Abstract
We hypothesized that the suppression of uninvolved immunoglobulin in monoclonal gammopathy of undetermined significance (MGUS) as detected by suppression of the isotype-specific heavy and light chain (HLC-pair suppression) increases the risk of progression to malignancy. This approach required quantitation of individual heavy/light chains (for example, IgGλ in IgGκ MGUS patients). Of 1384 MGUS patients from Southeastern Minnesota seen at the Mayo Clinic from 1960 to 1994, baseline serum samples obtained within 30 days of diagnosis were available in 999 persons. We identified HLC-pair suppression in 27% of MGUS patient samples compared with 11% of patients with suppression of uninvolved IgG, IgA or IgM. HLC-pair suppression was a significant risk factor for progression (hazard ratio (HR), 2.3; 95% confidence interval (CI) 1.5–3.7; P<0.001). On multivariate analysis, HLC-pair suppression was an independent risk factor for progression to malignancy in combination with serum M-spike size, heavy chain isotype and free light chain ratio (HR, 1.8; 95% CI, 1.1–3.00; P=0.018). The finding that HLC-pair suppression predicts progression in MGUS and occurs several years before malignant transformation has implications for myeloma biology.
Similar content being viewed by others
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a common premalignant plasma cell proliferative disorder that is a precursor of multiple myeloma (MM).1, 2, 3 A number of prognostic factors for progression of MGUS to MM have been identified. These include the size of the M-spike, heavy chain isotype, detection of urinary monoclonal light chain, percentage of bone marrow plasma cells, isotype suppression of uninvolved immunoglobulins, and serum-free light chain (FLC) κ/λ ratio.4, 5, 6, 7 By combining three of these variables (M-spike size, heavy chain isotype and serum FLC ratio), we have constructed a model that provides approximately a 10-fold difference in risk for MGUS progression.6 By contrast, suppression of uninvolved, polyclonal immunoglobulins (immunoparesis) has not been a consistent predictor of progression in MGUS.7, 8 Immunoparesis has always been defined as suppression of uninvolved immunoglobulins (for example, suppression of IgM and IgA in a patient with IgG MGUS). The effect on normal, polyclonal IgG could not be ascertained in a patient with IgG MGUS, as standard nephelometric assays do not differentiate between monoclonal and polyclonal IgG. However, we hypothesized that immunoglobulin heavy chain isotype-specific suppression (for example, IgG λ suppression in the case of IgG κ MGUS) is a marker of a more clonally advanced MGUS stage and will be associated with a greater risk of progression to MM. The novel Hevylite assay now enables us to accurately measure each isotype-specific heavy and light chain (HLC) (that is, IgGκ, IgGλ, IgAκ, IgAλ, IgMκ and IgMλ).9 Thus, for the first time, we can measure isotype-specific suppression of the uninvolved HLC-pair in order to more broadly test the impact of immunoparesis. The purpose of this study was to determine the prognostic value of isotype-specific suppression of immunoglobulins in a large population-based cohort of patients with MGUS. Identification of additional biomarkers that predict the risk of progression in MGUS is needed not just from a clinical stand point but also from a biological stand point to better understand the pathogenesis of myeloma.
Materials and methods
The study population was derived from a cohort of 1384 southeastern Minnesota patients with MGUS who were seen at the Mayo Clinic from 1 January 1960 through 31 December 1994; the characteristics of this group have previously been described.4 Our study group consisted of 999 of the MGUS cohort on whom cryopreserved serum samples collected within 30 days of initial diagnosis were available for assay (Table 1).
