Abstract
Despite the advances in cancer therapies in the past century, malignant melanoma continues to present a significant clinical challenge due to lack of chemotherapeutic response. Systemic therapy with immunostimulatory agents such as interferon and interleukin-2 (IL-2) has shown some promise, though each is associated with significant side effects. Over the past 50 years, oncolytic Newcastle disease virus (NDV) has emerged as an alternative candidate for cancer therapy. The establishment of reverse-genetics systems for the virus has allowed us to further manipulate the virus to enhance its oncolytic activity. Introduction of immunomodulatory molecules, especially IL-2, into the NDV genome was shown to enhance the oncolytic potential of the virus in a murine syngeneic colon carcinoma model. We hypothesize that a recombinant NDV expressing IL-2 would be an effective agent for therapy of malignant melanoma. We show that recombinant NDV possesses a strong cytolytic activity against multiple melanoma cell lines, and is effective in clearing established syngeneic melanoma tumors in mice. Moreover, introduction of murine IL-2 into NDV significantly enhanced its activity against syngeneic melanomas, resulting in increased overall animal survival and generation of antitumor immunity. These findings warrant further investigations of IL-2-expressing NDV as an antimelanoma agent in humans.
Similar content being viewed by others
Introduction
Malignant melanoma is an aggressive malignancy of melanocytes and is the fifth most common cancer in men and the sixth most common cancer in women in the United States.1 The incidence of melanoma is rapidly rising, best exemplified by a 270% increase from 1973 to 2002 in the United States. The increase in incidence was paralleled by an increase in mortality, which, despite aggressive interventions, has shown little improvement since the 1990s. The treatment is primarily surgical, which is often not possible in the late-stage disease. To date, dacarbazine interferon (IFN)-α2b and interleukin-2 (IL-2) remain the sole FDA-approved agents for treatment of late-stage melanoma, although neither of the agents has been shown to be effective in prolongation of overall survival.1
Advances in the understanding of melanoma biology have led to a development of a variety of novel therapeutic approaches, some of which have already shown promising results. Those include therapeutic cancer vaccines, angiogenesis inhibitors, novel cytotoxic agents, immunoregulatory strategies and oncolytic virus therapies.2
Newcastle disease virus (NDV) is a member of the Avulavirus genus in the Paramyxoviridae family, which has been shown to infect a number of avian species.3, 4 Naturally occurring attenuated NDV viruses have been previously shown to be effective oncolytic agents against a variety of experimental cancers.5, 6, 7
Promising results with utilization of naturally occurring NDV for oncolytic therapy of malignant melanoma have been reported by Cassel and Murray.8, 9 In a study using historical controls, patients with stage II malignant melanoma were immunized with autologous or allogeneic NDV viral oncolysates. Immunization reduced number of relapses, with over 60% survival reported at 10 years.9
A subsequent randomized phase III trial utilizing vaccinia virus melanoma oncolysates revealed no benefit in overall survival, though a survival benefit was observed in the patients in earlier stages.10 Later on, several of the naturally occurring NDV strains, rather than viral oncolysates, were directly used in clinical trials for other tumors, with promising results being reported for a minority of patients with advanced tumors.11, 12, 13, 14
To date, high-dose IFN-α2b and high-dose IL-2 (HDI) are the only immunological agents approved by the FDA for melanoma therapy. IL-2 was originally shown to grow and expand activated T cells, and to enhance the cytotoxicity of antigen-specific cytotoxic T cells and natural killer cells in vitro.15, 16 The ability of IL-2-activated cytolytic T cells to induce tumor regressions in preclinical animal tumor models became the foundation for the first clinical applications of IL-2.17 HDI was later shown to induce durable responses in 5–10% of patients with metastatic melanoma and renal cell carcinoma.18 Systemic administration of HDI, however, is associated with significant toxicity and expense, and only a minority of patients benefit from their use in terms of long-term survival.
Based on these findings, we introduced a sequence encoding murine IL-2 into the genome of fusogenic NDV and showed that the resultant virus was enhanced in its ability to clear established CT26 flank colon tumors in a syngeneic mouse model.19 Given the history of the effectiveness of NDV oncolysates in treatment of human melanomas and the use of IL-2 as an adjunct to melanoma therapy, we hypothesized that a recombinant NDV expressing IL-2 would present an attractive therapeutic strategy for melanoma. It this study, we tested the efficacy of the NDV-IL-2 virus both in vitro and in vivo. We show that the virus possesses a significant oncolytic activity against multiple human melanoma cell lines and is an effective oncolytic agent in a syngeneic murine B16-F10 melanoma model. The results of this study warrant further investigation of NDV-IL-2 as a therapeutic agent for human cancers.
Results
NDV effectively lyses human and mouse melanoma cell lines
For the current studies, we used the attenuated NDV of Hitchner B1 strain (NDV(B1)), which was genetically modified to express a highly fusogenic fusion (F) protein. We have previously shown that the resultant virus (termed NDV(F3aa)) was enhanced in its ability to form syncytia in the infected cells.19 To explore the oncolytic potential of the fusogenic NDV in treatment of malignant melanoma, we selected the human melanoma cell lines SkMel-2, SkMel-119, SkMel-197, DRO90-01 and the mouse melanoma cell line B16-F10 and infected them with NDV(B1) and NDV(F3aa) viruses at multiplicity of infection (MOI) 0.1. Cytotoxicity was assessed by lactate dehydrogenase (LDH) release assays at 24, 48 and 72 h postinfection. The results of this study are summarized in Figure 1a. NDV was effective against all melanoma cell lines tested, with NDV(F3aa) being more cytolytic than the parental nonfusogenic NDV(B1) virus for the majority of cell lines. Statistical significance was determined by Student's paired two-tailed t-test analysis. Infection of melanoma cell lines with NDV(F3aa) virus revealed that the virus effectively formed large syncytia, which was likely responsible for its enhanced cytolytic activity (Figure 1b).
