Abstract
Background:
Low glycemic index (GI) carbohydrates have been linked to increased satiety. The drive to eat may be mediated by postprandial changes in glucose, insulin and gut peptides.
Objective:
To investigate the effect of a low and a high GI diet on day-long (10 h) blood concentrations of glucose, insulin, cholecystokinin (CCK) and ghrelin (GHR).
Design:
Subjects (n=12) consumed a high and a low GI diet in a randomized, crossover design, consisting of four meals that were matched for macronutrients and fibre, and differed only in carbohydrate quality (GI). Blood was sampled every 30–60 min and assayed for glucose, insulin, CCK and GHR.
Results:
The high GI diet resulted in significantly higher glucose and insulin mean incremental areas under the curve (IAUC, P=0.027 and P=0.001 respectively). CCK concentration was 59% higher during the first 7 h of the low GI diet (394±95 pmol/l min) vs the high GI diet (163±38 pmol/l min, P=0.046), but there was no difference over 10 h (P=0.224). GHR concentration was inversely correlated with insulin concentration (Pearson correlation −0.48, P=0.007), but did not differ significantly between the low and high GI diets.
Conclusions:
Mixed meals of lower GI are associated with lower day-long concentrations of glucose and insulin, and higher CCK after breakfast, morning tea and lunch. This metabolic profile could mediate differences in satiety and hunger seen in some, but not all, studies.
Similar content being viewed by others
Introduction
Twin epidemics of obesity and diabetes have prompted research into the physiology and psychology of hunger and satiety. While the acute control of feeding is partly mediated by gut peptides such as cholecystokinin (CCK) and ghrelin (GHR) (Tschop et al., 2000; Wren and Bloom, 2007), the macronutrient composition of a food is a recognized determinant of feeding drive. Dietary protein is the most satiating macronutrient in experimental meal settings (Poppitt et al., 1998), with high-protein meals increasing blood concentrations of CCK (Blom et al., 2006; Bowen et al., 2006). Fat (Matzinger et al., 1999) and to a lesser extent carbohydrate (Holt et al., 1992; Parker et al., 2005) also stimulate CCK production from I cells in the duodenum and jejunum (Buffa et al., 1978).
CCK is an established satiety factor which binds to CCK-1 and -2 receptors concentrated in the gut and brain respectively (Wank, 1995). Exogenous administration of CCK was found to dose-dependently inhibit food intake in rats (Gibbs et al., 1973), findings that were later confirmed in humans (Kissileff et al., 1981). Nearly all short-term studies of CCK have demonstrated its ability to reduce food intake and/or subjective appetite (De Graaf, 1993) with longer-term evidence of a role in the regulation of body weight (McLaughlin et al., 1985).
Conversely, GHR is an orexigenic peptide largely produced by parietal cells in the stomach fundus and a ligand for growth hormone secretagogue receptors (Kojima et al., 1999; Tanaka-Shintani and Watanabe, 2005). Exogenous administration of GHR increases food intake in rats (Tschop et al., 2000) and humans (Wren et al., 2001). GHR concentrations increase preprandially and decrease postprandially (Cummings et al., 2001), particularly after carbohydrate-rich meals (Erdmann et al., 2004; Overduin et al., 2005). In some studies, GHR concentrations are inversely correlated to insulin concentrations (Blom et al., 2005; Little et al., 2006).
Changes in glucose and insulin may independently mediate hunger and satiety. Mayer's glucostatic theory of food intake regulation postulates transient declines in blood glucose are the most important determinant of feeding drive (Mayer, 1953). There is substantial evidence supporting the detection of low blood glucose by the central nervous system as a signal for meal initiation and/or hunger in both rats and humans (Campfield and Smith, 2003). Insulin itself may also influence acute satiety (Holt et al., 1992) and act as a long-term adiposity signal (Woods et al., 2006).
This study was undertaken to investigate the effect of differences in carbohydrate quality (glycemic index, GI) on the blood concentrations of glucose, insulin, CCK and GHR, over the course of a whole day. We hypothesized that low GI meals would be associated with lower glucose and insulin, higher CCK and lower GHR.
