Abstract
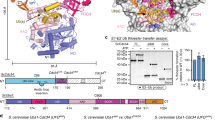
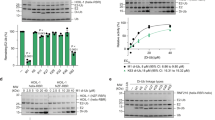
Post-translational modification by ubiquitin-like proteins (Ublps) is an essential cellular regulatory mechanism1,2,3. The Ublp NEDD8 regulates cell division, signalling and embryogenesis4,5,6. Ublps are conjugated to their targets by the sequential action of E1, E2 and often E3 enzymes3. Each Ublp has a dedicated E1, or activating enzyme, that initiates its conjugation cascade1,3,7,8,9,10. First, E1 associates with the Ublp and catalyses adenylation of the carboxy terminus of the Ublp. Second, E1 forms a thioester between its catalytic cysteine and the Ublp. Next, E1 is loaded with a second Ublp molecule, adenylating the C terminus of this second Ublp while still carrying the first thioester-bound Ublp. Last, E1 binds E2 and promotes Ublp transfer to the catalytic cysteine of E2. We report here the structure and mutational analysis of human APPBP1–UBA3, the heterodimeric E1 enzyme for NEDD8 (ref. 11). Each E1 activity is specified by a domain: an adenylation domain resembling bacterial adenylating enzymes12, an E1-specific domain organized around the catalytic cysteine, and a domain involved in E2 recognition resembling ubiquitin. The domains are arranged around two clefts that coordinate protein and nucleotide binding so that each of E1's reactions drives the next, in an assembly-line fashion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hochstrasser, M. Biochemistry. All in the ubiquitin family. Science 289, 563–564 (2000)
Jentsch, S. & Pyrowolakis, G. Ubiquitin and its kin: how close are the family ties? Trends Cell Biol. 10, 335–342 (2000)
Pickart, C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001)
Lammer, D. et al. Modification of yeast Cdc53p by the ubiquitin-related protein Rub1p affects function of the SCFCdc4 complex. Genes Dev. 12, 914–926 (1998)
Pozo, J. C., Timpte, C., Tan, S., Callis, J. & Estelle, M. The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science 280, 1760–1763 (1998)
Kurz, T. et al. Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science 295, 1294–1298 (2002)
Haas, A. L., Warms, J. V., Hershko, A. & Rose, I. A. Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J. Biol. Chem. 257, 2543–2548 (1982)
Haas, A. L. & Rose, I. A. The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J. Biol. Chem. 257, 10329–10337 (1982)
Hershko, A., Heller, H., Elias, S. & Ciechanover, A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J. Biol. Chem. 258, 8206–8214 (1983)
Haas, A. L., Bright, P. M. & Jackson, V. E. Functional diversity among putative E2 isozymes in the mechanism of ubiquitin-histone ligation. J. Biol. Chem. 263, 13268–13275 (1988)
Osaka, F. et al. A new NEDD8-ligating system for cullin-4A. Genes Dev. 12, 2263–2268 (1998)
Lake, M. W., Wuebbens, M. M., Rajagopalan, K. V. & Schindelin, H. Mechanism of ubiquitin activation revealed by the structure of a bacterial MoeB–MoaD complex. Nature 414, 325–329 (2001)
Finley, D., Ciechanover, A. & Varshavsky, A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell 37, 43–55 (1984)
Hatfield, P. M., Callis, J. & Vierstra, R. D. Cloning of ubiquitin activating enzyme from wheat and expression of a functional protein in Escherichia coli. J. Biol. Chem. 265, 15813–15817 (1990)
Johnson, E. S., Schwienhorst, I., Dohmen, R. J. & Blobel, G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509–5519 (1997)
Liakopoulos, D., Doenges, G., Matuschewski, K. & Jentsch, S. A novel protein modification pathway related to the ubiquitin system. EMBO J. 17, 2208–2214 (1998)
Whitby, F. G., Xia, G., Pickart, C. M. & Hill, C. P. Crystal structure of the human ubiquitin-like protein NEDD8 and interactions with ubiquitin pathway enzymes. J. Biol. Chem. 273, 34983–34991 (1998)
Sloper-Mould, K. E., Jemc, J. C., Pickart, C. M. & Hicke, L. Distinct functional surface regions on ubiquitin. J. Biol. Chem. 276, 30483–30489 (2001)
Beal, R., Deveraux, Q., Xia, G., Rechsteiner, M. & Pickart, C. Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc. Natl Acad. Sci. USA 93, 861–866 (1996)
Burch, T. J. & Haas, A. L. Site-directed mutagenesis of ubiquitin. Differential roles for arginine in the interaction with ubiquitin-activating enzyme. Biochemistry 33, 7300–7308 (1994)
Pickart, C. M., Kasperek, E. M., Beal, R. & Kim, A. Substrate properties of site-specific mutant ubiquitin protein (G76A) reveal unexpected mechanistic features of ubiquitin-activating enzyme (E1). J. Biol. Chem. 269, 7115–7123 (1994)
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998)
Stebbins, C. E., Kaelin, W. G. Jr & Pavletich, N. P. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumour suppressor function. Science 284, 455–461 (1999)
Bencsath, K. P., Podgorski, M. S., Pagala, V. R., Slaughter, C. A. & Schulman, B. A. Identification of a multifunctional binding site on Ubc9p required for Smt3p conjugation. J. Biol. Chem. 277, 47938–47945 (2002)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 176, 307–326 (1997)
Terwilliger, T. C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallogr. D 55, 849–861 (1999)
de la Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276, 472–494 (1997)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brünger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Scheffner, M., Huibregtse, J. M., Vierstra, R. D. & Howley, P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75, 495–505 (1993)
Acknowledgements
We thank P. Jeffrey, D. Minor, J. Berger, J. Huibregste and C. Ross for advice and assistance; M. Becker and staff at National Synchrotron Light Source Beamline X25, and T. Earnest, G. McDermott and staff at Advanced Light Source Beamline 5.0.2 for help with data collection; and P. Murray, T. Izard and L. Hendershot for comments on the manuscript. This work was supported by ALSAC, the NCI Cancer Center (CORE), and a Pew Scholar in Biomedical Sciences Award to B.A.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Walden, H., Podgorski, M. & Schulman, B. Insights into the ubiquitin transfer cascade from the structure of the activating enzyme for NEDD8. Nature 422, 330–334 (2003). https://doi.org/10.1038/nature01456
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01456
This article is cited by
-
Targeting NEDD8-activating enzyme for cancer therapy: developments, clinical trials, challenges and future research directions
Journal of Hematology & Oncology (2023)
-
Atg7 senses ATP levels and regulates AKT1-PDCD4 phosphorylation-ubiquitination axis to promote survival during metabolic stress
Communications Biology (2023)
-
Cryo-EM structures of Uba7 reveal the molecular basis for ISG15 activation and E1-E2 thioester transfer
Nature Communications (2023)
-
Targeting neddylation E2s: a novel therapeutic strategy in cancer
Journal of Hematology & Oncology (2021)
-
SUMO conjugating enzyme: a vital player of SUMO pathway in plants
Physiology and Molecular Biology of Plants (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.