Abstract
The emergence of drug resistance in bacterial pathogens is a growing clinical problem that poses difficult challenges in patient management. To exacerbate this problem, there is currently a serious lack of antibacterial agents that are designed to target extremely drug-resistant bacterial strains. Here we describe the design, synthesis and antibacterial testing of a series of 40 novel indole core derivatives, which are predicated by molecular modeling to be potential glycosyltransferase inhibitors. Twenty of these derivatives were found to show in vitro inhibition of Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus. Four of these strains showed additional activity against Gram-negative bacteria, including extended-spectrum beta-lactamase producing Enterobacteriaceae, imipenem-resistant Klebsiella pneumoniae and multidrug-resistant Acinetobacter baumanii, and against Mycobacterium tuberculosis H37Ra. These four compounds are candidates for developing into broad-spectrum anti-infective agents.
Similar content being viewed by others
Introduction
Antibiotic resistance in bacterial pathogens has become a global public health threat associated with grave human and economic loss. This problem, previously characteristic of nosocomial infections, is now increasingly seen in community-acquired infections as well. Notable examples include the Gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci and drug-resistant Streptococcus pneumoniae, as well as multiply resistant Gram-negative bacilli, such as Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, Enterobacter cloacae and Escherichia coli. These bacteria are common nosocomiants, but the emergence of multidrug resistance via multiple resistance mechanisms has made them deadly pathogens in a wide range of infections.1, 2, 3, 4, 5, 6, 7 With the escalation of multidrug resistance, the need for new antibiotics is urgent. Unfortunately, very few antibiotics currently available can combat multidrug resistance and also very few are in the pipeline for future development.
Peptidoglycan is an essential component to keep the bacterial cell wall’s strength and shape. The peptidoglycan glycosyltransferase (GT) and transpeptidase (TP) enzymes have a major role together in the synthesis of peptidoglycan. These enzymes are suitable targets for antibacterial drugs because they are not present in mammalian cells, and hence, drugs inhibiting them will not affect normal mammalian cells.8, 9
TP enzymes, also called penicillin-binding proteins, are well-known targets of β-lactam antibiotics. GT inhibitors, on the other hand, have been less studied for their antibacterial activity. The best known peptidoglycan GT inhibitors are moenomycins, which are a family of phosphoglycolipids produced by the Streptomyces genus. These inhibitors have been used as growth promoters in animal feed but have not been developed as antibiotics for human infections.10 Their activity against multidrug-resistant bacteria has not been reported.
Indoles are natural organic compounds with an aromatic heterocyclic structure that are widely used in the synthesis of pharmaceutical agents. Many synthesized derivatives have potent activity against fungi, viruses and Leishmania parasites as well as Gram-positive and Gram-negative bacteria and mycobacterial species.11, 12, 13, 14 This wide-spectrum activity makes them suitable candidates for the development of novel anti-infectives so urgently needed to overcome the current global problem of multidrug resistance.
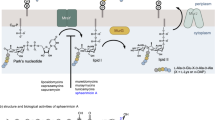
Here we describe the discovery of new indole core derivatives showing activity against bacterial pathogens commonly isolated from clinical specimens. We synthesized indole compounds with substituted side-chains to act as potential GT inhibitors. In the bacterial cell wall, TP and GT work as bifunctional proteins, in which the TP domain is located in the C-terminal and the GT domain in the N-terminal, with a small linker region separating the two domains. The crystal structure of the GT-binding site of S. aureus complexed with moenomycin (PDB 2OLV) has been reported.15 The moenomycin-binding site is a relatively big site. As Figure 1a shows, from studies on the crystal structure and drug-like properties of moenomycin, it appears unnecessary to design inhibitors to occupy the whole binding site. Hence, we chose the segment of the binding site composed by Asp156, Glu114, Pro231, Tyr196 and Phe158 (area in the red circle) as the target for our new indole derivatives. Based on the structure of the GT binding site (Figure 1b), it was noticed that Asp156, Glu114 and Pro231 form a hydrophilic pocket, which should be occupied by the positive-charged group to form strong static electricity interaction with the negative-charged residues (Asp156 and Glu114). Considering the Tyr196 in the binding site, it was believed that an aromatic ring around it should form strong π–π interactions, and another aromatic ring should be added to form same interactions with Phe158. Recently, much attention has been paid to indole core derivatives due to their diverse biological activities, for instance, the inhibition of bacteria,12 viruses,13 leishmania parasites14 and cancerous cells.16 More recently, it has been discovered that aminoguanidine or 2-hydrazino-2-imidazoline derivatives display antitumor activities17 and aminoguanidine derivatives show antibacterial activities.18, 19 Using this information, we designed a template compound (Figure 1c), which contains the indole core to form π–π interactions with Tyr196 (area 2), the aminoguanidine to form static electricity interactions with Asp156 and Glu114 and the N-1 substituted benzyl to form a potential π–π interaction with Phe158 (area 3). Based on the template compound, we noticed by the molecular docking results that aminoguanidine may form additional hydrogen bonds with Pro231 and Tyr191, and by adding methoxycarbonyl group on the indole core, hydrogen bonds may be formed with Asn235 (area 4). By further considering Pro231, which contains five-membered aromatic ring, it was thought an aromatic ring close by should increase the binding affinity. Therefore, in our second series, we used 2-(4,5-dihydro-1H-imidazol-2-yl)hydrazone to form an extra π–π interaction with Pro231. Subsequently, we prepared indole core derivatives by combining 3-acylindoles with either aminoguanidine or 2-hydrazino-2-imidazoline (Figure 1d).
The design of the glycosyltransferase inhibitors. (a) The binding site of moenomycin. The moenomycin is represented as a balls–sticks model, and the protein is representad as a ribbon; (b) the pharmacophore designed to fit the binding site; (c) the detailed interactions between the template compound and the GT-binding site; (d) combining bioactive molecules to generate target compounds. A full color version of this figure is available at The Journal of Antibiotics journal online.
Results and discussion
Chemistry
We synthesized a series of aminoguanidine derivatives of 3-acylindoles 4a-l, 7a-b, 13a-f and 2-(4,5-dihydro-1H-imidazol-2-yl)hydrazone derivatives of 3-acylindoles 5a-l, 8a-b and 14a-f as illustrated in Schemes 1,2,3. In Scheme 1, the substituted indoles 2a-b were generated in the presence of phosphorus oxychloride and dry dimethylformamide (DMF) by Vilsmeier–Haack reaction.20 Treatment of 2a-b with substituted benzyl bromide in the presence of sodium hydride in dry dimethyl sulfoxide (DMSO) generated 3a-l in moderate-to-good yields (51–96%). Finally, target compounds 4a-l were obtained by the reaction of 3a-l with aminoguanidine hydrochloride under pH 3–4 in dry methanol in 76–97% yields, which were purified by tutrition with ethyl ether. Target compounds 5a-l were generated by the reaction of 3a-l with 2-hydrazino-2-imidazoline hydrobromide in dry methanol under reflux, followed by purification by tutrition with ethyl ether in 82–96% yields.
In Scheme 2, treatment of compounds 2a-b with benzoyl chloride in the presence of sodium hydride in dry DMF generated 6a-b in 66–71% yields. Then target compounds 7a-b and 8a-b were obtained by the reaction of 6a-b separately with aminoguanidine hydrochloride under pH 3–4 and 2-hydrazino-2-imidazoline hydrobromide under reflux, followed by the purification by tutrition with ethyl ether in 70–92% yields.
In Scheme 3, compound 10 was generated from 9 in the presence of phosphorus oxychloride and dry DMF by Vilsmeier–Haack reaction.20 Treatment of 10 with benzenesulfonyl chloride in the presence of sodium hydride in dry DMF generated 11, which was reacted with substituted phenylboronic acid in the presence of PdCl2(dtbpf) and potassium carbonate in tetrahydrofuran/H2O by Suzuki reaction21 to give 12a-f in moderate-to-good yields (53–89%). Finally, the target compounds 13a-f and 14a-f were obtained by the reaction of 12a-f separately with aminoguanidine hydrochloride under pH 3–4 and 2-hydrazino-2-imidazoline hydrobromide under reflux, followed by the purification by tutrition with ethyl ether in 53–98% yields. All of the novel target compounds were characterized by 1H-NMR, MS, HRMS and m.p.
Determination of in vitro antibacterial activity
In the preliminary screen where all 40 compounds were tested for antibacterial activity against S. aureus, 20 compounds showed in vitro growth inhibition with minimum inhibitory concentrations (MICs) ranging from 3.13 to 100 μg ml−1 and a median MIC of 12.5 μg ml−1 (data not shown).
Subsequent MIC and minimum bactericidal concentration (MBC) studies on these 20 compounds showed the inhibition of both methicillin-susceptible and methicillin-resistant S. aureus with median MICs of 12.5 and 6.25 μg ml−1, respectively (Table 1). These six compounds (4a, 13a, 13c, 13d, 13e, 13f) showed antimethicillin-resistant Staphylococcus aureus MICs that were almost the same as that of vancomycin, the standard antibiotic for the treatment of methicillin-resistant Staphylococcus aureus infections. Eleven (58%) of the MICs and MBCs were within one doubling dilution difference, suggesting bactericidal activity from the majority of the compounds tested.
Four compounds, 4a, 4g, 5a and 5h, showed an MIC and MBC <100 μg ml−1 against a strain of multidrug-resistant A. baumanii, and two of them, 4a and 4g, also inhibited a strain of multidrug-resistant K. pneumoniae that carried the NDM-1 beta-lactamase gene.22 With these encouraging preliminary results, we expanded our MIC determination to a larger set of both Gram-positive and -negative bacteria along with Mycobacterium tuberculosis H37Ra. The results (Table 2) showed all four compounds inhibiting all Gram-positive bacteria tested, including reference and clinical strains of methicillin-susceptible and -resistant S. aureus, Streptococcus pyogenes and S. pneumoniae from respiratory tract infections, a strain of vancomycin-resistant enterococcus from a urinary tract infection, a coagulase-negative Staphylococcus, a Corynebacterium sp. and M. tuberculosis H37Ra. Against Gram-negative bacilli, all four compounds inhibited A. baumanii strains, which were resistant to multiple antibiotics, including carbapenem and imipenem, and were susceptible to only colistin, the ‘last-resort’ antibiotic for many multidrug-resistant bacteria. 4a and 4g inhibited E. coli and Klebsiella pneumoniae strains that were producers of extended-spectrum beta-lactamases. None of the compounds, however, showed activity against P. aeruginosa at concentrations <100 μg ml−1.
Molecular docking
The molecular docking research showed that all the molecules were docked into a relative small area, although the binding site indicated by Moenomycin A is much larger than the synthesized molecules (Figure 2). By comparing with the literature, it was observed that the binding site of the current study is similar to that published before,19 which is a site composed by Glu 114, TYR191, Gln 152, Asp 156, Ile 195, Phe 158, Gln 161, Tyr 196, Asn 235, Gln 232 and Pro234.
The compound 4a (represented by balls and sticks model with N atoms colored in blue, O atoms in red, C atoms in yellow and C1 atom in green) occupies the Moenomycin A-binding site (represented by blue mesh surface). A full color version of this figure is available at The Journal of Antibiotics journal online.
It was observed that, in the binding site, Asp156, Gln152, Tyr191, Glu114 and Gln232 form a hydrophilic and negative charged site, which is a perfect binding site for some positive charged groups to bind. For compound 4a (Figure 3), it was observed that the aminoguanidine forms strong electric effects with Glu114 and Asp156 and hydrogen bonds with Gln152 and Pro231. Tyr196, Ile195, Gln161 and Phe158 contribute large Vdw interactions for the binding of 4a, and Asn235 can form a potential link with the ester group. Chloride (Cl) on compound 4a has polar effects with Gln161 and some other polar groups. The binding pose of compound 4g is very similar to that of compound 4a, but the difference is on the position of ester group. When the ester group is located at the indole 6-position, the distance between the ester group and Asn235 is increased so that a hydrogen bond cannot be formed. The docking of compounds 5a and 5h shows binding poses similar to those of compounds 4a and 4g (Figure 3). They also form interactions similar to those of compounds 4a and 4g, but because of the 2-(4,5-dihydro-1H-imidazol-2-yl)hydrazone group, they can form π–π interactions with Pro231. For compound 5h, same as compound 4g, as the ester group is located on the indole 6-position, it cannot form hydrogen bond with Asn235.