Recombinant Newcastle disease virus (NDV) with enhanced fusogenic protein effectively lyses human and murine melanoma cell lines. (a) Cell lines (5 × 105 cells) were infected at multiplicity of infection (MOI) 0.1 in triplicate and lactate dehydrogenase (LDH) release assays were performed at 24, 48 and 72 h. Percentage of cells surviving at 24, 48 and 72 h is shown (*P=0.018, **P=0.005, ***P=0.067, ****P=0.01, *****P=0.0009). (b) Syncytia formation by the NDV(F3aa) virus. Cell lines tested in (a) were infected with NDV(B1) and NDV(F3aa) at MOI 0.01, fixed after 24 h and stained with dapi (blue) and anti-NDV polyclonal serum (green). Representative images from SkMel-2 and B16-F10 cells are shown. The color reproduction of this figure is available on the html full text version of the manuscript.
Recombinant NDV(F3aa)-IL-2 expresses IL-2 in the infected B16-F10 cells
After establishing that NDV(F3aa) virus is an effective agent against melanoma cell lines in vitro, we proceeded with the characterization of an NDV(F3aa) virus expressing murine IL-2 (NDV(F3aa)-IL-2). Construction of the NDV(F3aa)-IL-2 virus has been described previously.19 The murine IL-2 gene was cloned between the P and M genes of NDV.
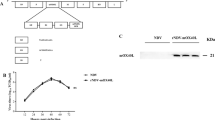
Replication of NDV (F3aa)-IL-2 virus was tested in the SkMel-2 cells and B16-F10 cells. Cells were infected at three different MOIs and virus production was assessed for 72 h. There was a statistical difference in replication between the three viruses in both cell lines (P<0.001 in SkMel-2 and P<0.001 in B16-F10 by analysis of variance (ANOVA)). The NDV(F3aa)-IL-2 virus was found to be slightly inferior in its replication ability to the parental NDV(F3aa), with the highest titers achieved on days 1 or 2 postinfection (P=0.009 in SkMel-2 cells, P=0.015 in B16-F10 cells, by paired Student's t-test). Viral titers peaked 24 or 48 h postinfection and then dropped off, presumably due to significant cytopathic effect and induction of the antiviral innate immune response. To assess the degree of cytolysis by the NDV(F3aa)-IL-2, the infected cells were collected 24, 48 and 72 h postinfection and assayed for LDH content. As can be seen from Figure 2b, there was a statistically significant difference in the cytolytic activity between the three viruses (P<0.001 in SkMel-2 cells and P=0.005 in B16-F10 cells by ANOVA). The NDV(F3aa)-IL-2 virus was comparable or lower in its cytolytic activity to the parental NDV(F3aa) virus (P=0.01 and P=0.39 in SkMel-2 and B16-F10 cells, respectively, determined by paired Student's t-test). Viral replication and cytotoxicity at different MOIs are shown in greater detail in Supplementary Figure 1.
Recombinant NDV(F3aa)-IL-2 replicates in melanoma cells and expresses interleukin-2 (IL-2). (a) SkMel-2 and B16-F10 cells were infected at the indicated multiplicity of infections (MOIs) and the viral titers in the supernatants were assessed at 24, 48, 72 and 96 h. Statistical significance in titer difference between NDV(F3aa) and NDV(F3aa)-IL-2 was determined by Student's t-test (*P=0.009, **P=0.015). (b) B16-F10 cells (right panel) and SkMel-2 cells (left panel) were infected with NDV(B1), NDV(F3aa) and NDV(F3aa)-IL-2 viruses at the indicated MOIs. Cytotoxicity was assessed at 24, 48 and 72 h by lactate dehydrogenase (LDH) release assays (*P=0.01, **P=0.39). Lower MOIs were used in SkMel-2 cells due to higher susceptibility of the cells to Newcastle disease virus (NDV). (c) B16-F10 cells were infected at MOI 2 with NDV(B1), NDV(F3aa), and NDV(F3aa)-IL-2 viruses and the supernatants were collected 24 h postinfection. Murine IL-2 production was determined by serial dilution of supernatants and ELISA.
To confirm that IL-2 was expressed in the infected melanoma cells, B16-F10 cells were infected at MOI 2 with NDV(F3aa) or NDV(F3aa)-IL-2 virus and the supernatants were assayed for IL-2 24 h postinfection. Infection with NDV(F3aa) virus resulted in no detectable IL-2 production, whereas the cells infected with NDV(F3aa)-IL-2 virus generated significant levels of IL-2 (Figure 2c).
NDV(F3aa)-IL-2 is an effective oncolytic agent in the syngeneic murine B16-F10 footpad melanoma model
To determine whether the NDV(F3aa)-IL-2 virus would be an effective antimelanoma agent in vivo, we used the syngeneic B16-F10 mouse footpad melanoma model. The B16-F10 cell line is known for its particularly aggressive tumor growth, early metastases and very poor response to therapy.20, 21, 22, 23, 24 The naturally occurring NDV(B1) virus was excluded from these studies, because the in vitro studies showed superior efficacy of the NDV(F3aa) viruses.
