Abstract
Elevated expression of heat shock protein 90 (HSP90) has been found in kidneys and serum of systemic lupus erythematosus (SLE) patients and MRL/Mp-Faslpr/Faslpr (MRL/lpr) autoimmune mice. We investigated if inhibition of HSP90 would reduce disease in MRL/lpr mice. In vitro, pretreatment of mesangial cells with HSP90 inhibitor Geldanamycin prior to immune-stimulation showed reduced expression of IL-6, IL-12 and NO. In vivo, we found HSP90 expression was elevated in MRL/lpr kidneys when compared to C57BL/6 mice and MRL/lpr mice treated with HSP90 inhibitor 17-DMAG. MRL/lpr mice treated with 17-DMAG showed decreased proteinuria and reduced serum anti-dsDNA antibody production. Glomerulonephritis and glomerular IgG and C3 were not significantly affected by administration of 17-DMAG in MRL/lpr. 17-DMAG increased CD8+ T cells, reduced double-negative T cells, decreased the CD4/CD8 ratio and reduced follicular B cells. These studies suggest that HSP90 may play a role in regulating T-cell differentiation and activation and that HSP90 inhibition may reduce inflammation in lupus.
Similar content being viewed by others
Introduction
Heat shock protein 90 (HSP90) is increasingly recognized as a potential therapeutic target in various diseases and has been shown to act as a signaling mediator for inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6) production as well as play a crucial role in modulation of Toll-like receptor (TLR) activation.1, 2, 3 HSP90 has also been found to be elevated in some subsets of systemic lupus erythematosus (SLE) patients, but its role in the disease is still unknown.4, 5, 6, 7 Elevated serum levels of HSP90 have been correlated to elevated levels of IL-6.8 Furthermore, the glomeruli of some SLE patients have been found to have deposits of HSP90.9 In addition, HSP90 and its endoplasmic reticulum homologue, glycoprotein 96 (gp96), have been linked to autoimmunity.3, 10, 11
There are several compounds in clinical trials that target HSP90 for the treatment of cancer.12, 13 The naturally occurring benzoquinoid ansamycin known as Geldanamycin (GA) has been found to be a potent and specific HSP90 inhibitor. It binds to an ATP binding site unique to HSP90.14 This binding prevents conformational changes in HSP90 that are required for chaperone function.15 Unfortunately, GA has low systemic duration (3–4 h) and causes acute hepatic necrosis when administered in vivo.16 However, the GA derivative 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) exhibits similar effectiveness at inhibiting HSP90 with significantly reduced hepatotoxicity, increased systemic duration (more than 24 h) and greater water solubility.17, 18, 19 GA has been shown to reduce the inflammatory response in a murine sepsis model.20 Recently, GA was also used to reduce inflammation as a treatment for rheumatoid arthritis.21 In addition to GA and 17-DMAG, HSP90 can also be inhibited by novobiocin, epigallocatechin gallate, cisplatin and others.22, 23 These alternative inhibitors differ from GA and 17-DMAG in that they exert additional effects on cellular mechanisms beyond the blockade of HSP90.24 Recently, a synthetic HSP90 inhibitor (EC144) was found to decrease disease severity in mouse and rat models of induced arthritis.25 Also, the gp96 inhibitor, (S)-methyl 2-(4,6-dimethoxypyrimidine-2-yloxy)-3-methylbutanoate, was shown to reduce the severity of SLE-like disease in transgenic mice.11
MRL/lpr mice serve as a model to study human SLE as they exhibit similar manifestations to the human disease including glomerulonephritis (GN), vasculitis and arthritis.26 Mesangial cells isolated from MRL/lpr mice exhibit increased sensitivity when immune-stimulated by inflammatory cytokines and chemokines such as tumor necrosis factor-α (TNF-α), IL-1β, interferon-γ (IFN-γ) or lipopolysaccharide (LPS).27, 28, 29 In SLE, increased sensitivity to immune stimulation leads to higher expression of TNF-α, IL-6, IL-12 and increased nitric oxide (NO) production.29, 30, 31
Lupus mice excrete elevated levels of protein in their urine and possess increased serum IgG antibodies to double-stranded DNA (anti-dsDNA) or anti-nuclear antibodies.32, 33, 34, 35, 36, 37 Renal histological markers of glomerular lesions and glomerular deposition of IgG and C3 can also indicate disease.36, 38, 39, 40, 41 Splenocyte populations are often altered in autoimmune mice including differences in T-cell subtypes (including T regulatory cells (TREG)) and also alterations in B-cell subtypes.36, 37, 42, 43 HSP90 has been implicated in studies investigating similar autoimmune diseases and it has been reported that T cells respond to extracellular HSP90 by increasing their anti-inflammatory cytokines.44 Other recent work has shown that T-cell activation by the T-cell receptor is dependent on functioning HSP90.45
Despite findings suggesting a role for HSP90 in SLE, or the anti-inflammatory effects of HSP90 inhibition, little has been published exploring the effect of HSP90 inhibition in SLE. Based on the evidence that suggests a possible link between HSP90 and lupus, we explored the relationship of HSP90 and SLE by studying the effect of HSP90 inhibition in the MRL/lpr lupus mouse model.
Materials and methods
Mesangial cell culture
SV40 MES13 cells (MES13 cells) were purchased from the American Type Culture Collection (Rockville, MD, USA) and cultured in a 3∶1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium with 14 mM HEPES, supplemented with fetal bovine serum and penicillin/streptomycin at a final concentration of 5% and 1%, respectively. MES13 cells between passages 10 and 20 were used for the experiments. Where appropriate, cells were rendered quiescent in Dulbecco's modified Eagle's medium containing 1% serum for a minimum of 2 h prior to stimulation.
In vitro inhibition of HSP90 using GA
Cells were pre-treated with GA (Santa Cruz, Santa Cruz, CA, USA) at concentrations of 0.01, 0.1 and 1 µM for 1 h prior to stimulation with the combination of 1 µg/ml LPS (Sigma, St Louis, MO, USA) and 300 units/ml IFN-γ (Accurate Chemical, Westbury, NY, USA). Cell culture media was collected 24 h after stimulation.
Cytokine ELISA and Griess assay
Supernatants were collected 24 h after stimulation and analyzed for IL-6 and NO. IL-6 levels were quantified by ELISA per the manufacturer's instructions (eBioscience, San Diego, CA, USA). Griess assay was used to quantify nitrite concentration (a stable reaction product of NO with oxygen).46 Briefly, supernatants were analyzed by mixing an equal volume of sample with Griess reagents (1% sulfanilamide and 0.1% naphthylethylenediamene in 2.5% H3PO4) in a 96-well plate. Absorbance was determined by a microplate reader measuring at a wavelength of 550 nm. The concentration of nitrite was calculated from a standard curve produced by the reaction of known quantities of control NaNO2 in the assay.
Mice
MRL/Mp-Faslpr/Faslpr (MRL/lpr) mice purchased from Jackson Laboratory (Bar Harbor, ME, USA) were bred and maintained at the Virginia–Maryland Regional College of Veterinary Medicine. Mice were treated in accordance with the Institutional Animal Care and Use Committee guidelines of Virginia Tech. Experiments were conducted in male and female mice. Baseline proteinuria, weight and blood data were collected at 12 weeks of age. Proteinuria and weight were recorded twice weekly and serum was collected every 2 weeks until mice were euthanized at 18 weeks of age.