Conlusions
Based on the crystal structure of GT, 40 novel indole core derivatives were designed and synthesized. Among these 40 compounds, 20 compounds showed anti-staphylococcal effects, of which 4 compounds can potentially be further developed into broad-spectrum anti-infective agents against common and multidrug-resistant bacterial and mycobacterial pathogens. Based on molecular modeling, we predicted that these compounds may target at GT, but further experiments are required for confirming this assumption, which is beyond our current research. Although β-lactam antibiotics inhibit the cross-linking of peptidoglycan chains by binding to the active site of the TP enzyme, GT inhibitors are expected to prevent the transfer of disaccharide peptides onto the growing glycan chains before the cross-linking step. Thus GT inhibitors are likely to act synergistically with β-lactam antibiotics to inhibit the polymerization and cross-linking of cell wall peptidoglycan.
Methods
Chemistry
All chemicals were purchased from commercial sources and used as supplied unless stated. Dry CH2Cl2 was distilled over calcium hydride and dry tetrahydrofuran was distilled over sodium benzophenone. TLC was carried out using silica gel 60 precoated aluminium plates (0.20 mm thickness) from Macherey-Nagel (Damstadt, Germany) with visualization by UV light (254 nm). Flash chromatography was performed on silica gel (particle size 40–63 μm). 1H-NMR spectra were obtained from Bruker AVANCE III 400 spectrometers (Fällanden, Switzerland). The chemical shifts, given as δ values, were quoted in p.p.m.; 1H-NMR chemical shifts were measured relative to internal tetramethylsilane; apparent coupling constants (absolute values), J, were measured in Hertz and multiplicities quoted as singlet (s), doublet (d), triplet (t), quartet (q) or combinations thereof as appropriate. Mass spectra were obtained from Agilent Technologies 6530 Accurate-Mass Q-TOF LC/MS (Agilent Technologies Inc., CA, USA). IR spectra were obtained from the solid phase using Bruker Tensor 27 spectrometer (Germany). Melting points were determined using WRS-1B melting point measurement (Shanghai, China) and were uncorrected.
Methyl 3-formyl-1H-indole-5-carboxylate (2a)
To a solution of 1a (5.00 g, 28.57 mmol) in dry DMF (20 ml) was added POCl3 (3.62 ml, 38.86 mol) at 0 °C under N2 and then stirred at room temperature for 2 h. The reaction was quenched with H2O (60 ml) at 0 °C and adjusted the pH to 7–8 with 2 M NaOH. The resulting solution was heated to 70 °C and stirred for 30 min. After cooling to room temperature, the precipitate was filtered and washed with MeOH to give 2a as a light pink solid (5.22 g, 90%), m.p. 231.4–233.0 °C; IR (KBr): υ max cm−1 3232 (NH), 1712 (C=O), 1649 (C=O), 1622 (C=C), 1531 (C=C), 1448 (C=C), 1281 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 12.44 (brs, 1H, NH), 9.98 (s, 1H, CHO), 8.77 (d, J=1.2, 1H, Ar-H), 8.44 (s, 1H, Ar-H), 7.88 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.61 (d, J=8.8, 1H, Ar-H), 3.87 (s, 3H, CH3); ES-MS 204.1 (M+H)+.
Methyl 3-formyl-1H-indole-6-carboxylate (2b)
2b was obtained by reaction of 1b as previously described for 1a as a white solid (0.875 g, 76%), m.p. 221.4–221.7 °C; IR (KBr): υ max cm−1 3218 (NH), 1707 (C=O), 1639 (C=O), 1572 (C=C), 1501 (C=C), 1430 (C=C), 1304 (C-O-C); 1H-NMR(400 MHz, DMSO): δ 12.43 (brs, 1H, NH), 9.98 (s, 1H, CHO), 8.51 (s, 1H, Ar-H), 8.18 (d, J=8.4, 1H, Ar-H), 8.14 (s, 1H, Ar-H), 7.83 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 3.87 (s, 3H, CH3); ES-MS 204.1 (M+H)+.
Methyl 1-(4-chlorobenzyl)-3-formyl-1H-indole-5-carboxylate (3a)
To a solution of 2a (500 mg, 2.46 mmol) in dry DMSO (8 ml) was added NaH (60%, 108 mg, 2.71 mmol) and stirred at room temperature for 1 h. 4-Chlorobenzyl bromide (557 mg, 2.71 mmol) was added and stirred at room temperature for 10 h. The reaction solution was quenched with saturated NH4Cl and extracted with CH2Cl2 (150 ml × 3), and the combined organic layers dried with Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel using CH2Cl2 as elunt to give 3a as a white solid (417 mg, 52%), m.p. 160.2–160.7 °C; IR (KBr): υ max cm−1 2360 (CH2), 1700 (C=O), 1656 (C=O), 1616 (C=C), 1534 (C=C), 1433 (C=C), 1278 (C-O-C), 744 (C-Cl); 1H-NMR (400 MHz, CDCl3): δ 10.06 (s, 1H, CHO), 9.03 (s, 1H, Ar-H), 8.02 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.78 (s, 1H, Ar-H), 7.35 (d, J=8.4, 2H, Ar-H), 7.33 (d, J=8.4, 1H, Ar-H), 7.10–7.12 (d, J=8.4, 2H, Ar-H), 5.37 (s, 2H, CH2), 3.95 (s, 3H, CH3); ES-MS 328.1 (M+H)+.
Methyl 1-(4-fluorobenzyl)-3-formyl-1H-indole-5-carboxylate (3b)
3b was obtained by reaction of 2a as previously described for 3a as a white solid (736 mg, 96%), m.p. 153.3–153.9 °C; IR (KBr): υ max cm−1 2360 (CH2), 1717 (C=O), 1658 (C=O), 1618 (C=C), 1532 (C=C), 1510 (C=C), 1273 (C-O-C), 1088 (C-F); 1H-NMR (400 MHz, CDCl3): δ 10.05 (s, 1H, CHO), 9.02 (s, 1H, Ar-H), 8.03 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.77 (s, 1H, Ar-H), 7.35 (d, J=8.4, 1H, Ar-H), 7.17 (dd, J1=8.8, J2=5.2, 2H, Ar-H), 7.06 (t, J=8.8, 2H, Ar-H), 5.36 (s, 2H, CH2), 3.94 (s, 3H, CH3); ES-MS 312.1 (M+H)+.
Methyl 3-formyl-1-(4-(methoxycarbonyl)benzyl)-1H-indole-5-carboxylate (3c)
3c was obtained by reaction of 2a as previously described for 3a as a white solid (767 mg, 92%), m.p. 203.6–204.0 °C; IR (KBr): υ max cm−1 2360 (CH2), 1716 (C=O), 1661 (C=O), 1615 (C=C), 1534 (C=C), 1436 (C=C), 1278 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.06 (s, 1H, CHO), 9.03 (d, J=1.2, 1H, Ar-H), 8.03 (d, J=8.4, 2H, Ar-H), 8.00 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.81 (s, 1H, Ar-H), 7.30 (d, J=8.8, 1H, Ar-H), 7.21 (d, J=8.4, 2H, Ar-H), 5.46 (s, 2H, CH2), 3.94 (s, 3H, CH3), 3.91 (s, 3H, CH3); ES-MS 352.1 (M+H)+.
Methyl 3-formyl-1-(3-(methoxycarbonyl)benzyl)-1H-indole-5-carboxylate (3d)
3d was obtained by reaction of 2a as previously described for 3a as a white solid (787 mg, 91%), m.p. 148.6–149.3 °C; IR (KBr): υ max cm−1 2360 (CH2), 1718 (C=O), 1667 (C=O), 1617 (C=C), 1532 (C=C), 1438 (C=C), 1292 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.06 (s, 1H, CHO), 9.03 (d, J=1.2, 1H, Ar-H), 8.02 (dd, J1=8.8, J2=1.2, 2H, Ar-H), 7.94 (s, 1H, Ar-H), 7.80 (s, 1H, Ar-H), 7.44 (t, J=8.0, 1H, Ar-H), 7.34 (d, J=8.8, 1H, Ar-H), 7.31 (d, J=8.0, 1H, Ar-H), 5.44 (s, 2H, CH2), 3.94 (s, 3H, CH3), 3.91 (s, 3H, CH3); ES-MS 352.1 (M+H)+.
Methyl 3-formyl-1-(4-nitrobenzyl)-1H-indole-5-carboxylate (3e)
3e was obtained by reaction of 2a as previously described for 3a as an yellow solid (355 mg, 86%), m.p. 219.2–219.6 °C; IR (KBr): υ max cm−1 2360 (CH2), 1715 (C=O), 1671 (C=O), 1636 (C=C), 1540 (C=C), 1521 (NO2), 1458 (C=C), 1339 (NO2), 1282 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 10.01 (s, 1H, CHO), 8.80 (d, J=1.2, 1H, Ar-H), 8.66 (s, 1H, Ar-H), 8.21 (d, J=8.8, 2H, Ar-H), 7.88 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.69 (d, J=8.4, 1H, Ar-H), 7.51 (d, J=8.8, 2H, Ar-H), 5.79 (s, 2H, CH2), 3.87 (s, 3H, CH3); ES-MS 339.1 (M+H)+.
Methyl 3-formyl-1-(3-nitrobenzyl)-1H-indole-5-carboxylate (3f)
3f was obtained by reaction of 2a as previously described for 3a as an yellow solid (588 mg, 71%), m.p. 190.9–193.9 °C; IR (KBr): υ max cm−1 2361 (CH2), 1712 (C=O), 1668 (C=O), 1617 (C=C), 1537 (C=C), 1521 (NO2), 1459 (C=C), 1351 (NO2), 1280 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.09 (s, 1H, CHO), 9.04(s, 1H, Ar-H), 8.21 (d, J=8.4, 1H, Ar-H), 8.11 (s, 1H, Ar-H), 8.03 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.85 (s, 1H, Ar-H), 7.55 (t, J=8.0, 1H, Ar-H), 7.41 (d, J=8.0, 1H, Ar-H), 7.30 (d, J=8.8 Hz, 1H, Ar-H), 5.52 (s, 2H, CH2), 3.95 (s, 3H, CH3); ES-MS 339.1 (M+H)+.
Methyl 1-(4-chlorobenzyl)-3-formyl-1H-indole-6-carboxylate (3g)
3g was obtained by reaction of 2b as previously described for 3a as a white solid (515 mg, 64%), m.p. 153.1–154.2 °C; IR (KBr): υ max cm−1 2924 (CH2), 1714 (C=O), 1666 (C=O), 1617 (C=C), 1529 (C=C), 1456 (C=C), 1273 (C-O-C), 748 (C-Cl); 1H-NMR (400 MHz, CDCl3): δ 10.03 (s, 1H, CHO), 8.36 (d, J=8.0, 1H, Ar-H), 8.09 (s, 1H, Ar-H), 8.02 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.82 (s, 1H, Ar-H), 7.35 (d, J=8.4, 2H, Ar-H), 7.12–7.14 (d, J=8.4, 2H, Ar-H), 5.40 (s, 2H, CH2), 3.94 (s, 3H, CH3); ES-MS 328.1 (M+H)+.