Subjects and methods
Subjects
Twelve male participants (23.2±s.d. 2.8 years with BMI 23.1±s.d. 1.9 kg/m2) were recruited by advertisement on the University of Sydney campus and were informed that the purpose of the study was to measure the effects of foods on blood glucose levels and other metabolic responses, namely hormones involved in appetite regulation. Inclusion criteria were: healthy weight (BMI 19–25 kg/m2), age 18–40 years and weight stable. Exclusion criteria were: smoking, medication, food allergy or intolerance, dieting, physical or mental illness, history of anaemia or family history of diabetes, exercising for more than 30 min more than 3 times a week and history of blood donation in previous 8 weeks or intention to donate blood in the next 8 weeks. The study was approved by the Sydney University Human Ethics Research Committee and the subjects gave informed, written consent.
Study design
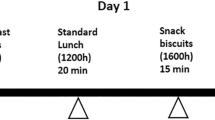
Four meals representing low or high GI diets were consumed over a 10 h period from 0800 to 1800 h. The energy content of each diet was based on 7500 kJ/d (1792 kcal), protein 15% energy, fat 30% energy, carbohydrate 55% energy and 30 g fibre per day. Breakfast comprised 20% of total intake per day (that is, energy, protein, fat, carbohydrate and fibre), morning snack 14%, lunch 28% and dinner 38%. Total energy intake was 88% of predicted energy intake for a restricted-energy diet (7500 kJ/1792 kcal per day, which is approximately 25% less than recommended by most international health authorities for adult males) (Table 1). Fewer kJ than normal were offered to accentuate feelings of hunger. Energy and nutrient compositions were calculated using Food Works software package 3.02 (Xyris Software, Highgate Hill, Australia), based on the Australian food composition tables. Differences in GI were achieved by varying the sources of carbohydrates (Table 1). The GI of each meal was calculated using the GI values of individual food components (Foster-Powell et al., 2002) and their relative contribution to total carbohydrate in the meal. Water (250 ml) was given with each meal and at 1200 and 1700 h (Figure 1). All meals were freshly prepared on the day of the study.
Study protocol
Subjects attended the testing suite on two occasions at least 7 days apart. On each visit, subjects fasted overnight (for at least 10 h) and refrained from alcohol and legume consumption the previous day. Weight (Sartorius BP 4100, Sartorius AG, Goettingen, Germany) and height (stretch stature) were measured in light clothing and without shoes. BMI was calculated as weight in kilograms divided by height in metres squared. A cannula was inserted into the antecubital vein of the lower arm by an experienced nurse and duplicate baseline fasting blood samples (0 min, 0800 h) were extracted into EDTA-coated Vacutainer tubes, and every 30 or 60 min thereafter for 10 h until 1800 h (at 0, 30, 60, 120, 180, 210, 240, 300, 330, 360, 420, 480, 540, 570 and 600 min, Figure 1). Subjective satiety was ascertained through a 100 mm-rating scale at the same time points, whereby opposing extremes of ‘extremely hungry’ to ‘extremely full’ were anchored at the left and right of the scale, respectively. Meals were given at 0, 180, 300 and 540 min—breakfast at 0800 h, morning snack at 1100 h, lunch at 1300 h and dinner at 1700 h respectively (Figure 1). Subjects were instructed to consume the meal and water within 20 min and record when they stopped eating. Palatability was ascertained using a 100 mm ratings scale after each meal, which listed opposing extremes of ‘extremely pleasant’ to ‘extremely unpleasant’ at the left and right of the scale, respectively. Subjects recorded their prospective food intake via a food diary from 1800 h on the test day until midnight the following day (kJ/30 h).
Blood analysis
Four vacutainers, each containing 3 ml of blood were collected at each time point. Vacutainer 1 was discarded. Vacutainers 2–4 were gently inverted 8–10 times and 0.3 ml (=3.6 TIU, trypsin inhibitor units) of aprotinin (0.6 TIU/ml, Phoenix Pharmaceuticals, Belmont, CA, USA) was added to vacutainers 2 and 3. Vacutainers 2–4 were then centrifuged (Eppendorf 5702R refrigerated centrifuge) at 1.6 rcf (1600 g) for 15 min at 4 °C, plasma aliquoted and stored at −80 °C until assay.
