Abstract
Background/Objectives:
Hypoglycemic effect of camel milk supplementation in experimental rat model and significant reduction in doses of insulin in type 1 diabetic patients have been observed in our previous studies. This long-term study was undertaken to assess the efficacy, safety and acceptability of camel milk as an adjunct to insulin therapy in type 1 diabetics.
Subjects/Methods:
In this 2-year randomized clinical, parallel design study, 24 type 1 diabetics were enrolled and divided into two groups. Group I (n=12) received usual care, that is, diet, exercise and insulin and Group II (n=12) received 500 ml camel milk in addition to the usual care. Insulin requirement was titrated weekly by blood glucose estimation. Results were analyzed by using the regression technique.
Results:
In camel milk group, there was decrease in mean blood glucose (118.58±19–93.16±17.06 mg/dl), hemoglobin A1c levels (7.81±1.39–5.44±0.81%) and insulin doses (32.50±9.99–17.50±12.09 U/day, P<0.05). Out of 12 subjects receiving camel milk, insulin requirement in 3 subjects reduced to zero. There was nonsignificant change in plasma insulin and anti-insulin antibodies in both the groups.
Conclusion:
It may be stated that camel milk is safe and efficacious in improving long-term glycemic control, with a significant reduction in the doses of insulin in type 1 diabetic patients.
Similar content being viewed by others
Introduction
Camel milk has emerged as a potent therapeutic alternative, which can help in reducing insulin doses. Primary treatment of type 1 diabetes mellitus is insulin replacement. Although conventional injectable insulin therapy meets this goal, it is associated with several therapeutic disadvantages, such as hyperinsulinemia, pain and inconvenience. Many attempts have been made to find alternative methods of insulin delivery through non-invasive routes (Owens, 2002; Gordberg and Gomez-Orellana, 2003; Cefalu, 2004). Among the alternative routes available for insulin delivery, oral administration is the most preferable, as it offers significant advantages in terms of therapeutic efficacy and patient acceptability. As orally delivered insulin undergoes a hepatic pass before entering the circulation, it has the potential to mimic the effects of pancreas-secreted insulin in terms of inhibition of hepatic gluconeogenesis (Lewis et al., 1996; Clement et al., 2002). Camel milk is one such alternative, as one of its proteins has many characteristics similar to insulin (Abu-Lehia et al., 1989), and it does not form coagulum in acidic environment, thus, safeguarding the viability of its components and making it available for absorption in intestine. Radioimmunoassay of camel milk has revealed that it contains high concentration of insulin, that is, 52.03 U/l (Singh, 2001) in comparison with cow milk, in which it is very low, that is, 16.32 U/l. There is significantly higher insulin (60.23 U/l) in human milk, but it is not available for absorption in intestine probably because of coagulation in stomach.
Taking all these evidences in account, a long-term study was undertaken to study the efficacy of camel milk in improving the glycemic control, consistent reduction in the doses of insulin and long-term safety profile in type 1 diabetes patients, with the aim that such an investigation would help to establish a more rational use of camel milk to control blood sugar levels in these patients.
Subjects and methods
Selection of subjects
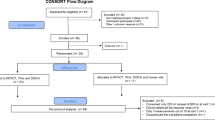
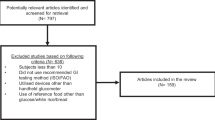
In total, 24 type 1 diabetic subjects were randomly selected from the outpatient diabetic clinic in the PBM Hospital, Bikaner, India. The patients were advised to follow a strict diet, that is, fiber rich and low-fat diet, with 3-km daily walk and taking insulin treatment for 1 month before randomization as well as during the course of study. During this period, frequent monitoring of blood sugar was done to maintain euglycemia. After 1 month the patients were randomly divided into two groups—Group I and Group II. Allocation to the intervention and control groups was matched regarding their age and male to female ratio. Mean body mass index (BMI) for both the groups were also compared before the study and was found nonsignificantly different. Randomization was computer generated. Both the groups were also matched for duration of diabetes diagnosis and duration of insulin therapy. Group I patients (n=12) served as control group receiving usual care, that is, diet, exercise and insulin, and Group II patients (n=12) were treated as study group receiving 500 ml of camel milk supplementation daily in addition to the recommended diet, exercise and insulin for 24 months. Patients were provided camel milk daily in the morning at the research center. Information about their compliance to camel milk consumption, and diet (by 24-h recall method) and exercise was taken regularly by dietician. Patients with any acute metabolic complications like hypoglycemia, ketoacidosis, cardiovascular event, renal or acute infection were not included in the study. Patients were not allowed to take non-insulin pharmacotherapies or any other concomitant medicine throughout the study period. All the subjects randomized were non-camel milk drinker. The local ethics committee of SP Medical College, Bikaner approved the study protocol. A written informed consent was obtained from the patients and in case of minors, from their parents.
