Abstract
Natural killer (NK) cells represent a heterogeneous population of innate lymphocytes with phenotypically and functionally distinct subsets. In particular, recent studies have identified a unique subset of NK cells residing within the liver that are maintained as tissue-resident cells, confer antigen-specific memory responses and exhibit different phenotypical and developmental characteristics compared with conventional NK (cNK) cells. These findings have encouraged researchers to uncover tissue-resident NK cells at other sites, and detailed analyses have revealed that these tissue-resident NK cells share many similarities with liver-resident NK cells and tissue-resident memory T cells. Here, we present a brief historical perspective on the discovery of liver-resident NK cells and discuss their relationship to cNK cells and other emerging NK cell subsets and their potential functions.
Similar content being viewed by others
Introduction
NK cells are an important member of the innate immune system, playing crucial roles in host defense against microbial infections and developing tumors.1, 2 Unlike T cells and B cells, NK cells can be rapidly activated to exert natural cytotoxicity against target cells without the necessity of priming, and this process is delicately controlled by multiple activating and inhibitory surface receptors.3 Additionally, NK cells also secrete an array of cytokines, among which IFNγ is mainly produced by NK cells during various physiological and pathological conditions.1, 4 By producing cytokines or direct cytotoxicity to immune cells, NK cells are emerging as important regulators of adaptive immune responses.4, 5, 6
NK cells are widely distributed throughout the body, including in lymphoid and non-lymphoid tissues. However, most of our knowledge concerning NK cells has been derived from studies performed on mouse splenic and human peripheral blood NK cells because of their ease of access.7 These NK cells, now referred to as conventional NK (cNK) cells, originate in the bone marrow and move through the circulation to different tissues and organs.7, 8
For decades, cNK cells were the only known innate lymphoid cells (ILCs). However, recent research into peripheral non-lymphoid tissues led to the discovery of diverse ILC subsets, which greatly expanded the knowledge of innate immunity and redefined our classic maps of the immune system.9 Over recent years, NK-cell heterogeneity has also been appreciated, and tissue-resident NK cells have come into the spotlight.8, 10, 11 In particular, we and others have recently found that the mutually exclusive expression of CD49a and DX5 could divide mouse liver NK cells into two distinct subsets. The CD49a−DX5+ subset resembles splenic NK cells and circulates in the blood; thus, they are called cNK cells. Another subset, liver-resident NK cells, selectively resides in the liver sinusoids and is characterized by the CD49a+DX5− phenotype.12, 13 These findings have encouraged the scanning of tissue-resident NK cells across the body, and tissue-resident NK cells in various tissues, such as the skin, uterus, salivary gland and adipose, have been successively discovered.13, 14, 15
Finding liver-resident NK cells
Early recognition of liver NK-cell heterogeneity
Previous studies have revealed that the liver contains a high frequency of NK cells relative to the spleen, peripheral blood and bone marrow and liver NK cells are phenotypically different from those in other tissues.16 In adult mice, liver NK cells highly express tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but express low levels of markers associated with NK cell maturation, such as DX5, CD11b and Ly49 receptors.17, 18, 19 By contrast, splenic NK cells are predominantly mature in phenotype. In early life, TRAIL+DX5−CD11blo phenotypically immature NK cells are predominant in the liver and abundant in the spleen.18 However, the numbers of these NK cells in the spleen sharply decline after birth, but only mildly decrease in the liver with age and these NK cells maintain a stable subpopulation in the adult murine liver.18 Consistent with these findings observed in mice, human liver NK cells also display unique features compared with their blood counterparts. For example, liver CD56+CD3− NK cells express relatively low levels of CD16, CD62L and inhibitory killer Ig-like receptors (KIRs) and high levels of CD69; moreover, human liver NK cells have weaker cytotoxic functions than blood NK cells.20 Overall, the early studies of liver NK cells in mice and humans provided evidence of heterogeneity among NK cells but not lineage divergence.