Gut peptides were measured in duplicate by competitive radioimmunoassay (RIA). CCK-8 was first extracted using C-18 separation columns (Strata C18-E, Phenomenex, Torrance, CA, USA) and buffers (Phoenix Pharmaceuticals, Belmont, CA, USA), extraction as per manufacturer's instructions, and a freeze dryer (Edwards RV vacuum pump, Scientific Instrument Services, NJ, USA and Dynavac housing, Dynavac Engineering, Australia) for evaporation to dryness of the decanted supernatant, before RIA using a commercially available kit (RK-069-04, Phoenix Pharmaceuticals, Belmont, CA, USA). Human CCK octapeptide 26–33 (non-sulfated) was assayed at a sensitivity of 0.6 pmol/l (or 1–128 pg per tube). The intra-assay coefficient of variation was 3.7% at 0.6 pmol/l and 10.5% at 20.4 pmol/l. Measurement of total GHR was determined by a commercially available RIA kit (GHRT-89HK, Linco Research, St Charles, MO, USA) involving detection of both acyl and des-acyl GHR isoforms at a sensitivity of 28 pmol/l (or 93 pg/ml). The intra-assay coefficient of variation was 3.6% at 35 pmol/l and 7.9% at 555 pmol/l. Insulin was measured in duplicate by competitive RIA (Coat-a-Count, Diagnostic Products, Los Angeles, CA, USA). Glucose was measured using a glucose hexokinase assay (Roche Diagnostics Corporation, Indianapolis, IN, USA).
Statistical analysis
Results are expressed as mean±s.e.m. of 12 subjects, unless otherwise stated. Primary end points were insulin, CCK and GHR concentrations. A power-based (90%) sample size calculation based on previous 10-h glucose data indicated that 10 subjects were needed to detect one SD difference in glucose incremental areas under the curve (IAUC) with P<0.05. The distance between the left end of the subjective satiety and palatability rating scales and each mark at each meal was measured (mm). 10 h IAUCs for subjective satiety, glucose, insulin and CCK were calculated using the trapezoidal equation for values above baseline (negative area below baseline was ignored) (Wolever, 2004). Multiple and separate two-tailed paired Student's t-tests were used to assess differences between 10 h IAUCs (subjective satiety, glucose, insulin and CCK), 7 h IAUCs (CCK only) and concentrations at 3 h (CCK only). A total palatability score was determined for each diet (by adding up the separate scores for each meal) and compared using a two-tailed paired Student's t-test. Energy in kJ for prospective food intake was calculated using Food Works software package 3.02 (Xyris Software, Highgate Hill, Australia) by inputting food type and quantity recorded for the 30 h post-diet (1800 h diet day to midnight the following day). Decrease from baseline (that is, change) was calculated for GHR (pmol/l) for each subject and mean 10 h change for 10 subjects was compared using two-tailed paired Student's t-tests (two subjects were excluded because of measurement errors at baseline). Pearson's correlations between all individual data points for glucose, insulin, CCK and GHR were calculated using SPSS 14 for Windows (SPSS Inc., Chicago, IL, USA). Differences are considered significant if P<0.05 and highly significant if P<0.01 or <0.001. Missing data and obvious outliers were substituted with either: an average of the values on either side of the missing/outlier value; or: a predicted value based on the average change of all other subjects from the previous time point.
Results
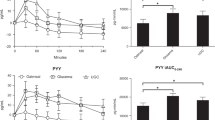
Fasting glucose and insulin concentration was similar on both days (glucose: low GI 5.1±0.1 mmol/l vs high GI 5.3±0.1 mmol/l, insulin: low GI 22.8±6.8 pmol/l vs high GI 19.0±4.0 pmol/l). Glucose and insulin profiles over the 10 h period were significantly lower during the low GI diet (Figure 2). Glucose and insulin concentrations increased after each meal and differences between diets were marked after the morning snack, lunch and dinner (Figure 2).
Glucose and insulin responses in venous plasma over 10 h on each diet (n=12). Bar charts show mean±s.e. 10 h incremental areas under the curve (IAUC). Glucose: Low glycemic index (GI) 10 h IAUC mean 221±s.e. 36 vs high GI mean 356±s.e. 49 mmol/l min, P=0.03, two-tailed paired Student's t-test. Insulin: Low GI 10 h IAUC mean 25150±s.e. 5940 vs high GI mean 63630±s.e. 13390 pmol/l min, P=0.001, two-tailed paired Student's t-test.
Fasting CCK concentration was similar on both days (low GI 2.3±0.2 pmol/l vs high GI 2.4±0.2 pmol/l). Serum CCK concentration increased postprandially and was higher on the low GI diet (Figure 3), with differences between diets more marked during the first 7 h (comprising breakfast, morning snack and lunch).