For toxicity studies, six C57/BL6 mice were inoculated subcutaneously or intravenously with 5 × 107 PFU of NDV(F3aa) and NDV(F3aa)-IL-2 viruses. Over the next 2 weeks, none of the animals exhibited signs of distress and continued to gain weight (data not shown). To investigate the efficiency of NDV(F3aa)-IL-2 virus in oncolytic therapy, we proceeded to use a low-dose treatment regimen (5 × 106 PFU) extended over 4–6 doses.
C57/BL6 mice from each group were inoculated into the right posterior footpad with 1 × 105 cultured B16-F10 cells. After 7 days, a pigmented tumor focus was visible in each of the animals. On day 7, the right posterior footpad of each animal was intratumorally injected with 5 × 106 PFU of NDV(F3aa), NDV(F3aa)-IL-2 or phosphate-buffered saline (PBS) control. Eight mice were included in the control group, whereas 12–13 mice were used for each virus treatment group. The inoculation with 5 × 106 PFU of each respective virus was repeated on days 9, 11 and 13 (total four injections).
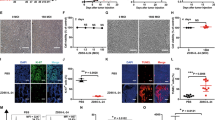
None of the animals exhibited significant weight loss over the study period (data not shown). The most common side effect was the development of localized swelling at the injection site, which subsided over the next few days after the last inoculation. On day 25 after tumor implantation, 8/8 control mice developed tumors of significant size and were killed. In addition, 6 animals from the NDV(F3aa) group and 5 animals from the NDV(F3aa)-IL-2 group were randomly selected and killed for analysis, whereas the rest of the animals continued to be monitored for tumor growth (see below). As can be seen from Figure 3a, tumors were undetectable in the animals in the NDV(F3aa)-IL-2 group, and were visible in only 2/13 animals in the NDV(F3aa) group. The visible tumors were significantly smaller than the majority of tumors in the control group.
NDV(F3aa)-IL-2 effectively suppresses tumor growth and promotes mouse survival in a syngeneic murine melanoma model. (a) Short-term tumor growth in B16-F10 melanoma-bearing mice treated with recombinant Newcastle disease virus (NDV) viruses. Six 8-week-old C57/BL6J mice were injected in the right posterior footpad with 105 of cultured B16-F10 cells. Seven days postinfection, the tumors were injected with 5 × 106 PFU of NDV(F3aa) or NDV(F3aa)-IL-2 virus (day 0). The injections were repeated on days 9, 11, and 13. Tumor measurements were recorded every 2 days. All eight mice from the control group and 5–6 randomly chosen mice from each virus group were killed on day 18 after first treatment for immune studies (see Figure 5). (b) Short-term tumor growth in mice treated at later stages. B16-F10 cells were injected into the right posterior footpads of mice (n=5 per group) and tumors were allowed to develop for 10 days. The mice were subsequently treated every other day with a total of six doses of 5 × 106 PFU of each virus, and tumors were measured every 2 days. When the largest tumors reached 8 mm in length, all the animals were killed.
To determine whether the viruses would be effective in clearing larger tumors, the experiment was repeated with the tumors being allowed to develop for a longer period of time. Five mice were included in each group. On day 10, the right posterior footpad of each animal was intratumorally injected with 5 × 106 PFU of NDV(F3aa), NDV(F3aa)-IL-2 or PBS control. Five mice were included in each group. The inoculations were repeated on days 12, 14, 16, 18 and 20 for a total of six injections. The animals were assessed for tumor size and weight loss until day 22, at which point all animals were killed because the tumor sizes of some animals in the control group reached the endpoint (see Materials and methods). As can be seen from Figure 3b, treatment with both NDV(F3aa) and NDV(F3aa)-IL-2 viruses markedly suppressed tumor growth in all animals, with only minor tumors being detectable on day 22. These results indicate that higher virus doses or increased number of treatments may be effective in clearing the tumors in later stages of development.
The remaining animals from the early tumor treatment group continued to be followed to determine the long-term efficacy of each viral treatment. Over the next 120 days, 4/7 animals in the NDV(F3aa) group developed significant tumors and needed to be killed, whereas only 1/8 animals in the NDV(F3aa)-IL-2 group developed a tumor that required animal killing (Figure 4a). The remaining animals in each group either completely cleared the tumor (1/3 in the NDV(F3aa) group and 2/7 in the NDV(F3aa)-IL-2 group), or had a persistent pigmented focus that did not change in size. The overall survival for the animals in the long-term study was 0/8 for the control group, 3/7 for the NDV(F3aa) group and 7/8 for the NDV(F3aa)-IL-2 group (Figure 4b; P=0.0002, when comparing control group to NDV(F3aa)-IL-2 group; P=0.0498, when comparing NDV(F3aa) group to NDV(F3aa)-IL-2 group, calculated using the logrank comparison).
Animals treated with NDV(F3aa)-IL-2 exhibit long-term tumor-free survival. (a) Long-term tumor growth follow-up in the treated mice. The remaining animals from each group in (a) were continued to be followed for 120 days, with tumor measurements being recorded every 2 days. (b) Summary of 120-day survival of the animals treated in (a). Mice were killed when the tumors reached 8 mm in length. For experimental groups, only the mice included in the long-term study (n=7 for each group) were included in the analysis (*P=0.0498, **P=0.0002).