Treatment of mice with 17-DMAG
I.P. injections of DMSO (control) or 17-DMAG (ChemieTek, Indianapolis, IN, USA) reconstituted in DMSO (treatment group) were administered at a frequency of 3 days/week (alternating days). Treatment of mice with 17-DMAG and vehicle began at 12 weeks of age and continued until mice exhibited signs of severe lupus at 18 weeks of age. While 17-DMAG is soluble in water, it has greater solubility in DMSO and to minimize the volume of vehicle required to treat the mice, we followed the work by Hertlein et al. and dissolved 17-DMAG in DMSO.47 Dosage of 5 mg/kg 17-DMAG was administered in a bolus of 50 µl per injection. To control for DMSO effects in the mice, control mice received a 50 µl bolus of DMSO at the same frequency as the 17-DMAG treated mice.
Histology of the kidney
At the time of euthanasia, the mice were weighed; kidneys were removed. One kidney was placed in buffered formalin, embedded in paraffin, sectioned, and stained by periodic acid-Schiff (PAS). Sections were assessed via light microscopy for glomerular proliferation, inflammation, size, number of nuclei per glomerulus, crescents, necrosis and fibrosis. Each of these parameters was graded for 0–3+ and an overall glomerular score derived. The pathology and morphometric analysis were performed by a pathologist blinded to the groups (Dr David Caudell).
The other kidney was embedded in OCT media (Miles, Elkhart, IN, USA) and frozen. Frozen kidneys were cut into 3-µm sections and stained with one of the following: goat anti-mouse IgG-conjugated to fluorescein isothiocyanate (FITC) diluted 1∶100 (Pierce, Rockford, IL, USA), goat anti-mouse C3-FITC diluted 1∶100 (Pierce), mouse anti-HSP90-DyLight 488 diluted 1∶500 or mouse anti-HSP70-DyLight 488 diluted 1∶500 (Enzo Life Sciences, Farmingdale, NY, USA). The severity of glomerulonephritis and immune complex deposition was determined in a blind manner. Scores ranged from 0 to 3+, where 0 corresponded to a non-autoimmune healthy mouse and 3+ to the maximal alteration observed in the study.
Measurement of proteinuria
Urine was collected twice a week and tested for proteinuria by a standard semiquantitative test using Siemens Uristix dipsticks (Siemens Healthcare, Deerfield, IL, USA). Results were quantified according to the manufacturer's instructions and scored as follows: Dipstick reading of 0 mg/dl=0, Trace=1, 30–100 mg/dl=2, 100–300 mg/dl=3, 300–2000 mg/dl=4 and 2000+ mg/dl=5.
Anti-dsDNA ELISA
Serum was collected at 12 weeks of age and at the time of euthanasia (18 weeks of age). Mice were bled from the retro-orbital sinus following inhalation of isoflurane anesthesia. Serum levels of antibodies to dsDNA were measured by ELISA as described in the literature.48 Briefly, ELISA plates (Corning Life Sciences, Lowell, MA, USA) were coated with 100 µl of 5 µg/ml calf thymus DNA (Sigma) and incubated at 37 °C overnight. After washing, the plates were blocked with BSA, then incubated sequentially for 45 min at room temperature with 1∶100 diluted serum followed by HRP-conjugated goat anti-mouse IgG gamma chain specific (1∶4000; Southern Biotech, Birmingham, AL, USA), and finally 3,3′,5,5′-tetramethylbenzidine was added (Pierce). A high titer serum was run in serial dilutions on each plate to allow quantification.
Flow cytometry
Flow cytometric analysis was performed using monoclonal antibodies of PerCP-CY5.5-conjugated anti-CD25, FITC-conjugated anti-CD21, PerCP–CY5.5 conjugated anti-CD19, phycoerythrin (PE)-conjugated anti-CD23 (BD Pharmingen, San Diego, CA, USA) and/or allophycocyanin (APC)-conjugated anti-CD3e, anti-CD4-FITC, anti-CD5-APC, eFluor450 (eF450)-conjugated anti-CD8a, anti-FoxP3-PE (eBioscience, San Diego, CA, USA). Splenic cells were isolated as previously described (173). Briefly, spleen lymphocytes from MRL/lpr mice at 18 weeks of age were aseptically dissociated, treated with red blood cell lysis buffer to remove erythrocytes, washed and suspended in RPMI media. Cells were stained with monoclonal antibodies and measured for fluorescence on a FACS Aria 1 (BD Biosciences, San Jose, CA, USA). Flow data were analyzed by FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4 software (GraphPad Software Inc.). Values are expressed as means plus or minus the standard error of the mean of seven mice. One-way ANOVA was used to compare data among groups and samples. Comparison of differences between groups was performed using paired, one-tailed t-tests (any use of unpaired t-tests is noted in figure captions). Proteinuria data was analyzed by linear regression. P values less than 0.05 were considered significant.
Results
HSP90 inhibition reduced inflammatory mediator production in stimulated mesangial cells
Mesangial cells are the resident kidney macrophage and regulate the immune response in the kidney by releasing pro-inflammatory cytokines that recruit other immune cells to migrate to the kidney.49 Because mesangial cells in lupus mice are hyper-responsive to immune stimulation, they are a valuable model for testing the role of HSP90 in the inflammatory response.50 Given that HSP90 is a chaperone for multiple proteins involved in the activation of inflammatory signal transduction cascade pathways, we sought to reduce inflammatory cytokine and molecule expression in mesangial cells through the inhibition of the HSP90 chaperone activity. Cultured mesangial cells were treated with GA for 24 h prior to stimulation with LPS/IFN-γ. Concentrations of 0.01–1 µM GA were used in these experiments based on the literature and our own unpublished observations showing minimal toxicity after 24-h treatments with concentrations of GA below 5 µM.51, 52 After 24 h of stimulation, supernatants were collected and assayed for NO, IL-6 and IL-12 production (Figure 1).
Inhibiting HSP90 reduced immune stimulated NO, IL-6 and IL-12 expression. (a) Nitrite concentrations measured by Griess assay show that post-stimulation NO expression was reduced by GA in a concentration dependent manner for concentrations greater than 0.1 µM. (b) IL-6 concentrations measured by ELISA showed that IL-6 expression stimulated by LPS/IFN-γ was reduced by GA in a concentration-dependent manner at concentrations greater than 0.1 µM. (c) Expression of IL-12 was reduced in all samples treated with GA, regardless of LPS/IFN-γ stimulation. One-way ANOVA, Tukey's multi-comparison tests: **P<0.01, ***P<0.001 for non-stimulated control vs. all other samples; # # #P<0.001 for comparisons between samples indicated by connecting bar; n.s. for not significant. GA, Geldanamycin; IFN, interferon; LPS, lipopolysaccharide; NO, nitric oxide.
Our results showed that GA decreased NO production in a concentration-dependent manner. Treatment with GA alone had no effect over baseline. LPS/IFN-γ stimulated NO production as expected (Figure 1a). With increasing concentrations of GA, the level of NO decreased. This decrease in NO was significantly different from cells receiving LPS/IFN-γ alone when GA was applied at concentrations of 0.1 and 1 µM (P<0.001) (Figure 1a).
IL-6 expression was also significantly reduced in LPS/IFN-γ stimulated cells pre-treated with GA (Figure 1b). Compared to the LPS/IFN-γ stimulated cells, we found that IL-6 was reduced by 0.1 and 1 µM concentrations of GA (P<0.001). GA treatment alone had no effect over baseline. Reductions in IL-12 were also determined to be dependent on GA concentrations and the reductions occurred in all concentrations of GA tested (P<0.001) (Figure 1c). GA also reduced IL-12 expression below baseline in cells not stimulated with LPS/IFN-γ (P<0.01).