Discovery of liver-resident NK cells
On the basis of earlier findings, we further researched the transcriptional, phenotypic and functional features of liver NK cells. Compared with NK cells in the bone marrow, peripheral blood and spleen, hepatic lymphocytes were enriched in DX5− NK cells, which accounted for approximately half of hepatic NK cells, but were rare in other examined tissues.12 Liver DX5+ NK cells were phenotypically similar to their splenic counterparts but distinct from liver DX5− NK cells.12 Genomic analysis further revealed that substantial differentially expressed genes existed between liver DX5− and DX5+ NK cells, including those encoding inhibitory and activating receptors, cytokines, homing molecules, cytotoxic effectors and transcription factors.12 Importantly, we found that liver DX5− NK cells selectively expressed CD49a, which is rarely expressed by liver DX5+ NK cells, and thus it was considered to be a specific marker for liver DX5− NK cells.12, 13
DX5− NK cells were previously regarded as an immature stage of cNK-cell development.17, 21 Through adoptive transfer experiments, however, we found that CD49a+DX5− NK cells from donor livers could not give rise to DX5+ NK cells and preferentially trafficked to the recipient liver after transfer, whereas donor CD49a−DX5+ NK cells exhibited non-discriminatory migration between different tissues.12 These findings suggest that CD49a+DX5− NK cells are not developmental intermediates but represent a stable population.
Of particular interest, when we analyzed the phenotype of NK cells in the blood flow from different vessels, we observed that neither afferent nor efferent blood of the liver contained CD49a+DX5− NK cells, which were instead present in the liver sinusoid blood.7, 12 By contrast, CD49a−DX5+ NK cells were found in all blood sources.7, 12 Parabiosis experiments between CD45.1+ and CD45.2+ mice showed that liver CD49a−DX5+ NK cells were confined to the host origin, whereas CD49a−DX5+ NK cells in the spleen and liver were derived from both the host and the other parabiont.12, 13 Taking into account the above findings, liver CD49a+DX5− NK cells were also called liver-resident NK cells, and CD49a−DX5+ NK cells represent circulating cNK cells.
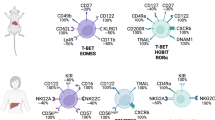
Efforts have recently been made to explore whether human liver contains liver-resident NK cells. Hudspeth et al. found that compared with peripheral blood, the liver tissue and liver perfusates from healthy humans contained significantly higher percentages of CD56bright NK cells that expressed high levels of CD69 and CXCR6 and low levels of CD62L, akin to murine liver-resident NK cells.22 Furthermore, a proportion of human liver CD56bright NK cells expressed CD49a, and these CD49a+ NK cells were T-bet+Eomes−,23 which is consistent with the transcription factor expression pattern found in murine liver CD49a+ NK cells. However, CD49a+CD56bright NK cells were present at a very low frequency (an average of 2.3%) and only identified in 12 of 29 livers examined.23 More recently, it has been revealed that CXCR6+ or CD49e− NK cells are highly enriched in the human liver but are rare in the circulation,24, 25, 26 suggesting that CXCR6 and CD49e might be potential markers identifying human liver-resident NK cells. However, unlike murine liver-resident NK cells, human liver CXCR6+ or CD49e− NK cells lacked T-bet expression and highly expressed Eomes (Table 1).24, 25, 26, 29
Development of liver-resident NK cells
Liver-resident NK cells exhibit fundamentally different profiles and requirements of transcription factors compared with cNK cells.30 For example, Hobit expression is low in cNK cells but specifically upregulated in liver-resident NK cells.31 Hobit deficiency results in a sharp reduction in the number of liver-resident NK cells but has little impact on cNK cells.31 Similarly, the T-box transcription factor T-bet is also strictly required for liver-resident NK cell development, while cNK cells are only moderately affected by the loss of T-bet.13, 27 By contrast, Eomes is highly expressed in cNK cells compared with liver-resident NK cells, and in Eomes-deficient mice, a complete loss of cNK cells was observed, while liver-resident NK cells were not affected.32 Acting upstream of Eomes, NFIL3 also exerts a more profound effect on cNK cells than on liver-resident NK cells.33
