Abstract
Recent fossil finds and experimental analysis of chick and mouse embryos highlighted the lateral fin fold theory, which suggests that two pairs of limbs in tetrapods evolved by subdivision of an elongated single fin1. Here we examine fin development in embryos of the primitive cartilaginous fish, Scyliorhinus canicula (dogfish) using scanning electron microscopy and investigate expression of genes known to be involved in limb positioning, identity and patterning in higher vertebrates. Although we did not detect lateral fin folds in dogfish embryos, Engrailed-1 expression suggests that the body is compartmentalized dorso-ventrally. Furthermore, specification of limb identity occurs through the Tbx4 and Tbx5 genes, as in higher vertebrates. In contrast, unlike higher vertebrates, we did not detect Shh transcripts in dogfish fin-buds, although dHand (a gene involved in establishing Shh) is expressed. In S. canicula, the main fin axis seems to lie parallel to the body axis. ‘Freeing’ fins from the body axis and establishing a separate ‘limb’ axis has been proposed to be a crucial step in evolution of tetrapod limbs2,3. We suggest that Shh plays a critical role in this process.
Similar content being viewed by others
Main
The continuous fin fold theory was once considered to be “more an established fact than a theory”3 but was subsequently questioned because of inconsistencies in the fossil record and in the embryology of cartilaginous fish4. Recently discovered fossils of the earliest-known Cambrian vertebrates, however, seem to have had lateral ribbon-shaped fins5, and recent analysis of muscle formation in dogfish has shown uniform budding from somites in both limb and inter-limb regions6. The embryos of some primitive cartilaginous fish have been reported to have lateral fin folds7.
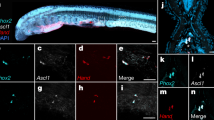
We therefore re-examined Scyliohinus canicula dogfish embryos from pre-fin-bud to fin-bud stages using scanning electron microscopy. In pre-fin-bud embryos (stage 22; ref. 8), no signs of lateral ridges could be detected (Fig. 1a). By stage 24 (about 3 days later), small shelf-like buds (pectoral fin buds) project out of the body wall just ventral to somites 5–13 (Fig. 1b). These buds are rimmed distally by a raised structure, the apical fold, and have discrete edges both anteriorly and posteriorly with no sign of continuation of the ‘shelf’ posteriorly along the flank. A thickening which will develop into pelvic fin buds is detected ventral to somites 27–36 (arrows; Fig. 1b). In later-stage embryos (stage 27, about 10–12 days older), pelvic fin buds (opposite somites 27–36) are present in addition to pectoral fin buds. Both types of bud are semicircular flaps rimmed with well defined apical folds. In between the buds, the body wall is quite smooth (Fig. 1c). Thus, we did not detect lateral folds in S. canicula embryos.
a, Stage 22; b, stage 24; and c, stage 27. a–c, Whole embryos. Pectoral and pelvic fins develop opposite somites 5–13 and 27–36 (arrows in b). Scale bars, 1 mm. d, Expression pattern of ScEngrailed-1 in S. canicula embryos at stage 23. ScEngrailed-1 expression is observed in the border of mesencephalon and metencephalon (arrowhead) and somites (arrows). Scale bar, 1 mm. e, f, Cross-sections of stage 23 and 26 embryos at pectoral fin-bud level. ScEngrailed-1 expression in presumptive fin buds region and fin buds indicated by arrows. Scale bars, 300 µm in e, 500 µm in f. g–j, ScTbx4 and ScTbx5 genes expression pattern in paired appendages in S. canicula. g, i, ScTbx5 expression at stage 28. g, Pectoral fin. i, Pelvic fin. h, j, ScTbx4 expression at stage 28. h, Pectoral fin. J, Pelvic fin. so, somite; pec, pectoral fin; pel, pelvic fin. Scale bars, 1 mm.