Proteinuria was reduced in mice treated with 17-DMAG
Having found that HSP90 facilitates cytokine and NO expression in mesangial cells in vitro, we tested the role of HSP90 in the development of SLE in vivo. To perform this test, we inhibited HSP90 in a lupus mouse model by administration of the HSP90 inhibitor 17-DMAG. We selected 17-DMAG over GA as our in vivo inhibitor based on published results showing reduced hepatotoxicity in vivo when compared to GA or 17-allylamino-17-demethoxygeldanamycin (17-AAG).16, 18, 53, 54 Dosages were selected based on studies showing effective treatment and minimal toxicity at dosages under 15 mg/kg administered 3 days per week for 3 weeks.55 We injected MRL/lpr mice with 17-DMAG 3 days a week on alternating days for 6 weeks, beginning at 12 weeks of age and lasting until euthanasia at 18 weeks of age. Disease progression during the study was measured by total body weight and proteinuria. We found that proteinuria increased with age in the control group, whereas the 17-DMAG treated group did not increase in proteinuria (Figure 2a). Linear regression of proteinuria showed a statistically significant increase in the control group but no significant increase in the group receiving 17-DMAG (P<0.01, control group; P<0.001, control vs. 17-DMAG). The groups maintained a comparable average weight throughout the study with no significant increase measured by linear regression (data not shown). Total body weight at the end of the study did not differ significantly between the groups at the time of euthanasia (Figure 2b).
Treatment with 17-DMAG decreased proteinuria. (a) Linear regression of grouped proteinuria scores showed control mice exhibited increased proteinuria, while mice treated with 17-DMAG did not increase in proteinuria. Linear regression for proteinuria in the control group was statistically significant with a P value less than 0.01. The difference between linear regression of proteinuria in the control and treated groups was also statistically significant (P<0.001). (b) Group average total body weight at euthanasia was not statistically different between control and treatment groups. 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin.
Treatment with HSP90 inhibitor 17-DMAG reduced serum anti-dsDNA antibodies but not total IgG
We measured autoantibodies and total IgG in sera of MRL/lpr mice treated with 17-DMAG. We found that mice treated with 17-DMAG had a significant reduction in anti-dsDNA antibodies when compared to their own baseline serum levels measured prior to treatment at 12 weeks of age (P<0.05, paired one-tail t-test) (Figure 3a). However, the difference between the control group and the treated group at 18 weeks of age was determined to not be significant. Total IgG in sera was measured at 12 and 18 weeks of age and was not found to be statistically significant (Figure 3b).
HSP90 inhibitor 17-DMAG reduced sera anti-dsDNA but not total IgG. (a) Anti-dsDNA antibodies in sera were reduced in the 17-DMAG group at 18 weeks of age when compared to the same group at 12 weeks of age. *P<0.05 (paired one-tailed t-test). (b) 17-DMAG did not have a statistically significant effect on serum IgG. Anti-dsDNA, antibodies to double-stranded DNA; 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin.
Renal deposition of IgG and C3 were unaffected by 17-DMAG treatment
Renal histological markers of glomerular lesions by PAS and/or hematoxylin and eosin staining and glomerular deposition of IgG and C3 are indications of the severity of GN and lupus in MRL/lpr mice.36, 38, 39, 40, 41 To assess the effect of HSP90 inhibition on renal pathology, kidneys were taken from MRL/lpr mice at 18 weeks of age after being treated with 17-DMAG. For PAS staining, kidneys were formalin-fixed and sections were stained by the PAS method. For C3 and IgG deposition, tissues were frozen in OCT media and 3 µM sections were stained with FITC-conjugated anti-IgG or anti-C3 antibodies (Figure 4). Histopathology sections were assessed in a blinded fashion and scored according to a rubric described in materials and methods. We found the glomerular pathology scores were not significantly different between the treated and control groups (scoring data not shown; representative images in Figure 4a). We also found that the C3 and IgG fluorescence intensity was not significantly different between treated and control groups as determined by a blinded assessment (scoring data not shown; representative images in Figure 4b and c).
Renal histopathology showed no significant differences in renal histopathology between the control and treated groups. Representative images of kidney sections stained with (a) PAS for renal histology assessment and GN scoring, (b) C3-FITC and DAPI stain, and (c) IgG and DAPI staining. Representative pictures shown at ×400 original magnification. FITC, fluorescein–isothiocyanate; GN, glomerulonephritis; PAS, periodic acid-Schiff.
HSP90 expression increased in untreated MRL/lpr mice, compared to MRL/lpr mice treated with 17-DMAG and untreated C57BL/6 mice
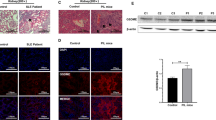
HSP90 expression has been shown to be altered in SLE patients.9 To verify that 17-DMAG has pharmacological effects on the murine renal organs, we performed immunofluorescence microscopy of renal tissue sections stained for HSP90 or HSP70. Inhibition of HSP90 by 17-DMAG has been shown to affect HSP90 and HSP70 expression both in vivo and clinically.53, 54 In addition, we questioned if HSP90 expression is altered in MRL/lpr mice compared to other mice strains such as the C57BL/6 strain. To answer these questions, we stained frozen kidney sections with DyLight 488-conjugated anti-HSP90 or anti-HSP70 antibodies and with DAPI nuclear stain. We found that HSP90 was expressed in the kidney at a low level in the C57BL/6 mice strain, but was greatly increased in the tubules and interstitium of untreated MRL/lpr mice; expression only slightly increased in the glomeruli (Figure 5a, top 2 rows of frames). In contrast, MRL/lpr mice treated with 17-DMAG exhibited low levels of HSP90 expression in all structures of the kidney, comparable to the C57BL/6 mice (Figure 5a, bottom row of frames). Examination of HSP70 expression showed that C57BL/6 mice expressed HSP70 relatively equally in tubules and glomeruli but MRL/lpr mice had decreased HSP70 expression in all renal structures (Figure 5b). Interestingly, HSP70 expression was not upregulated in the kidney of MRL/lpr mice treated with 17-DMAG (Figure 5b, bottom row of frames).
Comparison of renal HSP90 and HSP70 expression in C57BL/6 mice and MRL/lpr mice with and without 17-DMAG. Immunofluorescence microscopy of frozen tissue sections stained with DyLight 488-conjugated antibodies to HSP90 or HSP70 and DAPI nuclear stain. (a) C57BL/6 mice express low levels of HSP90 in tubules and glomeruli (top row of frames). Expression of HSP90 was increased in tubules but not glomeruli in diseased MRL/lpr control mice as compared to C57BL/6 mice (middle row of frames). MRL/lpr mice treated with 17-DMAG showed reduced HSP90 expression compared to MRL/lpr controls (bottom row of frames). (b) Renal expression of HSP70 was high in tubules and glomeruli C57BL/6 (top row of frames) compared to both groups of MRL/lpr mice (bottom two rows of frames). MRL/lpr mice treated with 17-DMAG did not exhibit elevated levels of HSP70 in the tubules or glomeruli of kidney sections. Representative images shown at ×400 original magnification. HSP90, Heat shock protein 90; 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin.
Reduction of splenomegaly by 17-DMAG
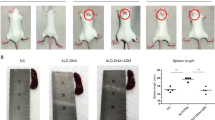
HSP90 inhibition has been shown to reduce cellular proliferation in cancer cells.56, 57 Splenomegaly is exhibited in the lymphoproliferative (lpr) model of MRL/lpr.58 To test the effect of HSP90 inhibition on splenomegaly in MRL/lpr mice, spleens were collected and weighed and the average mass of treatment groups was compared. We found a significant difference in spleen weights between the control and treated groups where P<0.05, unpaired one-tailed t-test (Figure 6).