Mice inoculated with NDV(F3aa)-IL-2 develop an immune response to B16-F10 cells
To assess for antitumor immune responses, we first proceeded to determine whether locoregional expression of IL-2 by the virus results in a differential immune cell infiltration.
Tumors from the killed mice from the late treatment group described above were collected on day 22, dissected and filtered, and stained for CD4 and CD8 antigen expression. As can be seen from Figure 5a, tumors from the animals treated with NDV(F3aa) and NDV(F3aa)-IL-2 viruses exhibited a high degree of both CD4 and CD8 cell infiltration (P<0.001 for CD4 and P<0.001 for CD8 by ANOVA). The percentage of the infiltrating CD4 and CD8 cells was significantly higher in the NDV(F3aa)-IL-2 group (P=0.025 and P=0.015, respectively) as determined by the Student's paired t-test. These findings suggested that the NDV(F3aa)-IL-2-treated animals develop a stronger locoregional immune response to the infection and/or tumor.
Newcastle disease virus (NDV) treatment leads to generation of protective antimelanoma immune responses. (a) Tumor infiltration by CD4 and CD8 cells. Tumors were collected from the animals on day 22 and were processed for CD4 and CD8 staining by FACS analysis. Statistical significance was determined by Student's paired two-tailed t-test analysis (*P=0.025, **P=0.015). (b) Interferon-γ (IFNγ) release from stimulated splenocytes. Splenocytes collected from the killed animals were cocultured with mitomycin-inactivated B16-F10 cells and IFNγ was measured in the supernatants on day 3 of coculture (*P=0.0015, **P=9.75 × 10−6). (c) Melanoma-specific cytotoxicity of the stimulated splenocytes. Stimulated splenocytes described in (b) on day 5 were cocultured with fresh B16-F10 cells for 4 h at the indicated ratios and the specific cytotoxic activity was determined by measurements of lactate dehydrogenase (LDH) release (*P=0.0078, **P<0.00015). (d) Survival of tumor challenge. Seven of surviving animals in the NDV(F3aa)-IL-2 group were challenged with 1 × 105 cultured B16-F10 cells into the left posterior footpad. Five naive animals were used as controls. Mice were killed when tumors reached 8 mm in length (*P=0.0424, **P=0.0035).
To determine whether the treated mice develop an adaptive immune response to melanoma cells, animals killed on day 25 were assessed for the development of cytotoxic T lymphocyte (CTL) responses against B16-F10 cells. Splenocytes from the animals were cocultured with mitomycin C-inactivated B16-F10 cells for 5 days and assessed for IFNγ release on day 3 and for B16-F10-specific CTL activity on day 5. As can be seen from Figures 5b and c, treatment with both NDV(F3aa) and NDV(F3aa)-IL-2 viruses resulted in enhanced IFNγ release and enhanced CTL activity (P<0.001 for IFNγ release and P<0.001 for CTL by ANOVA), when compared to the control. Of note, treatment with the NDV(F3aa)-IL-2 virus resulted in significantly higher levels of IFNγ release (P=0.0015) and a more significant CTL response (P=0.0078), when compared to that with NDV(F3aa). Statistical significance between NDV(F3aa) and NDV(F3aa)-IL-2 was determined by Student's paired two-tailed t-test analysis. These results suggested that tumor treatment with NDV resulted in generation of tumor-specific CTL responses, which may have contributed to the long-lasting antitumor effect of the virus.
To determine whether the mice treated with NDV(F3aa)-IL-2 virus developed protective systemic immune responses against the tumor cells, on day 100 after the initial tumor inoculation, all seven of the animals remaining in the group were challenged with the injection of cultured 1 × 105 B16-F10 cells into the left posterior footpad. Five naive C57/BL6 mice and the three surviving animals from the NDV(F3aa) group were used as controls. Mice were killed when the tumor size reached 8 mm in length. As can be seen from Figure 5d, 5/5 mice in the control group reached the endpoint within 25 days and required to be killed. The three animals in the NDV(F3aa) group demonstrated prolonged survival when compared to the control group, but all required to be killed by day 40. Five out of seven mice in the challenged NDV(F3aa)-IL-2 group demonstrated a prolonged survival, but eventually developed large tumors and were killed. The remaining two mice in the NDV(F3aa)-IL-2 group initially developed visible tumors which subsequently regressed and the animals remained tumor-free until the completion of the study. Overall, the mice previously treated with NDV(F3aa)-IL-2 survived the challenge longer (median survival of 42 days) than the naive control animals (median survival of 24 days; P=0.0035) or the NDV(F3aa)-treated animals (median survival of 32 days; P=0.0424). Statistical significance was calculated using the logrank comparison. Overall, these results suggest that the immune responses against the B16-F10 cells in the NDV(F3aa)-IL-2-treated group were strong enough to partially protect the animals from the tumor challenge and to offer complete protection to some animals.
Discussion
Malignant melanoma remains a disease with few effective treatments. Metastatic melanoma is rarely curable and current treatments for metastatic disease are primarily palliative in nature. Due to the predominant failure of cytotoxic chemotherapy, a number of alternative immunologic approaches have been devised; though overall the clinical efficacy of these therapies has been variable.