Each sample was tested for total immunoglobulin concentrations (that is, IgG, IgA and IgM), as well as for HLC concentration (that is, IgGκ, IgGλ, IgAκ, IgAλ, IgMκ and IgMλ). Total immunoglobulins were quantified by immunonephelometry (BNII, Siemens Diagnostics, Marburg, Germany). HLC concentrations were quantified by immunonephelometry using Hevylite reagents provided by the manufacturer (The Binding Site, Birmingham, UK). The IgG, IgA and IgM reference ranges are from the Mayo Clinic routine Immunology Laboratory. The reference ranges for each HLC and the HLC-pair ratios were derived from 129 normal serum donors aged 22–77 years. There was no apparent age or sex effect on the HLC reference ranges. An abnormal HLC-pair ratio was defined as being outside (either high or low) the reference range. Isotype-specific suppression of the uninvolved HLC (HLC-pair suppression) was defined as a concentration of the polyclonal member of the HLC-pair below the lower limit of the reference range concentration (for example, in a MGUS patient with a monoclonal IgGκ protein, suppression of IgGλ below the lower limit of the reference range for IgGλ). Suppression of uninvolved immunoglobulins was defined as either of the other two polyclonal heavy chain isotypes being below the lower limit of the reference range (for example, in a MGUS patient with a monoclonal IgG protein, suppression of IgA and/or IgM below the lower limits of their reference ranges).
Statistical considerations
The prognostic effect of HLC-pair suppression on the progression of MGUS was studied in the context of other known risk factors. The primary end point was progression to MM or a related disorder. Progression end points were examined both as a cumulative probability of progression and cumulative incidence. The former was computed using an ordinary Kaplan–Meier estimate10 where patients who died were censored; curves were compared using the log-rank test. The cumulative incidence curve, on the other hand, explicitly accounts for death from other causes (such as cardiovascular disease, cerebrovascular disease or unrelated malignancy) as a competing risk and was estimated using the method of Gooley.12 The effects of potential risk factors on progression rates were examined using a Cox proportional hazards model.11
Results
Clinical characteristics and reference levels
The adult 95% reference ranges for each HLC and HLC-pair ratio are shown on Table 2. No age or gender effects existed for any of these assays. The HLC ranges are similar to previously published ranges,9 and, similar to that study, the HLC-pair ratios were different for each heavy chain isotype. The median of the normal IgGκ/IgGλ ratio was 2.0, whereas the IgAκ/IgAλ ratio median was 1.4 and the IgMκ/IgMλ ratio median was 1.6.
HLC-pair ratio and HLC-pair suppression
Table 3 shows the occurrence of abnormal HLC-pair ratios, HLC-pair suppression and uninvolved isotype suppression segregated by MGUS heavy chain type and M-protein size. An abnormal HLC-pair ratio was detected in 66% of MGUS patients, but the frequency differed depending on the isotype of the M-protein. Patients with monoclonal IgG proteins presented with an abnormal HLC-pair ratio in only 56% of cases, whereas IgA and IgM MGUS had abnormal HLC-ratios in 97% and 90% of cases, respectively. As the monoclonal IgG concentration increased, the prevalence of an abnormal HLC-pair ratio also increased. Abnormal HLC-pair ratios were common in the IgA and IgM MGUS patients even at low M-spike concentrations.
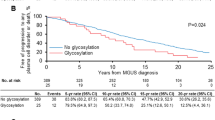
These same data were evaluated for HLC-pair suppression and uninvolved immunoglobulin suppression (Table 3). HLC-pair suppression was present in 27% of the MGUS patients, but uninvolved immunoglobulin suppression was observed in only 11% of the cases. The higher frequency of HLC-pair suppression compared with uninvolved immunoglobulin suppression was most obvious in the IgG MGUS patients, who had 29% HLC-pair suppression compared with 5% uninvolved isotype suppression.
Outcomes
For our initial assessment of the effect of HLC-pair suppression, we identified the lowest 5% HLC polyclonal concentrations in the IgG, IgA and IgM MGUS isotype groups and evaluated the effect on progression to MM. There were 13.5 expected progressions in this group but 22 observed progressions (1.6-fold increase over expected). This excess risk was seen in IgG MGUS (1.8-fold), IgA MGUS (1.2-fold) and IgM MGUS (1.7-fold). The effect of HLC-pair suppression, abnormal HLC-pair ratio, uninvolved immunoglobulin suppression, as well as previously identified variables of M-protein size, heavy chain isotype and FLC ratio, were tested in univariate analysis for their significance as prognostic factors for progression of MGUS (Table 4). All of these variables were significant in univariate analysis.
