Abstract
Background/Objectives:
Poor maternal diet in pregnancy can influence fetal growth and development. We tested the hypothesis that poor maternal diet quality during pregnancy would increase neonatal adiposity (percent fat mass (%FM)) at birth by increasing the fat mass (FM) component of neonatal body composition.
Methods:
Our analysis was conducted using a prebirth observational cohort of 1079 mother–offspring pairs. Pregnancy diet was assessed via repeated Automated Self-Administered 24-h dietary recalls, from which Healthy Eating Index-2010 (HEI-2010) scores were calculated for each mother. HEI-2010 was dichotomized into scores of ⩽57 and >57, with low scores representing poorer diet quality. Neonatal %FM was assessed within 72 h after birth with air displacement plethysmography. Using univariate and multivariate linear models, we analyzed the relationship between maternal diet quality and neonatal %FM, FM, and fat-free mass (FFM) while adjusting for prepregnancy body mass index (BMI), physical activity, maternal age, smoking, energy intake, preeclampsia, hypertension, infant sex and gestational age.
Results:
Total HEI-2010 score ranged between 18.2 and 89.5 (mean: 54.2, s.d.: 13.6). An HEI-2010 score of ⩽57 was significantly associated with higher neonatal %FM (β=0.58, 95% confidence interval (CI) 0.07–1.1, P<0.05) and FM (β=20.74; 95% CI 1.49–40.0; P<0.05) but no difference in FFM.
Conclusions:
Poor diet quality during pregnancy increases neonatal adiposity independent of maternal prepregnancy BMI and total caloric intake. This further implicates maternal diet as a potentially important exposure for fetal adiposity.
Similar content being viewed by others
Introduction
In the United States over 60% of women of reproductive age are overweight or obese.1 A significant focus of the research on developmental origins of health and disease has been on the impact of maternal overweight and obesity during pregnancy on infant outcomes. Large prospective cohort studies have consistently shown maternal overweight and obesity during pregnancy to be significant risk factors for higher birth weight and neonatal adiposity2, 3, 4 and for childhood obesity and later life metabolic dysregulation.5, 6 However, although effective interventions have been developed to promote healthy weight loss in the general adult population,7, 8, 9 it is challenging to implement interventions that can quickly and successfully help women to lose weight before pregnancy, due in large part to a significant number of pregnancies being unplanned. Therefore, a shift in research focus from reducing maternal weight before and during pregnancy to other interventions that could also impact fetal overgrowth and offspring adiposity, such as improving maternal diet and nutrition, is warranted.
Diet quality as measured by levels of macro- and micronutrients consumed, as well as by dietary patterns (for example, Western, Mediterranean) during pregnancy has demonstrated significant relationships with birth outcomes, implicating maternal nutritional exposures during pregnancy as important factors in fetal growth and development.10, 11, 12, 13, 14 Specifically, high-fat content in the diet during pregnancy has been shown to increase offspring birth weight and adiposity in several animal studies.15, 16 Evidence from nutrient-specific and dietary pattern analyses in human pregnancies remains inconsistent. Some studies report increased offspring adiposity given a maternal diet with a low protein-to-carbohydrate ratio.17 Other studies report growth restriction given a maternal ‘Western’ dietary pattern,12 or no relationship between maternal dietary patterns and offspring growth.18, 19 This lack of consistency may be due in part to the difficulty in replicating data-driven dietary patterns, such as factor and cluster analysis, across different populations20 and the use of different infant size and growth outcomes.
Measures of diet quality that are based a priori on national or international recommendations are a potential alternative for measuring the impact of nutrition during pregnancy on neonatal body composition. These standardized diet quality indices are generalizable across different cohorts.21 One such index, the Healthy Eating Index (HEI), measures the inadequacy, adequacy or excess of recommended intakes of food groups (for example, whole grains) and nutrients (for example, sodium), therefore quantifying the quality of the total diet. In the few studies that have used a diet quality index in developed countries, higher diet quality scores were positively associated with growth parameters such as birth weight and birth length.22, 23 However, no studies have used markers of offspring body composition such as adiposity.
