Abstract
Purpose
To assess the agreement of the ‘polygonal’ variable frame cell count option on a confocal microscope after keratoplasty, with planimetry as the reference method.
Methods
One hundred clear corneal grafts of 83 patients attending the cornea clinic at Gartnavel General Hospital in Glasgow underwent slit-scanning in vivoconfocal microscopy. Endothelial cell images were assessed with the Nidek Advanced Vision Information System (NAVIS), using the polygonal variable frame and the manual fixed-frame methods. Planimetry was used as the reference. The agreement between methods was assessed by Bland-Altman analysis.
Results
Planimetry provided a mean (±SD) endothelial cell density (ECD) of 1348±726 cells/mm2, a value that was very similar to that found by the polygonal method (1404±784 cells/mm2). The fixed-frame method provided lower cell counts with a mean ECD of 1026±610 cells/mm2 (P<0.001). When compared with the reference ECD, the polygonal method overestimated the ECD only very slightly with a mean difference of 58 cells/mm2 (limits of agreement, LoA, of −222 and 339 cells/mm2). Manual counting underestimated the ECD with a mean difference of −320 cells/mm2 (LoA −814 and 173 cell/mm2).
Conclusion
Following keratoplasty, endothelial cell counts with the NAVIS polygonal method are in good agreement with planimetry. The ‘polygonal’ option is proposed as the method of choice for clinical applications with this confocal microscope and a good compromise between reliability and ease of use.
Similar content being viewed by others
Introduction
The corneal endothelium can be viewed by a variety of techniques including high magnification slit lamp biomicroscopy, specular microscopy, and confocal microscopy.1, 2 The endothelial cell status is routinely assessed from estimating the endothelial cell density (ECD), and for some patients an accurate assessment of ECD is desired so that post-operative changes can be monitored.3 Various methods can be used to estimate the ECD in normal corneas and after surgical procedures such as corneal transplantation, and include automated, semi-automated, manual counts, and planimetry techniques.1, 2, 3, 4, 5, 6, 7, 8, 9, 10
For routine use in clinical practice, a method for the estimation of ECD needs to be reasonably reliable, quick to perform, and preferably require relatively little training as ophthalmic technicians often perform these assessments. A number of options have been developed to facilitate ECD assessments, most notably the availability of automated software methods9, 11, 12, 13 that have been designed to identify the cell domains (borders) across an endothelial image and then calculate an ECD estimate on the basis of averaged areas of these cell domains. There have been a number of studies comparing the outcome of different methods using images obtained from confocal or specular microscopes,7, 13, 14, 15, 16 studies that also indicate that the results can depend on the software used for automated analyses.2, 7, 13, 16 The most reliable method for ECD estimation is generally considered to be planimetry, even when used to assess abnormal endothelia.17 The technique is, however, time-consuming and cannot be implemented in routine clinical practice. Planimetry is based on identifying individual cells (for example, by drawing in the cell borders on an image), measuring the areas of these cells, and then an ECD is calculated from the average cell area value.
With issues being raised over the reliability of automated software methods but with the limited clinical applicability of planimetry, other software options need to be considered. One of these is to use a fixed-frame method by which a count is made of the number of cells within a pre-determined region of interest (ROI), by use of a mouse-based cursor.2, 3, 4 The method is easy to perform and can be undertaken very quickly. Manual fixed-frame counts also have their limitations in that some cells overlap the edges of the fixed frame and decisions have to be made as to which cells to include and which to leave out. As a result, a fixed-frame method can generate ECD estimates that are not always in very good agreement with the reference method of planimetry.2
A variable frame method offers a slightly different approach in that a set of cells is outlined (as opposed to individual cells) on the original on-screen image, and then the number of cells within that outline (the variable frame) is counted.18, 19, 20 With the area of the variable frame defined by the software and the number of cells counted, a calculation is then made to obtain the ECD in cells/mm2. By removing the step of needing to manually define each individual cell domain on an endothelial image, a variable frame software method may provide a good compromise between a time-consuming planimetry and unreliable results from either automated software or a fixed-frame manual cell count.2
The rationale for this study was to investigate the suitability of a variable frame ‘polygonal’ method, which is available on some specular or confocal microscopes, for providing reasonably reliable ECD estimates in routine surgical practice. The primary objective of the study was to evaluate the agreement of the NAVIS polygonal variable frame method incorporated into a scanning-slit confocal microscope with planimetry and with a manual fixed-frame counting technique in a sample of corneal grafts.