The prognostic effect of the risk factors significant on univariate analysis for the progression of MGUS to MM was then studied by multivariate analysis (Table 5). HLC-pair suppression remained significant when serum M-protein size and FLC ratio were analyzed in the multivariate model as continuous variables. By contrast, abnormal HLC-pair ratio (hazard ratio (HR)=1.4, P=0.38) and suppression of uninvolved immunoglobins (HR=1.1, P=0.74) were not significant for MGUS progression on multivariate analysis. When we analyzed the effect of HLC-pair suppression separately with each of the other risk factors in this multivariate model, the HR for HLC-pair suppression was 2.6 in combination with IgA or IgM heavy chain (P<0.001), 2.0 in combination with M-spike size (P<0.002), and 1.5 in combination with FLC ratio (P<0.082).
Risk stratification model
The effect of adding HLC-pair suppression to our previous risk assessment model6 is shown in Table 6. Except for the lowest risk group, the inclusion of HLC-pair suppression further divided the groups into lower and higher risk. We then developed a modified risk stratification model using the 4 variables of M-spike concentration, FLC ratio, heavy chain isotype and HLC-pair suppression is shown in Figure 1. The model has five groups (0, 1, 2, 3 or 4 adverse risk factors), and the probability of progression to MM increases with the number of risk factors.
Risk of progression of MGUS to MM using a risk stratification model that incorporates HLC-pair suppression, FLC κ/λ ratio, heavy chain isotype and size of the serum monoclonal protein. The top curve illustrates risk of progression in patients with all four risk factors, namely HLC-pair suppression, abnormal serum FLC κ/λ ratio, serum M-spike ⩾1.5 gm/dl and non-IgG MGUS; the second gives the risk of progression in patients with any three of these risk factors; the third curve illustrates the risk of progression with two of these risk factors; the forth curve illustrates the risk of progression with one of these risk factors; and the bottom curve is the risk of progression for patients with none of the risk factors.
Discussion
The term MGUS was first coined over 30 years ago.13 Studies have shown that MGUS almost always precedes MM,2, 3 and that the risk of progression of MGUS to MM or related malignancy is approximately 1% per year.4 Moreover, there is no decline in the risk of progression even after 25–35 years, raising the need for lifelong follow-up by primary-care providers necessary in all persons identified with MGUS. More accurate stratification of risk is essential if we are to consider early intervention strategies and better understand the mechanism of progression of MGUS to MM. Unfortunately, distinguishing the patients at high risk of progression is difficult when MGUS is initially recognized, as the distinction between MM and its precursor conditions is not based on histopathology or laboratory methods but on the manifestation of end organ damage.7 Consequently, patients are typically referred to hematologists in the face of a rising M-spike on follow-up or when other symptoms and signs suggestive of myeloma or related malignancy (for example, anemia, hypercalcemia, bone pain, lytic bone lesions and renal failure) have developed. However, this does not allow identification of candidates at highest risk of progression for possible intervention strategies, nor optimization of follow-up, as the efficacy and recommendations for monitoring vary with risk.14, 15
Our previous studies have indicated that the size of the M-spike, the heavy chain isotype and the secretion of excess FLC as detected by an abnormal serum FLC ratio are all predictive of progression, and they can be used to stratify risk at initial diagnosis of MGUS.6 In our earlier studies, we showed that these three variables each carry a 2–3-fold increased risk for progression. Moreover, combining all three variables identifies low- and high-risk patients with a 10-fold difference in risk: low-risk patients have only a 0.1%/year risk for progression to MM compared with high-risk patients with a 1.4%/year risk. In addition to the need for refining prognosis for optimal follow-up and preventive strategies, identification of additional risk factors may also provide important new insight into the biology and mechanisms of progression of MGUS to MM.
