The development of methods for polymerizing organic molecules on surfaces opens up fresh approaches for fabricating exceptionally robust two-dimensional materials1, including 2D forms of materials known as covalent organic frameworks (COFs). Writing in the Journal of the American Chemical Society, Cai et al.2 report the reversible formation of a single 2D layer of a COF on a graphite surface underneath the tip of a scanning tunnelling microscope (STM). The reaction occurs at room temperature, which is remarkably low for this type of transformation. The authors attribute their unexpected room-temperature polymerization to the large, highly localized electric field generated when a voltage is applied between the STM tip and the surface.
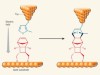
COFs are purely organic, permanently porous crystalline materials that have ultra-low densities because they do not contain heavy elements. In 2005, it was reported3 that a bulk form of a COF can be made from boronic acids — compounds that contain a B(OH)2 group. Boronic acids are prone to self-condensation reactions, in which three of them combine when heated to form boroxine rings (B3O3), which can constitute the three-pronged vertices of a COF (Fig. 1).

Figure 1 | A completely reversible 2D polymerization reaction. a, When heated, boronic acids can react to produce boroxine rings, expelling water as a side product. The reverse reaction can also occur on heating. R represents any chemical group. b, If a molecule contains more than one boronic acid group, they can react to form a 2D polymer called a covalent organic framework (COF). c, Cai et al.2 report that such a COF formation occurs at room temperature when the tip of a scanning tunnelling microscope (STM) passes across boronic acid molecules on a graphite surface, if there is a negative voltage on the graphite with respect to the tip. The reaction is completely reversed when the tip scans over the COF and the polarity of the voltage is reversed (not shown). The authors propose that the reactions are driven by the strong, localized electric field generated between the tip and the graphite.
Cai et al. have now studied three-lobed molecules that have a boronic acid group at each lobe, and have carried out condensation reactions at the interface between a solution of these compounds and graphite. The authors deposited the solution of their boronic acid onto graphite and scanned an STM tip across it while applying a positive sample bias (a voltage that causes electrons to ‘tunnel’ from the tip to the graphite). They observed the formation of highly ordered, 2D supramolecular assemblies of the molecules in the STM images. But when they scanned the same area at a negative sample bias (causing electrons to tunnel from the graphite to the tip), the assemblies transformed into regular networks of covalently linked molecules — small domains of a single-layer COF (Fig. 1).
The authors showed that polymerization occurred only in the scanned region — STM images of the surrounding non-scanned areas contained only unreacted molecules. Remarkably, when they then switched back to a positive sample bias, the COFs depolymerized, restoring the supramolecular assemblies. The ability to selectively drive either the polymerization or the depolymerization to completion at room temperature is unprecedented for COF-forming reactions, and clearly indicates the power of the non-thermal activation mechanism. Both reactions proceeded slowly enough for their kinetics to be monitored by normal STM imaging, with the depolymerization reaction being about ten times faster than the polymerization.
Reactions triggered electrically
The processes that produce highly ordered covalent materials such as COFs require dynamic covalent chemistry4 — reactions in which covalent bonds form reversibly, thereby allowing self-healing of defects in the nascent COF, and hence the formation of crystalline materials that have ‘long-range order’. The reversible condensation of boronic acids aids the formation both of bulk COFs3 and of single-layer COFs synthesized on surfaces5,6. In both cases, however, the polymerization required temperatures of around 100 °C; it was inconceivable that such COF formation could occur at room temperature.
Cai and colleagues’ work is therefore a stunning example of an underexplored phenomenon: how exceptional environmental conditions can alter the progress of chemical reactions. The authors accredit their astonishing finding to the influence of the strong, oriented electric field beneath the STM tip. Although the applied voltages in STMs are modest (just a few volts, at most), the close proximity of tip to the sample (less than 1 nanometre away) gives rise to strong, static electric fields of the order of 109 volts per metre, which are otherwise difficult to reach.
STMs have demonstrated a striking ability to induce individual molecules to undergo a variety of processes7, such as changing conformation, dissociation and chemical reactions. These processes could, in principle, be driven by the electric field. But the STM tip can also act as a powerful oxidizing or reducing agent, depending on the voltage between it and the surface that it scans across. Moreover, at liquid–solid interfaces, scanning of the STM tip causes ‘nano-stirring’ of the liquid. All of these tip effects could promote chemical reactions individually or in combination with each other, but the intimate connections between them make it difficult to separate out the effects that are at play in experiments.
So does the electric field definitely drive Cai and colleagues’ reactions, or could another mechanism be involved? Boronic acids act as Lewis acids (electron-pair acceptors), and so an alternative explanation is that electron-transfer processes are responsible. This possibility should now be explored.
Snapshots of vibrating molecules
Carrying out STM experiments at liquid–solid interfaces is relatively straightforward, facilitating future studies of the reported reactions. However, the reaction system itself is highly complex. For example, crucial factors that could affect the surface reactions include the solvent, the surrounding solute molecules (boronic acids that are still in solution, rather than on the graphite surface) and even dissolved impurities; none of these are easy to visualize using STMs, which can image only surface-bound molecules. The main challenge now, therefore, is to devise experiments or computational simulations that provide further clues about the mechanism. Cai et al. have taken the first step in this direction by studying the solvent dependence of the reactions. Future studies could also shed light on the role of water molecules, which are essential for the depolymerization reaction.
Since their development in 1981, STMs have enhanced our understanding of fundamental atomic processes on surfaces, and are still the sole analytical tool for tackling various ongoing research problems in surface science. Stunning experiments have also shown that STMs can be used to assemble atoms or molecules into precise nanostructures (see refs 8 and 9, for example), and to manipulate the chemical state of those structures10. But scaling up such experiments to make practically useful quantities of materials has been impossible, because the processes involved are inherently serial operations and atomic in scale.
Cai and colleagues’ work provides an intriguing test case for whether STM-induced processes can be translated to more-macroscopic scales. This would require comparably strong electric fields to be produced at least on the micrometre scale — which seems feasible, but challenging. If achieved, this would not only verify the proposed mechanism, but also constitute a milestone in bringing such unconventional chemistry closer to our macroscopic world.

 Reactions triggered electrically
Reactions triggered electrically
 Snapshots of vibrating molecules
Snapshots of vibrating molecules