Recent studies have identified common progenitors that are committed to diverse innate lymphoid cell (ILC) lineages. Both the α-lymphoid progenitors (αLPs), which differ from common lymphoid progenitors (CLPs) by their expression of α4β7 and CXCR6, and early innate lymphoid progenitor (EILPs) expressing TCF1 can give rise to all known ILCs, including cNK cells and liver-resident NK cells, but lack T- and B-lymphocyte potential.34, 35 Interestingly, multipotent progenitors that lose cNK-cell developmental potential but that can generate tissue-resident NK cells and other ILCs have been found,36, 37 suggesting that liver-resident NK cells are developmentally separate from cNK cells. Additionally, sharing many similarities with mucosal ILC1s in terms of their development pathway, liver-resident NK cells have also been categorized as ILC1s, regardless of their cytotoxic capacity.
Common features of tissue-resident lymphocytes
In addition to the liver, other non-lymphoid tissues have also been found to contain tissue-resident NK cells, which share many common features with liver-resident NK cells.38 The expression of CD49a and DX5 has been described as mutually exclusive to cNK cells and tissue-resident NK cells in the uterus, skin, kidney and liver.13, 39 CD49a+DX5− tissue-resident NK cells in the skin are T-bet-dependent and lack the expression of Eomes, like those found in the liver.13 By contrast, neither uterus-resident NK cells, which express high levels of Eomes, nor kidney-resident NK cells rely on T-bet for their development.13, 39, 40 CD49a is also a marker of the tissue-resident NK-cell subset residing in the salivary gland, but these cells are mostly DX5-positive and dependent on TGF-β signaling for their development.14 In addition to CD49a, CD69 is another tissue-resident marker, as shown by its constantly high expression among these NK cells.38
The concept of tissue residency has also been used to describe adaptive lymphocytes, which has been mostly driven by studies on CD8+ memory T cells.41 Tissue-resident memory T (Trm) cells share many markers with tissue-resident NK cells, including the high expression of CD49a and CD69 and low amounts of CD62L and S1PR1.42 Moreover, a recent study has found that the transcription factor Hobit is specifically up-regulated in tissue-resident lymphocytes, including Trm cells, liver-resident NK cells and NKT cells.31 Hobit and Blimp1 cooperate to specifically promote Trm- and NKT-cell development, while liver-resident NK cells are specifically dependent on Hobit.31 Further analysis revealed that Hobit and Blimp1 repressed a series of genes associated with tissue egress, including S1pr1, Ccr7 and Klf2, implicating a universal program of tissue residency shared by diverse tissue-resident lymphocyte populations.31, 43
Potential functions of liver-resident NK cells
NK cells have long been considered short-lived, non-antigen-specific effectors of innate immunity. However, several lines of evidence have unveiled that NK cells can mount antigen-specific secondary responses that resemble classical immunological memory.44 The first evidence for specific NK-cell responses to antigens was observed in a mouse model of contact hypersensitivity (CHS).45 Von Andrian and colleagues found that Rag2−/− and SCID mice, which lack T and B cells but possess NK cells, mediated CHS responses in a hapten-specific manner, while Rag2−/− x Il2rg−/ mice, which lack T, B, and NK cells, did not.45 In particular, only NK cells that are localized in the livers, and not those in the spleens, mediate memory responses upon sensitization with haptens or virus-like particles.28, 45 Moreover, memory responses mediated by hepatic NK cells are dependent on CXCR6,28 a chemokine receptor that is highly expressed by CD49a+DX5− liver-resident NK cells.12 Enlightened by these findings, we adoptively transferred liver-resident NK cells or cNK cells from hapten-sensitized mice to naïve mice and found that the recipients of sensitized liver-resident NK cells, but not cNK cells, displayed vigorous CHS responses after challenge.12 In addition, our recent study indicated that the adoptive transfer of liver CD49a+DX5− NK cells from influenza virus-infected mice into naive mice conferred protective immunity against secondary influenza virus infection, while transferred lung NK cells did not.46 These studies suggest that NK cells mediating antigen-specific responses are confined to liver CD49a+DX5− NK cells but the tissue residency of this subset makes migration events during NK-cell-mediated memory responses more complicated than expected. It has previously been hypothesized that naive NK cells traffic to draining lymph nodes where they are primed by antigen-presenting cells upon sensitization, then, they migrate to the liver where they persist as long-lived memory cells, and finally emigrate into the effector site to evoke CHS upon re-challenge.47 This model is largely based on memory T-cell theory. Direct evidence of each event is still lacking, and the mechanism underlying how tissue-resident NK cells exit from their initial place to other sites remains mysterious.
