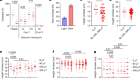
You are about to take the stage to speak in front of a large audience. As you wait, your heart starts to pound, your breathing quickens, your blood pressure rises and your palms sweat. These physiological responses are evolutionarily conserved mechanisms to prepare your body to fight against imminent dangers, or to run away quickly. Another key response is an increase in body temperature. Emotional stress can cause this psychogenic fever in many mammalian species, from rodents to humans1,2. What is the neural mechanism that underlies this phenomenon? Writing in Science, Kataoka et al.3 describe a key neural circuit in psychologically induced hyperthermia.
The current work builds on a long legacy of research by the same group, who began their quest for a neuronal circuit that triggers heat production in 2004, using brown fat tissue as an entry point4. Brown fat is a type of ‘good’ fat that can generate heat when needed. Blocking the activity of β3-adrenergic receptor proteins, which are abundant in brown fat and enable the tissue to respond to signals from neurons, attenuates stress-induced hyperthermia5.
In the 2004 study, the researchers injected viral ‘retrograde tracers’ into brown fat in rats; the tracers move through connected neurons, allowing the authors to identify brain regions from which neurons project to the fat4. This revealed that neurons in a brainstem area called the rostral medullary raphe (rMR) connect to brown fat. Later on, the same group identified2 the dorsomedial hypothalamus (DMH) as a key brain region upstream of the rMR. When the authors artificially activated the DMH-to-rMR pathway, they found an increase in neuronal activity and heat production in brown fat. Unexpectedly, activating this pathway also increased heart rate and blood pressure, suggesting that DMH–rMR could coordinate various physiological responses during stress.
Read the paper: A Central Master Driver of Psychosocial Stress Responses in the Rat
In humans, psychological stress often involves an understanding of complicated situations, and thus probably requires instructions from regions of the brain’s cortex, which is involved in cognition. In the current study, Kataoka et al. set out to identify the cortical regions that could send these instructions to the DMH. As in their previous work, the authors used retrograde tracers — this time, injected into the DMH — to look for neurons that link into their heat-generating circuit. They found that only one, little-studied, region of the cortex was strongly labelled by the tracer. This region, called the dorsal peduncular cortex and dorsal taenia tecta (DP/DTT), is also highly active in rats in the wake of social defeat (a hostile interaction in which the animal has lost a fight with another, dominant rat).
To examine the role of this region in stress responses, the authors impaired its connection to the DMH in three ways. They blocked activity throughout the DP/DTT using a chemical inhibitor; they used a virus to kill cells projecting from the DP/DTT to the DMH; and they used a sophisticated genetic approach to inhibit activity specifically in the projections that DP/DTT neurons send to the DMH. In each case, their intervention reduced stress-induced hyperthermia.
By contrast, artificial activation of the neuronal projections between the two regions elicited a battery of responses, including increases in heart rate, blood pressure, and heat production in brown fat. The group provided evidence that the DP/DTT neurons send excitatory signals to the DMH, and demonstrated that the projections from the DP/DTT terminate close to the DMH cells that, in turn, project to the rMR. Taken together, Kataoka and colleagues’ experiments support the idea of a DP/DTT–DMH–rMR–brown fat circuit for heat production in response to stress (Fig. 1).

Figure 1 | Stressful connections. Kataoka et al.3 report that, in rats, a brain region called the dorsal peduncular cortex and dorsal taenia tecta (DP/DTT) is involved in psychogenic fever — an increase in body temperature in response to social stress. Stress-related information reaches the DP/DTT from two other brain regions: the paraventricular (PVT) and mediodorsal (MD) thalamic nuclei. Neurons from the DP/DTT then project to and excite neurons in the brain’s dorsomedial hypothalamus (DMH), which in turn sends neuronal projections to the rostral medullary raphe (rMR). Finally, neurons from this region connect indirectly to brown fat tissue, which generates heat.
How does the stress-related information reach the DP/DTT? Further retrograde tracing experiments revealed that the strongest inputs to the DP/DTT are from the brain’s midline thalamic regions, including the paraventricular (PVT) and mediodorsal (MD) thalamic nuclei. The PVT is highly sensitive to various physical and psychological stressors, such as predator cues and pain6. By contrast, the MD interacts with the prefrontal cortex to mediate complex cognitive functions, such as rule learning, abstraction, evaluation and (in humans) imagination7. Thus, every possible stressor, from physical pain to anticipated legal trouble, can find their way to the DP/DTT. It remains unclear, however, how different stressors are encoded in the DP/DTT, whether the responses of the DP/DTT to stressors are influenced by experience, and whether deficits in DP/DTT cells could be responsible for abnormal physiological responses to stress. Future studies using electrophysiological or optical recordings of the DP/DTT cells will help to address these questions.
The philosopher and psychologist William James suggested that fear is an interpretation of physiological responses to threat, instead of the other way around8. In other words, rather than running from a bear because we are afraid, we are afraid because we are running from a bear. If James is right, rats should stop being afraid if their physiological responses to a threat are blocked. Kataoka et al. therefore asked whether inhibiting the DP/DTT–DMH pathway can suppress the fear that a rat shows when presented with an aggressive, dominant counterpart that has recently defeated it in a stressful social interaction.
Under normal conditions, a defeated animal will try to stay away from the aggressor to avoid incurring further damage. By contrast, naive animals that have not previously gone through a social defeat show no signs of fear, and investigate the dominant rat with great interest. Remarkably, when the authors blocked the DP/DTT–DMH pathway in rats that had been defeated, the animals behaved like naive rats.
Thus, the behavioural manifestation of fear, and perhaps the perception of fear (which can only be inferred from behaviours in rats), depends on bodily responses to threat. These data provide an indication of why taking a deep breath before that big public speech might help to calm us down. The data also suggest that suppressing physiological responses to stress could be an effective way to alleviate stressful feelings. Of importance in this context, non-stress-related thermoregulation — changes in internal temperature caused by infections or external temperature change, for instance — is mediated, not by the DP/DTT, but by another region upstream of the DMH, the preoptic area9. Blocking the DP/DTT–DMH pathway would therefore be expected to leave day-to-day regulation of temperature unchanged. It is early days, but manipulation of the DP/DTT could potentially be a way to curb chronic psychological stress.


 Eighty years of stress
Eighty years of stress
 Gut microbes regulate neurons to help mice forget their fear
Gut microbes regulate neurons to help mice forget their fear