Methyl 1-(4-fluorobenzyl)-3-formyl-1H-indole-6-carboxylate (3h)
3h was obtained by reaction of 2b as previously described for 3a as a white solid (170 mg, 56%), m.p. 155.6–155.7 °C; IR (KBr): υ max cm−1 2926 (CH2), 1713 (C=O), 1662 (C=O), 1618 (C=C), 1531 (C=C), 1439 (C=C), 1272 (C-O-C), 1095 (C-F); 1H-NMR (400 MHz, CDCl3): δ 10.03 (s, 1H, CHO), 8.35 (d, J=8.4, 1H, Ar-H), 8.11 (s, 1H, Ar-H), 8.01 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.81 (s, 1H, Ar-H), 7.20 (dd, J1=8.8, J2=5.2, 2H, Ar-H), 7.07 (t, J=8.4, 2H, Ar-H), 5.40 (s, 2H, CH2), 3.94 (s, 3H, CH3); ES-MS 312.1 (M+H)+.
Methyl 3-formyl-1-(4-(methoxycarbonyl)benzyl)-1H-indole-6-carboxylate (3i)
3i was obtained by reaction of 2b as previously described for 3a as a white solid (0.367 g, 58%), m.p. 169.9–171.1 °C; IR (KBr): υ max cm−1 3102 (CH2), 1714 (C=O), 1663 (C=O), 1616 (C=C), 1535 (C=C), 1437 (C=C), 1280 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.05 (s, 1H, CHO), 8.37 (d, J=8.0, 1H, Ar-H), 8.07 (s, 1H, Ar-H), 8.03 (d, J=8.4, 2H, Ar-H), 8.01–8.03 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.85 (s, 1H, Ar-H), 7.23 (d, J=8.4 Hz, 2H, Ar-H), 5.50 (s, 2H, CH2), 3.93 (s, 3H, CH3), 3.91 (s, 3H, CH3); ES-MS 352.1 (M+H)+.
Methyl 3-formyl-1-(3-(methoxycarbonyl)benzyl)-1H-indole-6-carboxylate (3j)
3j was obtained by reaction of 2b as previously described for 3a as a white solid (465 mg, 56%), m.p. 141.2–141.7 °C; IR (KBr): υ max cm−1 2953 (CH2), 1719 (C=O), 1659 (C=O), 1619 (C=C), 1531 (C=C), 1432 (C=C), 1279 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.03 (s, 1H, CHO), 8.36 (d, J=8.0, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 8.02 (d, J=8.0, 1H, Ar-H), 8.01 (dd, J1=8.0, J2=1.6, 1H, Ar-H), 7.94 (s, 1H, Ar-H), 7.84 (s, 1H, Ar-H), 7.45 (t, J=7.6, 1H, Ar-H), 7.34 (d, J=7.6, 1H, Ar-H), 5.47 (s, 2H, CH2), 3.93 (s, 3H, CH3), 3.91 (s, 3H, CH3); ES-MS 352.1 (M+H)+.
Methyl 3-formyl-1-(4-nitrobenzyl)-1H-indole-6-carboxylate (3k)
3k was obtained by reaction of 2b as previously described for 3a as an yellow solid (0.138 g, 80%), m.p. 184.5–184.9 °C; IR (KBr): υ max cm−1 2945 (CH2), 1711 (C=O), 1663 (C=O), 1616 (C=C), 1516 (NO2), 1436 (C=C), 1392 (C=C), 1344 (NO2), 1278 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.08 (s, 1H, CHO), 8.39 (d, J=8.0, 1H, Ar-H), 8.22 (d, J=8.8, 2H, Ar-H), 8.02–8.04 (m, 2H, Ar-H), 7.89 (s, 1H, Ar-H), 7.30 (d, J=8.8, 2H, Ar-H), 5.56 (s, 2H, CH2), 3.93 (s, 3H, CH3); ES-MS 339.1 (M+H)+.
Methyl 3-formyl-1-(3-nitrobenzyl)-1H-indole-6-carboxylate (3l)
3l was obtained by reaction of 2b as previously described for 3a as an yellow solid (580 mg, 70%), m.p. 153.8–155.3 °C; IR (KBr): υ max cm−1 3089 (CH2), 1701 (C=O), 1669 (C=O), 1618 (C=C), 1571 (C=C), 1523 (NO2), 1435 (C=C), 1348 (NO2), 1280 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.07 (s, 1H, CHO), 8.38 (d, J=8.4, 1H, Ar-H), 8.21 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 8.05 (s, 1H, Ar-H), 8.03 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.89 (s, 1H, Ar-H), 7.55 (t, J=8.0, 1H, Ar-H), 7.42–7.44 (d, J=8.0, 1H, Ar-H), 5.55 (s, 2H, CH2), 3.93 (s, 3H, CH3); ES-MS 339.1 (M+H)+.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-chlorobenzyl)-1H-indole-5-carboxylate hydrochloride (4a)
To a solution of 3a (200 mg, 0.61 mmol) in dry MeOH was added aminoguanidine hydrochloride (260 mg, 0.61 mmol) and then the reaction solution was adjusted pH to 3–4 and stirred at room temperature for 1.5 h. After the completion of the reaction, the solvent was removed under reduced pressure, and then the residue was tutrited in Et2O and filtered to give 4a as a white solid (223 mg, 87%), m.p. 241.1–242.1 °C; IR (KBr): υ max cm−1 3337 (NH), 3272 (NH), 3099 (NH), 2361 (CH2), 1709 (C=O), 1676 (C=O), 1641 (C=C), 1533 (C=C), 1433 (C=C), 1282 (C-O-C), 749 (C-Cl); 1H-NMR (400 MHz,DMSO): δ11.75 (s, 1H, NH), 8.75 (d, J=1.2, 1H, Ar-H), 8.43 (s, 1H, Ar-H), 8.21 (s, 1H, CH), 7.84 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.66 (d, J=8.4, 1H, Ar-H), 7.57 (s, 3H, NH), 7.40 (d, J=8.4, 2H, Ar-H), 7.27 (d, J=8.4, 2H, Ar-H), 5.54 (s, 2H, CH2), 3.85 (s, 3H, CH3); ES-MS 384.1 (M+H)+; HRMS calcd for C19H19ClN5O2+ 384.1227, found 384.1221.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-fluorobenzyl)-1H-indole-5-carboxylate hydrochloride (4b)
4b was obtained by reaction of 3b as previously described for 4a as a white solid (195 mg, 76%), m.p. 237.6–238.4 °C; IR (KBr): υ max cm−1 3479 (NH), 3275 (NH), 3096 (NH), 2362 (CH2), 1707 (C=O), 1674 (C=O), 1645 (C=C), 1533 (C=C), 1435 (C=C), 1280 (C-O-C), 1092 (C-F); 1H-NMR (400 MHz, DMSO): δ 11.72 (s, 1H, NH), 8.75 (d, J=1.6, 1H, Ar-H), 8.42 (s, 1H, Ar-H), 8.21 (s, 1H, CH), 7.83 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.69 (d, J=8.8, 1H, Ar-H), 7.56 (s, 3H, NH), 7.33 (dd, J1=8.8, J2=5.2, 2H, Ar-H), 7.15–7.20 (t, J=8.8, 2H, Ar-H), 5.52 (s, 2H, CH2), 3.85 (s, 3H, CH3); ES-MS 368.2 (M+H)+; HRMS calcd for C19H19FN5O2+ 368.1523, found 368.1514.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-(methoxycarbonyl)benz yl)-1H-indole-5-carboxylate hydrochloride (4c)
4c was obtained by reaction of 3c as previously described for 4a as an yellow solid (207 mg, 82%), m.p. 225.7–228.6 °C; IR (KBr): υ max cm−1 3465 (NH), 3344 (NH), 2949 (NH), 2361 (CH2), 1719 (C=O), 1672 (C=O), 1615 (C=C), 1537 (C=C), 1433 (C=C), 1281 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.72 (s, 1H, NH), 8.76 (d, J=1.2, 1H, Ar-H), 8.44 (s, 1H, Ar-H), 8.23 (s, 1H, CH), 7.92 (d, J=8.4, 2H, Ar-H), 7.82 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.63 (d, J=8.8, 1H, Ar-H), 7.56 (s, 3H, NH), 7.34 (d, J=8.0, 2H, Ar-H), 5.65 (s, 2H, CH2), 3.85 (s, 3H, CH3), 3.82 (s, 3H, CH3); ES-MS 408.2 (M+H)+; HRMS calcd for C21H22N5O4+ 408.1672, found 408.1663.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(3-(methoxycarbonyl)benz yl)-1H-indole-5-carboxylate hydrochloride (4d)
4d was obtained by reaction of 3d as previously described for 4a as an yellow solid (198 mg, 79%), m.p. 137.3–138.7 °C; IR (KBr): υ max cm−1 3365 (NH), 3155 (NH), 2949 (NH), 2361 (CH2), 1715 (C=O), 1672 (C=O), 1617 (C=C), 1536 (C=C), 1433 (C=C), 1281 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.70 (s, 1H, NH), 8.76 (d, J=1.2, 1H, Ar-H), 8.44 (s, 1H, Ar-H), 8.24 (s, 1H, CH), 7.86–7.88 (m, 2H, Ar-H), 7.83 (dd, J1=8.8, J2=1.6, 1H), 7.68 (d, J=8.8, 1H, Ar-H), 7.56 (s, 3H, NH), 7.50–7.52 (m, 2H, Ar-H), 5.64 (s, 2H, CH2), 3.85 (s, 3H, CH3), 3.81 (s, 3H, CH3); ES-MS 408.2 (M+H)+; HRMS calcd for C21H22N5O4+ 408.1672, found 408.1663.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-nitrobenzyl)-1H-indole-5-carboxylate hydrochloride (4e)
4e was obtained by reaction of 3e as previously described for 4a as an yellow solid (210 mg, 88%), m.p. 229.6–229.9 °C; IR (KBr): υ max cm−1 3363 (NH), 3113 (NH), 2950 (NH), 2361 (CH2), 1701 (C=O), 1674 (C=O), 1620 (C=C), 1520 (NO2), 1435 (C=C), 1392 (C=C), 1345 (NO2), 1281 (C-O-C); 1H-NMR (400 MHz,DMSO): δ 11.74 (s, 1H, NH), 8.77 (d, J=1.6, 1H, Ar-H), 8.44 (s, 1H, Ar-H), 8.25 (s, 1H, CH), 8.21 (d, J=8.8, 2H, Ar-H), 7.84 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.64 (d, J=8.8, 1H, Ar-H), 7.56 (s, 3H, NH), 7.45 (d, J=8.4, 2H, Ar-H), 5.73 (s, 2H, CH2), 3.85 (s, 3H, CH3); ES-MS 395.1 (M+H)+; HRMS calcd for C19H19N6O4+ 395.1468, found 395.1460.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(3-nitrobenzyl)-1H-indole-5-carboxylate hydrochloride (4f)
4f was obtained by reaction of 3f as previously described for 4a as an yellow solid (223 mg, 88%), m.p. 227.3–230.2 °C; IR (KBr): υ max cm−1 3368 (NH), 2361 (CH2), 1699 (C=O), 1639 (C=O), 1517 (NO2), 1435 (C=C), 1392 (C=C), 1355 (NO2), 1278 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.72 (s, 1H, NH), 8.77 (d, J=1.2, 1H, Ar-H), 8.44 (s, 1H, Ar-H), 8.27 (s, 1H, CH), 8.15 (m, 2H, Ar-H), 7.84 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.62–7.73 (m, 3H, Ar-H), 7.57 (s, 3H, NH), 5.71 (s, 2H, CH2), 3.85 (s, 3H, CH3); ES-MS 395.1 (M+H)+; HRMS calcd for C19H19N6O4+ 395.1468, found 395.1460.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-chlorobenzyl)-1H-indole-6-carboxylate hydrochloride (4g)
4g was obtained by reaction of 3g as previously described for 4a as a white solid (223 mg, 87%), m.p. 249.6–250.9 °C; IR (KBr): υ max cm−1 3470 (NH), 3326 (NH), 3088 (NH), 1718 (C=O), 1645 (C=O), 1622 (C=C), 1513 (C=C), 1438 (C=C), 1287 (C-O-C), 741 (C-Cl); 1H-NMR (400 MHz, DMSO): δ 11.71 (s, 1H, NH), 8.43–8.45 (d, J=8.4, 1H, Ar-H), 8.37 (s, 1H, Ar-H), 8.27 (s, 1H, CH), 8.17 (s, 1H, Ar-H), 7.76 (d, J=8.4, 1H, Ar-H), 7.57 (s, 3H, NH), 7.28 (dd, J1=8.4, J2=5.6, 2H, Ar-H), 7.18 (t, J=8.8, 2H, Ar-H), 5.59 (s, 2H, CH2), 3.86 (s, 3H, CH3); ES-MS 384.1 (M+H)+; HRMS calcd for C19H19ClN5O2+ 384.1227, found 384.1217.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-fluorobenzyl)-1H-indole-6-carboxylate hydrochloride (4h)