Treatment with 17-DMAG reduced splenomegaly. (a) The grouped average spleen weight was significantly lower for the 17-DMAG group compared to the control group (one-tail unpaired t-test, P<0.05). (b) Picture of representative spleens taken from both groups. 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin.
17-DMAG altered splenocyte profiles in MRL/lpr lupus mice
In addition to splenomegaly prevalent in MRL/lpr mice, disease pathogenesis of lupus in humans and murine models has been shown to include alterations in many T- and B-cell subtypes.59 The alterations in T- and B-cell subtypes has led to the investigation of many T and B cell-targeted therapies for the treatment of lupus, as recently reviewed.60, 61 We tested the effect of HSP90 on T and B cells by quantifying populations of splenocytes expressing markers for specific subtypes of T and B cells shown to be altered in MRL/lpr mice. Splenocytes were collected from 18-week-old mice treated with 17-DMAG for six weeks or treated with DMSO for six weeks (control), stained for appropriate markers and analyzed by flow cytometry.
Double-negative T cells decreased and CD8+ T cells increased in mice treated with 17-DMAG
First, we assessed CD4 and CD8 splenocyte profiles by staining with fluorescent-tagged antibodies to CD3, CD4 and CD8. We were able to show 17-DMAG decreased CD3+ CD4- CD8− or double-negative T (DNT) cells (P<0.05, paired one-tailed t-test) (Figure 7a, f and g). CD3+CD4+ T cells were not significantly different between groups (Figure 7b, f and g). 17-DMAG treatment produced an increase in CD3+CD4−CD8+ T cells (P<0.05, paired one-tailed t-test) (Figure 7c, f and g); CD3+CD4+CD8+ T cells were also not significantly different between groups (Figure 7d, f and g). We compared CD4 and CD8 expressing T cells by computing the ratio between CD3+CD4+CD8− and CD3+CD4−CD8+ T cells (CD4/CD8 ratio). We found the CD4/CD8 ratio decreased in mice treated with 17-DMAG (P<0.01, paired one-tailed t-test) (Figure 7e–g).
17-DMAG increased CD8+ T cells and reduced DNT cells (CD4−CD8−). Splenocytes were stained with fluorescent antibodies to CD3, CD4 and CD8 and analyzed by flow cytometry. (a) DNT cells were reduced in mice treated with 17-DMAG compared to the control (*P<0.05, paired one-tailed t-test). (b) CD3+CD4+CD8− T cells were not significantly affected by 17-DMAG treatment compared to the control. (c) CD4−CD8+ T cells were increased in mice receiving 17-DMAG treatments (*P<0.05, paired one-tailed t-test). (d) CD4+CD8+ T cells were not significantly altered in the group of mice receiving 17-DMAG. (e) The ratio of CD4+ T cells to CD8+ T cells was reduced in mice receiving 17-DMAG (**P<0.01, paired one-tailed t-test). (f, g) Representative images of flow cytometry data showing CD3+ gating of CD4 vs. CD8 splenocyte profiles for control and 17-DMAG treatment groups, respectively. DNT, double-negative T; 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin.
17-DMAG affected FoxP3- phenotypes in CD3+ T-cell subsets
CD8+ T cells and TREG cells CD4+CD25+ forkhead box P3+ (FoxP3+) have been shown to reduce autoimmune reactivity in SLE.62 We studied the effect of 17-DMAG on regulatory immune cells by profiling CD4, CD8 and FoxP3 expression in CD3+ T cells. We found no significant difference in CD4 and FoxP3 expressing populations between the control and treated groups (data not shown). However, CD8 and FoxP3 T cell profiles for the control and 17-DMAG groups did show that CD8 subsets of CD3+FoxP3− T cells were altered (Figure 8). 17-DMAG reduced the population of CD3+CD8−FoxP3− T cells (P<0.01, unpaired one-tailed t-test) (Figure 8a, e and f). Populations of CD3+CD8+FoxP3− T cells were increased in mice treated with 17-DMAG (P<0.05, unpaired one-tailed t- test) (Figure 8b, e and f). No significant difference was found between the groups for CD3+FoxP3+CD8− or CD3+FoxP3+CD8+ T cells (Figure 8c–f).
CD3+FoxP3− phenotypes were affected by 17-DMAG treatment in MRL/lpr mice. (a) A significant decrease in CD3+CD8−FoxP3− T cells was seen in the mice treated with 17-DMAG (**P<0.01, unpaired one-tailed t-test). (b) Treatment with 17-DMAG increased CD3+CD8+FoxP3− T cells (*P<0.05, unpaired one-tailed t-test). (c and d) No significant difference in CD3+CD8−FoxP3+ or CD3+CD8+FoxP3+ T cells between groups. (e and f) Representative images of flow cytometry data showing splenocyte profiles gated on CD3+, with axes corresponding to CD8 vs. FoxP3. 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin.
Mature B cells were decreased in mice treated with 17-DMAG
To test the effect of HSP90 inhibition on B-cell populations, we used flow cytometry to examine splenocytes stained with fluorescently-tagged antibodies to CD5, CD19, CD21 and CD23. We found follicular B cells (CD19+CD21−CD23+) were decreased in mice treated with 17-DMAG compared to the controls (P<0.05, paired one-tailed t-test) (Figure 9a, e and f). In contrast, populations of marginal zone B cells CD19+CD21+CD23− were found to be unchanged in the 17-DMAG group (Figure 9b, e and f). We examined B-1-cell populations and found that 17-DMAG had no effect on either the CD19+CD5+ B-1a cells (Figure 9c, g and h) or on the CD19+CD5− B-2a cells (Figure 9d, g and h).
Mice treated with 17-DMAG showed a decrease in CD19+CD21−CD23+ B-cell populations. (a) CD19+CD21−CD23+ follicular B cells decreased in mice treated with 17-DMAG (*P<0.05, paired one-tailed t-test). (b) CD19+CD21+CD23− marginal zone B cells were unchanged by 17-DMAG. (c) CD19+CD5+ B-1a cells were not affected by the treatment. (d) CD19+CD5− B-1b cells were not affected by the treatment. (e, f) Representative splenocyte flow cytometry gate: CD19+, plotting CD21 vs. CD23. (g, h) Representative splenocyte flow cytometry CD19 vs. CD5. 17-DMAG, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin.
Discussion
Several reports in the literature have demonstrated a link between HSP90 and lupus including studies showing increased HSP90 expression in SLE patients or antibody reactivity to extracellular HSP90.63 Recently investigations into the regulation of HSP90 in lupus has been renewed due to the ability of HSP90 to serve as a client protein for inflammatory signaling molecules such as phosphoinositide 3-kinase, mammalian target of rapamycin and Akt (protein kinase B).64, 65 Another example of HSP90 dependence in the inflammatory response is in the nuclear factor-κB (NF-κB) pathway which depends on functional HSP90 in order for upstream signal molecules to activate NF-κB.66, 67 TLR-mediated inflammation is regulated by HSP90 chaperoning of key TLR signal components that promote expression of TNF-α and IL-1β messenger RNA.2, 68 It has also been shown that several HSP90 homologs fold multiple TLRs.68 It has been shown that the severity of SLE can be diminished in mice and humans through downregulation of many of these HSP90-dependent inflammatory pathways.69, 70, 71, 72 Our current studies were undertaken to determine if modulation of HSP90 would decrease mesangial cell inflammatory mediator production and lessen renal disease in MRL/lpr lupus mice.