In chick embryos, the body ectoderm is compartmentalized with respect to the dorso-ventral axis in both limb-forming and flank regions9. This compartmentalization serves to position limbs laterally, because the apical ectodermal ridge, the thickened epithelium that mediates limb-bud outgrowth, arises at the compartment boundary. Engrailed-1 expression is restricted to the ventral compartment of the body ectoderm of both chick and mouse embryos at pre-limb-bud stages and then in the ventral ectoderm and the ventrical apical ridge of limb buds9,10. To gain insight into ectoderm compartmentalization and whether fin buds of S. canicula arise at an ectodermal boundary of Engrailed-1 expression, we isolated complementary DNA fragments of ScEngrailed-1 and carried out whole-mount in situ hybridization on dogfish embryos. At both pre-fin (stage 23; Fig. 1d) and fin-bud stages, ScEngrailed-1 transcripts were clearly seen at the border of the mesencephalon and metencephalon and in the somites. When these whole mounts were sectioned, ScEngrailed-1 was seen to be expressed in the ventral ectoderm of the body of dogfish embryos both in the presumptive pectoral fin-bud region (stage 23 embryo; Fig. 1e) and in the pectoral fin buds themselves (stage 26 embryo; Fig. 1f). At the later stage, it was clear that, in the pectoral fin, the boundary between ScEngrailed-1-expressing ectoderm and non-ScEngrailed-1-expressing ectoderm lies at the mid-point of the apical fold, thus respecting the same boundary as Engrailed-1 expression in chick and mouse limb buds. Thus, the pattern of Engrailed-1 expression in S. canicula in relation to fin development suggests that the body of the dogfish is compartmentalized as in higher vertebrates. The mechanism that determines the dorso-ventral position of tetrapod limbs appears to be an ancient feature of the gnathostome body plan and could form the basis for a lateral fin fold in an ancestral vertebrate (Fig. 2a).
a, Hypothetical ancestral vertebrate with lateral fin folds, expressing Tbx4/5, positioned at the Engrailed-1 expression boundary. b, Tbx4/5 cluster duplication took place before the ancestor of cartilaginous fishes (or in that lineage) and inter-limb formation was suppressed. Tbx5 and Tbx4 are expressed in the pectoral and pelvic fins, respectively. c, Following the acquisition of Shh expression, the main axes of paired fins are freed from the body wall and establish the proximo-distal axes of the appendages. D, dorsal; V, ventral; A, anterior; P, posterior; Pr, proximal; Di, distal.
According to fin fold theory, two pairs of tetrapod limbs evolved by subdivision of a single lateral fin. In contrast, other recent ideas suggest that an ancestral vertebrate had a single pair of fins; either posterior11 or anterior12. In tetrapods and teleosts, the identity of each pair of appendages is determined by expression of T-box genes Tbx5 and Tbx4, which are expressed in the anterior and posterior appendages, respectively13. In contrast, the cephalochordate Amphioxus has only one such gene, AmphiTbx4/5 (ref. 14), although its expression pattern has not been reported. Tbx4 and Tbx5 genes in tetrapods are thought to have arisen by duplication of this single ancestral gene. Therefore, we analysed the T-box gene complement in dogfish by employing polymerase chain reaction after reverse transcription of RNA (RT–PCR), using two sets of degenerate oligonucleotide primers. With each set of primers we obtained distinct PCR products, showing that the dogfish has both Tbx4 and Tbx5 genes. The deduced amino-acid sequences of S. canicula Tbx4 and Tbx5 T-box regions, aligned with those of zebrafish, chick, human homologues and the T-box region of AmphiTbx4/5 are shown in Supplementary Information. The T-box region alignment provides further evidence that ScTbx4 and ScTbx5 belong to Tbx4 and Tbx5 subfamilies respectively. Skate embryos may also have a Tbx5 gene13. In stage 28 dogfish embryos, ScTbx5 expression was observed in pectoral fin buds (Fig. 1g), dorsal eye and heart, but not in pelvic fin buds (Fig. 1i). On the other hand, ScTbx4 expression was not detected in pectoral fin buds (Fig. 1h), but was seen in pelvic fin buds (Fig. 1j). These results show that a Tbx4/5 gene cluster duplication occurred before the origin of cartilaginous fish (Fig. 2b). Furthermore, as in tetrapods and teleosts, Tbx5 and Tbx4 are expressed in the anterior and posterior appendages, respectively.