4h was obtained by reaction of 3h as previously described for 4a as a white solid (251 mg, 97%), m.p. 261.4–261.9 °C; IR (KBr): υ max cm−1 3376 (NH), 3225 (NH), 3177 (NH), 1712 (C=O), 1668 (C=O), 1633 (C=C), 1459 (C=C), 1394 (C=C), 1269 (C-O-C), 1100 (C-F); 1H-NMR (400 MHz, DMSO): δ 11.70 (s, 1H, NH), 8.45 (d, J=8.4 Hz, 1H, Ar-H), 8.38 (s, 1H, Ar-H), 8.28 (s, 1H, CH), 8.14 (s, 1H, Ar-H), 7.76 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.57 (s, 3H, NH), 7.41 (d, J=8.4, 2H, Ar-H), 7.23 (d, J=8.4, 2H, Ar-H), 5.61 (s, 2H, CH2), 3.85 (s, 3H, CH3); ES-MS 368.2 (M+H)+; HRMS calcd for C19H19FN5O2+ 368.1523, found 368.1513.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-(methoxycarbonyl)benzyl)-1H-indole-6-carboxylate hydrochloride (4i)
4i was obtained by reaction of 3i as previously described for 4a as a white solid (223 mg, 88%), m.p. 238.8–240.0 °C; IR (KBr): υ max cm−1 3471 (NH), 3334 (NH), 3159 (NH), 1715 (C=O), 1673 (C=O), 1616 (C=C), 1534 (C=C), 1438 (C=C), 1279 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.70 (s, 1H, NH), 8.45 (d, J=8.4, 1H, Ar-H), 8.39 (s, 1H, Ar-H), 8.30 (s, 1H, CH), 8.10 (s, 1H, Ar-H), 7.92 (d, J=8.0, 2H, Ar-H), 7.77 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.52 (s, 3H, NH), 7.30 (d, J=8.0, 2H, Ar-H), 5.72 (s, 2H, CH2), 3.84 (s, 3H, CH3), 3.82 (s, 3H, CH3); ES-MS 408.2 (M+H)+; HRMS calcd for C21H22N5O4+ 408.1672, found 408.1665.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(3-(methoxycarbonyl)benzyl)-1H-indole-6-carboxylate hydrochloride (4j)
4j was obtained by reaction of 3j as previously described for 4a as a white solid (225 mg, 89%), m.p. 228.8–230.9 °C; IR (KBr): υ max cm−1 3470 (NH), 2956 (NH), 2359 (CH2), 1717 (C=O), 1667 (C=O), 1640 (C=C), 1535 (C=C), 1435 (C=C), 1284 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.70 (s, 1H, NH), 8.45 (d, J=8.4, 1H, Ar-H), 8.39 (s, 1H, Ar-H), 8.31 (s, 1H, CH), 8.16 (s, 1H, Ar-H), 7.86–7.88 (d, J=6.8,1H, Ar-H), 7.83 (s, 1H, Ar-H), 7.77 (d, J=8.0, 1H, Ar-H), 7.65 (s, 3H, NH), 7.49–7.51 (m, 2H, Ar-H), 5.71 (s, 2H, CH2), 3.85 (s, 3H, CH3), 3.82 (s, 3H, CH3); ES-MS 408.2 (M+H)+; HRMS calcd for C21H22N5O4+ 408.1672, found 408.1666.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(4-nitrobenzyl)-1H-indole-6-carboxylate hydrochloride (4k)
4k was obtained by reaction of 3k as previously described for 4a as an yellow solid (230 mg, 96%), m.p. 259.8–260.8 °C; IR (KBr): υ max cm−1 3469 (NH), 2363 (CH2), 1711 (C=O), 1673 (C=O), 1644 (C=C), 1519 (NO2), 1436 (C=C), 1395 (C=C), 1349 (NO2), 1277 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.71 (s, 1H, NH), 8.47 (d, J=8.4, 1H, Ar-H), 8.39 (s, 1H, Ar-H), 8.31 (s, 1H, CH), 8.21 (d, J=8.8, 2H, Ar-H), 8.13 (s, 1H, Ar-H), 7.79 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7. 57 (s, 3H, NH), 7.41 (d, J=8.8, 2H, Ar-H), 5.80 (s, 2H, CH2), 3.84 (s, 3H, CH3); ES-MS 395.1 (M+H)+; HRMS calcd for C19H19N6O4+ 395.1468, found 395.1462.
Methyl (E)-3-((2-carbamimidoylhydrazono)methyl)-1-(3-nitrobenzyl)-1H-indole-6-carboxylate hydrochloride (4l)
4l was obtained by reaction of 3l as previously described for 4a as an yellow solid (199 mg, 79%), m.p. 248.9–249.3 °C; IR (KBr): υ max cm−1 3479 (NH), 3402 (NH), 3274 (NH), 2361 (CH2), 1707 (C=O), 1676 (C=O), 1643 (C=C), 1531 (NO2), 1439 (C=C), 1393 (C=C), 1348 (NO2), 1271 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.68 (s, 1H, NH), 8.47 (d, J=8.4, 1H, Ar-H), 8.39 (s, 1H, Ar-H), 8.33 (s, 1H, CH), 8.21 (s, 1H, Ar-H), 8.15 (dd, J1=5.6, J2=2.0, 1H, Ar-H), 8.12 (s, 1H, Ar-H), 7.78 (d, J=8.4, 1H, Ar-H), 7.64–7.67 (m, 2H, Ar-H), 7. 50 (s, 3H, NH), 5.79 (s, 2H, CH2), 3.85 (s, 3H, CH3); ES-MS 395.1 (M+H)+; HRMS calcd for C19H19N6O4+ 395.1468, found 395.1458.
Methyl (E)-1-(4-chlorobenzyl)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1H-indole-5-carboxylate hydrobromide (5a)
To a solution of 3a (50 mg, 0.15 mmol) in dry MeOH (2 ml) was added 2-hydrazino-2-imidazoline hydrobromide (28 mg, 0.15 mmol), and then the reaction solution was stirred at 75 °C. After the completion of the reaction, the solvent was removed under reduced pressure, and then the residue was washed by Et2O and filtered to give 4a as a white solid (65 mg, 87%), m.p. 243.6–244.3 °C; IR (KBr): υ max cm−1 3426 (NH), 3185 (NH), 2950 (NH), 2361 (CH2), 1708 (C=O), 1669 (C=O), 1603 (C=C), 1539 (C=C), 1453 (C=C), 1246 (C-O-C), 745 (C-Cl); 1H-NMR (400 MHz, DMSO): δ 11.95 (s, 1H, NH), 8.79 (d, J=1.2, 1H, Ar-H), 8.41 (s, 2H, CH, NH), 8.19 (s, 1H, Ar-H), 7.84 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.68 (d, J=8.8, 1H, Ar-H), 7.41 (d, J=8.4, 2H, Ar-H), 7.29 (d, J=8.4, 2H, Ar-H), 5.54 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.73 (s, 4H, CH2); ES-MS 410.1 (M+H)+; HRMS calcd for C21H21ClN5O2+ 410.1384, found 410.1374.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(4-fluorobenzyl)-1H-indole-5-carboxylate hydrobromide (5b)
5b was obtained by reaction of 3b as previously described for 5a as a white solid (279 mg, 92%), m.p. 241.5–243.7 °C; IR (KBr): υ max cm−1 3175 (NH), 3051 (NH), 2952 (NH), 2361 (CH2), 1706 (C=O), 1668 (C=O), 1602 (C=C), 1510 (C=C), 1451 (C=C), 1246 (C-O-C), 1096 (C-F); 1H-NMR (400 MHz, DMSO): δ 12.00 (s, 1H, NH), 8.79 (s, 1H, Ar-H), 8.41 (s, 2H, CH, NH), 8.19 (s, 1H, Ar-H), 7.84 (dd, J1=8.8, J2=1.2, 1H, Ar-H), 7.71 (d, J=8.4, 1H, Ar-H), 7.33–7.36 (dd, J1=8.4, J2=1.6, 2H, Ar-H), 7.16–7.20 (t, J=8.8, 2H, Ar-H), 5.52 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.73 (s, 4H, CH2); ES-MS 394.2 (M+H)+; HRMS calcd for C21H21FN5O2+ 394.1679, found 394.1670.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(4-(methoxycarbonyl)benzyl)-1H-indole-5-carboxylate hydrobromide (5c)
5c was obtained by reaction of 3c as previously described for 5a as a white solid (264 mg, 90%), m.p. 238.7 °C (dec); IR (KBr): υ max cm−1 3184 (NH), 2950 (NH), 1721 (C=O), 1666 (C=O), 1603 (C=C), 1538 (C=C), 1453 (C=C), 1281 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.99 (s, 1H, NH), 8.80 (d, J=0.8, 1H, Ar-H), 8.42 (s, 2H, CH, NH), 8.21 (s, 1H, Ar-H), 7.93 (d, J=8.0, 2H, Ar-H), 7.83 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.64 (d, J=8.4, 1H, Ar-H), 7.35 (d, J=8.4, 2H, Ar-H), 5.65 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.82 (s, 3H, CH3), 3.74 (s, 4H, CH2); ES-MS 434.2 (M+H)+; HRMS calcd for C23H24N5O4+ 434.1828, found 434.1817.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(3-(methoxycarbonyl)benzyl)-1H-indole-5-carboxylate hydrobromide (5d)
5d was obtained by reaction of 3d as previously described for 5a as a white solid (271 mg, 93%), m.p. 243.4–246.0 °C; IR (KBr): υ max cm−1 3192 (NH), 3086 (NH), 2951 (NH), 1704 (C=O), 1666 (C=O), 1601 (C=C), 1539 (C=C), 1449 (C=C), 1291 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.98 (s, 1H, NH), 8.80 (d, J=1.2, 1H, Ar-H), 8.42 (s, 2H, CH, NH), 8.23 (s, 1H, Ar-H), 7.83–7.89 (m, 3H, Ar-H), 7.69 (d, J=8.4, 1H, Ar-H), 7.51–7.53 (m, 2H, Ar-H), 5.64 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.81 (s, 3H, CH3), 3.74 (s, 4H, CH2); ES-MS 434.2 (M+H)+; HRMS calcd for C23H24N5O4+ 434.1828, found 434.1820.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(4-nitrobenzyl)-1H-indole-5-carboxylate hydrobromide (5e)
5e was obtained by reaction of 3e as previously described for 5a as an yellow solid (271 mg, 93%), m.p. 247.3–247.7 °C; IR (KBr): υ max cm−1 3463 (NH), 3268 (NH), 3107 (NH), 2362 (CH2), 1692 (C=O), 1661 (C=O), 1613 (C=C), 1539 (C=C), 1514 (NO2), 1435 (C=C), 1347 (NO2), 1248 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.99 (s, 1H, NH), 8.81 (s, 1H, Ar-H), 8.44 (s, 2H, CH, NH), 8.23 (s, 1H, Ar-H), 8.21 (d, J=8.8, 2H, Ar-H), 7.84 (dd, J1=8.8, J2=1.2, 1H, Ar-H), 7.65 (d, J=8.8, 1H, Ar-H), 7.46 (d, J=8.4, 2H, Ar-H), 5.73 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.74 (s, 4H, CH2); ES-MS 421.2 (M+H)+; HRMS calcd for C21H21N6O4+ 421.1624, found 421.1617.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(3-nitrobenzyl)-1H-indole-5-carboxylate hydrobromide (5f)
5f was obtained by reaction of 3f as previously described for 5a as an yellow solid (265 mg, 90%), m.p. 246.9–250.9 °C; IR (KBr): υ max cm−1 3424 (NH), 3198 (NH), 2361 (CH2), 1703 (C=O), 1665 (C=O), 1604 (C=C), 1532 (NO2), 1452 (C=C), 1395 (C=C), 1346 (NO2), 1248 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.99 (s, 1H, NH), 8.81 (s, 1H, Ar-H), 8.43 (s, 2H, CH, NH), 8.27 (s, 1H, Ar-H), 8.15 (m, 2H, Ar-H), 7.85 (d, J=8.4, 1H, Ar-H), 7.74–7.63 (m, 3H, Ar-H), 5.72 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.74 (s, 4H, CH2); ES-MS 421.2 (M+H)+; HRMS calcd for C21H21N6O4+ 421.1624, found 421.1615.