Mesangial cells are the principal resident immunoregulatory cells in the kidney. To assess the role of HSP90 in pro-inflammatory cytokine expression, we examined the effect of HSP90 inhibition in immune stimulated mesangial cells by measuring output of several pro-inflammatory cytokines (IL-6, IL-12 and the mediator NO). Each of these is known to play a role in SLE and known to be linked to HSP90. Mice and SLE patients exhibit elevated IL-6 and inhibition of IL-6 or the IL-6 receptor decreases anti-dsDNA and proteinuria.73, 74, 75 With HSP90 inhibition by GA, IL-6 expression was reduced in macrophages by preventing HSP90 from chaperoning newly transcripted cytokines.76 HSP90 complexes with iNOS and is required for iNOS to synthesize NO.77 It has also been found that HSP90 inhibition reduces iNOS function without reducing iNOS expression.78 Additionally, IL-12 promotes disease in MRL/lpr mice while a deficiency in IL-12 will delay GN in the same MRL mice.31 HSP90 has been linked to IL-12, with one example being that mature dendritic cells cultured in GA had their IL-12 expression significantly reduced.79 We have previously shown that HSP90 inhibition by 17-DMAG reduced inhibitor of κB kinase expression and decreased NF-κB translocation to the nucleus in J774 macrophage cells.80 Our results agree with the literature and we showed that HSP90 inhibition reduced mesangial cell responsiveness to inflammatory stimuli by decreasing NO, IL-6 and IL-12 expression. Changes in these cytokines would suggest the potential for an overall decrease of inflammation in SLE.
For initial in vitro experiments, our team focused on determining the mechanistic effects of HSP90 inhibition using the HSP90 inhibitor, GA. Having measured significant effects of HSP90 inhibition by GA, we set out to determine if HSP90 inhibition had in vivo effects in MRL/lpr mice. Our studies were focused on mechanism, and based on the in vivo toxicity of GA, we opted to use 17-DMAG, a derivative of GA with lower toxicity and similar mechanism of action, for the in vivo studies.53 Others have reported HSPs expressed in glomeruli and tubules of some SLE patients.9 However, our assessment of HSP90 expression in our control and HSP90-inhibited groups as well as C57BL/6 mouse kidneys showed that the diseased mice exhibited elevated HSP90 in tubules but not in glomeruli. While HSP90 inhibition reduced inflammatory mediator production in mesangial cells in vitro, the relatively lower levels of HSP90 in the glomeruli may suggest that the anti-inflammatory effects of HSP90 inhibition in the glomeruli may not be as critical to mesangial cell function as the in vitro studies would suggest.
Interestingly, HSP70 in C57BL/6 mice was higher than in either of the MRL/lpr groups we tested. Both 17-AAG and 17-DMAG have been shown to increase HSP70 expression in human macrophages and vascular smooth muscle cells after 4 h of treatment and is typically used as a marker for HSP90 inhibition.3, 81 Early preclinical study of 17-DMAG found that short-term (24-h) dosages of 17-DMAG increased renal and liver HSP70 expression. However, we found in our long-term (6-week) study that HSP70 did not increase in renal tissue following treatment with an HSP90 inhibitor (17-DMAG) in a lupus mouse model. It is possible that there is a compensation mechanism for HSP70 during long-term in vivo treatment with 17-DMAG that was beyond the scope of the present studies. Further work can be done to measure HSP70 expression during long term treatment with HSP90 inhibitors. Understanding HSP70 expression in SLE mice treated with HSP90 inhibitors may be instructive for several reasons. First, it has been shown that extracellular HSP70 is a ligand for TLR2 and TLR4 activation. Second, HSP70 activation of the TLR4 ligand on CD4+CD25+ T cells is attributed to an increase in suppressive activity of these T cells in atherosclerosis.82, 83 Furthermore, HSP70 has been indicated to have a negative feedback effect on NF-κB signaling activity.84 HSP70 has also been demonstrated to switch off TNF receptor-associated factor 6.85
Active lupus renal disease can be defined clinically or pathologically. As MRL/lpr mice age and disease progresses, they develop proteinuria. We report reductions in proteinuria in mice treated with 17-DMAG. Interestingly, we still found elevated levels of IgG and C3 deposition in both groups and the difference between the groups was not significantly different. We also found that there was no significant difference in glomerular pathology between the treated and control groups. Pathological assessment of MRL/lpr kidney sections typically exhibit elevated levels of IgG and C3 deposition in mice with active SLE.86 Therefore, we would expect to see some difference in C3 and IgG deposition or renal pathology due to the decrease in proteinuria and decrease in anti-dsDNA. However, it has been previously shown that C3 deposition in glomeruli can be uncoupled from the inflammatory response and that complement activation by immune complexes is not sufficient to trigger the inflammatory response.87 It was somewhat perplexing that proteinuria and glomerulonephritis were not as markedly increased in MRL/lpr mice at 18 weeks-of-age as many others have reported for the strain.88, 89 Possibly having the treatment regime progress for a longer period of time would have allowed us to determine more significant differences between the treated and the untreated groups. However, the downside of a prolonged treatment could either overshoot the window of therapeutic effects of 17-DMAG or risk the loss of untreated animals due to disease as several reports have shown 50% mortality for MRL/lpr mice after 20 weeks of age, with disease expression by the 14–16 week.32, 89, 90
Another pathological aspect of MRL/lpr mice is the presence of splenomegaly in mice exhibiting disease.59 We found that splenomegaly was reduced in mice treated with the HSP90 inhibitor 17-DMAG. We infer from this that HSP90 may be enabling the proliferation of T and B cells. In support of this hypothesis, it was recently reported that cells isolated from draining lymph nodes in experimental epidermolysis bullosa acquisita mice were shown to have significantly reduced proliferation of T cells when treated with 17-DMAG.91 The increased proliferation of T and B cells in SLE leads to increased expression of serum IgG with high levels of the IgG being anti-dsDNA. It has been shown that mice treated with 17-DMAG exhibit suppressed autoantibody production.91 We found that 17-DMAG did reduce anti-dsDNA, but we did not see a significant change in total IgG expression in the serum.
T-cell regulation has long been pursued as a potential therapy for lupus. There are a number of altered or distinct T-cell subtypes in lupus. The DNT cells are known to be upregulated in lupus mice and it is believed that this contributes to the disease state when they infiltrate target organs to produce pro-inflammatory cytokines and activate B-cell antibody production.59, 60 CD8+ T cells suppress autoimmune reactivity in SLE.62 Elevated CD4/CD8 ratios have been identified in murine SLE models.92, 93 We tested CD4 and CD8 profiles of splenic T cells and found that HSP90 inhibition decreased DNT cells, increased CD8+ T cells, but did not change CD4+ T cells. As a result, we found an overall decrease in the CD4/CD8 ratio. Recently, T cells from mice treated with an HSP90 inhibitor showed reduced responsiveness to activating antigens in the collagen induced arthritis model.25 Han et al.11 found that an inhibitor for the endoplasmic reticulum homologue of HSP90, gp96, reduced both the CD4+ memory T cells and activated CD4+ T cells in the spleen and lymph nodes. The effects of HSP90 inhibition on T-cell populations might be explained by the fact that stimulation of the T-cell receptor leading to T-cell activation requires HSP90 to stabilize lymphocyte-specific protein tyrosine kinase (Lck) in order to initiate activation.45 Furthermore, it has been shown that HSP90 is an essential regulator for gene expression of linker for activation of T cells (LAT) and that following inhibition of HSP90, LAT messenger RNA was decreased, followed by a decrease in total LAT protein. Taken together, HSP90 plays a role in activation of T cells which may be the mechanism behind the changes in T-cell subtypes we found in mice treated with an HSP90 inhibitor.