Cholecystokinin (CCK, n=12) and ghrelin (GHR, n=10) responses in venous plasma over 10 h on each diet. Bar charts show mean±s.e. 7 h incremental area under the curve (IAUC, CCK) and mean±s.e. 10 h change from baseline (GHR). CCK: Low glycemic index (GI) 7 h IAUC mean 394±s.e. 95 vs high GI mean 163±s.e. 38 pmol/l min, P=0.046. Low GI 10 h IAUC mean 472±s.e. 144 vs high mean GI 284±s.e. 65 pmol/l min, P=0.224. Concentration at 3 h was 55% higher low GI vs high GI, P=0.0498. All comparisons were made by two-tailed paired Student's t-tests. GHR: Low GI change in GHR from baseline mean −18±s.e. 29 vs high GI mean −34±s.e. 44 pmol/l, NS, two-tailed paired Student's t-test. GHR data points negatively correlated with insulin data points, r=−0.48, P=0.007, Pearson correlation.
Fasting GHR concentration was similar on both days (low GI 438±34 pmol/l vs high GI 459±63 pmol/l, n=10). As expected, serum GHR concentration decreased postprandially (Figure 3), but there was no difference in mean GHR change between diets or between GHR response to each meal. GHR on both diets separately and combined was inversely correlated to insulin (combined Pearson correlation r=−0.480, P=0.007), but not glucose (combined Pearson correlation r=−0.132, P=0.488).
There were no significant differences in palatability between the low and high GI diet (total day palatability 1199±2 vs 1217±2 mm, P=0.87; subjective satiety 10015±2234 vs 8879±1755 mm min respectively, P=0.82, Figure 4) or prospective food intake after the low vs high GI diet (13875±1966 vs 13437±1496 kJ/30 h, P=0.80; 3315±470 vs 3210±357 kcal/30 h respectively).
Discussion
Although studies on the GI have shown differences in glycemia and insulinemia over short time frames (2–4 h), few have examined day-long responses (McMillan-Price et al., 2006), and none has simultaneously documented differences in gut peptides. This study demonstrated that over the course of 10 h, four meals containing low GI carbohydrates produced significantly lower glucose and insulin concentrations and correspondingly higher concentrations of the ‘satiety’ hormone CCK, compared with nutrient-matched high GI meals. The concentration of the ‘hunger’ hormone, GHR, also rose and fell in a predictable meal-related pattern, but there was no detectable difference between the high and low GI diets. ‘Dynamic’ falls in blood glucose, however, have been associated with meal initiation in animal and human studies (Campfield and Smith, 2003) and a rise in counter-regulatory hormones and food energy intake in obese adolescents (Ludwig et al., 1999). The findings therefore provide a mechanism to explain higher satiety and greater falls in body fat on low GI diets in some settings.
The effect of GI on subjective satiety, prospective food intake and weight has been recently reviewed (Bornet et al., 2007). Low GI and low glycemic load diets were found to increase the rate of weight loss in some studies (Ludwig et al., 1999; Bouché et al., 2002; Brand-Miller et al., 2002; Ebbeling et al., 2007), but not others (Sloth et al., 2004; Das et al., 2007). As a consequence, the use of low GI foods to facilitate weight loss remains controversial (Sloth and Astrup, 2006).
The most novel finding is that average CCK concentrations were ∼60% higher during the first 7 h on the low GI diet compared with the high GI diet. Mourot et al. (Mourot et al., 1988) demonstrated an inverse relationship between gastric emptying half-time and the magnitude of the glycemic response to four foods: potatoes, bread, rice and spaghetti. In this study, the higher profile of CCK on the low GI diet may therefore be attributable to a slower rate of gastric emptying and hence entry of carbohydrate and other macronutrients into the proximal small intestine (<60 cm (Little et al., 2006)). This may prolong stimulation of I cells, which in turn may further decrease gastric emptying and maintain higher CCK production. Although the role of CCK in long-term weight regulation has not been fully elucidated, the evidence from animal studies is strong (McLaughlin et al., 1985; Meereis-Schwanke et al., 1998). In human studies, CCK appears to potentiate the effects of adiposity signals such as leptin and insulin (Moran, 2000).
