Abstract
Bacterial colonisation of the intestine has a major role in the post-natal development and maturation of the immune and endocrine systems. These processes are key factors underpinning central nervous system (CNS) signalling. Regulation of the microbiome–gut–brain axis is essential for maintaining homeostasis, including that of the CNS. However, there is a paucity of data pertaining to the influence of microbiome on the serotonergic system. Germ-free (GF) animals represent an effective preclinical tool to investigate such phenomena. Here we show that male GF animals have a significant elevation in the hippocampal concentration of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid, its main metabolite, compared with conventionally colonised control animals. Moreover, this alteration is sex specific in contrast with the immunological and neuroendocrine effects which are evident in both sexes. Concentrations of tryptophan, the precursor of serotonin, are increased in the plasma of male GF animals, suggesting a humoral route through which the microbiota can influence CNS serotonergic neurotransmission. Interestingly, colonisation of the GF animals post weaning is insufficient to reverse the CNS neurochemical consequences in adulthood of an absent microbiota in early life despite the peripheral availability of tryptophan being restored to baseline values. In addition, reduced anxiety in GF animals is also normalised following restoration of the intestinal microbiota. These results demonstrate that CNS neurotransmission can be profoundly disturbed by the absence of a normal gut microbiota and that this aberrant neurochemical, but not behavioural, profile is resistant to restoration of a normal gut flora in later life.
Similar content being viewed by others
Introduction
Gastrointestinal (GI) homeostasis is central to our general health and wellbeing.1, 2, 3 However, a specific role for gut health in the regulation of mood and behaviour is now emerging,4 particularly involving bidirectional communication along the microbiome–gut-brain axis.5, 6, 7, 8, 9 Bacterial colonisation of the intestine has a major role in the post-natal development and maturation of the endocrine and immune systems,2, 7 both of which underpin central nervous system (CNS) function6 and are of particular relevance to the developing serotonergic system.10, 11
Marrying these tenets together has led to the plausible hypothesis that the microbiota might have a key neurodevelopmental role. Indeed, studies exploring this concept have emerged demonstrating that germ-free (GF) animals had an exaggerated stress response compared with their conventionally colonised (CC) counterparts and that this aberrant endocrine profile could be at least partially reversed by reconstitution of the microbiota before a critical time window later in life.12 Moreover, hippocampal protein levels of brain-derived neurotrophic factor (BDNF) were also under the influence of the resident flora.
The functional behavioural consequences of growing up germ-free have been shown to reproducibly unmask a reduced anxiety phenotype compared with colonised animals in multiple independent laboratories and using different methods of assessment.13, 14, 15 A recent study that examined the effects of microbiota transplants between strains of mice with differential exploratory behaviour profiles offered further evidence of a microbial influence on anxiety levels.15 Challenging the GI tract with pathogenic microorganisms can also elicit an increased anxiety response.16, 17 Moreover, probiotic administration studies suggest that ingestion of Bifidobacteria or Lactobacilli can beneficially alter either anxiety or depressive-like behaviours, both under pathological conditions18 and in healthy animals.19, 20 Furthermore, we have previously shown that probiotic administration influences the availability of tryptophan, the serotonin precursor.21 Collectively, these studies suggest that microbiota manipulation could be a valid therapeutic strategy to modulate CNS signalling.6
There is limited data pertaining to the control exerted by the microbiota on the hippocampal serotonergic system, which is particularly relevant to stress, anxiety and depression.11, 13 This is despite the fact that microbiota-associated hippocampal alterations across multiple other neurobiological domains have been reported.6, 12, 14 The regulation of the serotonergic system is influenced by sex,22, 23 thus it is important to investigate the influence of microbiome–gut–brain axis in mice of both sexes. Indeed, it is worth noting that many GF studies have been only carried out in female mice, which are the sex of choice in immunological, but not neurobiological, studies.24, 25
Consequently, we focused on addressing whether the microbiota effects on the CNS extended to the serotonergic system in the hippocampus and whether such effects are sex dependent. Subsequently, we determined whether the alterations that we observed could be reversed following colonisation post weaning of animals previously lacking a gut microflora.
Materials and methods
Animals
GF and CC Swiss Webster breeding pairs were supplied by Taconic (Germantown, New York, USA) and first-generation offspring were used in all experiments. GF Swiss Webster mice were housed 4–5/cage in flexible film gnotobiotic isolators under a strict 12-h light/dark cycle. CC mice were similarly housed 4–5/cage in the standard animal facility under the same controlled conditions (temperature 20–21 °C, 55–60% humidity) on the same 12 h light/dark cycle. In all cases, both GF and CC mice received the same pelleted diet after it was autoclaved (sodium dodecyl sulphate diets, product code 801010). Experiments were conducted in accordance with the European Directive 86/609/EEC and the Recommendation 2007/526/65/EC, and were approved by the Animal Experimentation Ethics Committee of University College Cork.