The present analysis aimed to fill this information gap using the Healthy Start cohort, a prebirth, multiethnic cohort of 1410 mother–offspring pairs. Our goal was to test the hypothesis that neonates born to women with low diet quality during pregnancy have increased adiposity compared with those born to women with higher diet quality. We also tested whether diet quality modifies the effect of prepregnancy body mass index (BMI) on neonatal adiposity.
Participants and methods
Study population
Mother–infant pairs included in this analysis were enrolled in the Healthy Start study, an observational, longitudinal prebirth cohort study of ethnically diverse mothers. The Healthy Start study recruited 1410 pregnant women aged ⩾16 years before 24 weeks of gestation from the obstetrics clinics at the University of Colorado Hospital during 2010–2014. Women were excluded if they had prior diabetes, a history of prior premature birth or fetal death, asthma with active steroid management, serious psychiatric illness or a current multiple pregnancy. The Healthy Start study protocol was approved by the Colorado Multiple Institutional Review Board, and all women provided written informed consent before the first study visit. The Healthy Start study was registered as an observational study at clinicaltrials.gov as NCT02273297.
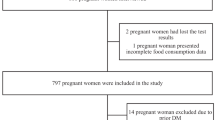
As of July 2014, 1410 women were enrolled in the Healthy Start cohort. Healthy Start participants were eligible for the current analysis if they had at least one dietary recall (N=1366). Women who had been diagnosed with gestational diabetes mellitus (n=53) were excluded because these women are encouraged to adopt special diets after diagnosis. Neonates born at <32 weeks of gestation or those without body composition measures at birth were also excluded from the eligible cohort to give a final sample size of 1079 for the analytic cohort used in this report.
Comparison of the analytic cohort with those who were excluded revealed no significant differences in maternal race/ethnicity (P=0.35), maternal age at delivery (P=0.67), prepregnancy BMI (P=0.25) and household income (P=0.31). As expected, the analytical cohort had significantly higher birth weight compared with the excluded participants (3255 vs 3007 g, P<0.001) given the exclusion of infants born very preterm (gestational age <32 weeks).
Data collection
Healthy Start mothers were invited to participate in two research visits during pregnancy. The first visit occurred between 8 and 24 weeks of gestation (median=17 weeks) and the second between 24 and 32 weeks of gestation (median=27 weeks). Maternal fasting blood samples were collected at each of the two pregnancy visits and demographic, behavioral, physical activity (energy expenditure) and dietary questionnaires were administered. A third visit occurred in the hospital, after delivery during which women were asked to complete questionnaires identical to those from the second pregnancy visit. Offspring’s birth length, weight, head circumference and skin-fold thickness were measured within 72 h after delivery, and neonatal body composition, fat mass (FM) and fat-free mass (FFM) were estimated from total mass and volume using air displacement plethysmography (PEA POD, Cosmed, Rome, Italy). Body composition was measured twice for each neonate with a third measurement taken if the first two percent body fat values were greater than two percentage points apart. Values used in this report are the average of the two closest measures.
Maternal prepregnant BMI was calculated using maternal height measured at the first research visit and prepregnant weight obtained from medical records (83.7%) or self-reported at the first research visit (16.2%). Prepregnant BMI was categorized as normal weight (BMI <25 kg m−2), overweight (25⩽BMI<30 kg m−2) and obese (BMI ⩾30 kg m−2). Physical activity in pregnancy was measured using the Pregnancy Physical Activity Questionnaire24 from which metabolic equivalent task (MET) values were estimated as described in detail elsewhere.24, 25
Infant sex, birth weight and gestational age at birth were abstracted from medical records. Information on race/ethnicity, household income, smoking during pregnancy and gravidity (0 vs prior pregnancies) was obtained from questionnaires administered to participants. Race/ethnicity was categorized into non-Hispanic white, non-Hispanic black, Hispanic and other. Household income was categorized into five levels: <$20 000, $20 000–$40 000, $40 000–$70 000, income >$70 000 and ‘don’t know’. Maternal age at delivery was calculated from offspring delivery date and maternal date of birth.