Meals high in carbohydrate are known to be a less potent stimulus for CCK release than meals that are high in fat and protein. Hence, to rule out confounding by differences in composition, the meals were carefully matched for macronutrients, including type of fat, fibre and energy. Even though carbohydrate was the major provisor of energy in both diets, the postprandial concentrations of CCK were comparable to those seen in other studies (Liddle et al., 1985; Kàlmar et al., 2006). The relative difference elicited by high and low GI meals was also similar to that found in a shorter 3-h study (Holt et al., 1992). The meals in the latter study, however, were not matched in composition, implying that factors other then GI could explain the observed difference in CCK.
This study also found that total GHR decreased postprandially, with concentrations and time of nadir (about 1 h) comparable to other studies (Erdmann et al., 2004; Poppitt et al., 2005), but there were no marked differences in GHR concentrations between the low and high GI diets. We speculated that the low GI diet might decrease GHR concentrations because earlier studies have linked low GI foods to a lower energy intake over a prolonged period (Ludwig et al., 1999). However, GHR production is partly mediated by lower intestinal osmolarity and insulin concentrations, not just the presence of nutrients in the stomach or duodenum (Cummings, 2006). Indeed, we found that GHR and insulin concentrations were inversely correlated, purporting previous findings for a role of insulin in the prandial suppression of GHR (Blom et al., 2005; Little et al., 2006). It has also been suggested that GHR may play a role in the adipocyte regulation of glucose homoeostasis through the direct potentiation of insulin-stimulated glucose uptake (Patel et al., 2006). There was no correlation, however, between GHR and blood glucose concentration in this study. In some studies, but not others (Blom et al., 2006), protein ingestion increased postprandial GHR secretion (Erdmann et al., 2006). The protein in the meals in this study may therefore have partly masked any inhibitory effect of carbohydrate. Lastly, GHR has been hypothesized to be a major regulator of hunger occurrence and prospective meal size rather than acute food intake (Saad et al., 2002) and for this reason, differences in GHR may be seen only over the longer term.
Our study has several limitations. Although meals were matched for total fibre, they were not matched for type of fibre. Fibre may affect satiety independent of effect on meal GI (Rigaud et al., 1998), especially if subjects are not accustomed to fibre intakes as high as those provided by study meals. Similarly, other lifestyle aspects on the days prior to the diets were not controlled for, for example, subjects were not restricted in how much protein, fat, E, etc they could consume, which may have potentially affected their satiety scores, blood variables and prospective food intake on the study days. Blood concentrations of CCK over the course of 1 day in lean male participants may not reflect the levels seen during chronic consumption by overweight individuals. Moreover, higher levels in the blood may not necessarily reflect increased production of CCK because of the complexity of binding and degradation, and the fact that CCK is secreted into the interstitial fluid (Walsh and Dockray, 1994). Our CCK assay recognized only CCK-8 and -33 isoforms and not other potentially biologically active forms (Rehfeld, 1998). Similarly, our GHR assay did not distinguish between acyl and des-acyl isoforms. Despite differences in CCK over the first 7 h, we found no difference in subjective satiety IAUC between the low GI and high GI days. Although this was unexpected, other studies have also failed to detect differences (Barkeling et al., 1995). Unfortunately, subjective assessment of satiety is an inherently blunt tool because uncontrolled factors in the environment can influence appetite and fullness. However, the hypocaloric nature of the meals could also have resulted in a masking of any GI-induced differences in satiety. Similarly, there was no difference in prospective food intake, a finding seen in some studies (Holt et al., 1992; Kaplan and Greenwood, 2002), but not others (Holt et al., 1996; Ludwig et al., 1999). Lastly, monitoring blood variables and subjective ratings for a longer period after dinner would have provided a more comprehensive picture of the effect of GI on appetitive responses.
In conclusion, energy-restricted mixed meals of lower GI are associated with lower day-long concentrations of glucose and insulin, and higher CCK after breakfast, morning tea and lunch. This metabolic profile could mediate differences in satiety and hunger seen in some (Holt et al., 1996; Ludwig et al., 1999), but not all (Holt et al., 1992; Barkeling et al., 1995), studies. Further research is needed to quantify the extent to which low GI meals and/or diets may reduce hunger and increase satiation or satiety over the longer term. These studies require large sample sizes and measurement of a wide range of gut peptides and adiposity signals in lean, overweight and obese subjects.