Materials and methods
One hundred corneal grafts of 83 subjects attending the cornea clinic at Gartnavel General Hospital in Glasgow were included in this analysis. All subjects attended for a routine assessment of their corneal grafts between September 2008 and May 2011, and underwent a comprehensive ophthalmic examination according to local protocols including best-corrected visual acuity, slit-lamp biomicroscopy, and Goldmann's tonometry. Following local topical anesthesia (Minims proxymetacaine 0.5%, Bausch & Lomb, Kingston upon Thames, UK), slit-scanning in vivo confocal microscopy (Confoscan 4, Nidek Technologies, Albignasego, Italy) was performed. The × 40 lens was used with a coupling gel (Viscotears, Novartis, Frimley, UK). Care was taken to obtain images from the center of the graft, and all subjects were instructed to focus on the small central fixation light inside the instrument. The cornea was scanned in ‘full-thickness’ and ‘auto-landing’ mode. The clearest image frame showing the corneal endothelium was selected for analysis. A scale marker of 50 μm length was inserted into each image for cross-calibration purposes.
Working on the displayed images, the endothelial cells were assessed with the Nidek Advanced Vision Information System (NAVIS; Nidek Technologies) variable frame (polygonal), and fixed-frame (manual) methods by one experienced observer (SJ). These analyses were performed under ambient illumination using a mouse-based cursor. For the variable frame method, the drawing tool was used to define the outermost edges of the most peripheral cells that were considered to be completely within the image (that is, no part of these cells extended beyond the edges of the image); this outlining generates a variable sized frame. Within this variable frame, all individual cells were then counted and marked with a small ‘spot’. On completing the count, the NAVIS system then calculates the ECD as the number of cells divided by the area of the variable frame. For the fixed-fame manual method, an ROI was defined based on the region of the image where as many cells as possible could be seen. An effort was made to try to obtain an ROI of 0.05 mm2 on each image. By definition, the fixed frame has straight borders and so different numbers of cells overlap these borders of the ROI. For the present studies, and following convention for such estimates, cells overlapping the upper and left border of the ROI were included in the cell count, but cells overlapping the right and lower borders were not included. Cells to be included within the count were again marked with a small red ‘spot’ using the mouse-based cursor. The software then calculates an ECD from the cell count within this fixed frame.
The endothelial images were downloaded, printed, and planimetry undertaken independently by another experienced investigator (MJD), as previously described.2 Briefly, the method involves manual outlining all of the cell borders on standardized endothelial image printouts. The areas of these cells were then measured using a Digipro digitiser pad (Elestree Computing Ltd., London, UK) in stream mode. Image calibration was checked against its internal system and against the scale marker on the original confocal images. The procedures described in this paper have been approved by the local Ethics committee.
Statistical analysis
Data were entered into spreadsheets and analyzed using the STATA SE software package version 11.1. (Stata Corporation, College Station, TX, USA) for generation of descriptive statistics and graphical outputs. Data sets were tested for normality of the distribution using the Shapiro-Wilk test, with a P<0.05 indicating a non-normal distribution of the data. Differences between sets of measurements were assessed with the non-parametric Wilcoxon signed-rank test, with statistical significance set at P<0.05. The agreement between methods was assessed with method comparison analysis (Bland-Altman). The mean differences and the 95% limits of agreement (LoA) were calculated as mean difference±1.96 SD. The difference between the data sets was also assessed in relation to the mean of two measurements by a linear regression analysis.21
Results
General cohort characteristics
The mean age of the subjects was 44±17 years, with a median of 40 years (range 16–82 years); the mean time since surgery was 77±85 months, with a median of 37 months (range 1–291 months). The mean number of cells that was analyzed per image frame was 53, 48, and 45 for manual fixed-frame method, planimetry, and the polygonal variable frame method, respectively.