TREG cells are marked by expression of the master TREG transcription factor, FoxP3. These cells are necessary for maintaining an immunological balance due to their suppressive role, but are typically low in SLE patients.60 Furthermore, TREG cell suppressive functions are reduced in SLE patients and MRL mice, and it is thought that this is due to elevated levels of IL-6, which inhibits TREG cell function.42, 94, 95 TREG cells have significantly higher expression of HSP90 than T cells. The high expression of HSP90 in TREG suggests a role for HSP90 in FoxP3 expression and may explain why we found higher levels of FoxP3−CD3+CD8+ T cells. However, we also found that CD4+CD25+FoxP3+ TREG cells were unaffected by 17-DMAG treatment. Nevertheless, HSP90 inhibitors have been shown to improve TREG suppressive functions. For example, TREG cells cocultured with T cells in the presence of HSP90 inhibitors exhibit an increased suppressive function. Furthermore, HSP90 inhibition (17-AAG) decreased homeostatic proliferation of T cells in spleens and lymph nodes after daily injection for a week.96 Taken together, we propose that HSP90 plays a role in regulating CD8+ T cells and DNT proliferation. This likely occurs through an IL-6 and TREG or FoxP3-mediated mechanism. Understanding the role of HSP90 in TREG cells may provide clues to how HSP90 contributes to SLE via a T cell-mediated mechanism.
B cells also play an important role in the development of lupus pathology.97, 98, 99 According to a recent review article, B cells act in lupus to present auto-antigens, induce T helper cells, CD8+ effector cells, and to inhibit TREG cells.61 Some B-cell therapy approaches being investigated include the therapeutic targeting of HSP90 client proteins such as TLR and phosphoinositide 3-kinase. When inhibited, these signal pathway proteins reduce B-cell activation and survival. It should also be noted that targeting IL-6 (an HSP90-dependent cytokine) reduces memory-cell differentiation.61 The inhibitor for gp96 was shown to reduce populations of mature B cells, B220+ and MHC class II+ cells.11 However, it has also been shown that splenic plasma cells and germinal center B cells are unaffected by 17-DMAG treatment.91 We found that only follicular B cells were affected by the HSP90 inhibition treatment, while marginal zone B cells (B1a and B1b) were not significantly affected. Current thinking in lupus literature suggests that follicular B cells are important for pathogenic autoantibody production, while the role for marginal zone B cells has yet to be established.100 B1 cells have been indicated as a possible source or activation of auto-reactive CD4 T cells and autoantibody production in the development of lupus. Taken together, our results suggest that HSP90 does not play a significant role in B cell regulation in lupus.
In summary, we have shown that HSP90 is important for the expression of the key lupus cytokines IL-6, IL-12 and the mediator NO in mesangial cells in vitro. Inhibition of HSP90 in the MRL/lpr mouse resulted in decreased proteinuria and decreased spleen size, but did not affect renal histopathology or C3 and IgG deposition. However, we show that HSP90 is upregulated in diseased MRL/lpr mice in the renal tubules, while HSP70 is downregulated in MRL/lpr mice, regardless of HSP90 inhibition. Furthermore, our data showed that anti-dsDNA can be decreased with HSP90 inhibition. We also found that DNT cells are downregulated, while CD8+ T cells are upregulated in mice treated with 17-DMAG. Some effects were also observed in TREG cells which express high levels of HSP90. Follicular B cells were decreased, but the role of follicular B cells has yet to be determined for lupus. Future work should focus on determining the mechanisms that link HSP90 to T-cell activation and TREG suppressive potency. Targeting HSP90 may be an effective treatment for SLE, especially if combined with other targeted therapeutic approaches.
References
Bandholtz L, Guo Y, Palmberg C, Mattsson K, Ohlsson B, High A et al. Hsp90 binds CpG oligonucleotides directly: implications for hsp90 as a missing link in CpG signaling and recognition. Cell Mol Life Sci 2003; 60: 422–429.
De Nardo D, Masendycz P, Ho S, Cross M, Fleetwood AJ, Reynolds EC et al. A central role for the Hsp90·Cdc37 molecular chaperone module in interleukin-1 receptor-associated-kinase-dependent signaling by Toll-like receptors. J Biol Chem 2005; 280: 9813–9822.
Dello Russo C, Polak PE, Mercado PR, Spagnolo A, Sharp A, Murphy P et al. The heat-shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin suppresses glial inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurochem 2006; 99: 1351–1362.
Dhillon VB, McCallum S, Norton P, Twomey BM, Erkeller-Yuksel F, Lydyard P et al. Differential heat shock protein overexpression and its clinical relevance in systemic lupus erythematosus. Ann Rheum Dis 1993; 52: 436–442.
Dhillon VB, McCallum S, Latchman DS, Isenberg DA . Elevation of the 90 kDa heat-shock protein in specific subsets of systemic lupus erythematosus. Q J Med 1994; 87: 215–222.
Latchman DS, Isenberg DA . The role of hsp90 in SLE. Autoimmunity 1994; 19: 211–218.
Faulds G, Conroy S, Madaio M, Isenberg D, Latchman D . Increased levels of antibodies to heat shock proteins with increasing age in Mrl/Mp-lpr/lpr mice. Br J Rheumatol 1995; 34: 610–615.
Ripley BJ, Isenberg DA, Latchman DS . Elevated levels of the 90 kDa heat shock protein (hsp90) in SLE correlate with levels of IL-6 and autoantibodies to hsp90. J Autoimmun 2001; 17: 341–346.
Kenderov A, Minkova V, Mihailova D, Giltiay N, Kyurkchiev S, Kehayov I et al. Lupus-specific kidney deposits of HSP90 are associated with altered IgG idiotypic interactions of anti-HSP90 autoantibodies. Clin Exp Immunol 2002; 129: 169–176.
Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z . Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proc Natl Acad Sci USA 2003; 100: 15824–15829.
Han JM, Kwon NH, Lee JY, Jeong SJ, Jung HJ, Kim HR et al. Identification of gp96 as a novel target for treatment of autoimmune disease in mice. PLoS ONE 2010; 5: e9792.
Taldone T, Gozman A, Maharaj R, Chiosis G . Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol 2008; 8: 370–374.
Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L et al. Update on Hsp90 Inhibitors in Clinical Trial. Curr Top Med Chem 2009; 9: 1479–1492.
Sõti C, Nagy E, Giricz Z, Vígh L, Csermely P, Ferdinandy P . Heat shock proteins as emerging therapeutic targets. Br J Pharmacol 2005; 146: 769–780.
Garnier C, Lafitte D, Tsvetkov PO, Barbier P, Leclerc-Devin J, Millot JM, et al. Binding of ATP to Heat Shock Protein 90. J Biol Chem 2002; 277: 12208–12214.
Supko JG, Hickman RL, Grever MR, Malspeis L . Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol 1995; 36: 305–315.
Blagg BS, Kerr TD . Hsp90 inhibitors: small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev 2006; 26: 310–338.
Egorin M, Lagattuta T, Hamburger D, Covey J, White K, Musser S et al. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats. Cancer Chemother Pharmacol 2002; 49: 7–19.
Egorin MJ, Zuhowski EG, Rosen DM, Sentz DL, Covey JM, Eiseman JL . Plasma pharmacokinetics and tissue distribution of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) in CD2F1 mice1. Cancer Chemother Pharmacol 2001; 47: 291–302.
Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J et al. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med 2007; 176: 667–675.
Rice JW, Veal JM, Fadden RP, Barabasz AF, Partridge JM, Barta TE et al. Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum 2008; 58: 3765–3775.