Experimental design
Both the GF and CC mice were euthanized in adulthood at 6–9 weeks. Cohorts of both animal types (male and female) were randomly allocated to three groups: one for high-performance liquid chromatography (HPLC) and quantitative real-time PCR (qRT-PCR) analysis (n=9–10), a second for novel-environment stress-induced corticosterone production (n=9–10) and a third for immunological assessment including splenocyte stimulation (n=5–8). In a subsequent study, a group of male GF mice were colonised (n=9) by removing them from the GF facility post weaning (3 weeks) at which time they were allowed grow to adulthood in the conventional animal facility in cages with bedding and faecal matter from CC mice, a protocol that has previously demonstrated to be effective at restoring a normal microbiota.26, 27 All the three groups (GF, CC, and colonised GF) were assessed for anxiety behaviour in the light–dark box apparatus 24 h before sacrifice for HPLC analysis.
Sample collection and analysis
Unanaesthetised animals were euthanized in a random order by cervical dislocation. The brain was rapidly removed from the cranium, the hippocampus dissected out on an ice-cold plate and stored in either chilled HPLC homogenisation buffer as previously described28 or RNA later (as per the manufacturer's instructions) and stored at −80 °C for subsequent analysis. The spleen was carefully dissected out for splenocyte stimulation studies (see Supplementary information (SI) online for more details). Trunk blood was collected in tubes with ethylenediaminetetraacetic acid (EDTA) as anticoagulant, centrifuged (3500 g, 15 min, 4 °C) and the plasma stored at −80 °C for later analysis. The HPLC assay for 5-hydroxytryptamine/5-hydroxyindoleacetic acid (5-HT/5-HIAA) was based on a previously used method29 as was the HPLC assay for tryptophan and its kynurenine pathway metabolites30 (see Supplementary Information online for more details). qRT-PCR was carried out as described previously29, 31 as was the protocol for the light–dark box32 (see Supplementary Information online for more details). All samples were collected from 0900 to 1300 hours.
Novel-environment stress
CC and GF mice were individually removed from the home cage and placed in a novel cage (33 cm × 16 cm × 13 cm, L × H × W) for 30 min before sacrifice with subsequent collection of plasma for corticosterone analysis (see Supplementary Information online for more details).
Statistical analysis
The sample size was determined by a power calculation and aimed at detecting differences between groups at the 0.05 level. Data was expressed as mean±s.e.m. Statistical analysis was carried out using SPSS 18 for Windows (SPSS, Chicago, ILL, US). Statistically significant alterations were determined using a two/three-way analysis of variance followed by Bonferroni post-hoc tests or by one-way analysis of variance followed by Bonferroni post-hoc tests.
Results
Both male and female GF animals have a blunted immune response
A two-way analysis of variance (ANOVA) revealed no interaction between sex and GF status in the production of tumour necrosis factor α (TNF-α) following splenocyte stimulation with lipopolysaccharide (LPS) (F1,22=0.2, P=0.64), with a significant effect of GF status (F1,22=17.3, P<0.001). Post-hoc analysis revealed a blunted production of tumour necrosis factor α in both male (P<0.05) and female (P<0.01) GF animals compared with their CC counterparts (Figure 1a).
(a) Blunted immune response in germ-free (GF) animals. Tumour necrosis factor α (TNFα) production (pg/ml) in GF and conventionally colonised (CC) animals (male and female) following stimulation of splenocytes with lipopolysaccharide (LPS; *P<0.05, male GF vs CC; $$P<0.01, female GF vs CC). (b) Exaggerated stress response in GF animals. Corticosterone release (pg/ml) following an acute stressor in male and female GF and CC animals. (**P<0.01, male GF vs CC; $$$P<0.001, female GF vs CC).
Both male and female GF animals have enhanced hypothalamic–pituitary–adrenal axis reactivity
There was no overall interaction between stress, sex and GF status in the release of corticosterone following a novel-environment stressor (F1,75=0.04, P=0.85). The same analysis indicated a significant interaction between stress and GF status (F1,75=21.84, P<0.001). Post-hoc analysis revealed an exaggerated production of corticosterone in both male (P<0.01) and female (P<0.001) GF animals compared with their CC counterparts (Figure 1b).
Decreased Bdnf expression in GF animals is sex specific
There was a significant GF × sex interaction for Bdnf expression levels in the hippocampus (F1,39=12.526, P=0.001). Post-hoc analysis revealed a significant decrease in the GF animals compared with their CC counterparts in male animals only (P<0.01, Figure 2a).