As an immune tolerant organ, the liver is predisposed to chronic infections and tumorigenesis, and the crosstalk between liver-resident antigen-presenting cells and circulating lymphocytes has been implicated in this process.48, 49, 50, 51 To date, however, little is known about the role of liver-resident NK cells in liver tolerance and diseases, as most previous studies have utilized bulk liver NK cells as research subjects. These studies have indicated that hepatic NK cells may play dual roles, protective or pathogenic, in different disease settings.48, 52 Accumulating evidence suggests that in the pathogenesis of various liver diseases, such as acute viral hepatitis and liver fibrosis, NK cells could accumulate in the liver and display enhanced reactivity,53, 54, 55, 56 which can be beneficial in inhibiting viral infection and tumor development, but which may also induce hepatocellular damage and inhibit liver regeneration.52 In certain liver diseases, such as chronic hepatitis B virus (HBV) infection and tumor, the impairment of NK cell function has been described,57, 58, 59 suggesting the interrelation of NK-cell dysfunction and chronic liver disease progression. Regarding distinct liver NK cell subsets, they may play different roles in liver diseases based on the different expression pattern of effector molecules. For example, compared with cNK cells, liver-resident NK cells are efficient in producing IFNγ, TNFα and GM-CSF, which are key players in inflammatory responses.13, 27 On the other hand, liver-resident NK cells constitutively express high levels of TRAIL, which has been reported to contribute to the NK-cell-mediated elimination of activated CD4+ T cells or virus-specific CD8+ T cells during chronic viral infection,6, 60 and thus may exert negative regulatory functions in anti-viral immune responses. Moreover, a recent study has reported that the absence of NKG2A, which is highly expressed by liver-resident NK cells, resulted in the expansion of virus-specific CD8+ T cells,61 but whether this immunosuppressive effect was mediated by liver-resident NK cells has not been established. Therefore, despite substantial differences identified between liver-resident NK cells and cNK cells, whether these two subsets play differential roles in the pathogenesis of various liver diseases remains elusive.
Conclusions
Recent studies have indicated that the liver contains a unique NK-cell population, called liver-resident NK cells, that displays distinct phenotypical and developmental features compared with cNK cells. In the CHS model and certain viral infection models, liver-resident NK cells, not cNK cells, may mediate antigen-specific memory responses, but the precise underlying mechanisms still await further investigation. The identification of liver-resident NK cells has led to the discoveries of tissue-resident NK cells in various non-lymphoid tissues, and these newly identified NK cell subsets from different tissues share many common features. Further studies should investigate the functions of these tissue-resident NK cells, especially in diseases, pathology and maintaining local homeostasis.
References
Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331: 44–49.
Sun C, Sun H, Zhang C, Tian Z . NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol 2015; 12: 292–302.