As noted above, the progression of MGUS to MM is defined by the manifestation of clinical symptoms due to the clone of monoclonal plasma cells. Suppression of uninvolved immunoglobulins is seen in many patients with MM, but it is not part of current diagnostic criteria, and there have been conflicting reports about the prognostic value of uninvolved immunoglobulin concentrations in MGUS.4, 5 We hypothesized that suppression of non-clonal plasma cells is a high-risk marker for progression, and that the inconsistent prognostic results seen previously were due to the relative insensitivity of total uninvolved immunoglobulin suppression for identifying this phenomenon. The availability of reagents to separately quantitate the κ- and λ-containing proteins of each immunoglobulin isotype now provides a unique tool to study polyclonal isotype-specific immunoglobulin suppression in patients with MGUS. In this paper, we demonstrate the independent prognostic value of HLC-pair suppression in MGUS. We find that the suppression of the isotype-specific HLC (for example, suppression of the IgGλ level below the reference range in IgGκ MGUS) is associated with a 2-fold excess risk of progression to MM after adjusting for other known risk factors, namely the size and type of the serum M-protein and the FLC ratio. When added to the risk groups defined by M-spike size, heavy chain isotype and FLC ratio, the HLC-pair suppression made added prognostic value to each of the subgroups with the exception of the lowest risk group.
When HLC-pair suppression was tested by multivariate analysis in combination with only M-spike size or only FLC ratio, we noted a slight reduction in risk due to HLC-pair suppression. As IgG half-life can be reduced by saturation of the IgG Fc receptors, it is possible that some of this reduction in risk is simply due to larger M-spikes resulting in shorter half-life for the uninvolved IgG HLC.16 Similarly, the FLC ratio may already contain some information due to immune suppression. However, these interactions did not abrogate the overall prognostic value of the HLC-pair suppression. On multivariate analysis, the HLC-pair suppression contributed unique prognostic information, independent of the serum M-spike size, heavy chain type and FLC ratio.
This study was possible because we had retained serum samples from a large cohort of MGUS patients, collected at the time of first diagnosis, which could subsequently be tested for HLC concentrations using the new Hevylite assay. There are a number of important biologic and clinical implications of our findings. First, we show that isotype-specific suppression of polyclonal HLC is a risk factor for progression of MGUS to MM. Second, we find that the different isotypes have different potential for polyclonal immune suppression. For example, IgG MGUS patients rarely cause uninvolved isotype suppression as detected by IgA or IgM levels, but can efficiently suppress polyclonal IgG as detected by the uninvolved HLC-pair concentration. The observation that IgG monoclonal plasma cell clones are able to more efficiently suppress the IgG populations of the opposite light type, compared with immunoglobulins of other heavy chain types, is unexplained. Whether this is due to different suppressive mechanism or simple proximity of plasma cell clones of different isotypes is not known. Third, all the MGUS sera evaluated in this study were initial diagnostic samples, and it is striking that the ‘progression-phenotype’ and the suppressive effect on uninvolved immunoglobulin synthesis is established so early in the disease course. The suppression of polyclonal HLC-pairs that we documented in this study occurred several years (median, 10 years) before the eventual disease manifestation and progression to MM.
Besides the main findings related to MGUS prognosis described above, there are other results that need to be highlighted, as they will be of value for future studies with this assay. The reagents used in the Hevylite assay are antibodies that detect epitopes at the interface of immunoglobulin heavy chain and light chain, and whose specificity is therefore defined by structures on both peptides.9 As previously noted and confirmed here, each heavy chain isotype apparently makes use of a different repertoire of light chains as indicated by the different reference ranges for light chain ratios of each HLC-pair. Thus, the median value for the IgGκ/IgGλ ratio is 2.0 compared with IgMκ/IgMλ and IgAκ/IgAλ ratios of 1.6 and 1.4, respectively. In addition, we found that IgG MGUS is different from IgA and IgM regarding isotype suppression and also differs from IgA and IgM in the sensitivity of the HLC-pair ratio for detecting monoclonality. The sensitivity of detecting an abnormal IgGκ/IgGλ ratio was dependent on the concentration of the monoclonal protein and had only 56% sensitivity for detecting monoclonality in the IgG MGUS patients. We assume that this relative insensitivity is due to the higher concentration of the polyclonal IgG background compared with IgA and IgM.