Study design and analysis
This study was a randomized, open clinical, parallel design single-center study. Blood sugar level was measured twice a week before breakfast and before dinner, and insulin doses were titrated weekly according to mean blood sugar levels. Patients were instructed to inform immediately for any untoward event like hypo- or hyperglycemia and other complications. All patients were trained and provided with a one-touch profile memory glucometer (Lifescan, Milpitas, CA, USA), along with strips for self-monitoring of blood glucose concentrations, and were instructed to record the glucose readings and insulin doses. Vital signs, body weight, hematological and laboratory parameters, hemoglobin A1c (HbA1c) levels were monitored throughout the study. Patients were also monitored for symptoms of hypoglycemia, and glucose readings were obtained when hypoglycemic symptoms occurred. Anti-insulin antibodies detection, brucellosis agglutination test and enzyme-linked immunosorbent assay for tuberculosis were carried out annually. Safety evaluations included detail physical examinations including vital signs and laboratory parameters.
Assays
Blood sugar concentration was measured using the glucose oxidase method (Trinder, 1969; Kaplan, 1984). Plasma insulin and C-peptide were estimated by fully automated chemiluminescence analyzer (Immulite, DPC, Siemens Healthcare Diagnostics, Deerfield, IL, USA) (Clark, 1999). Anti-insulin antibodies were estimated by radioimmunoassay (Desbuquois and Aurbach, 1971). HbA1c level was measured by ion-exchange chromatography using DS-5 HbA1c analyzer (Drew Scientific Inc., Dallas, TX, USA) (Cohen et al., 1993).
Statistical analysis
Statistical analysis was carried out by using the SPSS version 10.0 (SPSS South Asia Pvt. Ltd, Bangalore, India). The baseline difference between the two groups was analyzed using t-test for independent samples assuming heteroscedastic variance. Changes from baseline to end point were analyzed using analysis of variance. The groups were taken as independent variables. Insulin dose, mean blood sugar and HbA1c were taken as dependent variables and analyzed independently. Statistical significance was assigned for P-values <0.05.
Results and discussion
The mean age observed in Group I and II was 14.50±8.43 and 16.67±8.89 years, respectively, (difference found was statistically nonsignificant). Male to female ratio in Group I and II was 7:5 and 10:2, respectively. The baseline characteristics of the study groups were similar in terms of BMI, fasting blood glucose, HbA1c, dose of insulin, plasma insulin, C-peptide and anti-insulin antibody (Table 1). After 2 years of study, improvement in BMI was observed in both the groups, which may be attributed to adolescent growth along with good glycemic control (Table 1). Decrease in mean fasting blood glucose levels was observed in Group II, with a significant reduction in the doses of insulin requirement (Table 1, Figure 1), whereas in Group I there was significant increase in insulin requirement. In three diabetic patients of Group II, insulin requirement reduced to zero in the later phase of study. Improvement in HbA1c levels was found more pronounced in Group II as compared with the control group. There was significant change in C-peptide levels in both the groups, as all the patients were on insulin therapy, whereas no change was observed in plasma insulin and anti-insulin antibodies (Table 1). Anti-insulin antibody titers were <20% even after 2 years. No significant treatment-emergent adverse events were reported in either group. When both the groups were analyzed statistically before and after treatment to see the effect of treatment in between groups over the study period, there was a reduction in fasting blood glucose levels, HbA1c and the doses of insulin required (P<0.05) in camel milk group as compared with control group (Table 1). Acceptability to camel milk was assessed by the occurrence of hypoglycemic and untoward event. No significant hypoglycemic event as well as untoward event was observed in the group receiving camel milk, thus, showing good acceptability of camel milk.
The goal of oral insulin therapy is to mimic the effects of pancreas-secreted insulin in order to control postprandial hyperglycemia and to prevent hypoglycemia between meals. In previous separate clinical trials for 3 months, 6 months and 1 year, the efficacy of camel milk causing significant hypoglycemic effect was reported when given as adjunctive therapy. This effect was presumably due to the presence of insulin/insulin-like protein and because of lack of coagulum formation of camel milk in an acidic environment (Agrawal et al., 2002, 2003, 2005). The present study demonstrated that there was statistically significant improvement in mean BMI in both the groups. In this study, all the subjects were receiving insulin, conferring in better glycemic control, which in turn improved the mean BMI.