References
Barkeling B, Granfelt Y, Bjorck I, Rossner S (1995). Effects of carbohydrates in the form of pasta and bread on food intake and satiety in man. Nutr Res 15, 467–476 (10).
Blom WA, Lluch A, Vinoy S, Stafleu A, Van den Berg R, Holst JJ et al. (2006). Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr 83, 211–220.
Blom WA, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HF (2005). Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr 81, 367–375.
Bornet FR, Jardy-Gennetier AE, Jacquet N, Stowell J (2007). Glycaemic response to foods: Impact on satiety and long-term weight regulation. Appetite 49, 535–553.
Bouché C, Rizkalla SW, Luo J, Vidal H, Veronese A, Pacher N et al. (2002). Five-week, low-glycemic index diet decreases total fat mass and improves plasma lipid profile in moderately overweight nondiabetic men. Diabetes Care 25, 822–828.
Bowen J, Noakes M, Trenerry C, Clifton PM (2006). Energy intake, ghrelin, and cholecysokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab 91, 1477–1483.
Brand-Miller JC, Holt SH, Pawlak DB, McMillan J (2002). Glycemic index and obesity. Am J Clin Nutr 76 (suppl 1), 281S–285S.
Buffa R, Capella C, Fontana P, Usellini L, Solcia E (1978). Types of endocrine cells in the human colon and rectum. Cell Tissue Res 192, 227–240.
Campfield LA, Smith FJ (2003). Blood glucose dynamics and control of meal initiation: a pattern detection and recognition theory. Physiol Rev 83, 25–58.
Cummings DE (2006). Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89, 71–84.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001). A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719.
Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA et al. (2007). Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85, 1023–1030.
De Graaf C (1993). The validity of appetite ratings. Appetite 21, 156–160.
Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS (2007). Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA 297, 2092–2102.
Erdmann J, Leibl M, Wagenpfeil S, Lippl F, Schusdziarra V (2006). Ghrelin response to protein and carbohydrate meals in relation to food intake and glycerol levels in obese subjects. Regul Pept 135, 23–29.
Erdmann J, Topsch R, Lippl F, Gussmann P, Schusdziarra V (2004). Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab 89, 3048–3054.
Foster-Powell K, Holt SH, Brand-Miller JC (2002). International Table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76, 5–56.
Gibbs J, Young RC, Smith GP (1973). Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84, 488–495.
Holt S, Brand J, Soveny C, Hansky J (1992). Relationship of satiety to postprandial glycaemic, insulin and cholecystokinin responses. Appetite 18, 129–141.
Holt S, Brand-Miller JC, Petocz P (1996). Interrelationships among postprandial satiety, glucose and insulin responses and changes in subsequent food intake. Eur J Clin Nutr 50, 788–797.
Kàlmar K, Nemeth J, Kelemen D, Agoston E, Horvath OP (2006). Postprandial gastrointestinal hormone production is different, depending on the type of reconstruction following total gastrectomy. Ann Surg 243, 465–471.
Kaplan RJ, Greenwood CE (2002). Influence of dietary carbohydrates and glycaemic response on subjective appetite and food intake in healthy elderly persons. Int J Food Sci Nutr 53, 305–316.
Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP (1981). C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 34, 154–160.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660.
Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA (1985). Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding and relationship to gallbladder contraction. J Clin Invest 75, 1144–1152.
Little TJ, Doran S, Meyer JH, Smout AJ, O'Donovan DG, Wu KL et al. (2006). The release of GLP-1 and ghrelin, but not GIP and cholecystokinin, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab 291, E647–E655.
Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB (1999). High glycemic index foods, overeating, and obesity. Pediatrics 103, E26.
Matzinger D, Gutzwiller JP, Drewe J, Orban A, Engel R, D'Amato M et al. (1999). Inhibition of food intake in response to intestinal lipid is mediated by cholecystokinin in humans. Am J Physiol 277 (6 Pt 2), R1718–R1724.
Mayer J (1953). Glucostatic mechanism of regulation of food intake. N Engl J Med 249, 13–16.
McLaughlin CL, Baile CA, Buonomo FC (1985). Effect of cholecystokinin antibodies on food intake and weight gain in Zucker rats. Physiol Behav 34, 277–282.
McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K et al. (2006). Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med 166, 1466–1475.
