Abstract
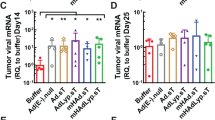
The IGF-I receptor (IGF-IR) has an important role in malignant disease and is the target of several drugs presently in clinical trials. Gene therapy has been explored as cancer treatment, mainly for delivery of genes that induce cell death or enhance the immunological response to cancer. Previously, we have shown that the implantation of autologous bone-marrow stromal cells producing a soluble form of IGF-IR (sIGFIR) inhibited experimental liver metastasis of several tumor types in mice. Here, we evaluated the utility of adenovirus-based gene delivery for generating therapeutically effective plasma levels of this decoy. We constructed a third generation gutless adenovirus expressing sIGFIR and found that HEK-293 cells transduced by this, but not control adenoviruses, secreted soluble receptor protein that blocked IGF-I-induced tumor cell migration, proliferation and survival in vitro. Following virus injection in vivo, viral DNA was detectable by PCR in several host organs, particularly the liver, and this resulted in the production of measurable sIGFIR plasma levels for up to 21 days post injection. In mice producing virus-encoded sIGFIR, experimental liver metastasis was inhibited, indicating that sIGFIR levels were therapeutically effective. The results show that adenovirus-based delivery of inhibitory soluble proteins can provide an effective anticancer strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S . Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006; 13: 668–676.
Samani AA, Yakar S, LeRoith D, Brodt P . The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 2007; 28: 20–47.
Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. Embo J. 1986; 5: 2503–2512.
Garrett TP, McKern NM, Lou M, Frenkel MJ, Bentley JD, Lovrecz GO et al. Crystal structure of the first three domains of the type-1 insulin-like growth factor receptor. Nature 1998; 394: 395–399.
LeRoith D, Werner H, Beitner-Johnson D, Roberts CT . Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev 1995; 16: 143–163.
Sachdev D, Yee D . Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 2007; 6: 1–12.
Rodon J, DeSantos V, Ferry RJ, Kurzrock R . Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther 2008; 7: 2575–2588.
Zha J, Lackner MR . Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin Cancer Res 2010; 16: 2512–2517.
Gao J, Chang YS, Jallal B, Viner J . Targeting the insulin-like growth factor axis for the development of novel therapeutics in oncology. Cancer Research 2012; 72: 3–12.
Becker CM, Farnebo FA, Iordanescu I, Behonick DJ, Shih MC, Dunning P et al. Gene therapy of prostate cancer with the soluble vascular endothelial growth factor receptor Flk1. Cancer Biol Ther 2002; 1: 548–553.
Trieu Y, Wen XY, Skinnider BF, Bray MR, Li Z, Claudio JO et al. Soluble interleukin-13Ralpha2 decoy receptor inhibits Hodgkin's lymphoma growth in vitro and in vivo. Cancer Res 2004; 64: 3271–3275.
Tseng JF, Farnebo FA, Kisker O, Becker CM, Kuo CJ, Folkman J et al. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery 2002; 132: 857–865.
Rudge JS, Thurston G, Davis S, Papadopoulos N, Gale N, Wiegand SJ et al. VEGF trap as a novel antiangiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene- based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol 2005; 70: 411–418.
Ferry N, Heard JM . Liver-directed gene transfer vectors. Hum Gene Ther 1998; 9: 1975–1981.
Kochanek S, Schiedner G, Volpers C . High-capacity 'gutless' adenoviral vectors. Curr Opin Mol Therap 2001; 3: 454–463.
Reddy PS, Sakhuja K, Ganesh S, Yang L, Kayda D, Brann T et al. Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol Ther 2002; 5: 63–73.
Vetrini F, Ng P . Gene therapy with helper-dependent adenoviral vectors: current advances and future perspectives. Viruses 2010; 2: 1886–1917.
Belalcazar LM, Merched A, Carr B, Oka K, Chen KH, Pastore L et al. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation 2003; 107: 2726–2732.
Schiedner G, Morral N, Parks RJ, Wu Y, Koopmans SC, Langston C et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet 1998; 18: 180–183.
Samani AA, Chevet E, Fallavollita L, Galipeau J, Brodt P . Loss of tumorigenicity and metastatic potential in carcinoma cells expressing the extracellular domain of the type 1 insulin-like growth factor receptor. Cancer Res 2004; 64: 3380–3385.
Wang N, Fallavollita L, Nguyen L, Burnier J, Rafei M, Galipeau J et al. Autologous bone marrow stromal cells genetically engineered to secrete an igf-I receptor decoy prevent the growth of liver metastases. Mol Ther 2009; 17: 1241–1249.
Brodt P . Characterization of two highly metastatic variants of Lewis lung carcinoma with different organ specificities. Cancer Res 1986; 46: 2442–2448.