A fundamental problem in treatment of malignant melanoma lies in the inadequate host immune response to the melanoma antigens.25 The mechanism of this immunosuppression is not completely clear, but has been shown to involve a loss or downregulation of HLA expression on the surface of tumor cells, loss of expression of tumor-specific antigens, and lack of appropriate costimulatory signals for T-cell activation.26 Indeed, treatment of patients with antibodies to the immunosuppressive receptor CTL antigen-4 (CTLA4) has shown promising results in clinical benefit against metastatic melanoma.25 As a different approach for antimelanoma immune enhancement, high doses of IL-2 have been approved for treatment of metastatic melanoma on the basis of the induction of durable complete responses in a minority of patients.27
The interest in NDV as an oncolytic therapeutic agent has emerged about half a century ago, when the virus was reported to effectively lyse Ehrlich ascites tumor cells.28 Since then, NDV has been shown to selectively replicate in a variety of tumor cell lines, whereas sparing noncancerous cells. NDV has, in addition, been reported to be an effective oncolytic agent in a variety of animal tumor models.29 Previous studies showed the selectivity of NDV for human tumor cells.5 The oncolytic specificity of NDV stems in large from its sensitivity to the antiviral effects of type I IFN. Many tumor cell types exhibit deficient IFN response to viral infection, which is responsible for oncolytic viruses such as NDV and VSV replicating selectively in tumor cells but not in normal cells.30 Moreover, it was previously shown that species-specificity of the NDV is partially dependent on its inability to overcome the human type I IFN response.31 The oncolytic effect of NDV was also shown to be dependent on activation of apoptotic pathways through endoplasmic reticulum stress, independent of p53 status.32
Naturally occurring NDV has been used effectively in vaccination with tumor-cell oncolysates in people with head and neck squamous-cell carcinomas, tumors of digestive tract, glioblastoma multiforme, malignant melanoma, colorectal carcinoma and other advanced cancers8, 10, 33, 34, 35. The effectiveness of antimelanoma vaccination with NDV oncolysates is based on the development of effective cellular immunity against the tumor. Indeed, infection of tumor cells with NDV has been previously shown to upregulate HLA and cell adhesion molecules and induce IFN, chemokines and apoptosis.36 These studies demonstrate the mechanism behind the immunostimulatory properties of NDV and explain why the virus-infected tumor cells stimulate activation of immune response against the tumor. Indeed, NDV-generated tumor oncolysates have been shown to be more effective stimulators of immune response than oncolysates generated by other methods.37 Studies also indicate that the viral HN and F proteins modify the surface of the infected cells to allow for better lymphocyte adhesion.38, 39 In addition, viral infection stimulates the local production of cytokines and chemokines, which enhance antitumor responses. Recent studies suggest that nitric oxide synthesis by the macrophages also contributes to the antitumor effects of NDV.40
Despite the studies that suggested a high efficiency of NDV oncolysate vaccination in prevention of tumors, there were very few studies utilizing viruses as direct antimelanoma treatment agents. A study utilizing melanoma oncolysates generated by recombinant vaccinia virus expressing IL-2 showed that dendritic cells pulsed with the oncolysate are capable of expressing of melanoma-associated antigens on their surface.41 The study, however, did not evaluate the efficiency of the virus or oncolysates in melanoma therapy in animal models. Based on these findings, we proceeded to investigate whether the treatment with genetically engineered NDV expressing IL-2 would be an effective approach in therapy of melanoma and in generation of antimelanoma immune responses. To ensure efficient replication of the vector, the generated NDV-IL-2 virus possessed an additional modification in the F protein, which was previously shown to enhance the oncolytic efficacy of NDV through formation of syncytia.19 The cleavage site of the NDV F protein has been postulated to be a major determinant of virulence.42, 43, 44 Modification of the cleavage site of the NDV F protein to a polybasic amino-acid sequence (F3aa) allows the protein to be cleaved by intracellular proteases, exposing the fusion peptide and making the virus more effective in entering cells and forming syncytia.42, 43, 44 In fact, introduction of the fusogenic NDV F into the VSV genome increased its syncytia-forming ability, which enhanced its oncolytic potential in head and neck squamous carcinomas.45 Interestingly, in our studies, SkMel-119 cell line did not show significant difference in oncolysis by NDV(F3aa) and NDV(B1). We speculate that the efficiency of intracellular F protein cleavage in the SkMel-119 cell line is higher than in other cell lines, resulting in a similar oncolytic efficiency between the NDV(B1) and NDV(F3aa) viruses.
We show that the generated NDV(F3aa)-IL-2 virus was capable of efficient replication and lysis of human and mouse melanoma cell lines in vitro, as well as resulted in high levels of murine IL-2 expression and secretion from the infected cells. To determine whether the expression of the cytokine would enhance the antitumor properties of NDV therapy, we proceeded to investigate the oncolytic efficacy of the NDV(F3aa)-IL-2 virus in the aggressive syngeneic murine B16-F10 melanoma model. The virus was significantly more effective than its parental NDV(F3aa) counterpart, and resulted in tumor regressions in the majority of the animals. Moreover, treatment with the NDV(F3aa)-IL-2 virus resulted in generation of specific CTL responses against melanoma cells, which was stronger than that in the animals treated with NDV(F3aa). We speculate that the immunostimulatory properties of IL-2 resulted in the observed CTL enhancement and increased tumor clearance. Indeed, our previous studies of the virus in the CT26 colon cancer model demonstrated increased tumor infiltration with CD4 and CD8 cells, suggesting that the observed tumor clearance is mediated not just by direct viral infection, but also through generation of an immune response to the tumor.
