE that controls FE.
The data obtained in the present work in spinal cord-transected and urethane-anaesthetised male rats show that stimulation of α1-adrenoceptors by methoxamine, as well as blockade of α2-adrenoceptors by yohimbine, initiate and modulate the rhythmic expression of GMPE, accompanied by the strong expulsion of the urethral contents and penile movements. On the other side, activation of α2-adrenoceptors with clonidine or blockade of α1-adrenoceptors by prazosin prevents the activity of the SGE, without inducing any other genital motor activity. Thus, present data suggest that the noradrenergic agents used in this study could target the SGE involved in the control of ejaculation.
The activational effect of methoxamine and yohimbine, at the doses tested, appears to be centred on the SGE, since no other fictive motor patterns such as micturition or locomotion were turned on. Nevertheless, the possibility that other spinal generators could be affected in a subthreshold manner cannot be excluded since, it has been reported that yohimbine, at doses higher than those employed in the present work, is capable of triggering fictive locomotion patterns in spinal cats.7
The fact that the pharmacologically-induced GMPE in our preparation was always associated to the strong expulsion of urethral contents and penile movements evidences that the expulsive activity in the striated bulbospongiosus muscle is elicited by the drugs acting at the SGE. Thus, it is proposed that both methoxamine and yohimbine specifically increase the excitability of the SGE to promote the expression of FE.
In addition to the activational effects of these compounds (initiation of FE superimposed to the intrinsic spinal rhythm), it was also observed that methoxamine and yohimbine modulated the expression of the rhythmic genital motor activity. Modulation of the GMPE by yohimbine and methoxamine consisted of the facilitation of the expression of complete GMPEs, evidenced by the presence of after-discharge activity. The movement repertoire of spinal animals contains the essential features of the movement patterns, but lacks the refinement observed in an intact animal.1, 2 This refinement is provided, at least partially, by the afferent inputs and has been observed in spinal animals after sensory stimulation,1, 21 pharmacological blockade of the major inhibitory processes22 or after direct pharmacological stimulation.23 The presence of after-discharge activity in an ejaculatory motor sequence has been considered to represent the refinement of the ejaculatory response.23 Thus, it is possible that pharmacological stimulation by methoxamine or yohimbine, could activate the SGE in a similar manner to that elicited by sensorial stimulation; modulating the expression of the GMPE until it reaches features of the ejaculatory process observed in intact animals.24
On the other side, the present series of experiments revealed that blockade of α1-adrenoceptors by prazosin or activation of α2-adrenoceptors with clonidine prevents the activity of the SGE. The prazosin-induced blockade of FE coincides with the effect reported for this drug on other fictive motor patterns.9 However, for the effect of clonidine, contrasting results have been described for other fictive motor acts. Thus, clonidine has been found to induce fictive walking and, at the same time, to abolish fast paw shaking in spinal cats.23 This last effect would correspond to the blocking action of clonidine on FE observed in the present study. Several studies have demonstrated that male rat ejaculatory behaviour can be significantly influenced by both α1- and α2-adrenoceptor agonists and antagonists.11, 16 The main physiological effects reported for these drugs in intact, behaving rats are in line with present data and include a dose-related suppression of ejaculatory behaviour after clonidine, a significant increase in the ejaculation latency after prazosin11 and a dramatic decrease in the duration of the ejaculation latency by both methoxamine and yohimbine.10, 16 Although it is clear that α-adrenoceptor agonists and antagonists affect male rat ejaculation, the site(s) of drugs' action has not been fully identified.14 Present results revealing that methoxamine and yohimbine can initiate and modulate the ejaculatory motor pattern in a spinal preparation and that prazosin and clonidine consistently block the expression of the GMPE suggest that main effects of these adrenergic agents could be exerted at the SGE. Thus, it could be thought that in copulating male rats the effects of these adrenergic drugs on ejaculation might be mediated, at least partially, by the spinal noradrenergic system.