Dietary assessment
Maternal diet was assessed several times throughout pregnancy using the Automated Self-Administered 24-h Dietary Recall (ASA24), an online platform developed and hosted by the National Cancer Institute (ASA24-Beta and ASA24-2011, Bethesda, MD, USA). Healthy Start participants were asked to complete up to six ASA24 dietary recalls beginning at their first pregnancy visit (approximately one per month). On average, participants completed 2 recalls over the pregnancy period (range: 1–8), with 76% (n=1038) of the eligible cohort (n=1366) having at least 2 diet recalls. Monthly calls were made by the University of North Carolina at Chapel Hill to remind participants to complete their dietary recalls at home. Trained, bilingual study staff members administered recalls in-person for Spanish-speaking participants (n=60) at study visits and over the phone between visits. Data from the ASA24 were collected (except for the Spanish interviews) and processed by the University of North Carolina.
The Healthy Eating Index
The Healthy Eating Index-2010 (HEI-2010) is a diet quality scoring system developed by the US Department of Agriculture, Center for Nutrition Policy and Promotion and the National Cancer Institute (NCI) designed to assess adherence to the 2010 Dietary Guidelines for Americans. This tool is a valid and reliable measure of diet quality26 and consists of 12 components (total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium and empty calories).27 The 12 components are scored per 1000 kcal to give a maximum possible total HEI-2010 score of 100.
We calculated average food group servings across the multiple ASA24 recalls for each participant for use in calculating the HEI-2010 component and total scores. Average intakes of total energy, saturated fat, mono- and polyunsaturated fats and sodium across the repeated recalls were also used for calculation of the HEI-2010 scores. Alcohol was not included in the total HEI-2010 score because participants were all under 13 g per 1000 kcal of alcohol consumption for each recall, the threshold for inclusion of calories from alcohol. Publically available NCI SAS macro code from the NCI website (http://appliedresearch.cancer.gov/hei/tools.html) was used to generate the HEI-2010 total and component scores from the average food groups and nutrient values for each participant.
Statistical analysis
Different studies have used varying ways to categorize the HEI-2010 total score. However, many analyses use quintile categorization.28, 29, 30 We plotted the observed mean neonatal %FM against the quintiles of the HEI-2010 total score (Q1: HEI-2010 ⩽42; Q2: 42
Maternal and neonatal descriptive statistics were generated, and differences between the two HEI-2010 total score categories were tested using Satterthwaite t-tests for continuous variables and Cochran Mantel–Haenszel tests for categorical variables. Average predicted estimates of usual intake of macro- and micronutrients and average HEI-2010 component scores were compared between the HEI-2010 categories using t-tests to demonstrate the differences in nutrient and food group intakes related to the two levels of diet quality.
We fit a general linear multivariable model, and a planned, backward stepwise approach to examine the effects of HEI-2010 total score category (⩽57 versus >57), maternal prepregnancy BMI category and their interaction on neonatal %FM. Covariates for inclusion in all models were chosen based on the literature and included maternal age, race/ethnicity, infant sex, gestational age at birth, household income, usual daily energy intake (kcal per day), smoking in pregnancy and average energy expenditure (METs per week) over the pregnancy period. We also adjusted for pregnancy complications that could impact neonatal body composition such as chronic hypertension (yes/no), gestational hypertension (yes/no) and preeclampsia (yes/no) as diagnosed by a physician and reported in the medical record. As a sensitivity analysis we included gestational weight gain as a covariate to test whether the relationship between diet quality and infant adiposity was independent of this pregnancy exposure.