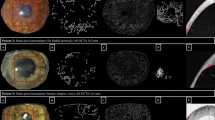
Figure 1a shows a representative image of the corneal graft endothelium in this cohort, with the cells on the image outlined as undertaken for the planimetry. For this graft image, the ECD was 771 cells/mm2. For the set of 100 graft images, between 15 and 90 cells were measured by planimetry (average 48 per image) to obtain a set of ECD values ranging from 336 to 2941 cells/mm2 (Figure 1b). The histogram shows that the data was positively skewed (P<0.05). The group mean ECD value was 1346±726 cells/mm2 (mean±SD), whereas the median ECD was 1088 cells/mm2.
An example of the outcome of NAVIS polygonal variable frame method on the same graft is shown in Figure 2a. Comparison with Figure 1a shows that most of the same cells were identified in the application of the variable frame. The ECD was 806 cells/mm2. The set of polygonal ECD values of the entire sample is shown in Figure 2b, again revealing a skewed distribution (P<0.05). The group mean ECD was 1404±784 cells/mm2, and the median ECD value was 1121 cells/mm2.
The application of the manual fixed-frame method on the same graft is shown in Figure 3a. Most of the same cells are again counted, but there are additional partial cells overlapping the upper and left borders of the ROI. The calculated ECD was lower at just 645 cells/mm2. The distribution of ECD values was now more dominated by lower counts (Figure 3b) and was again skewed (P<0.05). The group mean ECD (±SD) for the 100 grafts in the cohort was 1026±610 cells/mm2 for the fixed-frame method, and the median value was 780 cells/mm2.
Agreement between methods
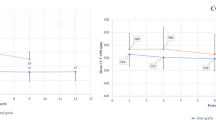
As shown in Figure 4, the set of ECD data using a polygonal variable frame method was similar to that independently obtained by the reference planimetry method. The fairly broad±25% inter-quartile intervals and the extent of the whiskers results from the data sets being not only including a wide range of ECD values, but also because they were skewed (Figures 1, 2 and 3). There were, however, no obvious outliers. By comparison, the set of ECD data from the manual fixed-frame method is notably different to that from the planimetry reference method with a lower median value. The ECD data obtained by the three methods are summarized in Table 1.
Bland-Altman analyses revealed that when compared with the planimetric reference method, independent application of the polygonal variable frame method yielded a slightly higher ECD with a mean difference of 58 cells/mm2 (LoA −222 and 339 cells/mm2) (Figure 5a). There was a slight, but statistically significant trend in that the difference between polygonal and planimetry ECD was slightly greater in higher density grafts (r=0.401, R2=0.161, P<0.001).
(a) Method comparison: polygonal and planimetry analysis. (b) Method comparison: fixed-frame and planimetry analysis. The x axis represents the mean of the polygonal and the planimetry ECD, the y axis shows the mean difference between the methods. The upper and the lower horizontal lines represent the upper and the lower limit of agreement (mean difference±1.96 SD), respectively. The middle line represents the mean difference between the methods.
In contrast, the manual counting fixed-frame method applied to the same images, as used for the polygonal method, resulted in predictably lower ECD values compared with the reference method (Figure 5b). The mean difference between the manual fixed-frame method and planimetry was −320 cells/mm2 (LoA −814 and 173 cells/mm2). Analyses of the difference in relation to overall cell density revealed a negative trend with the lower ECD estimates from the fixed-frame method being found in higher density grafts (r=−0.477, R2=0.228, P<0.001). When manual fixed-frame and planimetric ECD were compared (Figure 5b), six extreme data points were noted that lay well outside the 95% LoA. These data points were assumed to have influenced the results of the regression analysis. Excluding these leverage values from the regression analysis reduced the strength of the aforementioned association (r=−0.326, R2=0.106), but it was still statistically significant (P=0.001).