Methyl (E)-1-(4-chlorobenzyl)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1H-indole-6-carboxylate hydrobromide (5g)
5g was obtained by reaction of 3g as previously described for 5a as a white solid (281 mg, 94%), m.p. 176.7–179.6 °C; IR (KBr): υ max cm−1 3408 (NH), 2951 (NH), 2361 (CH2), 1713 (C=O), 1667 (C=O), 1617 (C=C), 1511 (C=C), 1437 (C=C), 1277 (C-O-C), 748 (C-Cl); 1H-NMR (400 MHz, DMSO): δ 12.00 (s, 1H, NH), 8.58 (brs, 1H, NH), 8.48 (d, J=8.4, 1H, Ar-H), 8.38 (s, 1H, Ar-H), 8.28 (s, 1H, CH), 8.18 (s, 1H, Ar-H), 7.77 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.30 (dd, J1=8.4, J2=1.6, 2H, Ar-H), 7.19 (t, J=8.8, 2H, Ar-H), 5.59 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.74 (s, 4H, CH2); ES-MS 410.1 (M+H)+; HRMS calcd for C21H21ClN5O2+ 410.1384, found 410.1374.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(4-fluorobenzyl)-1H-indole-6-carboxylate hydrobromide (5h)
5h was obtained by reaction of 3h as previously described for 5a as a white solid (257 mg, 85%), m.p. 213.4–214.6 °C; IR (KBr): υ max cm−1 3403 (NH), 3168 (NH), 2948 (NH), 2361 (CH2), 1709 (C=O), 1665 (C=O), 1617 (C=C), 1511 (C=C), 1437 (C=C), 1277 (C-O-C), 1097 (C-F); 1H-NMR (400 MHz, DMSO): δ 11.98 (s, 1H, NH), 8.58 (brs, 1H, NH), 8.48 (d, J=8.4, 1H, Ar-H), 8.38 (s, 1H, Ar-H), 8.28 (s, 1H, CH), 8.18 (s, 1H, Ar-H), 7.78 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.30 (dd, J1=8.4, J2=5.6, 2H, Ar-H), 7.19 (t, J=8.8, 2H, Ar-H), 5.59 (s, 2H, CH2), 3.86 (s, 3H, CH3), 3.75 (s, 4H, CH2); ES-MS 394.2 (M+H)+; HRMS calcd for C21H21FN5O2+ 394.1679, found 394.1670.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(4-(methoxycarbonyl)benzyl)-1H-indole-6-carboxylate hydrobromide (5i)
5i was obtained by reaction of 3i as previously described for 5a as a white solid (280 mg, 96%), m.p. 239.1 °C (dec); IR (KBr): υ max cm−1 3416 (NH), 2951 (NH), 2361 (CH2), 1718 (C=O), 1670 (C=O), 1617 (C=C), 1459 (C=C), 1436 (C=C), 1281 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.99 (s, 1H, NH), 8.49 (d, J=8.4, 2H, Ar-H, NH), 8.39 (s, 1H, Ar-H), 8.30 (s, 1H, CH), 8.12 (s, 1H, Ar-H), 7.93 (d, J=8.4, 2H, Ar-H), 7.78 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.31 (d, J=8.0, 2H, Ar-H), 5.72 (s, 2H, CH2), 3.84 (s, 3H, CH3), 3.82 (s, 3H, CH3), 3.75 (s, 4H, CH2); ES-MS 434.2 (M+H)+; HRMS calcd for C23H24N5O4+ 434.1828, found 434.1816.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(3-(methoxycarbonyl)benzyl)-1H-indole-6-carboxylate hydrobromide (5j)
5j was obtained by reaction of 3j as previously described for 5a as a white solid (240 mg, 82%), m.p. 203.7–206.2 °C; IR (KBr): υ max cm−1 3405 (NH), 2950 (NH), 2361 (CH2), 1716 (C=O), 1665 (C=O), 1618 (C=C), 1536 (C=C), 1457 (C=C), 1287 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.98 (s, 1H, NH), 8.49 (d, J=8.4, 2H, Ar-H, NH), 8.40 (s, 1H, Ar-H), 8.31 (s, 1H, CH), 8.17 (s, 1H, Ar-H), 7.89–7.87 (m, 1H, Ar-H), 7.84 (s, 1H, Ar-H), 7.78 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.52–7.50 (m, 2H, Ar-H), 5.71 (s, 2H, CH2), 3.85 (s, 3H, CH3), 3.82 (s, 3H, CH3), 3.75 (s, 4H, CH2); ES-MS 434.2 (M+H)+; HRMS calcd for C23H24N5O4+ 434.1828, found 434.1821.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(4-nitrobenzyl)-1H-indole-6-carboxylate hydrobromide (5k)
5k was obtained by reaction of 3k as previously described for 5a as an yellow solid (260 mg, 88%), m.p. 253.6–255.6 °C; IR (KBr): υ max cm−1 3404 (NH), 3108 (NH), 2910 (NH), 2361 (CH2), 1712 (C=O), 1662 (C=O), 1615 (C=C), 1522 (NO2), 1490 (C=C), 1391 (C=C), 1350 (NO2), 1276 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 12.01 (d, J=3.6, 1H, NH), 8.51 (d, J=8.4, 2H, Ar-H, NH), 8.40 (s, 1H, CH), 8.32 (s, 1H, Ar-H), 8.22 (d, J=8.8, 2H, Ar-H), 8.14 (s, 1H, Ar-H), 7.79 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.42 (d, J=8.8, 2H, Ar-H), 5.81 (s, 2H, CH2), 3.84 (s, 3H, CH3), 3.75 (s, 4H, CH2); ES-MS 421.2 (M+H)+; HRMS calcd for C21H21N6O4+ 421.1624, found 421.1618.
Methyl (E)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(3-nitrobenzyl)-1H-indole-6-carboxylate hydrobromide (5l)
5l was obtained by reaction of 3l as previously described for 5a as an yellow solid (278 mg, 94%), m.p. 252.0–253.8 °C; IR (KBr): υ max cm−1 3422 (NH), 3234 (NH), 2361 (CH2), 1708 (C=O), 1661 (C=O), 1619 (C=C), 1531 (NO2), 1462 (C=C), 1438 (C=C), 1351 (NO2), 1286 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 12.01 (s, 1H, NH), 8.51 (d, J=8.4, 2H, Ar-H, NH), 8.40 (s, 1H, Ar-H), 8.34 (s, 1H, CH), 8.22 (s, 1H, Ar-H), 8.15–8.17 (m, 1H, Ar-H), 8.12 (s, 1H, Ar-H), 7.79 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 7.67–7.65 (m, 2H, Ar-H), 5.79 (s, 2H, CH2), 3.85 (s, 3H, CH3), 3.75 (s, 4H, CH2); ES-MS 421.2 (M+H)+; HRMS calcd for C21H21N6O4+ 421.1624, found 421.1618.
Methyl 1-benzoyl-3-formyl-1H-indole-5-carboxylate (6a)
To a solution of 2a (100 mg, 0.49 mmol) in dry DMF (2 ml) was added NaH (60%, 22 mg, 0.54 mmol) at 0 °C under argon and then stirred at room temperature for 1 h. The reaction solution was cooled to 0 °C, benzoyl chloride (0.07 ml, 0.54 mmol) was added and then stirred at room temperature overnight. The reaction solution was poured into H2O (20 ml), and the precipitate was filtered. The crude product was purified by column chromatography on silica gel using EtOAc/PE (1/8, V/V) as elute to give 6a as a white solid (107 mg, 71%), m.p. 172.7–173.3 °C; IR (KBr): υ max cm−1 1721 (C=O), 1675 (C=O), 1611 (C=C), 1552 (C=C), 1452 (C=C), 1267 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.10 (s, 1H, CHO), 9.00 (d, J=1.2, 1H, Ar-H), 8.35 (d, J=8.8, 1H, Ar-H), 8.18 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 8.03 (s, 1H, Ar-H), 7.81–7.79 (m, 2H, Ar-H), 7.71 (t, J=7.6, 1H, Ar-H), 7.61 (t, J=7.6, 2H, Ar-H), 3.98 (s, 3H, CH3); ES-MS 308.1 (M+H)+.
Methyl 1-benzoyl-3-formyl-1H-indole-6-carboxylate (6b)
6b was obtained by reaction of 2b as previously described for 6a as a white solid (99 mg, 66%), m.p. 176.1–176.4 °C; IR (KBr): υ max cm−1 1718 (C=O), 1672 (C=O), 1612 (C=C), 1547 (C=C), 1431 (C=C), 1283 (C-O-C); 1H-NMR (400 MHz, CDCl3): δ 10.09 (s, 1H, CHO), 9.00 (s, 1H, Ar-H), 8.38 (d, J=8.0, 1H, Ar-H), 8.15 (dd, J1=8.4, J2=1.2, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 7.81–7.79 (m, 2H, Ar-H), 7.73 (t, J=7.6, 1H, Ar-H), 7.62 (t, J=7.6, 2H, Ar-H), 3.97 (s, 3H, CH3); ES-MS 308.1 (M+H)+.
Methyl (E)-1-benzoyl-3-((2-carbamimidoylhydrazono)methyl)-1H-indole-5-carboxylate hydrochloride (7a)
7a was obtained by reaction of 6a as previously described for 4a as a white solid (245 mg, 94%), m.p. 204.6–206.0 °C; IR (KBr): υ max cm−1 3446 (NH), 1685 (C=O), 1627 (C=O), 1551 (C=C), 1451 (C=C), 1371 (C=C), 1298 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.92 (s, 1H, NH), 8.82 (s, 1H, Ar-H), 8.43 (s, 1H, Ar-H), 8.0 (d, J=8.8, 1H, Ar-H), 8.20 (s, 1H, CH), 8.07 (d, J=8.4, 1H, Ar-H), 7.85 (d, J=7.6, 2H, Ar-H), 7.63–7.77 (m, 6H, Ar-H, NH), 3.90 (s, 3H, CH3); ES-MS 364.1 (M+H)+; HRMS calcd for C19H18N5O3+ 364.1410, found 364.1403.