The spinal circuitry modulating sexual function receives a dense noradrenergic innervation from either the lateral tegmental or the locus coerulean noradrenergic cell groups.25, 26, 27, 28, 29 This innervation is sexually dimorphic27 and particularly dense in the pudendal nucleus of motoneurones which supply the striated genital muscles involved in ejaculation.25, 26 Evidence has been presented that some of the noradrenergic innervation of the spinal cord may also originate from spinal cells.30 Neurones with contra-lateral axons in the spinal cord play an important role in motor coordination.31 For rhythmic motor synchrony, CPG must maintain the proper left–right coordination for locomotor activity. Neurones can be rhythmically excited via crossed pathways to elicit locomotor synchrony.31, 32 Interestingly, pudendal motoneurones possess prominent contra laterally projecting dendritic arborisations33 and exhibit immunoreactivity to α2A- and α2C-adrenoceptor subtypes.34 Thus, it is possible that coordinated rhythmic behaviour of the muscles involved in ejaculation could be mediated by noradrenergic pathways that cross the neurones of the spinal generator on the homonymous side to release ejaculation and that could be influenced by the noradrenergic agents acting at both α1- and α2-adrenoceptor subtypes. In support of this notion, the bilateral organisation of unilaterally generated activity in the spinal nucleus of the bulbocavernosus muscle has been demonstrated in a recent study.35 On the other side, it is important to state the possibility that the adrenergic agents here employed might have acted upon the SGE activating in turn sympathetic, parasympathetic and somatic neurones involved in penile erection and ejaculation. Further studies are necessary to dissect the individual actions of these drugs in the dynamic of FE in spinal male rats.
Methoxamine and yohimbine facilitate male rat ejaculation through the activation of α1-adrenoceptors and blockade of α2-adrenoceptors, respectively.14, 15 Several studies have demonstrated that α1-adrenoceptors are located on postsynaptic neural elements and α2-adrenoceptors on both presynaptic and postsynaptic sites.36 Thus, it can be suggested that the initiation and/or modulation of the GMPE could be modulated by the enhancement of noradrenergic transmission, through the yohimbine-mediated blockade of inhibitory autoreceptors, and by stimulation of postsynaptic α1-adrenoceptors, as observed after methoxamine administration. In line with present data, in a previous study, we demonstrated that the inhibition of the GMPE resulting from its repeated elicitation can be surmounted by the activation of α1-adrenoceptors as well as by the blockade of α2-adrenoceptors.18
Physiological and behavioural studies have suggested that the central noradrenergic system plays an important role in the modulation of motor patterns involved in ejaculation.12, 18 Experimental manipulations have shown that the noradrenergic system plays a facilitatory role in the control of ejaculation.10, 13, 14, 15 Thus, it could be thought that enhancement of noradrenergic transmission and/or direct α1-adrenoceptor stimulation might serve as potential therapeutic targets to favour the human male ejaculatory response. Needless to mention specific studies should be carried out to address this issue. As a whole, present findings provide the evidence that the GMPE might be importantly influenced by the noradrenergic system, which exerts a facilitatory control on the activity of the SGE.
References
Delcomyn F . Neural basis of rhythmic behavior in animals. Science 1980; 210: 492–498.
Grillner S . Neurobiological bases of the rhythmic motor acts in vertebrates. Science 1985; 228: 143–149.
Arshavsky I . Cellular and network properties in the functioning of the nervous system: from central pattern generators to cognition. Brain Res Rev 2003; 41: 229–267.
Carro-Juárez M, Cruz SL, Rodríguez-Manzo G . Evidence for the involvement of a spinal pattern generator in the control of the genital motor pattern of ejaculation. Brain Res 2003; 975: 222–228.
Holmes GH, Chapple WD, Leipheimer RE, Sachs BD . Electromyographic analysis of the male rat perineal uscles during copulation and reflexive erections. Physiol Behav 1991; 49: 1235–1246.
Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H et al. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann NY Acad Sci 1999; 860: 346–359.
Barbeau H, Chau C, Rossignol S . Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res Bull 1993; 30: 387–393.
Chau C, Barbeau H, Rossignol S . Effects of intrathecal α1- and α2-noradrenergic agonist and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol 1998; 79: 2941–2963.
Marcoux J, Rossignol S . Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. J Neurosci 2000; 20: 8577–8585.
Clark JT, Smith ER, Davidson JM . Enhancement of sexual motivation in male rats by yohimbine. Science 1984; 225: 847–849.
Clark JT, Smith ER, Davidson JM . Evidence for the modulation of sexual behavior by α-adrenoceptors in male rats. Neuroendocrinol 1985; 41: 36–43.
Hernández GM, Oropeza MV, Guevara MA, Cervantes M, Moralí G . Effects of intrathecal administration of adrenergic agonists on the frequency of copulatory pelvic thrusting of the male rat. Arch Med Res 1994; 25: 419–425.
McIntosh TK, Barfield RJ . Brain monoaminergic control of male reproductive behavior. III norepinephrine and the post-ejaculatory refractory period. Behav Brain Res 1984; 12: 275–281.
Meisel EL, Sachs BD . The physiology of male sexual behavior. In: Knobil E, Neill JD (eds.), The Physiology of Reproduction Vol. II, second edn. Raven Press Ltd: New York, 1994, pp. 4–106.
Rampin O . Pharmacology of alpha-adrenoceptors in male sexual function. Eur Urol 1999; 36: 103–106.