Narni-Mancinelli E, Ugolini S, Vivier E . Tuning the threshold of natural killer cell responses. Curr Opin Immunol 2013; 25: 53–58.
Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 2004; 5: 1260–1265.
Crome SQ, Lang PA, Lang KS, Ohashi PS . Natural killer cells regulate diverse T cell responses. Trends Immunol 2013; 34: 342–349.
Schuster IS, Wikstrom ME, Brizard G, Coudert JD, Estcourt MJ, Manzur M et al. TRAIL+ NK cells control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity 2014; 41: 646–656.
Yokoyama WM, Sojka DK, Peng H, Tian Z . Tissue-resident natural killer cells. Cold Spring Harb Symp Quant Biol 2013; 78: 149–156.
Sojka DK, Tian Z, Yokoyama WM . Tissue-resident natural killer cells and their potential diversity. Semin Immunol 2014; 26: 127–131.
Spits H, Di Santo JP . The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 2011; 12: 21–27.
Spits H, Bernink JH, Lanier L . NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol 2016; 17: 758–764.
Erick TK, Brossay L . Phenotype and functions of conventional and non-conventional NK cells. Curr Opin Immunol 2016; 38: 67–74.
Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest 2013; 123: 1444–1456.
Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife 2014; 3: e01659.
Cortez VS, Cervantes-Barragan L, Robinette ML, Bando JK, Wang Y, Geiger TL et al. Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity 2016; 44: 1127–1139.
O'Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM et al. Adipose-Resident Group 1 Innate Lymphoid Cells Promote Obesity-Associated Insulin Resistance. Immunity 2016; 45: 428–441.
Peng H, Wisse E, Tian Z . Liver natural killer cells: subsets and roles in liver immunity. Cell Mol Immunol 2016; 13: 328–336.
Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 2002; 3: 523–528.
Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood 2005; 105: 2082–2089.
Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012; 36: 55–67.
Burt BM, Plitas G, Zhao Z, Bamboat ZM, Nguyen HM, Dupont B et al. The lytic potential of human liver NK cells is restricted by their limited expression of inhibitory killer Ig-like receptors. J Immunol 2009; 183: 1789–1796.
Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP . Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol 2001; 31: 1900–1909.
Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun 2016; 66: 40–50.
Marquardt N, Beziat V, Nystrom S, Hengst J, Ivarsson MA, Kekalainen E et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J Immunol 2015; 194: 2467–2471.
Stegmann KA, Robertson F, Hansi N, Gill U, Pallant C, Christophides T et al. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci Rep 2016; 6: 26157.
Harmon C, Robinson MW, Fahey R, Whelan S, Houlihan DD, Geoghegan J et al. Tissue-resident Eomes(hi) T-bet(lo) CD56(bright) NK cells with reduced proinflammatory potential are enriched in the adult human liver. Eur J Immunol 2016; 46: 2111–2120.
Aw Yeang HX, Piersma SJ, Lin Y, Yang L, Malkova ON, Miner C et al. Cutting edge: human CD49e- NK cells are tissue resident in the liver. J Immunol 2017; 198: 1417–1422.
Tang L, Peng H, Zhou J, Chen Y, Wei H, Sun R et al. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J Autoimmun 2016; 67: 29–35.
Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 2010; 11: 1127–1135.
Cuff AO, Robertson FP, Stegmann KA, Pallett LJ, Maini MK, Davidson BR et al. Eomeshi NK cells in human liver are long-lived and do not recirculate but can be replenished from the circulation. J Immunol 2016; 197: 4283–4291.
Peng H, Tian Z . Re-examining the origin and function of liver-resident NK cells. Trends Immunol 2015; 36: 293–299.
Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016; 352: 459–463.
Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med 2014; 211: 563–577.
Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med 2014; 211: 635–642.
Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife 2014; 3.