An important recent finding with respect to the fin fold theory is that the inter-limb region in tetrapods has the potential to form limbs. Balinsky first discovered this in newts15 but limb-forming potential has now been described in both chick and mouse embryos16,17. This remarkable property is associated with the potential of flank cells to form a polarizing region17. The polarizing region is the major limb ‘organizer’ and consists of mesenchyme at the posterior limb bud margin that expresses Shh18. We isolated an ScShh fragment using degenerate PCR and used this fragment to examine the ScShh expression pattern in stage 27 (Fig. 3a–c) and 28 (not shown) dogfish embryos. ScShh expression was observed in the floor plate, the notochord (Fig. 3b), the branchial arches (Fig. 3c), the brain and the optic vesicle, but surprisingly we could not detect Shh expression in either pectoral (Fig. 3a) or pelvic fin buds. We extended the length of the ScShh probe using another set of primers, but even with this longer probe we were unable to detect a signal in fin buds. We further attempted to detect ScShh transcripts in stage 27 and 28 embryos by RT–PCR but although we were able to detect ScShh in the body, ScShh was not detected in either pectoral or pelvic fin buds (Fig. 3d). In zebrafish (Danio rerio), another member of the hedgehog gene family, Tiggy-winkle hedgehog (Twhh), has been reported19. It seems unlikely that the Shh we have isolated is Twhh because Twhh is expressed only in the floorplate in zebrafish embryos19 and ScShh transcripts were observed in both the floorplate and the notochord in S. canicula embryos (Fig. 3b). Thus it appears that Shh must be expressed at very low levels, if at all, in the fin buds of dogfish embryos.
a–c, Expression pattern of ScShh in stage 27 S. canicula embryos. ScShh is expressed in the floor plate, the notochord and the branchial arches (b and c) , but not in the pectoral fin bud (a). Scale bars, 500 µm. d, ScShh expression of the stage 27 embryo was analysed by RT–PCR. ScShh expression was observed from body tissue (lane 1), but not from either pectoral (lane 2) nor pelvic fin-bud tissue (lane 3). 18S rRNA expression is shown as an internal control. e, f, Expression pattern of ScdHand in stage 27 S. canicula embryo. ScdHand expression was observed in the posterior part of paired fin buds (arrows) and the dorsal and anal fin buds (arrowheads). Scale bars, 1 mm. g, Expression pattern of ScBmp4 in stage 28 S. canicula embryo. ScBmp4 expression was observed throughout paired fin buds (arrows) and dorsal and anal fin buds (arrowheads). pec, pectoral fin; fp, floor plate; nc, notochord; mc, mouth cavity; br, branchial arch. Scale bar, 1 mm.
In higher vertebrates, genes that are involved in regulation of Shh expression (for example dHand20) and that are targets of Shh signalling (for example, Bmp4; ref. 21) have been identified. We cloned these two genes from dogfish by degenerate PCR and examined their expression patterns. ScdHand transcripts were found in the same regions as in higher vertebrates, including the heart, the head and the posterior part of the fin buds where the polarizing region normally develops (Fig. 3e and f). In addition, ScdHand was detected at the posterior margins of the dorsal and anal fin buds (Fig. 3e). In higher vertebrates, Bmp4 transcripts are abundant in anterior mesenchyme and expression is negatively regulated by Shh21. In dogfish fin buds, ScBmp4 transcripts were detected throughout the fin bud (Fig. 3g), as might be predicted if ScShh expression is absent.