(a) Brain-derived neutrotrophic factor (Bdnf) expression in the hippocampus. Decreased Bdnf expression (fold change) in the hippocampus of male germ-free (GF) mice compared with their conventionally colonised (CC) counterparts (**P<0.01, male GF vs CC). (b and c) Sex-specific alterations in serotonergic system. (b) Increased 5-hydroxytryptamine (5-HT) concentration (ng/g tissue) in the hippocampus of male GF mice compared with their CC counterparts (**P<0.01, male GF vs CC). (c) Increased 5-hydroxyindoleacetic acid (5-HIAA) concentration (ng/g tissue) in the hippocampus of male GF mice compared with their CC counterparts (***P<0.001, male GF vs CC). (d and e) Altered tryptophan availability and metabolism in GF animals. (d) Increased plasma tryptophan concentrations (ng/ml) in male GF animals compared with their CC controls (***P<0.001, male GF vs CC). (e) Decreased plasma kynurenine:tryptophan ratio in both male and female GF animals compared with their CC controls (***P<0.001, male GF vs CC; $$$P<0.001, female GF vs CC).
Hippocampal weight
A two-way ANOVA analysis of hippocampal weight indicated no effect of sex (F1,39=1.9, P=0.18), GF status (F1,39=0.02, P=0.9) or GF × sex interaction (F1,39=0.34, P=0.565) for this brain region.
Increased 5-HT concentrations in GF animals are sex specific
There was a trend towards a GF × sex interaction for 5-HT concentrations in the hippocampus (F1,39=3.86, P=0.057). The same analysis indicated a significant effect for GF status (F1,39=5.31, P=0.027) and sex (F1,39=4.61, P=0.038). Post-hoc analysis revealed a significant increase in 5-HT in GF compared with their CC counterparts (P<0.01) in male animals only (Figure 2b).
There was a significant interaction between GF status and sex for 5-HIAA concentrations, the main metabolite of 5-HT, in the hippocampus (F1,39=9.46, P=0.004). The same analysis revealed a significant effect for both GF status (F1,39=6.87, P=0.013) and sex (F1,39=13.43, P=0.0008). Post-hoc analysis indicated significantly increased 5-HIAA concentrations in GF compared with CC in male animals only (P<0.001, Figure 2c).
Tryptophan availability and metabolism is altered in GF animals
Although there was no significant GF × sex interaction for plasma tryptophan concentrations (F1,39=1.131, P=0.29), there was both a significant GF (F1,39=17.97, P=0.0001) and sex effect (F1,39=25.78, P<0.0001). Post-hoc analysis revealed a significant increase in plasma tryptophan availability in male (P<0.001), but not female (P=0.09), GF mice compared with their CC controls (Figure 2d).
Similarly, there was no significant GF × sex interaction (F1,39=1.3, P=0.26) in the kynurenine:tryptophan ratio, an indicator of tryptophan metabolism along the kynurenine pathway. However, there was both a significant GF (F1,39=64.78, P<0.0001) and sex effect (F1,39=14.63, P=0.0005) on this ratio. Post-hoc analysis revealed a significant decrease in this ratio in both male (P<0.001) and female (P<0.001) GF animals compared with their CC controls (Figure 2e).
GF status and serotonergic gene expression
There was an interaction between GF status and sex only for the 5-HT2C receptor gene (Table 1). Post-hoc analysis demonstrated no difference between GF animals of either sex and their respective CC controls for any of the targets assessed (Table 1).
GF status and body weight
A two-way ANOVA analysis of body weight data indicates an effect of sex (F1,39=60.269, P<0.001) but not GF status (F1,39=0.4, P=0.5). There was also a significant interaction between sex and GF status (F1,39=11.68, P=0.002). A Bonferroni post-hoc analysis revealed that there was no body weight difference between male GF animals and their CC counterparts (27.3±0.4 g vs 25.98±0.61 g, t=1.97, P>0.05) but female GF animals had a lower body weight than their CC counterparts (22.0±0.39 g vs 23.92±0.44 g, t=2.86, P<0.05).
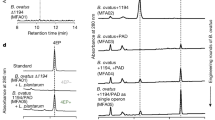
Increased 5-HT and 5-HIAA concentration in GF animals is resistant to colonisation
There was a significant overall group effect for 5-HT concentrations in the hippocampus (F2,24=4.527, P=0.0215). A post-hoc analysis demonstrated that both GF (P<0.05) and colonised GF mice (P<0.05) had elevated hippocampal 5-HT concentrations compared with their CC control animals (Figure 3a).