The results of this study warrant further investigation of NDV and NDV(F3aa)-IL-2 in the therapy of human melanomas. The efficiency of lysis of human melanoma cell lines by the recombinant NDV suggests their inherent susceptibility to the virus. Several additional aspects of the virus, in particular, define it as a strong candidate to be used in oncolytic melanoma therapy. First of all, replication of NDV in tumor cells results in primarily locoregional expression of the IL-2 gene, which may limit the systemic toxicity seen with IL-2 administration in humans. Secondly, the modular nature of the NDV genome allows for incorporation and stable expression of foreign genes,46, 47 ensuring the stability of NDV(F3aa)-IL-2 construct. Third, NDV infection has been shown to upregulate expression of MHC I and to elicit strong CTL responses in tumors. Its ability to induce strong cellular immune responses makes it an attractive vector for presentation of foreign antigens and tumor-associated antigens.29, 36, 48, 49 Fourth, NDV is an avian pathogen, which avoids the problem of preexisting immunity to the virus in humans. Fifth, NDV can infect humans without inducing severe symptoms. Several of the naturally occurring strains of NDV have been used in multiple clinical trials against advanced human cancers, confirming safety of the virus in human administration.11, 13, 14, 29, 50 A derivative of the NDV(B1) strain, used for construction of the vectors utilized in this study, has been previously used in clinical trials under the name of NDV-HUJ.14
The results of this study provide evidence that the aggressive B16-F10 murine melanoma strongly responds to treatment with NDV expressing IL-2. Based on these findings and the previous success with NDV oncolysates in melanoma therapy, we speculate that a similar strategy in treatment of malignant melanoma in humans may improve the outcomes of the late-stage disease.
Materials and methods
Cell lines, antibodies and other reagents
SkMel-2, SkMel-119 and SkMel-197 cells were maintained in RPMI medium supplemented with penicillin, streptomycin and 10% fetal calf serum (FCS). B16-F10 and DRO90-01 cells were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM) medium supplemented with 10% FCS, penicillin and streptomycin. Polyclonal serum to NDV virus was generated by immunizing a rabbit with NDV lysate, as described previously.31 Fluorochrome-conjugated secondary anti-mouse and anti-rabbit antibodies for microscopy were from Molecular Probes (Eugene, OR, USA). Cytotox LDH release assay kits were purchased from Promega (Madison, WI, USA).
Virus cloning and rescue
The NDV mutant viruses with modified F cleavage site (NDV(F3aa)) were generated as previously described.42 To generate NDV(F3aa) virus expressing murine IL-2, the cDNA fragment encoding the murine IL-2 protein was inserted into the XbaI site created between the P and M genes of pT7NDV/F3aa. Viruses were rescued from cDNA using methods described previously46 and sequenced by reverse transcription-PCR for insert fidelity. Expression of the IL-2 in the NDV(F3aa)-IL-2 infected cells was confirmed by ELISA.
LDH release assays
Cells were infected in 12-well plates for 24, 48 and 72 h in triplicate for each condition. At each time point, the media were aspirated and the cells were washed with 1 ml of PBS. Cells were subsequently incubated with 1% Triton X-100 at 37 °C for 30 min. LDH activity in the lysates was determined using the Promega CytoTox 96 assay kit, according to the manufacturer's instructions. Briefly, 50 μl of cell lysates were incubated with 50 μl of assay reagent and the absorbance was recorded at 490 nm. Percentage of surviving cells was calculated through the ratio of the corrected absorbance from the infected cells to the absorbance in the noninfected controls.
Infections and virus titers
Cells of interest were incubated at room temperature with the virus in 12-well culture dishes at indicated MOIs in a total volume of 100 μl. One hour after the incubation, the infection media were aspirated and the cells were incubated at 37 °C in 1 ml of DMEM with 0.3% bovine serum albumin. To the cells infected with wild-type NDV(B1) virus, 10% chick allantoic fluid was added to the medium to allow for F protein activation. After 24, 48, 72 and 96 h, the supernatants were collected and the virus titers were determined by serial dilution and immunofluorescence in Vero cells.
Murine IL-2 ELISA
B16-F10 cells were infected with NDV(F3aa)-IL-2 at MOI 2 and the infection supernatants were harvested 24 h later. The supernatants were diluted 1:10, 1:100, 1:1000 and 1:10 000 and analyzed by ELISA for presence of murine IL-2 using Quantikine M kit (R&D Systems, Minneapolis, MN, USA).
Microscopy
Cells were cultured on 10 mm round cover slips and infected with viruses of interest at an MOI of 0.001. Twenty hours later, the cells were fixed with 5% formaldehyde in PBS and permeabilized with 1% Triton X-100. Proteins of interest were visualized by indirect immunofluorescence. Cells were probed with specific primary antibody for 2 h at room temperature, washed and labeled with secondary antibody conjugated to a specific fluorophore. Labeled cells were visualized by laser scanning confocal microscopy (Leica TCS-SP) with TCS-SP software for image capture.
Mouse experiments
Cultured B16-F10 cells (1 × 105) were inoculated into the right posterior footpad of 6- to 8–week-old C57/BL6J mice in a total volume of 50 μl. On day 7 postinoculation, the mice were treated by intratumoral injection of 5 × 106 NDV virus of interest or PBS, in a total volume of 50 μl. In each treatment group, 12 mice were included, with 8 mice used in the control group. The treatments were repeated on days 9, 11 and 13, for a total of four treatments. Tumor sizes and mouse weights were recorded every other day. According to the institutional protocols, the animals were killed when the tumors reached 8 mm in length. On day 25, all eight animals from the control group and five animals from each treatment group were killed, and their spleens, popliteal lymph nodes and tumors were collected. The remaining mice in each treatment group were observed for 120 days with measurement of tumor sizes every other day.