Our inability to detect Shh expression in dogfish fin buds was at first surprising. It could be that the acquisition of Shh expression in appendages was of primary importance for evolution of the distal regions. However, another possibility is that Shh could play a role in the ‘freeing’ of fins and the establishment of a proximo-distal limb axis (Fig. 2c). In S. canicula, as in many other cartilaginous fish, the metapterygium—the main long bone of the fin—develops parallel to the main body axis, in the proximal part of the fin bud next to the body wall (Fig. 4A). One hypotheses, which is still controversial, is that the metapterygial axis rotated outwards from the body wall during evolution2,3 (Fig. 4A). Fate maps of cells at the base of a chick wing-bud show that anterior cells remain at the proximal part of the limb, but cells in the middle and posterior regions extend posteriorly and distally as the wing bud grows out (Fig. 4B–D). The relative displacement of these populations is illustrated by the curved red line in Fig. 4C. Support for the idea that Shh is involved in this reorientation comes unexpectedly from a recent detailed analysis of Shh null mutant mouse embryos, which described the limbs as being ‘trapped’ within the body wall22.
The red line represents the position of the major axis. In S. canicula, the metapterygial stem has retained its original position in the body wall; in the fossils Xenacanthus and Neoceratodus, the metapterygial stem has became freed from the body wall and forms the principal axis3,28. B, Diagram of stage 20 chick wing-bud showing positions at which DiI was injected. Scale bar, 250 µm. C, Map of representative results obtained in chick wing at 96 h for populations of labelled cells. Scale bar, 500 µm. The relative displacement of cells is illustrated by the red line. D, Micrographs showing fate maps of wing buds 96 h after DiI injection at the positions a–d respectively indicated in B and C. Scale bars 500 µm.
Our data suggest that the dogfish may represent an important intermediate form in the evolution of tetrapod limbs (Fig. 2b). Although we did not detect lateral fin folds in dogfish, we suggest that an ancestral vertebrate had already been compartmentalized dorso-ventrally with Engrailed-1 expression ventrally and had lateral fin folds that expressed a single Tbx4/5 gene (Fig. 2a). This assumes, however, that a lateral fin fold is likely to be primitive. It will be interesting to characterize these features of the body plan in hagfish and lampreys. Our view of the origin of tetrapod limbs also suggests that more attention should be given to mechanisms that inhibit limb development. Many genes that are thought to be involved in limb formation in higher vertebrates, including Fgf10 and Hoxd9, are initially expressed in both limb and inter-limb regions and then ‘switch off’ in the inter-limb regions.
Methods
Scanning electron microscopy
S. canicula eggs were incubated at 16 °C in sea water and staged according to ref. 8. Embryos were fixed in Peter's fixative (1.25% glutaraldehyde, 1% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.2) at 4 °C. After post-fixation in 1% osmium in cacodylate buffer, specimens were dehydrated in graded ethanol, placed in acetone, critically point-dried, sputter-coated with gold/palladium and viewed on a Hitachi S-4700 field-emission scanning electron microscope.
Identification of S. canicula gene homologues
We identified fragments of S. canicula Tbx4 (275 base pairs, bp), Tbx5 (278 bp), Engrailed-1 (326 bp), dHand (267 bp), Bmp4 (243 bp) and Shh (297 bp) from complementary DNA pools prepared from stage 24–28 embryos8 using degenerate primers. The amino-acid sequences used for degenerate primers were: ScTbx4, KFCDNKWM and TAFCTHVF; ScTbx5, YKFADNKW and TAFCTHVF; ScEngrailed-1, WPAWVYCT and MAQGLYNH; ScdHand, ECIPNVP and WPQHVWA; ScBmp4, WIVAPPG and DMVVEG. To avoid amplification of Dhh and Ihh, the ScShh fragment was obtained by nested PCR. The amino-acid sequences used for degenerate primers were: ScShh first PCR, KQFIPNVA and AHIHCSV ScShh second, nested PCR, PNYNPDI and GFDWVYYE. The longer ScShh (348 bp) fragment was obtained from cDNA pools of stage 27 S. canicula embryos by RT–PCR using a degenerate forward primer and a ScShh-specific reverse primer (GRYEGKIT and TTCGTAGTAGACCCAGTC). The nucleotide sequences of ScEn1, ScShh, ScTbx4, ScTbx5, ScdHand and ScBmp4 cDNA are deposited in the GenBank database under the accession numbers: AF393834–AF393837, AY057890 and AY057891.