Given that no significant interaction between HEI-2010 category and maternal prepregnant BMI was noted, we then tested the main effect of the two HEI-2010 categories, and the main effect of BMI categories, while controlling for covariates. This same modeling approach was applied for the general multivariate linear model that tested the effect of HEI-2010 and BMI categories on FM and FFM. An alpha-spending approach was used to control the overall Type I error. We used the Hotelling–Lawley test to assess the significance of the association between BMI and HEI-2010 with both FM and FFM at P<0.03, and, if the overall test was significant, we planned to use P<0.01 for each step-down test of association between BMI and HEI-2010, and FM and FFM, respectively.
Results
The HEI-2010 total score in the Healthy Start cohort ranged between 18 and 89 with a mean of 54.2 (s.d.=13.6). Maternal and neonatal characteristics are presented by HEI-2010 category in Table 1. Women with an HEI-2010 total score of ⩽57 (lower diet quality) were significantly more likely to be obese, to have reported smoking during pregnancy and to have a household income of <$20 000 (P<0.001 for all, respectively). Lower diet quality was also significantly related to younger maternal age (P<0.001), shorter length of gestation (P=0.02), higher gravidity (P=0.01) and higher energy expenditure (P<0.001). Furthermore, neonates born to women with an HEI-2010 total score of ⩽57 had significantly lower birth weight (3226 vs 3297 g, P=0.01) and FFM (2804 g vs 2872 g, P<0.01). There was no difference in %FM (9.2% vs 8.8%, P=0.11), birth head circumference (P=0.09) or birth length (P=0.47) between the two HEI-2010 groups.
The pattern of macro- and micronutrient average daily intakes (Table 2) as well as HEI-2010 component scores (Table 3) were as expected between the HEI-2010 categories. Although small differences were seen between groups, the percent of total energy as fat (P<0.001) and as saturated fat (P<0.001) were significantly higher in the group with an HEI total score of ⩽57. As expected, average HEI-2010 component scores for all of the components were significantly lower in the group with an HEI-2010 total score of ⩽57 (P<0.001 for all, respectively). However, empty calories were significantly lower in this group (P<0.001) that was not expected.
Table 4 shows the results of the regression analyses assessing the relationship between HEI-2010 total score categories and neonatal body composition, adjusting for covariates. Having an HEI-2010 score of ⩽57 was significantly associated with higher %FM (P<0.05), independent of maternal BMI. Among women with an HEI-2010 total score of ⩽57 during pregnancy, the %FM of their neonate was on average 0.58 percentage points higher compared with neonates born to women with an HEI-2010 total score of >57 (β=0.58, 95% confidence interval (CI) 0.07–1.1). Furthermore, the HEI-2010 score of ⩽57 was associated with significantly higher FM (g) (β=20.74; 95% CI 1.49–40.0; P<0.05) but not with significantly different FFM (β=7.30; 95% CI −29.71 to 44.31; P=0.97), indicating that the increased %FM associated with lower maternal diet quality reflects an increase in neonatal FM rather than a decrease in FFM. This compartmentalization of the effect of diet quality on infant body composition is further supported by the nonsignificant effect of HEI-2010 total score category on overall birth weight (β=27.86; 95% CI–21.16, 76.89; P=0.35). Maternal prepregnancy BMI was a significant predictor of higher %FM, FM, FFM and birth weight independent of maternal diet quality (P<0.001, P<0.001, P<0.05 and P<0.001, respectively). Other independent predictors of increased FM and/or %FM were older maternal age and lower household income, whereas gestational smoking was independently associated with lower %FM and FM. Interestingly, girls had higher FM (and %FM) but lower FFM as compared with boys, whereas infants of non-Hispanic Black women had both lower FM and FFM as compared with infants born to non-Hispanic white women. In addition, our sensitivity analysis showed no significant change in the results when including gestational weight gain in the model.
Discussion
In this prospective, multiethnic, prebirth cohort we have shown that diet quality has a significant impact on neonatal adiposity, and that this effect is independent of the mother’s prepregnancy BMI. Furthermore, the association between poorer maternal diet quality and higher neonatal adiposity is primarily because of an effect of lower diet quality on the fat compartment of neonatal body composition.