(a and b) Hippocampal serotonergic alterations in male germ-free (GF) animals are resistant to the effects of colonization. (a) Increased hippocampal 5-hydroxytryptamine (5-HT) concentrations (ng/g tissue) are maintained in male colonised animals (*P<0.05, conventionally colonised (CC) vs GF, colonised GF). (b) Increased hippocampal 5-hydroxyindoleacetic acid (5-HIAA) concentrations (ng/g tissue) are maintained in male colonised animals (*P<0.05, CC vs GF, colonised GF). (c and d) Tryptophan availability and metabolism is restored to control levels following colonisation. (c) Colonisation of male GF mice restores plasma tryptophan concentrations (ng/ml) to control levels (*P<0.05, GF vs CC; #P<0.05, colonised GF vs GF). (d) Colonisation of male GF mice restores the plasma kynurenine:tryptophan ratio to control values (**P<0.001, GF vs CC; ###P<0.001, colonised GF vs GF). (e) Anxiety levels depend on GF status. Colonisation of male GF mice restores the number of transitions in the light–dark box to control values (**P<0.01, GF vs CC; ##P<0.01, colonised GF vs GF).
There was a significant overall group effect for 5-HIAA concentrations in the hippocampus (F2,22=4.904, P=0.0173). A post-hoc analysis demonstrated that both GF (P<0.05) and colonised GF mice (P<0.05) had elevated hippocampal 5-HIAA concentrations compared with their CC control animals (Figure 3b).
Colonisation restores tryptophan availability/metabolism to control levels
There was an overall group effect for plasma tryptophan concentrations (F2,25=4.293, P=0.026). A post-hoc analysis demonstrated that GF animals had elevated plasma tryptophan concentrations compared with CC control animals (P<0.05). Colonisation of the GF animals reduced plasma tryptophan concentrations compared with GF levels (P<0.05, Figure 3c).
There was an overall group effect for the kynurenine:tryptophan ratio (F2,25=18.5, P<0.0001). A post-hoc analysis demonstrated that GF animals had a reduction in this ratio compared with the CC control animals (P<0.01). Colonisation of the GF animals increased the kynurenine:tryptophan ratio compared with GF animals (P<0.001, Figure 3d).
Reduced anxiety-like behaviour in GF animals is reversed following colonisation
There was an overall group effect for the number of transitions in the light–dark box (F2,25=6.974, P=0.004). A post-hoc analysis revealed that the GF animals were less anxious than their CC controls as evidenced by an increased number of transitions (P<0.01). Colonised GF mice displayed a reduced number of transitions compared with the GF animals (P<0.01, Figure 3e).
Discussion
Bacterial colonisation of the GI tract is integral to a variety of structural, metabolic and protective functions.7 Emerging evidence now also supports a role for the microbiota in the development of the CNS.6, 12, 14, 15 In our present work, we show for, what is to our knowledge, the first time that this remit extends to a marked influence on the hippocampal serotonergic system. Our results also highlight a sex-specific effect of the microbiota on this brain region, a factor that should inform future studies in GF animals seeking to explore the CNS consequences of an absent microbiota. In addition, we have demonstrated that these neurochemical, but not behavioural, alterations are resistant to a restoration of the GI microenvironment later in life.
As previous studies had demonstrated hippocampal alterations at both the protein and gene expression levels in GF animals,12, 14 we focused on this important brain region in our assessment of the impact of the microbiota on serotonergic function. We initially demonstrated that the consequences of growing up GF extends to a clear increase in both 5-HT and 5-HIAA, its main metabolite, over the normal levels of these neurochemicals present in the hippocampus following conventional colonisation of the GI tract. The fact that a chronic absence of the microbiota can elicit a 1.3-fold increase in 5-HT, a degree of magnitude not dissimilar to more traditional CNS-directed interventions (escitalopram and lithium),33 adds weight to recent speculation that the resident flora might represent a valid therapeutic target for CNS disorders.6 This should be tempered by our subsequent results, which demonstrated that this increase in hippocampal 5-HT concentration was maintained despite colonisation of the GF animals with a normal microbiota post weaning. In this regard, determining the impact of colonisation with specific strains of bacteria will be particularly informative.12
We then determined whether the neurochemical effects we demonstrated could also be observed at the gene expression level for key indices of serotonergic functionality. Despite the increased 5-HT concentration observed, we did not find an altered expression of the Tph2 gene, the key CNS isoform of the enzyme responsible for the synthesis of 5-HT from tryptophan,34, 35, 36 its amino-acid precursor, in either the male or female GF animals compared with their respective CC counterparts. However, this does not preclude the possibility that the intrinsic activity of this enzyme might be altered in GF animals. It is also noteworthy that the enzyme is not saturated at normal tryptophan concentrations so that enzyme-specific perturbations are not required per se to result in an increased 5-HT synthesis.37 Metabolomic studies indicate that colonisation of GF mice by the gut microbiota results in a 2.8-fold increase in plasma serotonin levels, which could theoretically also be due to a GI microbiota-mediated alteration in tryptophan metabolism.38 These findings are in agreement with the more generalised findings from metagenomic studies, which suggest the microbiome significantly enriches the metabolism of amino acids.39
Similarly, no alteration in gene expression level was found for either the SERT (serotonin transporter) gene or the range of serotonergic receptor genes evaluated (5-HT1A, 5-HT6 and 5-HT2C). Interestingly, decreased 5-HT1A receptor gene expression has been reported specifically in the dentate granule layer of the hippocampus for female GF animals14 using in-situ hybridisation. It is possible that more subtle changes in the hippocampal serotonergic system may occur at the gene expression level in male GF animals than our qRT-PCR approach can detect in whole-tissue homogenates.