Methyl (E)-1-benzoyl-3-((2-carbamimidoylhydrazono)methyl)-1H-indole-6-carboxylate hydrochloride (7b)
7b was obtained by reaction of 6b as previously described for 4a as a white solid (239 mg, 92%), m.p. 229.0 °C (dec); IR (KBr): υ max cm−1 3423 (NH), 2361 (CH2), 1680 (C=O), 1632 (C=O), 1545 (C=C), 1432 (C=C), 1372 (C=C), 1242 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 11.93 (s, 1H, NH), 8.94 (d, J=1.2, 1H, Ar-H), 8.59 (d, J=8.4, 1H, Ar-H), 8.38 (s, 1H, Ar-H), 8.28 (s, 1H, CH), 7.99 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.83–7.85(m, 2H, Ar-H), 7.63–7.77 (m, 6H, Ar-H, NH), 3.92 (s, 3H, CH3); ES-MS 364.1 (M+H)+; HRMS calcd for C19H18N5O3+ 364.1410, found 364.1404.
Methyl (E)-1-benzoyl-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1H-indole-5-carboxylate hydrobromide (8a)
8a was obtained by reaction of 6a as previously described for 5a as a white solid (212 mg, 70%), m.p. 215.7–216.7 °C; IR (KBr): υ max cm−1 3407 (NH), 3291(NH), 2950 (NH), 1699 (C=O), 1664 (C=O), 1615 (C=C), 1447 (C=C), 1369 (C=C), 1272 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 12.24 (s, 1H, NH), 8.86 (d, J=0.8, 1H, Ar-H), 8.42–8.39 (m, 2H, Ar-H, CH), 8.18 (s, 1H, Ar-H), 8.07 (dd, J1=8.8, J2=1.6, 1H, Ar-H), 7.85 (d, J=7.2, 2H, Ar-H), 7.76 (t, J=7.2, 1H, Ar-H), 7.65 (t, J=7.6, 2H, Ar-H), 3.91 (s, 3H, CH3), 3.75 (s, 4H, CH2); ES-MS 390.2 (M+H)+; HRMS calcd for C21H20N5O3+ 390.1566, found 390.1562.
Methyl (E)-1-benzoyl-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)- 1H-indole-6-carboxylate hydrobromide (8b)
8b was obtained by reaction of 6b as previously described for 5a as a white solid (217 mg, 80%), m.p. 218.6–219.6 °C; IR (KBr): υ max cm−1 3428 (NH), 3366(NH), 2360 (CH2), 1712 (C=O), 1661 (C=O), 1613 (C=C), 1432 (C=C), 1380 (C=C), 1230 (C-O-C); 1H-NMR (400 MHz, DMSO): δ 12.28 (s, 1H, NH), 8.95 (d, J=0.8, 1H, Ar-H), 8.61 (d, J=8.4, 1H, Ar-H), 8.37 (s, 1H, CH), 8.27 (s, 1H, Ar-H), 7.99 (dd, J1=8.4, J2=1.6, 1H, Ar-H), 7.86–7.84 (m, 2H, Ar-H), 7.76 (t, J=7.6, 1H, Ar-H), 7.65 (t, J=7.4, 2H, Ar-H), 3.92 (s, 3H, CH3), 3.77 (s, 4H, CH2); ES-MS 390.2 (M+H)+; HRMS calcd for C21H20N5O3+ 390.1566, found 390.1599.
5-Bromo-1H-indole-3-carbaldehyde (10)
To a solution of 9 (5.00 g, 25.5 mmol) in dry DMF (20 ml) was added POCl3 (3.22 ml, 34.68 mol) at 0 °C under N2 and then stirred at room temperature for 2 h. The reaction was quenched with H2O (60 ml) at 0 °C and adjusted the pH to 7–8 with 2 M NaOH. The resulting solution was heated to 70 °C and stirred 30 min. After cooling to room temperature, the precipitate was filtered and washed with MeOH to give 10 as a white solid (5.19 g, 99%), m.p. 203.5–204.3 °C; IR (KBr): υ max cm−1 3217 (NH), 2361 (C-N), 1645 (C=O), 1523 (C=C), 1437 (C=C), 1395 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.28 (s, 1H, NH), 9.93 (s, 1H, CHO), 8.35 (s, 1H, Ar-H), 8.21 (d, J=2.0, 1H, Ar-H), 7.49 (d, J=8.4, 1H, Ar-H), 7.43 (dd, J1=8.8, J2=2.0, 1H, Ar-H); ES-MS 225.1 (M+H)+.
5-Bromo-1-(phenylsulfonyl)-1H-indole-3-carbaldehyde (11)
To a solution of 10 (2 g, 8.88 mmol) in dry DMSO (15 ml) was added NaH (60%, 0.392 g, 9.77 mmol) and stirred at room temperature for 1 h. After the reaction solution was cooled to 0 °C, benzenesulfonyl chloride (1.36 ml, 10.66 mmol) was added and then stirred at room temperature overnight. The reaction solution was quenched with sat. NH4Cl and extracted with CH2Cl2 (200 ml x 3), and the combined organic layers were dried with Na2SO4, filtered and concentrated. The residue was recrystallized by acetone/hexane to give 11 as a white solid (2.67 g, 83%), m.p. 235.2–239.3 °C; IR (KBr): υ max cm−1 2361 (C-N), 1677 (C=O), 1539 (C=C), 1441 (C=C), 1376 (C=C); 1H-NMR (400 MHz, DMSO): δ 10.06 (s, 1H, CHO), 8.96 (s, 1H, Ar-H), 8.22 (d, J=2.0, 1H, Ar-H), 8.14–8.12 (m, 2H, Ar-H), 7.95 (d, J=8.8, 1H, Ar-H), 7.81–7.77 (m, 1H, Ar-H), 7.69–7.65 (m, 2H, Ar-H), 7.62 (dd, J1=8.8, J2=2.0, 1H, Ar-H); ES-MS 365.2 (M+H)+.
4-(3-Formyl-1-(phenylsulfonyl)-1H-indol-5-yl)phenyl acetate (12a)
To a solution of 11 (0.5 g, 1.37 mmol) in a solution of tetrahydrofuran/H2O (20 ml, 1/1, v/v) was added K2CO3 (0.28 g, 2.05 mmol), 4-(methoxycarbonyl)benzeneboronic acid (0.24 g, 1.64 mmol) and Pd(dbpf)Cl2 (8.9 mg, 0.0137 mmol) and then stirred at 60 °C for 8 h. The reaction solution was extracted with EtOAc (100 ml × 3), and the combined organic layers were dried with Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel using EtOAc/PE (1/7, v/v) as elunt to give 12a as a white solid (620 mg, 86%), m.p. 166.5–166.8 °C; IR (KBr): υ max cm−1 2361 (C-N), 1720 (C=O), 1678 (C=O), 1541 (C=C), 1455 (C=C), 1378 (C=C); 1H-NMR (400 MHz, CDCl3): δ 10.13 (s, 1H, CHO), 8.52 (d, J=2.0, 1H, Ar-H), 8.27 (s, 1H, Ar-H), 8.11 (d, J=8.4, 2H, Ar-H), 8.04 (d, J=8.8, 1H, Ar-H), 8.02–7.99 (m, 2H, Ar-H), 7.70–7.63 (m, 4H, Ar-H), 7.57–7.53 (m, 2H, Ar-H), 3.94 (s, 3H, OCH3); ES-MS 420.5 (M+H)+.
Methyl 3-(3-formyl-1-(phenylsulfonyl)-1H-indol-5-yl)benzoate (12b)
12b was obtained by reaction of 11 as previously described for 12a as a white solid (328 mg, 53%), m.p. 186.8–187.1 °C; IR (KBr): υ max cm−1 2361 (C-N), 1714 (C=O), 1681 (C=O), 1542 (C=C), 1453 (C=C), 1379 (C=C); 1H NMR (400 MHz, CDCl3): δ 10.13 (s, 1H, CHO), 8.50 (d, J=1.6, 1H, Ar-H), 8.28–8.27 (m, 2H, Ar-H), 8.05–7.99 (m, 4H, Ar-H), 7.83–7.80 (m, 1H, Ar-H), 7.69 (dd, J1=8.8, J2=2.0, 1H, Ar-H), 7.66–7.63 (m, 1H, Ar-H), 7.57–7.50 (m, 3H, Ar-H), 3.95 (s, 3H, OCH3); ES-MS 420.5 (M+H)+.
1-(Phenylsulfonyl)-5-(4-(trifluoromethoxy)phenyl)-1H-indole-3-carbaldehyde (12c)
12c was obtained by reaction of 11 as previously described for 12a as a white solid (360 mg, 89%), m.p. 180.4–181.3 °C; IR (KBr): υ max cm−1 2361 (C-N), 1678 (C=O), 1542 (C=C), 1457 (C=C), 1380 (C=C); 1H NMR (400 MHz, DMSO): δ 10.12 (s, 1H, CHO), 8.98 (s, 1H, Ar-H), 8.34 (d, J=1.6, 1H, Ar-H), 8.18–8.16 (m, 2H, Ar-H), 8.07 (d, J=8.8, 1H, Ar-H), 7.80–7.75 (m, 2H, Ar-H), 7.69–7.65 (m, 3H, Ar-H), 7.61–7.57(m, 2H, Ar-H), 7. 37 (d, J=8.4, 1H, Ar-H); ES-MS 446.4 (M+H)+.
1-(Phenylsulfonyl)-5-(3-(trifluoromethoxy)phenyl)-1H-indole-3-carbaldehyde (12d)
12d was obtained by reaction of 11 as previously described for 12a as a white solid (300 mg, 74%), m.p. 150.4–151.0 °C; IR (KBr): υ max cm−1 2361 (C-N), 1677 (C=O), 1541 (C=C), 1450 (C=C), 1379 (C=C); 1H NMR (400 MHz, DMSO): δ 10.12 (s, 1H, CHO), 8.98 (s, 1H, Ar-H), 8.33 (d, J=1.6, 1H, Ar-H), 8.18–8.16 (m, 2H, Ar-H), 8.07 (d, J=8.8, 1H, Ar-H), 7.78–7.74 (m, 4H, Ar-H), 7.70–7.66 (m, 2H, Ar-H), 7.45 (d, J=8.0, 2H, Ar-H); ES-MS 446.4 (M+H)+.
1-(Phenylsulfonyl)-5-(4-(trifluoromethyl)phenyl)-1H-indole-3-carbaldehyde (12e)
12e was obtained by reaction of 11 as previously described for 12a as a white solid (85 mg, 67%), m.p. 216.5–216.8 °C; IR (KBr): υ max cm−1 2361 (C-N), 1677 (C=O), 1542 (C=C), 1451 (C=C), 1380 (C=C); 1H NMR (400 MHz, CDCl3): δ 10.13 (s, 1H, CHO), 8.50 (d, J=1.6, 1H, Ar-H), 8.28 (s, 1H, Ar-H), 8.05 (d, J=8.8, 1H, Ar-H), 8.03–8.00 (m, 2H, Ar-H), 7.74–7.68 (m, 4H, Ar-H), 7.68–7.63(m, 2H, Ar-H), 7.58–7.53 (m, 2H, Ar-H); ES-MS 430.4 (M+H)+.
5-(3-Chloro-4-fluorophenyl)-1-(phenylsulfonyl)-1H-indole-3-carbaldehyde (12f)
12f was obtained by reaction of 11 as previously described for 12a as a white solid (510 mg, 83%), m.p. 171.1–171.6 °C; IR (KBr): υ max cm−1 2361 (C-N), 1677 (C=O), 1542 (C=C), 1453 (C=C), 1379 (C=C); 1H NMR (400 MHz, CDCl3): δ 10.12 (s, 1H, CHO), 8.41 (d, J=1.6, 1H, Ar-H), 8.27 (s, 1H, Ar-H), 8.03–7.99 (m, 3H, Ar-H), 7.67–7.62 (m, 2H, Ar-H), 7.58–7.53 (m, 3H, Ar-H), 7.46 (ddd, J1=8.6, J2=4.4, J3=2.4, 1H, Ar-H), 7.21 (t, J=8.6, 1H, Ar-H); ES-MS 414.9 (M+H)+.