The important observation of the present study was a significant reduction in insulin requirement (46.15%) at the end of 2 years in patients consuming camel milk, in comparison with control group. Among the 12 subjects in camel milk-consuming group, each patient had significant response to reduction in insulin requirement, but the remarkable observation was gradual reduction upto zero in three subjects. These three subjects reached to this level on 12th, 18th and 21st month of the study and returned to this ‘no insulin requirement status’ upto the end of the study, with some intermittent deviations. Two out of these three subjects maintained this zero level continuously, from 18th to 21st month, till the end of study. Exact reason behind reduction in insulin requirement upto zero in these three subjects is not known. However, it may be attributed to the immunopotentiating effect or improvement in β-cell function due to camel milk supplementation, as rest of the factors like diet and exercise were similar for all the subjects. Researchers, exploring the field of non-invasive insulin delivery, have been continuously making efforts to administer insulin through different systems including oral delivery. Hexyl-insulin-monoconjugate-2 is considered as the most promising oral insulin to date, with bioavailability of ∼5% (Agamy et al. (1992). The insulin-requirement-lowering effect due to camel milk supplementation in this study suggests emergence of camel milk as a new oral insulin, which can give new dimensions to the therapeutic insulin era. This may be strongly recommended, as insulin requirement reduced to zero in three diabetic subjects.
The lack of hypoglycemic events in this study is encouraging and it looks more acceptable for patients with type 1 diabetes. The promising data in this study are in consistent with study conducted by Clement et al. (2002) using oral insulin product. The significant reduction was observed in HbA1c levels in camel-milk receiving group (7.81–5.44%). HbA1c is marker of glycemic control and improvement in its values clearly indicates better glycemic control. Fasting plasma insulin levels did not reveal any significant change in each group, though significant increase in C-peptide levels in camel milk group (0.16–0.21 ng/ml) may reflect improvement in β-cell function. In our previous study, after 1 year of camel milk supplementation, the C-peptide level increased from 0.18 to 0.24 (Agrawal et al., 2005). Fasting insulin per se cannot be considered as an accurate surrogate for insulin sensitivity. It can simply be reported as a quantitative reflection of insulin resistance.
Several studies have shown that patients treated with insulin, develop significant insulin antibodies after some period and these subjects occasionally experience prolongation of insulin action, which produces nocturnal hypoglycemia and insulin resistance during the day period (Jeandidier et al., 1995). In the present study, we did not observe marked changes in the positive proportion of insulin antibodies level.
The exact cause of hypoglycemic effect of camel milk is not known. It is possibly due to insulin-like activity, regulatory and immunomodulatory function of β cells (Lutz, 2002). Agamy et al. (1992) found good amount of lysozyme, lactoferrin, lactoperoxidase, immunoglobulin G and secretory immunoglobulin A in camel milk. The lack of coagulum formation of camel milk may also be helpful in taking the insulin present in it unchanged to intestine where it can be absorbed even if some amount is destroyed in the passage. Beg et al. (1986) have found that amino-acid sequence of some of the camel milk protein is rich in half-cystine, which has superficial similarity with insulin family of peptides.
Anecdotal reports also suggested that camel milk consumption is associated with low prevalence of diabetes. In an epidemiological observation, Agrawal et al. (2004) studied the prevalence of diabetes and glucose intolerance in the Raica community (a desert tribe of North-west Rajasthan) consuming camel milk habitually as a part of staple diet. They reported a very low prevalence of both variables, which may be due to the protective influence of camel milk. The results of the present study are in agreement with the previous observation of positive effects of supplementation of camel milk as an adjunctive to insulin therapy in type 1 diabetic patients (Agrawal et al., 2003, 2005).
Despite the limited size of the study population, we were able to demonstrate a significant association between camel milk consumption and gradual reduction in insulin requirement in type 1 diabetic subjects. Further studies are needed to verify these observations. In conclusion, the results of the present study throw a light on the potential use of camel milk as an adjunct to insulin therapy, for better glycemic control and good quality of life.
References
Abu-Lehia IH, Al-Mohizea IS, El-Behary M (1989). Studies on the production of ice cream from camel milk products. Aust J Dairy Technol 44, 31–34.
Agamy El, Ruppanner R, Ismail A (1992). Antibacterial and antiviral activity of camel milk protective proteins. J Dairy Res 59, 169–175.