Splenocyte collection, IFNγ release and CTL assays
Spleens were removed from the killed animals and splenocytes were isolated by passing the spleens through 80 μm nylon mesh filters. Cultured B16-F10 cells (5 × 105) were incubated with 50 μg ml−1 of mitomycin C for 2 h at 37 °C to induce cell-cycle arrest. After the incubation, the cells were washed with PBS and incubated with 1 × 107 splenocytes in RPMI with 10% FCS for 5 days. On day 3, the supernatants were collected and tested for IFNγ release by ELISA using Quantikine M kit (R&D Systems). On day 5, the splenocytes were collected, washed, counted, and cocultured for 4 h with 1 × 103 B16-F10 cells at the stimulator/effector ratios of 1:1.25, 1:2.5, 1:5, 1:10, 1:20 and 1:40. Specific CTL activity was determined by LDH release from the target cells utilizing the CytoTox 96 LDH kit from Promega according to the manufacturer's instructions.
Statistical analysis
Statistical significance was determined utilizing two-tailed Student's t-test with the help of Microsoft Excel software. One-way ANOVA, Kaplan–Meier and logrank statistical analyses were performed utilizing MedCalc software.
References
Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc 2007; 82: 364–380.
Lejeune FJ, Rimoldi D, Speiser D . New approaches in metastatic melanoma: biological and molecular targeted therapies. Expert Rev Anticancer Ther 2007; 7: 701–713.
Alexander DJ . Newcastle Disease, Newcastle Disease Virus—An Avian Paramyxovirus. Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp 1–22.
Seal BS, King DJ, Sellers HS . The avian response to Newcastle disease virus. Dev Comp Immunol 2000; 24: 257–268.
Reichard KW, Lorence RM, Cascino CJ, Peeples ME, Walter RJ, Fernando MB et al. Newcastle disease virus selectively kills human tumor cells. J Surg Res 1992; 52: 448–453.
Lorence RM, Reichard KW, Katubig BB, Reyes HM, Phuangsab A, Mitchell BR et al. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst 1994; 86: 1228–1233.
Tzadok-David Y, Metzkin-Eizenberg M, Zakay-Rones Z . The effect of a mesogenic and a lentogenic Newcastle disease virus strain on Burkitt lymphoma Daudi cells. J Cancer Res Clin Oncol 1995; 121: 169–174.
Cassel WA, Murray DR . Treatment of stage II malignant melanoma patients with a Newcastle disease virus oncolysate. Nat Immun Cell Growth Regul 1988; 7: 351–352.
Cassel WA, Murray DR . A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother 1992; 9: 169–171.
Wallack MK, Sivanandham M, Balch CM, Urist MM, Bland KI, Murray D et al. Surgical adjuvant active specific immunotherapy for patients with stage III melanoma: the final analysis of data from a phase III, randomized, double-blind, multicenter vaccinia melanoma oncolysate trial. J Am Coll Surg 1998; 187: 69–77; discussion 77–79.
Csatary LK, Gosztonyi G, Szeberenyi J, Fabian Z, Liszka V, Bodey B et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol 2004; 67: 83–93.
Csatary LK, Moss RW, Beuth J, Torocsik B, Szeberenyi J, Bakacs T . Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H). Anticancer Res 1999; 19: 635–638.
Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol 2002; 20: 2251–2266.
Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther 2006; 13: 221–228.
Morgan DA, Ruscetti FW, Gallo R . Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976; 193: 1007–1008.
Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA . Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res 1981; 41: 4420–4425.
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985; 313: 1485–1492.
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17: 2105–2116.
Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res 2007; 67: 8285–8292.
Poste G, Doll J, Hart IR, Fidler IJ . In vitro selection of murine B16 melanoma variants with enhanced tissue-invasive properties. Cancer Res 1980; 40: 1636–1644.
Lee YS, Kim JH, Choi KJ, Choi IK, Kim H, Cho S et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin Cancer Res 2006; 12: 5859–5868.
Entin I, Plotnikov A, Korenstein R, Keisari Y . Tumor growth retardation, cure, and induction of antitumor immunity in B16 melanoma-bearing mice by low electric field-enhanced chemotherapy. Clin Cancer Res 2003; 9: 3190–3197.
Rochlitz C, Dreno B, Jantscheff P, Cavalli F, Squiban P, Acres B et al. Immunotherapy of metastatic melanoma by intratumoral injections of Vero cells producing human IL-2: phase II randomized study comparing two dose levels. Cancer Gene Ther 2002; 9: 289–295.
Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C . Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res 2001; 61: 1095–1099.
Cranmer LD, Hersh E . The role of the CTLA4 blockade in the treatment of malignant melanoma. Cancer Invest 2007; 25: 613–631.
Khong HT, Restifo NP . Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat Immunol 2002; 3: 999–1005.
Tarhini AA, Agarwala SS . Novel agents in development for the treatment of melanoma. Expert Opin Investig Drugs 2005; 14: 885–892.
Prince AM, Ginsberg HS . Studies on the cytotoxic effect of Newcastle disease virus (NDV) on Ehrlich ascites tumor cells. I. Characteristics of the virus-cell interaction. J Immunol 1957; 79: 94–106.