Methyl (E)-4-(3-((2-carbamimidoylhydrazono)methyl)-1-(phenylsulfonyl)-1H-indol-5-yl)benzoate hydrochloride (13a)
To a solution of 12a (100 mg, 0.22 mmol) in dry MeOH was added aminoguanidine hydrochloride (24 mg, 0.22 mmol), and then the reaction solution was adjusted pH to 3–4 and stirred at room temperature for 1.5 h. After completion of the reaction, the solvent was removed under reduced pressure and then the residue was tutrited in EtOAc and filtered to give 13a as a white solid (100 mg, 89%), m.p. 229.4–232.4 °C; IR (KBr): υ max cm−1 3736 (NH), 3420 (NH), 3276 (NH), 2361 (C-N), 1677 (C=O), 1540 (C=C), 1452 (C=C), 1379 (C=C); 1H NMR (400 MHz, DMSO): δ 11.90 (s, 1H, NH), 8.56 (s, 1H, Ar-H), 8.49 (d, J=1.5, 1H, Ar-H), 8.42 (s, 1H, CH), 8.02–8.09 (m, 5H, Ar-H), 7.89 (d, J=8.5, 2H, Ar-H), 7.61–7.82 (m, 7H, Ar-H and NH), 3.88 (s, 3H, CH3); ES-MS 476.1 (M+H)+; HRMS calcd for C24H22N5O4S+ 476.1393, found 476.1383.
Methyl (E)-3-(3-((2-carbamimidoylhydrazono)methyl)-1-(phenylsulfonyl)-1H-indol-5-yl)benzoate hydrochloride (13b)
13b was obtained by reaction of 12b as previously described for 13a as a white solid (56 mg, 73%), m.p. 206.6–208.6 °C; IR (KBr): υ max cm−1 3735 (NH), 3396 (NH), 3275 (NH), 2361 (C-N), 1673 (C=O), 1539 (C=C), 1453 (C=C), 1377 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.08 (s, 1H, NH), 8.55 (s, 1H, Ar-H), 8.47 (s, 1H, Ar-H), 8.44 (s, 1H, CH), 8.23 (s, 1H, Ar-H),7.99–8.10 (m, 4H, Ar-H), 7.95 (d, J=7.7, 1H, Ar-H), 7.67–7.87 (m, 5H, Ar-H and NH), 7.60–7.66 (m, 3H, Ar-H), 3.89 (s, 3H, CH3); ES-MS 476.1 (M+H)+; HRMS calcd for C24H22N5O4S+ 476.1393, found 476.1388.
(E)-2-((1-(Phenylsulfonyl)-5-(4-(trifluoromethoxy)phenyl)-1H-indol-3-yl)methylene)hydrazine-1-carboximidamide hydrochloride (13c)
13c was obtained by reaction of 12c as previously described for 13a as a white solid (134 mg, 83%), m.p. 268.9–269.4 °C; IR (KBr): υ max cm−1 3735 (NH), 3488 (NH), 3255 (NH), 2361 (C-N), 1674 (C=O), 1540 (C=C), 1455 (C=C), 1380 (C=C); 1H-NMR (400 MHz, DMSO): δ 11.93 (s, 1H, NH), 8.55 (s, 1H, Ar-H), 8.43 (d, J=1.5, 1H, Ar-H), 8.41 (s, 1H, CH), 8.02–8.10 (m, 3H, Ar-H), 7.81–7.87 (m, 2H, Ar-H), 7.60–7.80 (m, 7H, Ar-H and NH), 7.44 (d, J=8.0, 2H, Ar-H); ES-MS 502.1 (M+H)+; HRMS calcd for C23H19F3N5O3S+ 502.1161, found 502.1152.
(E)-2-((1-(Phenylsulfonyl)-5-(3-(trifluoromethoxy)phenyl)-1H-indol-3-yl)methylene)hydrazine-1-carboximidamide hydrochloride (13d)
13d was obtained by reaction of 12d as previously described for 13a as a white solid (170 mg, 65%), m.p. 218.6–220.8 °C; IR (KBr): υ max cm−1 3468 (NH), 3255 (NH), 3128 (NH), 2361 (C-N), 1674 (C=O), 1538 (C=C), 1452 (C=C), 1380 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.08 (s, 1H, NH), 8.55 (s, 1H, Ar-H), 8.45 (d, J=1.5, 1H, Ar-H), 8.43 (s, 1H, CH), 8.05–8.07 (m, 3H, Ar-H), 7.70–7.81 (m, 6H, Ar-H and NH), 7.56–7.70 (m, 4H, Ar-H), 7.36 (d, J=8.2, 1H, Ar-H); ES-MS 502.1 (M+H)+; HRMS calcd for C23H19F3N5O3S+ 502.1161, found 502.1156.
(E)-2-((1-(Phenylsulfonyl)-5-(4-(trifluoromethyl)phenyl)-1H-indol-3-yl)methylene)hydrazine-1-carboximidamide hydrochloride (13e)
13e was obtained by reaction of 12e as previously described for 13a as a white solid (55 mg, 89%), m.p. 249.0–249.3 °C; IR (KBr): υ max cm−1 3735 (NH), 3396 (NH), 3216 (NH), 2361 (C-N), 1673 (C=O), 1539 (C=C), 1454 (C=C), 1379 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.05 (s, 1H, NH), 8.57 (s, 1H, Ar-H), 8.49 (d, J=1.5, 1H, Ar-H), 8.43 (s, 1H, CH), 8.02–8.13 (m, 3H, Ar-H), 7.95 (d, J=8.1, 2H, Ar-H), 7.70–7.85 (m, 7H, Ar-H and NH), 7.64 (t, J=7.8, 2H, Ar-H); ES-MS 486.1 (M+H)+; HRMS calcd for C21H19F3N5O2S+ 486.1212, found 486.1201.
(E)-2-((5-(3-Chloro-4-fluorophenyl)-1-(phenylsulfonyl)-1H-indol-3-yl)methylene)hydrazine-1-carboximidamide hydrochloride (13f)
13f was obtained by reaction of 12f as previously described for 13a as a white solid (110 mg, 98%), m.p. 234.2–237.3 °C; IR (KBr): υ max cm−1 3736 (NH), 3462 (NH), 3128 (NH), 2361 (C-N), 1672 (C=O), 1539 (C=C), 1455 (C=C), 1379 (C=C); 1H-NMR (400 MHz, DMSO): δ 11.91 (s, 1H, NH), 8.55 (s, 1H, Ar-H), 8.41 (s, 1H, Ar-H), 8.40 (s, 1H, CH), 8.02–8.07 (m, 3H, Ar-H), 7.95 (dd, J=7.1, 1H, Ar-H), 7.70–7.77 (m, 5H, Ar-H and NH), 7.63–7.66 (m, 3H, Ar-H), 7.50 (t, J=9.1, 1H, Ar-H); ES-MS 470.1 (M+H)+; HRMS calcd for C22H18ClFN5O2S+ 470.0854, found 470.0849.
Methyl (E)-4-(3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(phenylsulfonyl)-1H-indol-5-yl)benzoate dihydrobromide (14a)
To a solution of 12a (50 mg, 0.11 mmol) in dry MeOH (2 ml) was added 2-hydrazino-2-imidazoline hydrobromide (20 mg, 0.11 mmol), and then the reaction solution was stirred at 75 °C. After the completion of the reaction, the solvent was removed under reduced pressure and then the residue was tutrited in EtOAc and filtered to give 14a as a white solid (48 mg, 88%), m.p. 228.9–230.3 °C; IR (KBr): υ max cm−1 3735 (NH), 3439 (NH), 3174 (NH), 2361 (C-N), 1661 (C=O), 1541 (C=C), 1454 (C=C), 1376 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.39 (s, 1H, NH), 8.55 (s, 1H, Ar-H), 8.51 (d, J=1.6, 1H, Ar-H), 8.42 (s, 1H, CH), 8.11–8.01 (m, 5H, Ar-H), 7.88 (d, J=8.5, 2H, Ar-H), 7.79 (dd, J1=8.6, J2=1.8, 1H, Ar-H), 7.75 (d, J=7.6, 1H, Ar-H), 7.65 (t, J=7.8, 2H, Ar-H), 3.88 (s, 3H, CH3), 3.72 (s, 4H, CH2); ES-MS 502.2 (M+H)+; HRMS calcd for C26H24N5O4S+ 502.1549, found 502.1538.
Methyl (E)-3-(3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(phenylsulfonyl)-1H-indol-5-yl)benzoate hydrobromide (14b)
14b was obtained by reaction of 12b as previously described for 14a as a white solid (187 mg, 97%), m.p. 237.4–240.4 °C; IR (KBr): υ max cm−1 3736 (NH), 3459 (NH), 3123 (NH), 2361 (C-N), 1665 (C=O), 1541 (C=C), 1452 (C=C), 1378 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.31 (s, 1H, NH), 8.56 (s, 1H, Ar-H), 8.49 (s, 1H, Ar-H), 8.43 (s, 1H, CH), 8.25 (s, 1H, Ar-H), 8.07–8.09 (m, 3H, Ar-H), 8.01 (d, J=8, 1H, Ar-H), 7.96 (d, J=7.6, 1H, Ar-H), 7.76 (t, J=7.6, 2H, Ar-H), 7.61–7.67 (m, 3H, Ar-H), 3.89 (s, 3H, OCH3), 3.74 (s, 4H, CH2); ES-MS 502.2 (M+H)+; HRMS calcd for C26H24N5O4S+ 502.1549, found 502.1539.
(E)-3-((2-(4,5-Dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(phenylsulfonyl)-5-(4-(trifluoromethoxy)phenyl)-1H-indole hydrobromide (14c)
14c was obtained by reaction of 12c as previously described for 14a as a white solid (100 mg, 60%), m.p. 254.4–254.5 °C; IR (KBr): υ max cm−1 3732 (NH), 3445 (NH), 3179 (NH), 2361 (C-N), 1667 (C=O), 1541 (C=C), 1455 (C=C), 1378 (C=C); 1H NMR (400 MHz, DMSO): δ 12.28 (s, 1H, NH), 8.55 (s, 1H, Ar-H), 8.44(s, 1H, Ar-H), 8.42 (s, 1H, CH), 8.06–8.09 (m, 3H, Ar-H), 7.83 (d, J=8.6, 2H, Ar-H), 7.62–7.77 (m, 4H, Ar-H), 7.46 (d, J=8.1, 2H, Ar-H), 3.74 (s, 4H, CH2 CH2); ES-MS 528.1 (M+H)+; HRMS calcd for C25H21F3N5O3S+ 528.1317, found 528.1306.
(E)-3-((2-(4,5-Dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(phenylsulfonyl)-5-(3-(trifluoromethoxy)phenyl)-1H-indole hydrobromide (14d)
14d was obtained by reaction of 12d as previously described for 14a as a white solid (183 mg, 53%), m.p. 243.2 °C; IR (KBr): υ max cm−1 3736 (NH), 3435 (NH), 3174 (NH), 2361 (C-N), 1666 (C=O), 1541 (C=C), 1452 (C=C), 1376 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.33 (s, 1H, NH), 8.57 (s, 1H, Ar-H), 8.47 (d, J=1.2, 1H, Ar-H), 8.42 (s, 1H, CH), 8.06–8.09 (m, 3H, Ar-H), 7.74–7.78 (m, 4H, Ar-H), 7.58–7.68 (m, 3H, Ar-H), 7.37 (d, J=8.4, 1H, Ar-H), 3.73 (s, 4H, CH2); ES-MS 528.1 (M+H)+; HRMS calcd for C25H21F3N5O3S+ 528.1317, found 528.1305.