Although maternal nutrition during pregnancy has been previously studied in relation to birth outcomes, to the best of our knowledge this is the first study to investigate the effect of maternal diet quality during pregnancy on neonatal body composition. Furthermore, few studies of maternal diet during pregnancy have used the HEI. In addition, these studies have not targeted neonatal adiposity as the outcome specifically; rather they have used birth weight. For example, Rodríguez-Bernal and colleagues23 found that increasing quintiles of the Alternative Healthy Eating Index for Pregnancy (AHEI-P) score31 was associated with higher birth weight in a cohort of Spanish women. However, in a similar study of pregnant Spanish women, Gesteiro et al.32 did not see a significant difference in birth weight of infants born to mothers with an HEI-1995 total score of ⩽70 compared with those with an HEI-1995 total score of >70. Gesteiro et al.32 implemented the 1995 version of the Healthy Eating Index, and therefore the inconsistency between findings may be because of differences between these versions. Poon et al.19 also did not see a significant association between maternal diet quality and infant birth weight or other markers of growth using the AHEI-P.
The discrepancies may also be because of the timing of dietary assessment or a combination of differences in the methods of dietary assessment (use of a single Food Frequency Questionnaire versus repeated ASA24 dietary recalls) and model adjustment for potential confounders. In the Spanish cohort used by Rodríguez-Bernal and colleagues23(2010) and Gesteiro et al.32 (2012), diet was assessed only once and in the first trimester and Poon et al.19 also measured diet once but in the third trimester, whereas the Healthy Start study assessed diet repeatedly (82% of sample used in this analysis had ⩾2 diet recalls in pregnancy) to estimate the HEI-2010 total score over the observed pregnancy period. The degree to which we adjusted for additional potential confounders above that of these other studies may also explain differences in the findings. Given the lack of studies that parallel our dietary assessment methods and analysis, our findings warrant further investigation and replication in other large, diverse birth cohorts.
The HEI-2010 categorization used in this analysis to denote a ‘poorer quality diet’ reflects what we would expect in terms of macronutrients for such a diet given the Institute of Medicine’s report on Acceptable Macronutrient Distribution Ranges.33 Women with an HEI-2010 score of ⩽57 had higher total and saturated fat intake (Table 2). Although total fat and saturated fat have been shown to effect offspring size and adiposity in animal studies,15, 16 their effect in studies of human development have not been demonstrated,17 suggesting a potentially different mechanism in humans. Together, our findings that poorer diet quality is positively associated with infant adiposity and that higher intakes of total fat and saturated fat are characteristics of this poorer diet quality suggest that the deleterious effect of these specific nutrients on human neonatal size and body composition may be the result of multiple nutrients interacting. This highlights the importance of using a measure of diet quality that reflects the whole diet, likely accounting for the synergistic effects of foods and nutrients on neonatal body composition that may not be explained by a single nutrition factor.
Maternal BMI is also an established risk factor for accelerated fetal growth and increased birth weight and size.2, 3, 34, 35 Furthermore, our group recently reported that the %FM at birth of neonates born to women with a prepregnancy BMI in the overweight or obese categories was significantly higher than the %FM of neonates born to women in the normal BMI category, a finding that sustained when further adjusted by total energy and diet quality in this analysis, further supporting the link between maternal BMI and infant adiposity.4 The consistent and robust data supporting the independent effects of maternal diet during pregnancy (broadly measured) and maternal BMI on fetal growth and size provide clues to potential pathways and mechanisms that need to be further explored.
We purposefully did not include gestational weight gain as a covariate in our model, hypothesizing that it may be part of the causal pathway linking maternal diet quality to neonatal adiposity. Interestingly, sensitivity analyses adjusting for maternal weight gain during pregnancy resulted in similar findings, suggesting that the effect of maternal diet quality on neonatal adiposity is independent of gestational weight gain.