Yang Q, Li F, Harly C, Xing S, Ye L, Xia X et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol 2015; 16: 1044–1050.
Constantinides MG, Gudjonson H, McDonald BD, Ishizuka IE, Verhoef PA, Dinner AR et al. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci USA 2015; 112: 5123–5128.
Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014; 157: 340–356.
Fan X, Rudensky AY . Hallmarks of tissue-resident lymphocytes. Cell 2016; 164: 1198–1211.
Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D et al. Tissue-resident NK cells mediate ischemic kidney injury and are not depleted by anti-Asialo-GM1 antibody. J Immunol 2015; 195: 4973–4985.
Tayade C, Fang Y, Black GP, V Jr AP, Erlebacher A, Croy BA . Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J Leukoc Biol 2005; 78: 1347–1355.
Mueller SN, Mackay LK . Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 2016; 16: 79–89.
Schenkel JM, Masopust D . Tissue-resident memory T cells. Immunity 2014; 41: 886–897.
Mackay LK, Kallies A . Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol 2017; 38: 94–103.
Marcus A, Raulet DH . Evidence for natural killer cell memory. Curr Biol 2013; 23: R817–R820.
O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH . T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 2006; 7: 507–516.
Li T, Wang J, Wang Y, Chen Y, Wei H, Sun R et al. Respiratory influenza virus infection induces memory-like liver NK cells in mice. J Immunol 2017; 198: 1242–1252.
Paust S, von Andrian UH . Natural killer cell memory. Nat Immunol 2011; 12: 500–508.
Horst AK, Neumann K, Diehl L, Tiegs G . Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol 2016; 13: 277–292.
Grakoui A, Crispe IN . Presentation of hepatocellular antigens. Cell Mol Immunol 2016; 13: 293–300.
Ju C, Tacke F . Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol 2016; 13: 316–327.
Shuai Z, Leung MW, He X, Zhang W, Yang G, Leung PS et al. Adaptive immunity in the liver. Cell Mol Immunol 2016; 13: 354–368.
Tian Z, Chen Y, Gao B . Natural killer cells in liver disease. Hepatology 2013; 57: 1654–1662.
Hou X, Zhou R, Wei H, Sun R, Tian Z . NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis. Hepatology 2009; 49: 940–949.
Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B . Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006; 130: 435–452.
Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut 2012; 61: 885–893.
Gao B, Radaeva S, Park O . Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009; 86: 513–528.
Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129: 428–437.
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50: 799–807.
Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J . Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol 2017; 14: 465–475.
Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med 2013; 210: 99–114.
Krueger PD, Narayanan S, Surette FA, Brown MG, Sung SJ, Hahn YS . Murine liver-resident group 1 innate lymphoid cells regulate optimal priming of anti-viral CD8+ T cells. J Leukoc Biol 2017; 101: 329–338.
Acknowledgements
This work was supported by the Natural Science Foundation of China (#81761128013, 81571522, 91642105, 81361120388, 31300727, 91542114, 91442112, 91542000 and 81330071) and the Ministry of Science and Technology of China (973 Basic Science Project 2013CB944902).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Peng, H., Sun, R. Liver-resident NK cells and their potential functions. Cell Mol Immunol 14, 890–894 (2017). https://doi.org/10.1038/cmi.2017.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2017.72
This article is cited by
-
Early life gut microbiota sustains liver-resident natural killer cells maturation via the butyrate-IL-18 axis
Nature Communications (2023)
-
Synergistic anti-inflammatory effect of gut microbiota and lithocholic acid on liver fibrosis
Inflammation Research (2022)
-
Innate-like Lymphocytes and Innate Lymphoid Cells in Asthma
Clinical Reviews in Allergy & Immunology (2020)
-
Tissue-resident memory-like ILCs: innate counterparts of TRM cells
Protein & Cell (2020)
-
Tissue-resident lymphocytes: from adaptive to innate immunity
Cellular & Molecular Immunology (2019)