It is noteworthy that many of the CNS alterations we found occurred in a sex-specific manner. Only male GF animals exhibited the serotonergic alterations described above as well as the decreased Bdnf expression. Interestingly, this sex specificity does not extend to peripheral immunological or neuroendocrine measures with both male and female GF mice having a blunted immune response to LPS stimulation and an exaggerated release of corticosterone following an acute stressor compared with their CC counterparts. The latter findings are in line with the available literature on GF status.7, 12 Future studies seeking to exploit the GF paradigm should take this into account and choose the appropriate sex of animal according to the outputs under investigation.
The mechanism surrounding these sex differences are not well understood but may relate to the well-known, but complex, influence of the oestrous cycle hormones on the CNS serotonergic system.40 For example, estrogen and its receptor (ERβ) is known to have a role in the modulation of hippocampal serotonin concentrations.41 It is possible then that this system exerts a larger influence over CNS serotonin concentrations than the resident microflora. Clearly, further studies are required to confirm this hypothesis and the use of ovarietomized animals, notwithstanding the challenges of such an approach when working with GF mice, may be necessary to address this important issue.
Only male GF animals exhibited a decrease in hippocampal Bdnf mRNA expression which is in agreement with previous data in male animals, albeit at the protein level,12 but is discordant with recent data showing increased Bdnf mRNA expression in the hippocampus of female GF mice.14 One potentially significant design difference in the latter study by Neufeld and colleagues is that their experiments were carried out on commercially sourced GF animals just 48 h following arrival at their facility.6, 14 This caveat notwithstanding, these data once again highlight the importance of sex on the modulatory CNS effects of the microbiota. Increased emphasis is being placed on the role of sex in modulating neurotransmitter systems relevant to stress, anxiety and depression.42, 43 Nonetheless, caution should be exercised in any attempt to translate sex differences in rodents to human populations.24, 25 It is also worth noting that although BNDF is an important neurotrophin for hippocampal development and repair, we did not observe any weight differences in this brain region between the GF and CC animals, regardless of sex. Nevertheless, the decreases in hippocampal BDNF in the male GF animals may be a compensatory response to the persistent elevation in 5-HT concentrations in that brain region.
We next investigated whether alterations in the peripheral availability of tryptophan might be at the root of the serotonergic alterations we observed. In support of this theory, we found that plasma tryptophan concentrations were significantly elevated in male GF animals only. Since CNS concentrations of tryptophan are predominantly determined by this circulating pool,37 our data suggests an increased supply of this amino acid precursor of serotonin to the CNS and a potential humoral route through which the microbiota can influence the CNS in addition to the recently validated neural mechanism of action.19 Previous studies have demonstrated alterations in tryptophan availability following probiotic administration.21 The fact that a similarly significant increase in plasma tryptophan concentration is not evident in the female GF animals reinforces the importance of investigating sex differences at different nodes of the microbiome–gut–brain axis and may explain why we did not see any increases in hippocampal serotonergic concentrations in our female GF animals.
Degradation of tryptophan along the kynurenine pathway actually represents the dominant metabolic fate for this amino acid37 and the ratio of kynurenine, the main metabolite, to the parent compound has been extensively used as an index of activity along this metabolic cascade.30, 44, 45, 46 In our study, this ratio indicated a reduced metabolism of tryptophan along the kynurenine pathway in male GF animals compared with their CC controls. Interestingly, this was also evident in the female GF animals where, as discussed above, it did not result in significantly altered tryptophan concentrations.