In situ hybridization
S. canicula embryos were removed from their egg casings and dissected from the yolk mass. Wholemount in situ hybridization on younger S. canicula embryos was carried out as previously described23 and this is based on methods used for other vertebrate embryos24. Older embryos were treated with dimethyl sulphoxide (DMSO) instead of proteinase K treatment by placing them in 2 ml of DMSO/methanol (1:1) on ice until they sank. Then 0.5 ml of 10% Triton X-100 (Sigma) in distilled water was added, and the embryos were incubated for an additional 20 minutes at room temperature25,26. After washing in PBT (1% Tween 20 (Sigma) in PBS), embryos were hybridized with probes as described previously for chick embryos24. Some whole-mount in situ samples were embedded in gelatin, and frozen sections were cut.
RT–PCR
RT–PCR was performed as previously described27. The primers used for PCR amplification of ScShh (172 bp) were 5′-GAGCTGACAGGCTGATGACAC-3′ and 5′-TGGTGATGTCCACAGCTCGGC-3′. The PCR cycle was at 96 °C for 20 seconds, 55 °C for 40 seconds and 72 °C for 1 minute for 32 cycles. Relative levels of transcripts were compared to levels of internal control using 18S ribosomal RNA primers (Ambion). Both ScShh and 18S rRNA primers were added into the same reaction solution.
Observation of cartilaginous pattern
S. canicula embryos to be stained for cartilage were fixed in 5% TCA (trichloroacetic acid), stained in 0.1% Alcian blue in 70% acid alcohol, dehydrated in ethanol and cleared in methyl salicylate.
DiI labelling
DiI (1,1-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchloride; Molecular Probes; 3 mg ml-1 in DMSO) was injected into chick wing-bud using a micropipette to label a small group of cells. Embryos were then incubated for 96 h and fixed in 4% paraformaldehyde in PBS. The average size of the initial DiI injected dot was 40–50 µm.
References
Thacher, J. K. Median and paired fins, a contribution to the history of vertebrate limbs. Trans. Conn. Acad. 3, 281–310 (1877).
Jarvik, E. in Basic Structure and Evolution of Vertebrates 109–131 (Academic, London, 1980).
Moy-Thomas, J. A. The evolution of the pectoral fins of fishes and the tetrapod forelimb. School Sci. Rev. 36, 592–599 (1936).
Coates, M. I. The origin of vertebrate limbs. Development (1994 Suppl.), 169–180 (1994).
Shu, D.-G. et al. Lower Cambrian vertebrates from South China. Nature 402, 42–46 (1999).
Neyt, C. et al. Evolutionary origins of vertebrate appendicular muscle. Nature 408, 82–86 (2000).
Balfour, F. M. The development of elasmobranch fishes. J. Anat. Physiol. Lond. 11, 128–172 (1876).
Ballard, W. W., Mellinger, J. & Lechenault, H. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliohinidae). J. Exp. Zool. 267, 318–336 (1993).
Altabef, M., Clarke, J. D. & Tickle, C. Dorso-ventral ectodermal compartments and origin of apical ectodermal ridge in developing chick limb. Development 124, 4547–4556 (1997).
Tanaka, M. et al. Apical ectodermal ridge induction by the transplantation of En-1-overexpressing ectoderm in chick limb bud. Dev. Growth Differ. 40, 423–429 (1998).
Tabin, C. & Laufer, E. Hox genes and serial homology. Nature 361, 692–693 (1993).
Coates, M. I. Hox genes, fin folds and symmetry. Nature 364, 195–196 (1993).
Tamura, K. et al. Evolutionary aspects of positioning and identification of vertebrate limbs. J. Anat. 199, 195–204 (2001).