Agrawal RP, Beniwal R, Sharma S, Kochar DK, Tuteja FC, Ghorui SK et al. (2005). Camel milk as an adjunct to insulin therapy improves long-term glycemic control and reduction in doses of insulin in patients with type-1 diabetes – a 1 year randomized controlled trial. Diabetes Res Clin Prac 68, 176–177.
Agrawal RP, Singh G, Nayak KC, Kochar DK, Sharma RC, Beniwal R et al. (2004). Prevalence of diabetes in camel milk consuming ‘RAICA’ rural community of North West Rajasthan. Int J Diab Dev Countries 24, 109–114.
Agrawal RP, Swami SC, Beniwal R, Kochar DK, Sahani MS, Tuteja FC et al. (2003). Effect of camel milk on glycemic control, lipid profile and diabetes quality of life in type-1 diabetes: a randomised prospective controlled cross over study. Indian J Anim Sci 73, 1105–1110.
Agrawal RP, Swami SC, Beniwal R, Kochar DK, Kothari RP (2002). Effect of camel milk on glycemic control, risk factors and diabetes quality of life in type-1 diabetes: a randomised prospective controlled study. Int J Diab Dev Countries 22, 70–74.
Beg OU, Von Bahr-Lind Strom H, Zaidi ZH, Jornvall H (1986). A camel milk protein rich in half cystine. Primary structure assessment of variations, internal repeat patterns and relationship with neurophysin and other active polypeptides. Eur J Biochem 15, 195–201.
Cefalu WT (2004). Concept, strategies and feasibility of non-invasive insulin delivery. Diabetes Care 27, 239–246.
Clark PM (1999). Assays for insuin, proinsulin(s) and C-peptide. Ann Clin Biochem 36 (Suppl 5), 541–564.
Clement S, Still G, Kosutic G, McAllister RG (2002). Oral insulin product hexyl-insulin monoconjugate 2 (HIM2) in type 1 diabetes mellitus: the glucose stabilization effects of HIM2. Diabetes Technol Therap 4, 459–466.
Cohen MP, Witt J, Wu VY (1993). Purified haemoglobin preparations in the evaluation of HbA1c determination by ion exchange chromatography. Ann Clin Biochem 30, 265–271.
Desbuquois B, Aurbach GD (1971). Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassay. J Clin Endocrinol Metab 33, 732–738.
Gordberg M, Gomez-Orellana I (2003). Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov 4, 289–295.
Jeandidier N, Boivin S, Sapin R, Rosart-Ortega F, Uring-Lambert B, Réville P et al. (1995). Immunogenicity of intraperitoneal insulin infusion using programmable implantable devices. Diabetologia 38, 577–584.
Kaplan LA (1984). Glucose. Clin Chem. The CV Mosby Co.: St Louis, Toronto, Princeton, pp 1032–1036.
Lewis GF, Zinman B, Groenewound Y, Vranic M, Giacca A (1996). Hepatic glucose production is regulated both by direct hepatic and extrahepatic effects of insulin in humans. Diabetes 45, 454–462.
Lutz B (2002). Insulin and anti diabetic activity of camel milk. J Camel Res Pract 9, 43–45.
Owens DR (2002). New horizons-alternative routes for insulin therapy. Nat Rev Drug Discov 1, 529–540.
Singh R (2001). Annual Report of National Research Centre on Camel. National Research Centre on Camel: Rajasthan, India.
Trinder P (1969). Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22 (Suppl 2), 158–161.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Agrawal, R., Jain, S., Shah, S. et al. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur J Clin Nutr 65, 1048–1052 (2011). https://doi.org/10.1038/ejcn.2011.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2011.98
Keywords
This article is cited by
-
Udder, teat, and milk vein measurements of Indian dromedary camel and its relationship with milkability traits
Tropical Animal Health and Production (2023)
-
Brief report first report of the in vitro ovicidal activity of camel milk and its fractions on zoonotic-liver fluke (Fasciola gigantica) eggs
Veterinary Research Communications (2023)
-
The association between dairy products consumption with risk of type 1 diabetes mellitus in children: a meta-analysis of observational studies
International Journal of Diabetes in Developing Countries (2021)
-
Comparison of knowledge, attitude, and practices of animal and human brucellosis between nomadic pastoralists and non-pastoralists in Kenya
BMC Public Health (2020)
-
Short term therapeutic efficacy of camel milk Vis-À-Vis buffalo milk in Alloxan® induced diabetic rabbits
Journal of Diabetes & Metabolic Disorders (2020)