Given that the majority of central effects of GF status were unmasked in male animals, we used these for our colonisation studies where we sought to determine if restoration of a normal microbiota later in life could also reinstate normal CNS 5-HT concentrations. As indicated earlier, this proved not to be the case with the aberrant serotonergic profile maintained in the colonised GF animals. Conversely, circulating tryptophan levels were restored to control levels, accompanied by a normalisation of the kynurenine:tryptophan ratio. This reversal of plasma tryptophan availability, while reaffirming microbiota manipulation as a viable strategy for tryptophan and kynurenine pathway modulation, was apparently insufficient to impact on the hippocampal 5-HT aberrations. Thus, it appears that the neurochemical consequences of early-life intestinal microflora absence, during which time the hippocampus is particularly susceptible to stressors,47 are much more difficult to reverse with an unbiased adjustment of the GI milieu later in life. This observation also extends to the serotonergic system whose neurons are among the first to develop and may consequently be most at risk to early-life disturbances in intestinal homeostasis.10 Indeed this time period, including early adolescence, represents a critical window during which the brain is programmed for later life.48, 49, 50 However, we cannot rule out the possibility that the changes in the hippocampal serotonergic system may take a longer time to reverse than the peripheral tryptophan levels.
GF status has been most consistently associated with reductions in anxiety-like behaviours, a feature which is consistent across multiple independent laboratories using different assessment strategies.6, 13, 14, 15 Here we show once again that the male GF mice display less anxiety-like behaviours than their CC counterparts, using the light–dark box paradigm. We also report that, in contrast to the neurochemical alterations described, colonisation of the GF animals restored normal anxiety-like behaviours. Previously, this has only been demonstrated for the progeny of what have been termed ‘recolonised animals’,13 not directly in the ‘recolonised’ animals themselves. Conversely, reconstitution of the intestinal microbiota has proved insufficient to reverse the alteration in anxiety at all in female mice.51 The former study used a different mouse strain, making direct comparison with our results difficult. Irrespective of these methodological and other differences,6 the current dataset support a role for the microbiota in the regulation of anxiety-like behaviours and further implicates our resident flora as a viable therapeutic target. Further behavioural studies are urgently required to elaborate on these findings, particularly in relation to whether GF animals display altered locomotor activity or reduced behavioural inhibition but also to characterise depression-related behaviours and cognitive performance.
Collectively, the current report and data from other GF studies indicate that there is a discordance in the increased stress reactivity physiologically and the reduction in anxiety behaviourally, which has now been observed in three different research groups using different tasks.13, 14, 15 Interestingly, chronic treatment with a Lactobacillus strain has been shown to reduce anxiety and also reduce stress-induced corticosterone responses when given to adult CC animals.19 This suggests that the effect on anxiety is probably not due to a general adult miscommunication in the microbiome–gut–brain axis but may be developmentally primed.
Despite the fact that 5-HT has been shown to regulate the developmental ontogeny of anxiety at critical time windows during development,10, 52, 53 our current data could be interpreted as exemplifying a dissociation between the elevations in hippocampal serotonin concentrations and anxiety-like behaviours in the light–dark box. However, the relationship between serotonin and anxiety is far from being straight forward with both an anxiogenic and anxiolytic role being proposed for elevations in 5-HT, much debate still existing over the neurocircuitry involved54, 55 and yet selective serotonin reuptake inhibitors being the first choice treatment in certain anxiety disorders.56 Moreover, it is worth noting that dietary depletion of tryptophan or administration of SSRIs has been shown to have no or modest effects on light–dark box behaviour that further supports a dissociation between hippocampal 5-HT and anxiety-like behaviours in this task.57, 58, 59 Finally, as exemplified by studies with SERT knockout mice, serotonin-mediated alterations in anxiety during development can be mouse-strain dependent,60 and thus future studies, where logistically possible, should focus on whether data generated generalise to other mouse strains.
The microbiota has a broad and critical role in supporting normal digestion and host metabolism and it is interesting to consider how this function might contribute to the results presented here. A significant energy source for humans is the bacterial metabolism of dietary fiber to short-chain fatty acids, agents that are of importance for gut motility, have a trophic effect on epithelial cells, impact on immune system development and modulate enterendocrine hormone secretion.2 Moreover, systemic administration of butyrate, a histone deacetylase inhibitor, has been shown to influence BDNF expression in the hippocampus.4 However, other alternatives are also possible. For example, certain microorganisms, including Lactobacilli, are able to convert nitrate to nitric oxide, a potent regulator of both the immune and nervous systems, while others can produce neuroactive amino acids such as γ-aminobutyric acid (GABA).4 Indeed, it is likely that multiple mechanisms, including but not limited to the examples quoted above, may contribute to our results. However, it should also be noted that there was no difference in the dietary availability of proteins, fatty acids, amino acids or vitamins between the GF and CC groups.