Sinkovics JG, Horvath JC . Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol 2000; 16: 1–15.
Krishnamurthy S, Takimoto T, Scroggs RA, Portner A . Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol 2006; 80: 5145–5155.
Park MS, Garcia-Sastre A, Cros JF, Basler CF, Palese P . Newcastle disease virus V protein is a determinant of host range restriction. J Virol 2003; 77: 9522–9532.
Fabian Z, Csatary CM, Szeberenyi J, Csatary LK . p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J Virol 2007; 81: 2817–2830.
Karcher J, Dyckhoff G, Beckhove P, Reisser C, Brysch M, Ziouta Y et al. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res 2004; 64: 8057–8061.
Liang W, Wang H, Sun TM, Yao WQ, Chen LL, Jin Y et al. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J Gastroenterol 2003; 9: 495–498.
Csatary LK, Eckhardt S, Bukosza I, Czegledi F, Fenyvesi C, Gergely P et al. Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect Prev 1993; 17: 619–627.
Washburn B, Schirrmacher V . Human tumor cell infection by Newcastle disease virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol 2002; 21: 85–93.
Schirrmacher V, Ahlert T, Probstle T, Steiner HH, Herold-Mende C, Gerhards R et al. Immunization with virus-modified tumor cells. Semin Oncol 1998; 25: 677–696.
Schirrmacher V, Haas C, Bonifer R, Ahlert T, Gerhards R, Ertel C . Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle disease virus. Gene Therapy 1999; 6: 63–73.
Haas C, Ertel C, Gerhards R, Schirrmacher V . Introduction of adhesive and costimulatory immune functions into tumor cells by infection with Newcastle Disease Virus. Int J Oncol 1998; 13: 1105–1115.
Hrabak A, Csuka I, Bajor T, Csatary LK . The cytotoxic anti-tumor effect of MTH-68/H, a live attenuated Newcastle disease virus is mediated by the induction of nitric oxide synthesis in rat peritoneal macrophages in vitro. Cancer Lett 2006; 231: 279–289.
Aydin N, Jack A, Montenegro G, Boyes C, Alam K, Wallack MK . Expression of melanoma-associated antigens in human dendritic cells pulsed with an interleukin-2 gene encoded vaccinia melanoma oncolysate (rIL-2VMO). Cancer Biol Ther 2006; 5: 1654–1657.
Park MS, Steel J, Garcia-Sastre A, Swayne D, Palese P . Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc Natl Acad Sci USA 2006; 103: 8203–8208.
de Leeuw OS, Koch G, Hartog L, Ravenshorst N, Peeters BP . Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin-neuraminidase protein. J Gen Virol 2005; 86: 1759–1769.
Peeters BP, de Leeuw OS, Koch G, Gielkens AL . Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J Virol 1999; 73: 5001–5009.
Shin EJ, Chang JI, Choi B, Wanna G, Ebert O, Genden EM et al. Fusogenic vesicular stomatitis virus for the treatment of head and neck squamous carcinomas. Otolaryngol Head Neck Surg 2007; 136: 811–817.
Nakaya T, Cros J, Park MS, Nakaya Y, Zheng H, Sagrera A et al. Recombinant Newcastle disease virus as a vaccine vector. J Virol 2001; 75: 11868–11873.
Engel-Herbert I, Werner O, Teifke JP, Mebatsion T, Mettenleiter TC, Romer-Oberdorfer A . Characterization of a recombinant Newcastle disease virus expressing the green fluorescent protein. J Virol Methods 2003; 108: 19–28.
Schirrmacher V . In situ analysis of tumor-specific CTL effector and memory responses elicited by tumor vaccination. Int J Oncol 1999; 15: 217–227.
Vigil A, Martinez O, Chua MA, Garcia-Sastre A . Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther 2008; 16: 1883–1890.
Hotte SJ, Lorence RM, Hirte HW, Polawski SR, Bamat MK, O’Neil JD et al. An optimized clinical regimen for the oncolytic virus PV701. Clin Cancer Res 2007; 13: 977–985.
Acknowledgements
We thank Gang Sheng for technical support. Partial support of this work was from the Northeast Biodefense Grant U54 AI057158 (PP), NIH training Grant T32AI07647 (DZ), Mount Sinai Department of Medicine Training Grant (DZ), Bill and Melinda Gates Foundation Grant 38648 (PP and AGS) and Award from the Flight Attendant Medical Research Institute (YF). Mount Sinai School of Medicine owns patent positions for reverse genetics of Newcastle disease viruses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Supplementary information
Rights and permissions
About this article
Cite this article
Zamarin, D., Vigil, A., Kelly, K. et al. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther 16, 796–804 (2009). https://doi.org/10.1038/gt.2009.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.14
Keywords
This article is cited by
-
Rapid Generation of a Recombinant Genotype VIII Newcastle Disease Virus (NDV) Using Full-Length Synthetic cDNA
Current Microbiology (2021)
-
The Role of Oncolytic Viruses in the Treatment of Melanoma
Current Oncology Reports (2018)
-
Recombinant Newcastle disease virus expressing P53 demonstrates promising antitumor efficiency in hepatoma model
Journal of Biomedical Science (2016)
-
Molecular characterization of an apoptotic strain of Newcastle disease virus isolated from an outbreak in India
Cancer Gene Therapy (2015)
-
Oncolysis by paramyxoviruses: multiple mechanisms contribute to therapeutic efficiency
Molecular Therapy - Oncolytics (2015)