Whitesell L, Lindquist SL . HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5: 761–762.
Zhang H, Burrows F . Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berl) 2004; 82: 488–499.
Donnelly A, Blagg BS . Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr Med Chem 2008; 15: 2702–2717.
Yun TJ, Harning EK, Giza K, Rabah D, Li P, Arndt JW et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol 2011; 186: 563–575.
Suzuka H, Yoshifusa H, Nakamura Y, Miyawaki S, Shibata Y . Morphological analysis of autoimmune disease in MRL–lpr,Yaa male mice with rapidly progressive systemic lupus erythematosus. Autoimmunity 1993; 14: 275–282.
Ka SM, Cheng CW, Shui HA, Wu WM, Chang DM, Lin YC et al. Mesangial cells of lupus-prone mice are sensitive to chemokine production. Arthritis Res Ther 2007; 9: R67.
Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR . The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res 2001; 3: 136–141.
Aringer M, Smolen JS . Cytokine expression in lupus kidneys. Lupus 2005; 14: 13–18.
Reilly CM, Oates JC, Sudian J, Crosby MB, Halushka PV, Gilkeson GS . Prostaglandin J2 inhibition of mesangial cell iNOS expression. Clin Immunol 2001; 98: 337–345.
Kikawada E, Lenda DM, Kelley VR . IL-12 deficiency in MRL–Faslpr mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol 2003; 170: 3915–3925.
Santiago-Raber ML, Laporte C, Reininger L, Izui S . Genetic basis of murine lupus. Autoimmun Rev 2004; 3: 33–39.
Theofilopoulos AN, Kono DH . Mechanisms and genetics of autoimmunity. Ann NY Acad Sci 1998; 841: 225–235.
Singh AK . Lupus in the Fas lane? J R Coll Physicians Lond 1995; 29: 475–478.
Deng GM, Liu L, Tsokos GC . Targeted tumor necrosis factor receptor I preligand assembly domain improves skin lesions in MRL/lpr mice. Arthritis Rheum 2010; 62: 2424–2431.
Ichinose K, Juang YT, Crispín JC, Kis-Toth K, Tsokos GC . Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis Rheum 2011; 63: 523–529.
Xie C, Patel R, Wu T, Zhu J, Henry T, Bhaskarabhatla M et al. PI3K/AKT/mTOR hypersignaling in autoimmune lymphoproliferative disease engendered by the epistatic interplay of Sle1b and FASlpr. Int Immunol 2007; 19: 509–522.
Sekine H, Reilly CM, Molano ID, Garnier G, Circolo A, Ruiz P et al. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J Immunol 2001; 166: 6444–6451.
Xie C, Sharma R, Wang H, Zhou XJ, Mohan C . Strain distribution pattern of susceptibility to immune-mediated nephritis. J Immunol 2004; 172: 5047–5055.
Xie C, Zhou XJ, Liu X, Mohan C . Enhanced susceptibility to end-organ disease in the lupus-facilitating NZW mouse strain. Arthritis Rheum 2003; 48: 1080–1092.
Du Y, Fu Y, Mohan C . Experimental anti-GBM nephritis as an analytical tool for studying spontaneous lupus nephritis. Arch Immunol Ther Exp (Warsz) 2008; 56: 31–40.
Divekar AA, Dubey S, Gangalum PR, Singh RR . Dicer insufficiency and microRNA-155 overexpression in lupus regulatory T cells: an apparent paradox in the setting of an inflammatory milieu. J Immunol 2011; 186: 924–930.
Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 2009; 60: 1472–1483.
Wieten L, Broere F, van der Zee R, Koerkamp EK, Wagenaar J, van Eden W . Cell stress induced HSP are targets of regulatory T cells: a role for HSP inducing compounds as anti-inflammatory immuno-modulators? FEBS Lett 2007; 581: 3716–3722.
Giannini A, Bijlmakers MJ . Regulation of the Src family kinase Lck by Hsp90 and ubiquitination. Mol Cell Biol 2004; 24: 5667–5676.
Gilkeson G, Cannon C, Oates J, Reilly C, Goldman D, Petri M . Correlation of serum measures of nitric oxide production with lupus disease activity. J Rheumatol 1999; 26: 318–324.
Hertlein E, Wagner AJ, Jones J, Lin TS, Maddocks KJ, Towns WH 3rd et al. 17-DMAG targets the nuclear factor-kappaB family of proteins to induce apoptosis in chronic lymphocytic leukemia: clinical implications of HSP90 inhibition. Blood 2010; 116: 45–53.
Gilkeson GS, Pippen AM, Pisetsky DS . Induction of cross-reactive anti-dsDNA antibodies in preautoimmune NZB/NZW mice by immunization with bacterial DNA. J Clin Invest 1995; 95: 1398–1402.
Schwarz M, Wahl M, Resch K, Radeke HH . IFNγ induces functional chemokine receptor expression in human mesangial cells. Clin Exp Immunol 2002; 128: 285–294.
Teramoto K, Negoro N, Kitamoto K, Iwai T, Iwao H, Okamura M et al. Microarray analysis of glomerular gene expression in murine lupus nephritis. J Pharmacol Sci 2008; 106: 56–67.
Jeon YK, Park CH, Kim KY, Li YC, Kim J, Kim YA et al. The heat-shock protein 90 inhibitor, geldanamycin, induces apoptotic cell death in Epstein–Barr virus-positive NK/T-cell lymphoma by Akt down-regulation. J Pathol 2007; 213: 170–179.
Dey A, Cederbaum AI . Geldanamycin, an inhibitor of Hsp90, potentiates cytochrome P4502E1-mediated toxicity in HepG2 cells. J Pharmacol Exp Ther 2006; 317: 1391–1399.
Eiseman JL, Lan J, Lagattuta TF, Hamburger DR, Joseph E, Covey JM et al. Pharmacokinetics and pharmacodynamics of 17-demethoxy 17-[[(2-dimethylamino)ethyl]amino]geldanamycin (17DMAG, NSC 707545) in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer xenografts. Cancer Chemother Pharmacol 2005; 55: 21–32.
Ramanathan RK, Egorin MJ, Erlichman C, Remick SC, Ramalingam SS, Naret C et al. Phase I pharmacokinetic and pharmacodynamic study of 17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor of heat-shock protein 90, in patients with advanced solid tumors. J Clin Oncol 2010; 28: 1520–1526.
Hollingshead M, Alley M, Burger AM, Borgel S, Pacula-Cox C, Fiebig HH et al. In vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother Pharmacol 2005; 56: 115–125.
Babchia N, Calipel A, Mouriaux F, Faussat AM, Mascarelli F . 17-AAG and 17-DMAG-induced inhibition of cell proliferation through B-Raf downregulation in WTB-Raf-expressing uveal melanoma cell lines. Invest Ophthalmol Vis Sci 2008; 49: 2348–2356.
Bishop SC, Burlison JA, Blagg BS . Hsp90: a novel target for the disruption of multiple signaling cascades. Curr Cancer Drug Targets 2007; 7: 369–388.
Vidal S, Kono DH, Theofilopoulos AN . Loci predisposing to autoimmunity in MRL–Fas lpr and C57BL/6–Faslpr mice. J Clin Invest 1998; 101: 696–702.
Reilly CM, Mishra N, Miller JM, Joshi D, Ruiz P, Richon VM et al. Modulation of renal disease in MRL/lpr mice by suberoylanilide hydroxamic acid. J Immunol 2004; 173: 4171–4178.
Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC . T cells as therapeutic targets in SLE. Nat Rev Rheumatol 2010; 6: 317–325.