It is also worth considering whether the differences we have reported might be related to the influence of GF status on maternal care, a factor which can have an important role in shaping behaviour in adulthood.61 However, previous reports by Sudo and colleagues,12 who assessed maternal care and did not find any effect of GF status, suggest that this does not contribute to the repertoire of perturbations we have reported. Another caveat relates to the well-known influence of cyclical female sex hormone variation on behavioural and neurochemical parameters.62 Although this can be important, the short duration of the oestrous cycle in mice coupled with the duration of the study and the fact that female mice housed together can phase into the same cycle stage63, 64 would most likely result in not having a differential predominance of animals in any one particular phase of the cycle across groups.
The current study is limited by the common difficulty all GF studies have in directly translating the results to the clinical situation where no equivalent obliteration of the microbiota can be said to exist.6 Nevertheless, it may have direct relevance to neonatal care units, where current hygienic standards in developed countries coupled to the frequent use of broad spectrum antibiotics65 may be the closest approximation to the early-life GF environment under investigation in our experiments. Indeed, the altered commensal colonisation pattern engendered by such a scenario has already been linked to the risk of developing immune-mediated diseases in western infants.65 Our studies suggest that the broader implications for the CNS should now also be considered.
In conclusion, it is emerging that a more complete understanding of the molecular, cellular and physiological basis of microbiome–gut–brain communication is required before any potential therapeutic benefits of microbiota manipulation strategies can be generated.6 We have demonstrated a sex-specific role for the microbiota in the regulation of CNS serotonergic neurotransmission profiles. These studies offer further proof of the importance of the resident flora, both as an aetiological factor and in the development of novel treatment modalities for microbiome–gut–brain axis disorders.
References
Bischoff SC . ‘Gut health’: a new objective in medicine? BMC Med 2010; 9: 24.
Grenham S, Clarke G, Cryan J, Dinan TG . Brain-gut-microbe communication in health and disease. Front Gastrointest Sci 2011; 2: 94.
Lyte M . The microbial organ in the gut as a driver of homeostasis and disease. Med Hypotheses 2010; 74: 634–638.
Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J . Mood and gut feelings. Brain Behav Immun 2010; 24: 9–16.
Mayer EA . Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 2011; 12: 453–466.
Cryan JF, O’Mahony SM . The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 2011; 23: 187–192.
O’Hara AM, Shanahan F . The gut flora as a forgotten organ. EMBO Rep 2006; 7: 688–693.
Rhee SH, Pothoulakis C, Mayer EA . Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009; 6: 306–314.
Bercik P, Collins SM, Verdu EF . Microbes and the gut-brain axis. Neurogastroenterol Motil 2012; 24: 405–413.
Gaspar P, Cases O, Maroteaux L . The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 2003; 4: 1002–1012.
Leonard BE . HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation 2006; 13: 268–276.
Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004; 558 (Part 1): 263–275.
Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 2011; 108: 3047–3052.
Neufeld KM, Kang N, Bienenstock J, Foster JA . Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 2010; 23: 255–264, e119.
Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011; 141: 599–609.e593.
Goehler LE, Lyte M, Gaykema RP . Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav Immun 2007; 21: 721–726.
Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP . Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun 2008; 22: 354–366.
Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG . Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010; 170: 1179–1188.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci 2011; 108: 16050–16055.
Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011; 105: 755–764.
Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG . The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 2008; 43: 164–174.
Jones MD, Lucki I . Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology 2005; 30: 1039–1047.
Maswood S, Truitt W, Hotema M, Caldarola-Pastuszka M, Uphouse L . Estrous cycle modulation of extracellular serotonin in mediobasal hypothalamus: role of the serotonin transporter and terminal autoreceptors. Brain Res 1999; 831: 146–154.
Cryan JF, Mombereau C . In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry 2004; 9: 326–357.
Palanza P . Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev 2001; 25: 219–233.
Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T et al. Patterns of early gut colonization shape future immune responses of the host. PLoS One 2012; 7: e34043.
O’Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci USA 2011; 108: 11217–11222.
Harkin A, Connor TJ, Mulrooney J, Kelly JP, Leonard BE . Prior exposure to methylenedioxyamphetamine (MDA) induces serotonergic loss and changes in spontaneous exploratory and amphetamine-induced behaviors in rats. Life Sci 2001; 68: 1367–1382.
O’Mahony S, Chua AS, Quigley EM, Clarke G, Shanahan F, Keeling PW et al. Evidence of an enhanced central 5HT response in irritable bowel syndrome and in the rat maternal separation model. Neurogastroenterol Motil 2008; 20: 680–688.