Discussion
This study was designed to assess the agreement of the NAVIS polygonal variable frame ECD analysis option to planimetry as the reference method. On the 100 clear corneal grafts included in the study, this variable frame method of the NAVIS system was found to be in good agreement with planimetry. The results indicate that the polygonal method can be used in clinical and research applications, as it avoids any substantial and clinically relevant over- or underestimation of the ECD. In particular, it avoids the very substantial overestimation of the ECD that has been reported for the fully automated analysis for the same NAVIS system after keratoplasty.2 This polygonal variable frame method is reasonably quick to perform, making it a useful cost- and time-effective alternative to planimetry.
As noted in the introduction,3 the importance of reliable assessments of ECD can be surmised from the number of previous studies that have compared endothelial image analysis outcomes from confocal and contact or non-contact specular microscopes.7, 13, 14, 15, 16 Klais et al13compared confocal microscope images with those obtained by contact specular microscopy. For images from each instrument, image analysis was carried out by three different methods, using standard software programs namely manual, automated, and semi-automated techniques. The endothelial cell densities were not significantly different between confocal and specular microscopy. Differences between the methods of analysis were, however, noted, indicating that the method of endothelial cell analysis has a greater impact on the results than the type of the microscope. Non-contact specular microscopy has been compared with an earlier version (Confoscan 3) of the confocal instrument used in the present study.16 Images from both microscopes were analyzed using the corners method of a separate analysis system. The confocal images were also assessed by an automated system (with and without manual correction) incorporated into the instrument. No significant differences in ECD were noted for images from the two microscopes when the same corners method was used. The authors concluded that the instruments may be used interchangeably when the same software and method could be used for morphological analysis.16 Sheng et al15 also used a non-contact specular microscope and the earlier version of the confocal microscope used in the present study to obtain images from the central cornea. The images from the specular microscope were analyzed by a ‘dot’ technique (also referred to as a corners method) on a set of contiguous cells, the results from which were compared with images from a confocal instrument analyzed using an automated and a semi-automated method. The comparison of confocal and specular images provided similar mean ECDs, with a semi-automated confocal analysis method showing the best agreement with the analysis system incorporated into the specular microscope. Non-contact specular microscopy and laser-scanning confocal microscopy were used to assess the cornea in another recent study. ECD was estimated using two different methods, namely an automated software option (on the specular microscope) and a ‘dotting’ method (incorporated into the confocal microscope). A just statistically significant difference in ECD (P=0.04) of on average ∼3% was noted between the instruments, with the confocal instrument giving slightly higher readings than the specular microscope.7 The authors report that, based on observation of the method comparison plots, the differences between the instruments were significant and systematic, but that the relatively small difference was indeed caused by the application of two different analysis methods rather than the use of two types of microscopes. Another study used an image analysis approach, based on a center method, to assess images from non-contact specular, non-contact confocal, and contact confocal microscopy. The resultant ECD values were considered to be in good agreement for all three microscopy techniques.14
From these comparative studies, it can be concluded that the type of microscope, that is, whether a confocal or specular microscope is being used, does not affect the outcome of endothelial cell density assessment. It could, however, be argued that for assessments of corneal grafts the use of a confocal microscope is preferable because of the better image quality these systems can provide.14
Variable frame methods or versions of such techniques have been used in earlier studies, including large multi-center studies with a centralized reading center such as the ‘Specular microscopy ancillary study’.20 One advantage of analyzing endothelial images with a variable frame method include the capacity of such a method to provide reasonably reliable results even in cases with less than optimal image quality, resulting in a higher proportion of images that can be included in the analyses.20
A recent study compared a so-called flex-center method to a standard corner method, and found that the flex-center method can be used interchangeably with the reference method after corneal transplantation.5 The flex-center method involves tracing of a boundary around a set of cells on the endothelial image, and this tracing seems to be similar to drawing the boundary around cells that is part of the polygonal method used in this study. The mean difference in ECD between the flex-center and the standard corner method after keratoplasty was −21 cells/mm2 (LoA −69 and 27 cells), but the sample size was small. In this study, we noted a greater mean difference and a broader LoA interval between the polygonal variable frame and the reference method. A possible reason for this difference might have been the inclusion of PK and DALK grafts in the analysis. However, the inclusion of both types of grafts not only expands the relevance of the study but also provides ECD values across the entire spectrum that can be expected in keratoplasties. The utility of the polygonal method has been demonstrated over a wide range of ECD values.