Sanz I, Lee FE . B cells as therapeutic targets in SLE. Nat Rev Rheumatol 2010; 6: 326–337.
La Cava A . The busy life of regulatory T cells in systemic lupus erythematosus. Discov Med 2009; 8: 13–17.
Staub HL, Dal Ben ERR, Bisi MC, Keiserman MW . Revisiting anti-hsp90 antibodies in systemic lupus erythematosus. Clin Exp Rheumatol 2010; 28: 928.
Neckers L . Heat shock protein 90: the cancer chaperone. J Biosci 2007; 32: 517–530.
Neckers L . Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med 2002; 8: S55–S61.
Karkoulis PK, Stravopodis DJ, Margaritis LH, Voutsinas GE . 17-Allylamino-17-demethoxygeldanamycin induces downregulation of critical Hsp90 protein clients and results in cell cycle arrest and apoptosis of human urinary bladder cancer cells. BMC Cancer 2010; 10: 481.
Broemer M, Krappmann D, Scheidereit C . Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene 2004; 23: 5378–5386.
Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y et al. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun 2010; 1: 79.
Patel RK, Mohan C . PI3K/AKT signaling and systemic autoimmunity. Immunol Res 2005; 31: 47–55.
Wu T, Mohan C . The AKT axis as a therapeutic target in autoimmune diseases. Endocr Metab Immune Disord Drug Targets 2009; 9: 145–150.
Fernandez D, Perl A . mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med 2010; 9: 173–178.
Liu J, Beller DI . Distinct pathways for NF-kappa B regulation are associated with aberrant macrophage IL-12 production in lupus- and diabetes-prone mouse strains. J Immunol 2003; 170: 4489–4496.
Finck BK, Chan B, Wofsy D . Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest 1994; 94: 585–991.
Mihara M, Takagi N, Takeda Y, Ohsugi Y . IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol 1998; 112: 397–402.
Tsai CY, Wu TH, Yu CL, Lu JY, Tsai YY . Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm–Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron 2000; 85: 207–214.
Wax S, Piecyk M, Maritim B, Anderson P . Geldanamycin inhibits the production of inflammatory cytokines in activated macrophages by reducing the stability and translation of cytokine transcripts. Arthritis Rheum 2003; 48: 541–550.
Antonova G, Lichtenbeld H, Xia T, Chatterjee A, Dimitropoulou C, Catravas JD . Functional significance of hsp90 complexes with NOS and sGC in endothelial cells. Clin Hemorheol Microcirc 2007; 37: 19–35.
Yoshida M, Xia Y . Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J Biol Chem 2003; 278: 36953–36958.
Bae J, Mitsiades C, Tai YT, Bertheau R, Shammas M, Batchu RB et al. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol 2007; 178: 7730–7737.
Shimp SK III, Parson CB, Regna NL, Thomas A, Chafin CB, Reilly CM et al. HSP90 inhibition by 17-DMAG reduces inflammation in J774 macrophages through suppression of Akt and NF-κB pathways. Inflamm Res 2012; in press.
Madrigal-Matute J, Lopez-Franco O, Blanco-Colio LM, Munoz-Garcia B, Ramos-Mozo P, Ortega L et al. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res 2010; 86: 330–337.
Dasu MR, Devaraj S, Park S, Jialal I . Increased Toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010; 33: 861–868.
Pockley AG, Calderwood SK, Multhoff G . The atheroprotective properties of Hsp70: a role for Hsp70–endothelial interactions? Cell Stress Chaperones 2009; 14: 545–553.
de Jong PR, Schadenberg AW, Jansen NJ, Prakken BJ . Hsp70 and cardiac surgery: molecular chaperone and inflammatory regulator with compartmentalized effects. Cell Stress Chaperones 2009; 14: 117–131.
Chen H, Wu Y, Zhang Y, Jin L, Luo L, Xue B et al. Hsp70 inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett 2006; 580: 3145–3152.
Julkunen H, Ekblom-Kullberg S, Miettinen A . Nonrenal and renal activity of systemic lupus erythematosus: a comparison of two anti-C1q and five anti-dsDNA assays and complement C3 and C4. Rheumatology Int 2011; 1–7
Clynes R, Dumitru C, Ravetch JV . Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 1998; 279: 1052–1054.
Sekine H, Kinser TT, Qiao F, Martinez E, Paulling E, Ruiz P et al. The benefit of targeted and selective inhibition of the alternative complement pathway for modulating autoimmunity and renal disease in MRL/lpr mice. Arthritis Rheum 2011; 63: 1076–1085.
Sun L, Zhou L, Chen M, Zhong R, Liu J . Amelioration of systemic lupus erythematosus by withangulatin A in MRL/lpr mice. J Cell Biochem 2011; 112: 2376–2382.
Wenderfer SE, Wang H, Ke B, Wetsel RA, Braun MC . C3a receptor deficiency accelerates the onset of renal injury in the MRL/lpr mouse. Mol Immunol 2009; 46: 1397–1404.
Kasperkiewicz M, Muller R, Manz R, Magens M, Hammers CM, Somlai C et al. Heat-shock protein 90 inhibition in autoimmunity to type VII collagen: evidence that nonmalignant plasma cells are not therapeutic targets. Blood 2011; 117: 6135–6142.
Sobel ES, Morel L, Baert R, Mohan C, Schiffenbauer J, Wakeland EK . Genetic dissection of systemic lupus erythematosus pathogenesis: evidence for functional expression of Sle3/5 by non-T cells. J Immunol 2002; 169: 4025–4032.
Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES et al. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest 2005; 115: 1869–1878.
Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR . Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol 1991; 147: 117–123.
Wan S, Xia C, Morel L . IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol 2007; 178: 271–279.
de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3+ T-regulatory cells. Mol Cell Biol 2011; 31: 2066–2078.
Madaio MP . B cells and autoantibodies in the pathogenesis of lupus nephritis. Immunol Res 1998; 17: 123–132.
Chan OT, Madaio MP, Shlomchik MJ . The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev 1999; 169: 107–121.
Theofilopoulos AN, Singer PA, Kofler R, Kono DH, Duchosal MA, Balderas RS . B and T cell antigen receptor repertoires in lupus/arthritis murine models. Springer Semin Immunopathol 1989; 11: 335–368.
Grimaldi CM . Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr Opin Rheumatol 2006; 18: 456–461.
Acknowledgements
We would like to acknowledge the assistance that Melissa Makris provided with the flow cytometry. We would also like to thank the animal care staff at VMRCVM for their attention to our animals. We are appreciative of the support by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimp, S., Chafin, C., Regna, N. et al. Heat shock protein 90 inhibition by 17-DMAG lessens disease in the MRL/lpr mouse model of systemic lupus erythematosus. Cell Mol Immunol 9, 255–266 (2012). https://doi.org/10.1038/cmi.2012.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2012.5
Keywords
This article is cited by
-
HSP90 Exacerbates Bone Destruction in Rheumatoid Arthritis by Activating TRAF6/NFATc1 Signaling
Inflammation (2024)
-
Restoration of aberrant gene expression of monocytes in systemic lupus erythematosus via a combined transcriptome-reversal and network-based drug repurposing strategy
BMC Genomics (2023)
-
Integrating 3D genomic and epigenomic data to enhance target gene discovery and drug repurposing in transcriptome-wide association studies
Nature Communications (2022)
-
Heat shock protein 90 is a new potential target of anti-rejection therapy in allotransplantation
Cell Stress and Chaperones (2022)
-
DNA vaccine encoding heat shock protein 90 protects from murine lupus
Arthritis Research & Therapy (2020)