Umana P, Gerdes CA, Stone D, Davis JR, Ward D, Castro MG et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat Biotechnol 2001; 19: 582–585.
Gilbert R, Dudley RW, Liu AB, Petrof BJ, Nalbantoglu J, Karpati G . Prolonged dystrophin expression and functional correction of mdx mouse muscle following gene transfer with a helper-dependent (gutted) adenovirus-encoding murine dystrophin. Hum Mol Genet 2003; 12: 1287–1299.
Gilbert R, Liu A, Petrof B, Nalbantoglu J, Karpati G . Improved performance of a fully gutted adenovirus vector containing two full-length dystrophin cDNAs regulated by a strong promoter. Mol Ther 2002; 6: 501–509.
Meneses-Acosta A, Dormond E, Jacob D, Tom R, Bernier A, Perret S et al. Development of a suspension serum-free helper-dependent adenovirus production system and assessment of co-infection conditions. J Virol Methods 2008; 148: 106–114.
Gilbert R, Nalbantoglu J, Howell JM, Davies L, Fletcher S, Amalfitano A et al. Dystrophin expression in muscle following gene transfer with a fully deleted ("gutted") adenovirus is markedly improved by trans-acting adenoviral gene products. Hum Gene Ther 2001; 12: 1741–1755.
Dudley RW, Lu Y, Gilbert R, Matecki S, Nalbantoglu J, Petrof BJ et al. Sustained improvement of muscle function one year after full-length dystrophin gene transfer into mdx mice by a gutted helper-dependent adenoviral vector. Hum Gene Ther 2004; 15: 145–156.
Burnier JV, Wang N, Michel RP, Hassanain M, Li S, Lu Y et al. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene 2011; 30: 3766–3783.
Brunetti-Pierri N, Ng P . Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum Mol Genet 2011; 20: R7–13.
Brodt P, Fallavollita L, Khatib AM, Samani AA, Zhang D . Cooperative regulation of the invasive and metastatic phenotypes by different domains of the type I insulin-like growth factor receptor beta subunit. J Biol Chem 2001; 276: 33608–33615.
Candolfi M, Xiong W, Yagiz K, Liu C, Muhammad AKMG, Puntel M et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci 2010; 107: 20021–20026.
Kim CY, Park SH, Jeong M, Kwon OS, Doh H, Kang SH et al. Preclinical studies for pharmacokinetics and biodistribution of Ad-stTRAIL, an adenovirus-based gene therapy delivering secretable trimetic TRAIL. Exp Mol Med 2011; 43: 580–586.
Muhammad AK, Puntel M, Candolfi M, Salem A, Yagiz K, Farrokhi C et al. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin Pharmacol Ther 2010; 88: 204–213.
Auguste P, Fallavollita L, Wang N, Burnier J, Bikfalvi A, Brodt P . The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol 2007; 170: 1781–1792.
Khatib AM, Auguste P, Fallavollita L, Wang N, Samani A, Kontogiannea M et al. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol 2005; 167: 749–759.
Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, Brodt P . Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res 2002; 62: 5393–5398.
Wu Y, Brodt P, Sun H, Mejia W, Novosyadlyy R, Nunez N et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res 2010; 70: 57–67.
Kelley KW, Weigent DA, Kooijman R . Protein hormones and immunity. Brain Behav Immun 2007; 21: 384–392.
Gualberto A, Pollak M . Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene 2009; 28: 3009–3021.
Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol 2009; 27: 2516–2522.
Karp DD, Pollak MN, Cohen RB, Eisenberg PD, Haluska P, Yin D et al. Safety, pharmacokinetics, and pharmacodynamics of the insulin-like growth factor type 1 receptor inhibitor figitumumab (CP-751,871) in combination with paclitaxel and carboplatin. J Thoracic Oncol 2009; 4: 1397–1403.
Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA 2001; 98: 4605–4610.
Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, Finegold MJ et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther 2007; 15: 732–740.
Acknowledgements
This study was supported by Canadian Institute for Health Research grants MOP-77677 and MOP-81201 (to PB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Rights and permissions
About this article
Cite this article
Wang, N., Lu, Y., Pinard, M. et al. Sustained production of a soluble IGF-I receptor by gutless adenovirus-transduced host cells protects from tumor growth in the liver. Cancer Gene Ther 20, 229–236 (2013). https://doi.org/10.1038/cgt.2013.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.10
Keywords
This article is cited by
-
Acute knockdown of the insulin receptor or its substrates Irs1 and 2 in 3T3-L1 adipocytes suppresses adiponectin production
Scientific Reports (2016)
-
Exogenous administration of protease-resistant, non-matrix-binding IGFBP-2 inhibits tumour growth in a murine model of breast cancer
British Journal of Cancer (2014)