Clarke G, Fitzgerald P, Cryan JF, Cassidy EM, Quigley EM, Dinan TG . Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol 2009; 9: 6.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25: 402–408.
O’Mahony CM, Sweeney FF, Daly E, Dinan TG, Cryan JF . Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav Brain Res 2010; 213: 148–154.
Jacobsen JP, Mork A . The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res 2004; 1024: 183–192.
Nakamura K, Hasegawa H . Developmental role of tryptophan hydroxylase in the nervous system. Mol Neurobiol 2007; 35: 45–54.
Walther DJ, Bader M . A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 2003; 66: 1673–1680.
Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003; 299: 76.
Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA . Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 2006; 8: 1–27.
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009; 106: 3698–3703.
Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS et al. Metagenomic analysis of the human distal gut microbiome. Science 2006; 312: 1355–1359.
Bethea CL, Lu NZ, Gundlah C, Streicher JM . Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 2002; 23: 41–100.
Imwalle DB, Gustafsson JA, Rissman EF . Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav 2005; 84: 157–163.
Painsipp E, Herzog H, Sperk G, Holzer P . Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol 2011; 163: 1302–1314.
Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 2010; 15: 877, 896–904.
Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D . Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 2006; 364: 82–90.
Myint AM, Kim YK, Verkerk R, Park SH, Scharpe S, Steinbusch HW et al. Tryptophan breakdown pathway in bipolar mania. J Affect Disord 2007; 102: 65–72.
Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B . Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 2007; 98: 143–151.
Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ . Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol 2003; 27: 121–136.
de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV . Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev 2005; 29: 271–281.
Schmidt MV . Molecular mechanisms of early life stress—lessons from mouse models. Neurosci Biobehav Rev 2010; 34: 845–852.
Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, Valentino R . Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology 2011; 36: 896–909.
Neufeld KA, Kang N, Bienenstock J, Foster JA . Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol 2011; 4: 492–494.
Ansorge MS, Morelli E, Gingrich JA . Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci 2008; 28: 199–207.
Gross C, Hen R . The developmental origins of anxiety. Nat Rev Neurosci 2004; 5: 545–552.
Gordon JA, Hen R . The serotonergic system and anxiety. Neuromolecular Med 2004; 5: 27–40.
Graeff FG . Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev 2004; 28: 239–259.
Nutt DJ . Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr 2005; 10: 49–56.
Borsini F, Podhorna J, Marazziti D . Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002; 163: 121–141.
Browne CA, Clarke G, Dinan TG, Cryan JF . An effective dietary method for chronic tryptophan depletion in two mouse strains illuminates a role for 5-HT in nesting behaviour. Neuropharmacology 2012; 62: 1903–1915.
Cryan JF, Sweeney FF . The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 2011; 164: 1129–1161.
Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN . Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav 2003; 2: 365–380.
Kaffman A, Meaney MJ . Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry 2007; 48: 224–244.
Simpson J, Kelly JP . An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res 2012; 229: 289–300.
Gangrade BK, Dominic CJ . Studies of the male-originating pheromones involved in the Whitten effect and Bruce effect in mice. Biol Reprod 1984; 31: 89–96.
Whitten WK . Occurrence of anoestrus in mice caged in groups. J Endocrinol 1959; 18: 102–107.
Adlerberth I, Wold AE . Establishment of the gut microbiota in Western infants. Acta Paediatr 2009; 98: 229–238.
Acknowledgements
The Alimentary Pharmabiotic Centre is a research centre funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. The authors and their work were supported by SFI (grant nos. 02/CE/B124 and 07/CE/B1368) and by GlaxoSmithKline. GC is in receipt of a research grant from the American Neurogastroenterology and Motility Society (ANMS). GC, JFC and TD are also funded by the Irish Health Research Board (HRB) Health Research Awards (grant no HRA_POR/2011/23). We acknowledge the contribution of Ms Frances O’Brien, Dr Monica Tramullas and Mr Kieran Davey to the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Clarke, G., Grenham, S., Scully, P. et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18, 666–673 (2013). https://doi.org/10.1038/mp.2012.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.77
Keywords
This article is cited by
-
Sex differences in a mouse model of diet-induced obesity: the role of the gut microbiome
Biology of Sex Differences (2024)
-
Tryptophan metabolites and gut microbiota play an important role in pediatric migraine diagnosis
The Journal of Headache and Pain (2024)
-
Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice
Molecular Neurobiology (2024)
-
Cellular and Molecular Roles of Immune Cells in the Gut-Brain Axis in Migraine
Molecular Neurobiology (2024)
-
Diosgenin alleviates alcohol-mediated escalation of social defeat stress and the neurobiological sequalae
Psychopharmacology (2024)