We conclude that HLC-pair suppression is an independent risk factor for progression in MGUS. Although the finding has major implications for the biology of MGUS progression, the clinical utility of this assay needs further study. Studies also need to be done to determine if immune suppression is more frequent closer to progression to malignant disease, and whether HLC-pair suppression can be reliably used as an early predictor of MM.
References
Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354: 1362–1369.
Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RE et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 2009; 113: 5412–5417.
Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM . A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113: 5418–5422.
Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med 2002; 346: 564–569.
Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol 2002; 20: 1625–1634.
Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, Bradwell AR, Clark RJ et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance (MGUS). Blood 2005; 106: 812–817.
Kyle RA, Rajkumar SV . Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23: 3–9.
Pérez-Persona E, María-Belén V, Mateo G, Garcia-Sanz R, Mateos M-V, Garcia de Coca A et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007; 110: 2586–2592.
Bradwell AR, Harding SJ, Fourrier NJ, Wallis GLF, Drayson MT, Carr-Smith HD et al. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clin Chem 2009; 55: 1646–1655.
Kaplan E, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life tables. J R Stat Soc 1972; 34: 187–202.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Kyle RA . Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am J Med 1978; 64: 814–826.
Kyle RA, Durie BGM, Rajkumar SV, Landgren O, Blade J, Merlini G et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010; 24: 1121–1127.
Bianchi G, Kyle RA, Colby CL, Larson DR, Kumar S, Katzmann JA et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance (MGUS) on early diagnosis and prevention of myeloma-related complications. Blood 2010; 116: 2019–2025.
Ward ES, Zhou J, Ghetie V, Ober RJ . Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol 2003; 15: 187–195.
Acknowledgements
This study was supported in part by the National Cancer Institute (CA107476, CA100707, CA83724), National Institutes of Health, US Public Health Service, Bethesda, MD and in part by the Jabbs Foundation, Birmingham, UK and the Henry J Predolin Foundation, USA.
Author contributions
JAK, SVR, RAK, ARB and AD designed the research. RC generated the data. DRL, TMT, JTB and CLC analyzed the data. JAK, SVR, LJM, OL, JRC, AD, SK and RAK wrote and edited the manuscript. All authors reviewed and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Bradwell is the founder of the Binding Site, manufacturer of the Hevylite assay and has a financial investment in the company. Dr Kyle has received honoraria from The Binding Site for educational lectures. None of the other authors have conflicts of interest pertinent to this manuscript.
Rights and permissions
About this article
Cite this article
Katzmann, J., Clark, R., Kyle, R. et al. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia 27, 208–212 (2013). https://doi.org/10.1038/leu.2012.189
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.189
Keywords
This article is cited by
-
Immunoparesis defined by heavy/light chain pair suppression in smoldering multiple myeloma shows initial isotype specificity and involves other isotypes in advanced disease
Annals of Hematology (2021)
-
Prevalence of monoclonal gammopathy of undetermined significance in a large population with annual medical check-ups in China
Blood Cancer Journal (2020)
-
Monoclonal Gammopathy of Undetermined Significance: Current Concepts and Future Prospects
Current Hematologic Malignancy Reports (2020)
-
Elevated IgM and abnormal free light chain ratio are increased in relatives from high-risk chronic lymphocytic leukemia pedigrees
Blood Cancer Journal (2019)
-
The multiple myelomas — current concepts in cytogenetic classification and therapy
Nature Reviews Clinical Oncology (2018)