Automated assessments of the ECD by a previous version of the NAVIS software have been shown not to be in agreement with an established reference method, leading to overestimation of the ECD.15 The authors also noted that, although manual editing of the automated output improved the agreement, such editing may have its limitations in corneas with ‘complicated endothelial images’, implying that manual editing is not entirely satisfactory when analyzing irregular endothelial cell mosaics, as commonly encountered after keratoplasty.
As shown in this study, manual fixed-frame cell counts can underestimate ECD. This approach systematically yields lower ECD estimates, likely because it is susceptible to the effect of a cell (or several cells) being excluded from the count.
The polygonal method of the NAVIS system described above and included in a commercially available confocal microscope largely avoids these problems and provides the clinician and researcher with ECD estimates that are very similar to those from planimetry. Planimetry is generally being considered to be the most robust method to estimate ECD, as it takes into consideration the individual cell to cell borders. A clear disadvantage of planimetry, however, is the fact that the technique is time-consuming and not very suitable for routine use in a busy clinic. The outcome of the present studies indicates that a variable frame method, available as a new ‘polygonal’ method, should be suitable for routine clinical assessments of the endothelium, using confocal microscopy.
Conclusions
After keratoplasty, endothelial cell counts with the NAVIS polygonal variable frame method are more closely related to the planimetric reference method than manual ECDs. The results indicate better reliability for the polygonal counts than for manual counts. The polygonal method is suggested as the method of choice with this confocal microscope for research and clinical purposes where accurate estimation of endothelial cell counts is required.

References
Doughty MJ . Toward a quantitative analysis of corneal endothelial cell morphology: a review of techniques and their application. Optom Vis Sci 1989; 66 (9): 626–642.
Jonuscheit S, Doughty MJ, Ramaesh K . In vivo confocal microscopy of the corneal endothelium: comparison of three morphometry methods after corneal transplantation. Eye (Lond) 2011; 25 (9): 1130–1137.
McCarey BE, Edelhauser HF, Lynn MJ . Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008; 27 (1): 1–16.
Benetz BA, Diaconu E, Bowlin SJ, Oak SS, Laing RA, Lass JH . Comparison of corneal endothelial image analysis by Konan SP8000 non contact and Bio-Optics Bambi systems. Cornea 1999; 18 (1): 67–72.
Patel SV, McLaren JW, Bachman LA, Bourne WM . Comparison of flex-center, center, and corner methods of corneal endothelial cell analysis. Cornea 2010; 29 (9): 1042–1047.
Deb-Joardar N, Thuret G, Zhao M, Acquart S, Péoc’h M, Garraud O et al. Comparison of two semi-automated methods for evaluating endothelial cells of eye bank corneas. Invest Ophthalmol Vis Sci 2007; 48 (7): 3077–3082.
Salvetat ML, Zeppieri M, Miani F, Parisi L, Felletti M, Brusini P . Comparison between laser scanning in vivo confocal microscopy and non contact specular microscopy in assessing corneal endothelial cell density and central corneal thickness. Cornea 2011; 30 (7): 754–759.