Meereis-Schwanke K, Klonowski-Stumpe H, Herberg L, Niederau C (1998. Long-term effects of cholesytokinin-agonist and -antagonist on food intake and body weight in Zucker lean and obese rats. Peptides 19, 291–299.
Moran TH (2000). Cholecystokinin and satiety: current perspectives. Nutrition 16, 858–865.
Mourot J, Thouvenot P, Couet C, Antoine JM, Krobicka A, Debry G (1988). Relationship between the rate of gastric emptying and glucose and insulin responses to starchy foods in young healthy adults. Am J Clin Nutr 48, 1035–1040.
Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE (2005). Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146, 845–850.
Parker BA, Doran S, Wishart J, Horowitz M, Chapman IM (2005). Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin Endocrinol (Oxf) 62, 539–546.
Patel AD, Stanley SA, Murphy KG, Frost GS, Gardiner JV, Kent AS et al. (2006). Ghrelin stimulates insulin-induced glucose uptake in adipocytes. Regul Pept 134, 17–22.
Poppitt SD, Leahy FE, Keogh GF, Wang Y, Mulvey TB, Stojkovic M et al. (2005). Effect of high-fat meals and fatty acid saturation on postprandial levels of the hormones ghrelin and leptin in healthy men. Eur J Clin Nutr 60, 77–84.
Poppitt SD, McCormack D, Buffenstein R (1998). Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav 64, 279–285.
Rehfeld JF (1998). Accurate measurement of cholecystokinin in plasma. Clin Chem 44, 991–1001.
Rigaud D, Paycha F, Meulemans A, Merrouche M, Mignon M (1998). Effect of psyllium on gastric emptying, hunger feeling and food intake in normal volunteers: a double blind study. Eur J Clin Nutr 52, 239–245.
Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E et al. (2002). Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab 87, 3997–4000.
Sloth B, Astrup A (2006). Low glycemic index diets and body weight. Int J Obes 30 (suppl 3), S47–S51.
Sloth B, Krod-Mikkelsen I, Flint A, Tetens I, Björck I, Vinoy S et al. (2004). No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am J Clin Nutr 80, 337–347.
Tanaka-Shintani M, Watanabe M (2005). Distribution of ghrelin-immunoreactive cells in human gastric mucosa: comparison with that of parietal cells. J Gastroenterol 40, 345–349.
Tschop M, Smiley DL, Heiman ML (2000). Ghrelin induces adiposity in rodents. Nature 407, 908–913.
Walsh JH, Dockray GJ (1994). Gut peptides: biochemistry and physiology. Raven Press: New York.
Wank SA (1995). Cholecystokinin receptors. Am J Physiol 269 (5 Pt 1), G628–G646.
Wolever TM (2004). Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br J Nutr 91, 295–301.
Woods SC, Lutz TA, Geary N, Langhans W (2006). Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci 361, 1219–1235.
Wren AM, Bloom SR (2007). Gut hormones and appetite control. Gastroenterology 132, 2116–2130.
Wren AM, Seal LJ, Cohen MA, Byrnes AE, Frost GS, Murphy KG et al. (2001). Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86, 5992.
Acknowledgements
We thank our volunteers and Mrs Jenny Phuyal and Ms Kai Lin Ek for technical assistance.
This study was internally funded. JBM is a coauthor of The Low GI Diet Revolution (Marlowe & Co, New York, NY, 2005) and of The New Glucose Revolution book series (Hodder and Stoughton, London, England; Marlowe & Co; and Hodder Headline, Sydney, and elsewhere).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reynolds, R., Stockmann, K., Atkinson, F. et al. Effect of the glycemic index of carbohydrates on day-long (10 h) profiles of plasma glucose, insulin, cholecystokinin and ghrelin. Eur J Clin Nutr 63, 872–878 (2009). https://doi.org/10.1038/ejcn.2008.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2008.52
Keywords
This article is cited by
-
Diet pattern may affect fasting insulin in a large sample of black and white adults
European Journal of Clinical Nutrition (2021)
-
Personalized nutrition by prediction of glycaemic responses: fact or fantasy?
European Journal of Clinical Nutrition (2016)
-
The Individual and Combined Effects of Glycemic Index and Protein on Glycemic Response, Hunger, and Energy Intake
Obesity (2011)
-
Optimal dietary approaches for prevention of type 2 diabetes: a life-course perspective
Diabetologia (2010)