In our large prebirth cohort we have demonstrated that lower maternal diet quality has a statistically significant impact on neonatal adiposity and that this effect is independent of prepregnancy BMI. Although the clinical relevance of this finding is unclear at this time, it is important to note that neonates of women with lower diet quality had, on average, 24.9 g more fat mass as compared with those whose mothers had higher diet quality. This is just half that of the effect of maternal obesity (47.5 g higher in neonates of obese vs normal weight mothers), and given the clinical emphasis on obesity in pregnancy, this suggests that maternal diet quality may be similarly important, clinically. Longitudinal follow up of this cohort, now ongoing, will provide much needed data on the clinical significance of increased adiposity at birth with respect to childhood obesity and other relevant outcomes.
Our analysis is not without limitations. Our use of the HEI-2010 precludes us from comparing our results with studies using other HEI versions (for example, HEI-1995) because of the different national dietary standards used in the calculation of these HEI scores. However, the HEI-2010 was designed to reflect the Dietary Guidelines for Americans and therefore we are confident that our results are generalizable to the overall population in the United States and to the pregnant population in this country. Future studies of diet in pregnancy and neonatal outcomes should employ the HEI so to allow comparison across pregnant populations and for replication of the findings from this analysis.
In conclusion, our study suggests that poor overall diet quality, as assessed by the HEI-2010, during pregnancy may lead to increased neonatal adiposity regardless of maternal BMI. This highlights the potential importance of dietary interventions during pregnancy, likely a more accessible time for clinicians and public health practitioners to communicate the importance of healthy eating to pregnant women.
References
Flegal KM, Carroll MD, Ogden CL, Curtin LR . Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010; 303: 235–241.
Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA . Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol 2008; 198: e1–e6.
Sewell MF, Huston-Presley L, Super DM, Catalano P . Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006; 195: 1100–1103.
Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr 2015; 101: 302–309.
Boney CM, Verma A, Tucker R, Vohr BR . Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005; 115: e290–296.
Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 2012; 125: 1381–1389.
Eriksson B, Löf M, Olausson H, Forsum E . Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br J Nutr 2010; 103: 50–57.
Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med 2013; 173: 113–121.
Weinstock RS, Trief PM, Cibula D, Morin PC, Delahanty LM . Weight loss success in metabolic syndrome by telephone interventions: results from the SHINE Study. J Gen Intern Med 2013; 28: 1620–1628.
Grieger JA, Grzeskowiak LE, Clifton VL . Preconception dietary patterns in human pregnancies are associated with preterm delivery. J Nutr 2014; 144: 1075–1080.
Hernandez TL, Van Pelt RE, Anderson MA, Reece MS, Reynolds RM, de la Houssaye BA et al. Women with gestational diabetes randomized to a higher complex carbohydrate/low fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study. Diabetes Care 2015; 39: 39–42.
Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, Olsen SF . Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr 2008; 62: 463–470.
Obermann-Borst SA, Vujkovic M, de Vries JH, Wildhagen MF, Looman CW, de Jonge R et al. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. BJOG 2011; 118: 1205–1215.
Food and Nutrition Board of the Institute of Medicine, The Board on Children Youth and Families of the Institute of Medicine, National Research Council Examining a Developmental Approach to Childhood Obesity: The Fetal and Early Childhood Years: Workshop in Brief. Examining a Developmental Approach to Childhood Obesity: The Fetal and Early Childhood Years. The National Academies: Washington, DC, 2015.
Ashino NG, Saito KN, Souza FD, Nakutz FS, Roman EA, Velloso LA et al. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem 2012; 23: 341–348.
Franco JG, Fernandes TP, Rocha CPD, Calviño C, Pazos-Moura CC, Lisboa PC et al. Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol 2012; 590: 5503–5518.
Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, Giles WB et al. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr 2012; 96: 1032–1041.
Bouwland-Both MI, Steegers-Theunissen RPM, Vujkovic M, Lesaffre EMEH, Mook-Kanamori DO, Hofman A et al. A periconceptional energy-rich dietary pattern is associated with early fetal growth: the Generation R study. BJOG 2013; 120: 435–445.