Ruvinsky, I., Silver, L. M. & Gibson-Brown, J. J. Phylogenetic analysis of T-box genes demonstrates the importance of amphioxus for understanding evolution of the vertebrate genome. Genetics 156, 1249–1257 (2000).
Balinsky, B. I. Das extremitaetenseitenfeld, seine ausdehnung und beschaffenheit. Roux Arch. Dev. Biol 130, 704–736 (1933).
Cohn, M. J., Izpisua-Belmonte, J. C., Abud, H., Heath, J. K. & Tickle, C. Fibroblast growth factors induce additional limb development from the flank of chick embryo. Cell 80, 739–746 (1995).
Tanaka, M. et al. Distribution of polarizing activity and potential for limb formation in mouse and chick embryos and possible relationship to polydactyly. Development 127, 4011–4021 (2000).
Riddle, R. D., Johnson, R. L., Laufer, E. & Tabin, C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401–1416 (1993).
Ekker, S. C. et al. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr. Biol. 5, 44–55 (1995).
Cohn, M. J. Giving limbs a hand. Nature 406, 953–954 (2000).
Tümpel, S. et al. Antero-posterior signaling in vertebrate limb development and stripes of Tbx3 expression. Dev. Biol (submitted).
Kraus, P., Fraidenraich, D. & Loomis, C. A. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech. Dev. 100, 45–58 (2001).
Mazan, S., Jaillard, D., Baratte, B. & Janvier, P. Otx1 gene-controlled morphogenesis of the horizontal semicircular canal and the origin of the gnathostome characteristics. Evol. Dev. 2, 186–193 (2000).
Wilkinson, D. G. In Situ Hybridization: A Practical Approach 75–83 (IRL Press/Oxford Univ. Press, Oxford, 1992).
Kuratani, S., Ueki, T., Aizawa, S. & Hirano, S. Peripheral development of cranial nerves in a cyclostome, Lampetra japonica: morphological distribution of nerve branches and the vertebrate body plan. J. Comp. Neurol. 384, 482–500 (1997).
Schlosser, G. & Roth, G. Evolution of nerve development in frogs. I. The development of the peripheral nervous system in Discoglossus pictus (Discoglossidae). Brain Behav. Evol. 50, 61–93 (1997).
Münsterberg, A. E., Kitajewski, J., Bumcrot, D. A., McMahon, A. P. & Lassar, A. B. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 9, 2911–2922 (1995).
Coates, M. I. Limb evolution. Fish fins or tetrapod limbs—a simple twist of fate? Curr. Biol. 5, 844–848 (1995).
Acknowledgements
We are grateful to A. Wells for his assistance in maintenance of S. canicula embryos, S. Kuratani for information about S. canicula developmental studies before publication, S. Mazan for technical advice and ScOtx1and ScOtx2 cDNA as positive control probes for establishing in situ hybridization methods and N. Helps for DNA sequencing. M.T. is supported by JSPS Postdoctoral Fellowships for Research Abroad, JSPS Research Fellowships for Young Scientists and the Inoue Research Award for Young Scientists. A.M. is supported by a Wellcome Trust research Career Development Award. C.T. is Foulerton Research Professor of The Royal Society.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Tanaka, M., Münsterberg, A., Anderson, W. et al. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature 416, 527–531 (2002). https://doi.org/10.1038/416527a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416527a
This article is cited by
-
Muscle development in the shark Scyliorhinus canicula: implications for the evolution of the gnathostome head and paired appendage musculature
Frontiers in Zoology (2017)
-
Similarity of morphological composition and developmental patterning in paired fins of the elephant shark
Scientific Reports (2017)
-
The fin-to-limb transition as the re-organization of a Turing pattern
Nature Communications (2016)
-
Genoarchitecture of the rostral hindbrain of a shark: basis for understanding the emergence of the cerebellum at the agnathan–gnathostome transition
Brain Structure and Function (2016)
-
Head, Body and Fins: Patterns of Morphological Integration and Modularity in Fishes
Evolutionary Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.