Clark JT, Karla SP, Karla PS . Effects of a selective alpha1 adrenoceptor agonist, methoxamine, on sexual behavior and penile reflexes. Physiol Behav 1987; 40: 747–753.
Clark JT . Suppression of copulatory behavior in male rats following central administration of clonidine. Neuropharmacol 1991; 30: 373–382.
Carro-Juárez M, Rodríguez-Manzo G . Yohimbine reverses the exhaustion of the coital reflex in spinal male rats. Behav Brain Res 2003; 141: 43–50.
Sachs BD, Garinello LD . Spinal pacemaker controlling sexual reflexes in male rats. Brain Res 1979; 171: 152–156.
McKenna KE . Ejaculation. In: Knobil E, Neill JD (eds.), Encyclopedia of Reproduction, Vol. 1. Academic Press: London, 1999, pp. 1002–1008.
Barbeau H, Chau C, Rossignol S . Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res Bull 1993; 30: 387–393.
Bracci E, Ballerini L, Nistri A . Spontaneous rhythmic burst induced by pharmacological block of inhibition in lumbar motoneurons of the neonatal rat spinal cord. J Neurophysiol 1996; 75: 640–647.
Barbeau H, Julien C, Rossignol S . The effects of clonidine and yohimbine on locomotion and cutaneous reflexes in the adult chronic spinal cat. Brain Res 1987; 437: 83–96.
Carro-Juárez M, Rodríguez-Manzo G . Sensory and motor aspects of the coital reflex in the spinal male rat. Behav Brain Res 2000; 108: 97–103.
Kojima M, Matsuura T, Amagai T, Iminashi I, Sano Y . Characteristic distribution of noradrenergic terminals on the anterior horn motoneurones innervating the perineal striated muscles in the rat. Anat Embriol 1985; 171: 267–273.
Lyons WE, Fristchy JM, Grzanna R . The noradrenergic neurotoxin-DSP4 eliminates the coerulospinal projection but spares projections of the A5 and A7 groups to the ventral horn of the spinal cord. J Neurosci 1989; 9: 1481–1498.
Monaghan EP, Breedlove SM . Brain sites projecting to the spinal nucleus of the bulbocavernosus. J Comp Neurol 1991; 307: 370–374.
Rajaofetra N, Ridet JL, Poulat P, Marlier L, Sandillon I, Geffard M et al. Immunocytochemical mapping of noradrenergic projections to the rat spinal cord with an antiserum against noradrenaline. J Neurocytol 1992; 21: 481–494.
Schroder HD, Skagerberg G . Catecholamine innervation of the caudal spinal cord in the rat. J Comp Neurol 1985; 242: 358–368.
Marshall KC . Catecholamines and their action in the spinal cord. In: Davidoff RA (ed.), Handbook of the Spinal Cord: Pharmacology, Vol. 1. Marcel Dekker: New York, Basel, 1993, pp. 275–328.
Kjaerulff O, Kiehn O . Crossed rhythmic synaptic input to motoneurones during selective activation of the contralateral spinal locomotor network. J Neurosci 1997; 17: 9433–9447.
Otah Y, Dubuc R, Grillner S . A new population of neurons with crossed axons in the lamprey spinal cord. Brain Res 1981; 564: 143–148.
Rose RD, Collins WF . Crossing dendrites may be a substrate for synchronized activation of penile motoneurones. Brain Res 1985; 337: 373–377.
Yaici ED, Rampin O, Calas A, Justin A, McKenna KE, Leclerc P et al. Giuliano F α2A and α2C adrenoceptors on spinal neurones controlling penile erection. Neuroscience 2002; 114: 945–960.
Foster AM, Sengelaub DR . Bilateral organization of unillaterally generated activity in lumbar spinal motoneurons of the rat. Brain Res 2004; 1009: 98–109.
Timmermans PBMW, van Zwieten PA . α2 adrenoceptors: classification, localization, mechanisms and targets for drugs. J Med Chem 1982; 25: 1389–1401.
Acknowledgements
We thank Mrs Angeles Ceja for technical assistance and animal caring.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carro-Juárez, M., Rodríguez-Manzo, G. α-Adrenergic agents modulate the activity of the spinal pattern generator for ejaculation. Int J Impot Res 18, 32–38 (2006). https://doi.org/10.1038/sj.ijir.3901393
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901393
Keywords
This article is cited by
-
Plant-Derived Supplements for Sexual Health and Problems: Part 1—Trends over the Past Decade
Current Sexual Health Reports (2019)
-
Effects of bupropion on the ejaculatory response of male rats
International Journal of Impotence Research (2014)
-
Effects of level and degree of spinal cord injury on male orgasm
Spinal Cord (2006)