(E)-3-((2-(4,5-Dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(phenylsulfonyl)-5-(4-(trifluoromethyl)phenyl)-1H-indole hydrobromide (14e)
14e was obtained by reaction of 12e as previously described for 14a as a white solid (204 mg, 90%), m.p. 249.2–249.7 °C; IR (KBr): υ max cm−1 3735 (NH), 3449 (NH), 3178 (NH), 2361 (C-N), 1666 (C=O), 1542 (C=C), 1451 (C=C), 1379 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.31 (s, 1H, NH), 8.58 (s, 1H, Ar-H), 8.49 (d, J=1.2, 1H, Ar-H), 8.43 (s, 1H, CH), 8.08–8.10 (m, 3H, Ar-H), 7.93 (d, J=8.0, 2H, Ar-H), 7.82 (d, J=8.4, 2H, Ar-H), 7.77 (m, 2H, Ar-H), 7.66 (t, J=7.8, 2H, Ar-H), 3.74 (s, 4H, CH2); ES-MS 512.1 (M+H)+; HRMS calcd for C23H18F3N5O2S+ 512.1368, found 512.1359.
(E)-5-(3-Chloro-4-fluorophenyl)-3-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1-(phenylsulfonyl)-1H-indole hydrobromide (14f)
14f was obtained by reaction of 12f as previously described for 14a as a white solid (205 mg, 91%), m.p. 256.3–256.5 °C; IR (KBr): υ max cm−1 3735 (NH), 3281 (NH), 2925 (NH), 2361 (C-N), 1664 (C=O), 1546 (C=C), 1457 (C=C), 1373 (C=C); 1H-NMR (400 MHz, DMSO): δ 12.31 (s, 1H, NH), 8.56 (s, 1H, Ar-H), 8.42 (d, J=1.6, 1H, Ar-H), 8.41 (s, 1H, CH), 8.03–8.08 (m, 3H, Ar-H), 7.94 (dd, J1=7.0, J2=2.2, 1H, Ar-H), 7.71–7.77 (m, 3H, Ar-H), 7.65 (t, J=7.8, 2H), 7.52 (t, J=8.8, 1H, Ar-H), 3.74 (s, 4H, CH2); ES-MS 496.1 (M+H)+; HRMS calcd for C24H20ClFN5O2S+ 496.1010, found 496.1001.
Biology
Bacterial strains
S. aureus strains ATCC 29213, ATCC 25923 and ATCC-S-6007 were purchased from the American Type Culture Collection (Manassas, VA, USA). When required for testing, these strains were retrieved from storage at −80 °C and subcultured on Luria-Bertani agar for viability and purity.
The clinical strains used for MIC determinations were isolated from hospital in-patients as part of their routine investigations for the diagnosis of various infections. They were identified by standard microbiological methods and tested for antibiotic susceptibilities by the Kirby Bauer method23, 24 as well as the semiautomated BD Phoenix system (Becton Dickinson, Franklin Lakes, NJ, USA).
As these strains were archived routine isolates made anonymous by the removal of all labels that could be traced back to the patient source, ethical approval for this study was exempted.
Determination of MIC and MBC
All bacterial strains were initially screened with 100 μg ml−1 of each compound in Trypticase Soy Broth with 1% DMSO. The compounds showing bactericidal activity at this concentration were then further tested in two-fold dilutions from 50 to 0.39 μg ml−1. The inoculum used was an overnight culture diluted 1:1000 for Gram-negative bacteria and 1:100 for Gram-positive bacteria, to make approximately 105 cfu ml−1.
Each well in a sterile 96-well microtiter plate was filled with 150 μl of compound in Trypticase Soy Broth or Middlebrook broth with 1% DMSO. Each compound concentration was pipetted into duplicate wells. The wells were then inoculated with 10 μl of culture, covered with a plate cover and incubated at 36 °C for 48 h. OD readings (at 600 nm) were taken at 0, 24 and 48 h. After 48 h of incubation, each well was subcultured onto a blood agar plate to demonstrate bacterial growth. Viability and 1% DMSO controls were used in every plate.
For the determination of MIC, OD readings for test wells were compared with those of the control wells; an arrested or at least 50% retarded increase in OD reading was taken to indicate bacterial growth inhibition. MBC was defined as the lowest compound concentration to cause complete absence of bacterial growth on subculture.
For Mycobacterium H37Ra, inoculations were made in Middlebrook 7H9 broth and incubated up to 4 weeks. Subcultures were made onto Middlebrook 7H10 agar at 2 weeks and again at 4 weeks of incubation.
Molecular docking
In total, 40 compounds were designed and synthesized, and molecular docking was performed by using GOLD (v 5.2.2 Genetic Optimization for Ligand Docking, Cambridge, UK). Each ligand was docked 10 times, starting each time from a different random population of ligand orientations and using the default automatic genetic algorithm parameter settings. The Moenomycin A in the crystal structure was used as the reference to indicate the binding site for the molecular docking. All torsion angles in each compound were allowed to rotate freely and the results of the different docking runs were ranked using Gold Score.

(a) POCl3 dry DMF; (b) substituted benzyl bromide, NaH, dry DMSO; (c) aminoguanidine hydrochloride, dry CH3OH; (d) 2-hydrazino-2-imidazoline hydrobromide, dry CH3OH.

(a) benzoyl chloride, NaH, dry DMF; (b) aminoguanidine hydrochloride, dry CH3OH; (c) 2-hydrazino-2-imidazoline hydrobromide, dry CH3OH.

(a) POCl3, dry DMF; (b) benzenesulfonyl chloride, NaH, dry DMF; (c) substituted phenylboronic acid, K2CO3, Pd(dbpf)Cl2, tetrahydrofuran/H2O; (d) aminoguanidine hydrochloride, dry CH3OH; (e) 2-hydrazino-2-imidazoline hydrobromide, dry CH3OH.
References
Yang, Z. et al. Proportions of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus in patients with surgical site infections in mainland China: a systematic review and meta-analysis. PLoS ONE 10, e0116079 (2015).
Kali, A. Antibiotics and bioactive natural products in treatment of methicilln resistant Staphylococcus aureus: a brief review. Pharmacogn. Rev. 9, 29–34 (2015).
Kumar, A., Verma, N., Agrawal, D. & Sharma, B. S. Endoscopic lavage for antibiotic unresponsive severe Acinetobacter baumanii ventriculitis: an unexplored treatment option. Acta Neurochir. (Wien) 157, 1225–1227 (2015).
Ahmed, M. U. et al. Sensitive, resistant and multi-drug resistant Acinetobacter baumanii at Saudi Arabia hospital eastern region. Pak. J. Pharm. Sci. 28, 825–832 (2015).
Lee, C. Y., Tsai, H. C., Lee, S. S. & Chen, Y. S. Concomitant emphysematous prostatic and periurethral abscesses due to Klebsiella pneumoniae: a case report and review of the literature. Southeast Asian J. Trop. Med. Public Health 45, 1099–1106 (2014).
Huang, C. H. et al. Risk factors for in-hospital mortality in patients with type 2 diabetes complicated by community-acquired Klebsiella pneumoniae bacteremia. J. Formos Med. Assoc. 114, 916–922 (2015).
Yaita, K., Komatsu, M., Oshiro, Y. & Yamaguchi, Y. Postoperative meningitis and epidural abscess due to extended-spectrum β-lactamase-producing Klebsiella pneumoniae: a case report and a review of the literature. Intern. Med. 51, 2645–2648 (2012).
Wright, G. D. A new target for antibiotic development. Science 315, 1373–1374 (2007).
Halliday, J., McKeveney, D., Muldoon, C., Rajaratnam, P. & Meutermans, W. Targeting the forgotten transglycosylases. Biochem. Pharmacol. 71, 957–967 (2006).
Bohdan, O. B. & Walker, S. Moenomycin family antibiotics: chemical synthesis, biosynthesis, biological activity. Nat. Prod. Rep. 27, 1594–1617 (2010).
Xu, H., Wang, Q. & Yang, W. B. Antifungal activities of some indole derivatives. Z. Naturforsch C. 65, 437–439 (2010).
Zhang, M. Z. et al. Synthesis and antifungal activity of 3-(1,3,4-oxadiazol-5-yl)-indoles and 3-(1,3,4-oxadiazol-5-yl)methyl-indoles. Eur. J. Med. Chem. 63, 22–32 (2013).
Zhao, F., Liu, N., Zhan, P., Jiang, X. & Liu, X. Discovery of HCV NS5B thumb site I inhibitors: core-refining from benzimidazole to indole scaffold. Eur. J. Med. Chem. 94, 218–228 (2015).
Giraud, F. et al. Design, synthesis and evaluation of 3-(imidazol-1-ylmethyl)indoles as antileishmanial agents. Part II. J. Enzyme Inhib. Med. Chem. 24, 1067–1075 (2009).
Andrew, L. L. et al. Structural insight into the transglycosylation step of bacterial cell-wall biosynthesis. Science 315, 1402–1405 (2007).
Penthala, N. R., Yerramreddy, T. R. & Crooks, P. A. Synthesis and in vitro screening of novel N-benzyl aplysinopsin analogs as potential anticancer agents. Bioorg. Med. Chem. Lett. 21, 1411–1413 (2011).
Andreani, A. et al. Imidazo[2,1-b]thiazole guanidinylhydrazones as RSK2 inhibitors. Eur. J. Med. Chem. 46, 4311–4323 (2011).
Mohammad, H. et al. Synthesis and antibacterial evaluation of a novel series of synthetic phenylthiazole compounds against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 94, 306–316 (2015).
Wang, Y. et al. Structure-based design, synthesis, and biological evaluation of isatin derivatives as potential glycosyltransferase inhibitors. Chem. Biol. Drug Des. 84, 685–696 (2014).
Xu, H. & Fan, L. Antifungal agents. Synthesis and antifungal activities of novel indole[1,2-c]-1,2,4-benzotriazine derivatives against phytopathogenic fungi in vitro. Eur. J. Med. Chem. 46, 364–369 (2011).
Moseley, J. D., Murray, P. M., Turp, E. R., Tyler, S. N. G. & Burn, R. T. A mild robust generic protocol for the Suzuki reaction using an air stable catalyst. Tetrahedron 68, 6010–6017 (2012).
Al-Marzooq, F., Ngeow, Y. F. & Tay, S. T. Emergence of Klebsiella pneumoniae producing dual carbapenemases (NDM-1 and OXA-232) and 16S rRNA methylase (armA) isolated from a Malaysian patient returning from India. Int. J. Antimicrob. Agents 45, 445–446 (2015).
Clinical and Laboratory Standards Institute. Approved Standard M2-A10. Performance Standards for Antimicrobial Disk Susceptibility Tests 10th edn. (CLSI, Wayne, PA, USA, (2009).
Clinical and Laboratory Standards Institute. M100-S21 (M2). Disk Diffusion Supplemental Tables (CLSI, Wayne, PA, USA, (2011).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81460538, 81660588) given to WH; IPSR/RMC/UTARRF/2015-C1/NO3 awarded to YFN by Universiti Tunku Abdul Rahman (UTAR), Kuala Lumpur, Malaysia; and University of Malaya-MOHE High Impact Research grant (UM.C/625/1/HIR/MOHE/DENT/22) given to ICP. The authors would like to thank Dr Charles Laughton at University of Nottingham, UK for offering the access to the computing facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hong, W., Li, J., Chang, Z. et al. Synthesis and biological evaluation of indole core-based derivatives with potent antibacterial activity against resistant bacterial pathogens. J Antibiot 70, 832–844 (2017). https://doi.org/10.1038/ja.2017.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.55
This article is cited by
-
A brief review of the biological potential of indole derivatives
Future Journal of Pharmaceutical Sciences (2020)
-
Synthesis of novel indole derivatives containing double 1,3,4-oxadiazole moiety as efficient bactericides against phytopathogenic bacterium Xanthomonas oryzae
Chemical Papers (2019)