Modis Jr L, Langenbucher A, Seitz B . Corneal endothelial cell density and pachymetry measured by contact and non contact specular microscopy. J Cataract Refract Surg 2002; 28 (10): 1763–1769.
Szalai E, Nemeth G, Berta A, Modis Jr L . Evaluation of the corneal endothelium using non contact and contact specular microscopy. Cornea 2011; 30 (5): 567–570.
Rieth S, Engel F, Buhner E, Uhlmann S, Wiedemann P, Foja C . Comparison of data from the rostock cornea module of the heidelberg retina tomograph, the oculus pentacam, and the endothelial cell microscope. Cornea 2010; 29 (3): 314–320.
Patel HY, Patel DV, McGhee CN . Identifying relationships between tomography-derived corneal thickness, curvature, and diameter and in vivo confocal microscopic assessment of the endothelium in healthy corneas of young adults. Eye (Lond) 2009; 23 (2): 270–278.
Imre L, Nagymihaly A . Reliability and reproducibility of corneal endothelial image analysis by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol 2001; 239 (5): 356–360.
Klais CM, Buhren J, Kohnen T . Comparison of endothelial cell count using confocal and contact specular microscopy. Ophthalmologica 2003; 217 (2): 99–103.
Raecker ME, McLaren JW, Kittleson KM, Patel SV . Endothelial image quality after descemet stripping with endothelial keratoplasty: a comparison of three microscopy techniques. Eye Contact Lens 2011; 37 (1): 6–10.
Sheng H, Parker EJ, Bullimore MA . An evaluation of the ConfoScan3 for corneal endothelial morphology analysis. Optom Vis Sci 2007; 84 (9): 888–895.
Kitzmann AS, Winter EJ, Nau CB, McLaren JW, Hodge DO, Bourne WM . Comparison of corneal endothelial cell images from a non contact specular microscope and a scanning confocal microscope. Cornea 2005; 24 (8): 980–984.
Doughty MJ, Jonuscheit S, Button NF . Assessment of the reliability of endothelial cell-density estimates in the presence of pseudoguttata. Graefes Arch Clin Exp Ophthalmol 2012; 250 (1): 111–121.
Okubo A, Inoda S, Ohara K . Effects of anterior segment inflammation on the corneal endothelium. Morphometric study with panoramic photography and cell shape analysis. Nihon Ganka Gakkai Zasshi 1983; 87 (11): 1233–1241.
Giasson CJ, Solomon LD, Polse KA . Morphometry of corneal endothelium in patients with corneal guttata. Ophthalmology 2007; 114 (8): 1469–1475.
Benetz BA, Gal RL, Ruedy KJ, Rice C, Beck RW, Kalajian AD et al. Specular microscopy ancillary study methods for donor endothelial cell density determination of Cornea Donor Study images. Curr Eye Res 2006; 31 (4): 319–327.
Bland JM, Altman DG . Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8 (2): 135–160.
Acknowledgements
This study was supported by an Ophthalmology Research Grant from the Royal College of Surgeons, Edinburgh (KR, MJD, SJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Part of the data was presented at the World Ophthalmology Congress, Berlin, 2010.
Rights and permissions
About this article
Cite this article
Jonuscheit, S., Doughty, M. & Ramaesh, K. Assessment of a variable frame (polygonal) method to estimate corneal endothelial cell counts after corneal transplantation. Eye 26, 803–809 (2012). https://doi.org/10.1038/eye.2012.42
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.42
Keywords
This article is cited by
-
Morphometry of organ cultured corneal endothelium using Voronoi segmentation
Cell and Tissue Banking (2017)
-
Fully automatic evaluation of the corneal endothelium from in vivo confocal microscopy
BMC Medical Imaging (2015)
-
Inherent errors of the fixed-frame counting method for corneal endothelial cell density in eye banks
Cell and Tissue Banking (2014)