Poon AK, Yeung E, Boghossian N, Albert PS, Zhang C . Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Scientifica 2013; 2013: 786409.
Ocké MC . Evaluation of methodologies for assessing the overall diet: dietary quality scores and dietary pattern analysis. Proc Nutr Soc 2013; 72: 191–199.
Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML et al. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr 2015; 145: 393–402.
Ferland S, O'Brien HT . Maternal dietary intake and pregnancy outcome. J Reprod Med 2003; 48: 86–94.
Rodríguez-Bernal CL, Rebagliato M, Iñiguez C, Vioque J, Navarrete-Muñoz EM, Murcia M et al. Diet quality in early pregnancy and its effects on fetal growth outcomes: the Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr 2010; 91: 1659–1666.
Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS . Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc 2004; 36: 1750–1760.
Harrod CS, Chasan-Taber L, Reynolds RM, Fingerlin TE, Glueck DH, Brinton JT et al. “Physical activity in pregnancy and neonatal body composition: the Healthy Start study. Obstet Gynecol 2014; 124 (2 Pt 1): 257–264.
Guenther PM, Kirkpatrick Si, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 2014; 144: 399–407.
Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HAB, Kuczynski KJ et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013; 113: 569–580.
George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014; 180: 616–625.
Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR et al. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr 2015; 101: 587–597.
Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML et al. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. (1541-6100 (Electronic)).
Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW . Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 2009; 109: 1004–1011.
Gesteiro E, Rodríguez Bernal B, Bastida S, Sánchez-Muniz FJ . Maternal diets with low healthy eating index or Mediterranean diet adherence scores are associated with high cord-blood insulin levels and insulin resistance markers at birth. Eur J Clin Nutr 2012; 66: 1008–1015.
Institute of Medicine Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC, 2005.
Catalano PM, Thomas A, Huston-Presley L, Amini SB . Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 2003; 189: 1698–1704.
Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR . The effect of maternal body mass index on perinatal outcomes in women with diabetes. Am J Perinatol 2014; 31: 249–256.
Acknowledgements
We thank all women and children who have taken part in the Healthy Start study. We also thank Mrs Mercedes Martinez, the Healthy Start Study Project Coordinator, Colorado School of Public Health, University of Colorado Denver and the Healthy Start team for their hard work and dedication. The Healthy Start study is supported by the R01 Grant from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) (R01 #DK076648; Principal Investigator: Dana Dabelea).
Author contributions
ALBS conducted the research, analyzed data and wrote the full draft of the manuscript as well as made revisions on subsequent drafts. BMR and DHG analyzed data and consulted on interpretation of statistical findings. JLK, TLC, APS, AMS-R and JMN made substantial contributions for the revision of manuscript drafts. LAB and JEF reviewed all manuscript drafts and made substantial contributions to the clinical and mechanistic interpretation of the study findings. DD designed the Healthy Start study, reviewed each manuscript draft and has primary responsibility for the final content of this manuscript. The Healthy Start research team collected all data, including diet data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shapiro, A., Kaar, J., Crume, T. et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes 40, 1056–1062 (2016). https://doi.org/10.1038/ijo.2016.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.79
This article is cited by
-
Maternal plasma vitamin D levels across pregnancy are not associated with neonatal birthweight: findings from an Australian cohort study of low-risk pregnant women
BMC Pregnancy and Childbirth (2023)
-
Neighborhood Walkability, Historical Redlining, and Childhood Obesity in Denver, Colorado
Journal of Urban Health (2023)
-
Prenatal exposure to tobacco and adverse birth outcomes: effect modification by folate intake during pregnancy
Maternal Health, Neonatology and Perinatology (2022)
-
Using non-parametric Bayes shrinkage to assess relationships between multiple environmental and social stressors and neonatal size and body composition in the Healthy Start cohort
Environmental Health (2022)
-
Association between 1st trimester diet quality & gestational weight gain rate among pregnant women in Dhulikhel, Nepal
BMC Nutrition (2022)