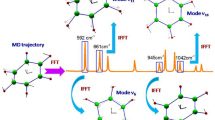
Abstract
The delineation of molecular properties that underlie reactivity and selectivity is at the core of physical organic chemistry1,2,3,4,5, and this knowledge can be used to inform the design of improved synthetic methods or identify new chemical transformations6,7,8,9. For this reason, the mathematical representation of properties affecting reactivity and selectivity trends, that is, molecular parameters, is paramount. Correlations produced by equating these molecular parameters with experimental outcomes are often defined as free-energy relationships and can be used to evaluate the origin of selectivity and to generate new, experimentally testable hypotheses6,10,11,12. The premise behind successful correlations of this type is that a systematically perturbed molecular property affects a transition-state interaction between the catalyst, substrate and any reaction components involved in the determination of selectivity10,11. Classic physical organic molecular descriptors, such as Hammett4, Taft3 or Charton5 parameters, seek to independently probe isolated electronic or steric effects3,4,5,6,13,14. However, these parameters cannot address simultaneous, non-additive variations to more than one molecular property, which limits their utility. Here we report a parameter system based on the vibrational response of a molecule to infrared radiation that can be used to mathematically model and predict selectivity trends for reactions with interlinked steric and electronic effects at positions of interest. The disclosed parameter system is mechanistically derived and should find broad use in the study of chemical and biological systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Williams, A. Free Energy Relationships in Organic and Bio-Organic Chemistry (Royal Society of Chemistry, 2003)
Verloop, A. &. Tipker, J. Physical basis of Sterimol and related steric constants. Pharmacochem. Libr. 10, 97–102 (1987)
Taft, R. W. Linear steric energy relationships. J. Am. Chem. Soc. 75, 4538–4539 (1953)
Hammett, L. P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 59, 96–103 (1937)
Charton, M. Steric effects. I. Esterification and acid-catalyzed hydrolysis of esters. J. Am. Chem. Soc. 97, 1552–1556 (1975)
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991)
Anslyn, E. V. & Dougherty, D. A. Modern Physical Organic Chemistry (University Science Books, 2006)
Jacobsen, E. N., Zhang, W. & Guler, M. L. Electronic tuning of asymmetric catalysts. J. Am. Chem. Soc. 113, 6703–6704 (1991)
Harper, K. C. & Sigman, M. S. Three-dimensional correlation of steric and electronic free energy relationships guides asymmetric propargylation. Science 333, 1875–1878 (2011)
Curtin, D. Y. Stereochemical control of organic reactions differences in behaviour of diastereoisomers. Rec. Chem. Prog. 15, 110–128 (1954)
Jaffe, H. H. A reexamination of the Hammett equation. Chem. Rev. 53, 191–261 (1953)
Hammett, L. P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 17, 125–136 (1935)
Charton, M. The application of the Hammett equation to ortho-substituted benzene reaction series. Can. J. Chem. 38, 2493–2499 (1960)
Harper, K. C., Bess, E. N. & Sigman, M. S. Multidimensional steric parameters in the analysis of asymmetric catalytic reactions. Nature Chem. 4, 366–374 (2012)
Peng, C. S., Baiz, C. R. & Tokmakoff, A. Direct observation of ground-state lactam-lactim tautomerization using temperature-jump transient 2D IR spectroscopy. Proc. Natl Acad. Sci. USA 110, 9243–9248 (2013)
Coates, J. in Encyclopedia of Analytical Chemistry (ed. Meyers, R. A. ) 10815–10837 (Wiley, 2000)
Meyer, M. P. New applications of isotope effects in the determination of organic reaction mechanisms. Adv. Phys. Org. Chem. 46, 57–120 (2012)
Harper, K. C., Vilardi, S. C. & Sigman, M. S. Prediction of catalyst and substrate performance in the enantioselective propargylation of aliphatic ketones by a multidimensional model of steric effects. J. Am. Chem. Soc. 135, 2482–2485 (2013)
Gustafson, J. L., Sigman, M. S. & Miller, S. J. Linear free-energy relationship analysis of a catalytic desymmetrization reaction of a diarylmethane-bis(phenol). Org. Lett. 12, 2794–2797 (2010)
Valero, R., Gomes, J. R. B., Truhlar, D. G. & Illas, F. Good performance of the M06 family of hybrid meta generalized gradient approximation density functionals on a difficult case: CO adsorption on MgO(001). J. Chem. Phys. 129, 124710 (2008)
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008)
Schäfer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829 (1994)
Schäfer, A., Horn, H. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 97, 2571 (1992)
Gaussian. 09 rev. C.01 (Gaussian Inc., Wallingford, 2009)
Bess, E. N. & Sigman, M. S. Distinctive meta-directing group effect for iridium-catalyzed 1,1-diarylalkene enantioselective hydrogenation. Org. Lett. 15, 646–649 (2013)
Dunitz, J. D. & Ibberson, R. M. Is deuterium always smaller than protium? Angew. Chem. Int. Ed. 47, 4208–4210 (2008)
Mei, T.-S., Werner, E. W., Burckle, A. J. & Sigman, M. S. Enantioselective redox-relay oxidative heck arylations of acyclic alkenyl alcohols using boronic acids. J. Am. Chem. Soc. 135, 6830–6833 (2013)
Werner, E. W., Mei, T. S., Burckle, A. J. & Sigman, M. S. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012)
Livingstone, D. Data Analysis for Chemists (Oxford Univ. Press, 1995)
Draper, N. R. & Smith, H. Applied Regression Analysis (Wiley, 1998)
Baldi, P. & Brunak, S. Bioinformatics: the Machine Learning Approach 2nd edn, 5–7 (MIT Press, 2001)
Goodman, S. A dirty dozen: twelve P-value misconceptions. Semin. Hematol. 45, 135–140 (2008)
MATLAB. student version v.7.14.0.739 (R2012a) (MathWorks Inc., 2012)
Jensen, K. H. & Sigman, M. S. Evaluation of catalyst acidity and substrate electronic effects in a hydrogen bond-catalyzed enantioselective reaction. J. Org. Chem. 75, 7194–7201 (2010)
Sigman, M. S. & Miller, J. J. Examination of the role of Taft-type steric parameters in asymmetric catalysis. J. Org. Chem. 74, 7633–7643 (2009)
Miller, J. J. & Sigman, M. S. Quantitatively correlating the effect of ligand-substituent size in asymmetric catalysis using linear free energy relationships. Angew. Chem. Int. Ed. 47, 771–774 (2008)
Harper, K. C. & Sigman, M. S. Using physical organic parameters to correlate asymmetric catalyst performance. J. Org. Chem. 78, 2813–2818 (2013)
Bess, E. N. & Sigman, M. S. in Asymmetric Synthesis II: More Methods and Applications (eds Christmann, M. & Bräse, S. ) 363–370 (Wiley-VCH, 2012)
Taft, R. W. Polar and steric substituent constants for aliphatic and o-benzoate groups from rates of esterification and hydrolysis of esters. J. Am. Chem. Soc. 74, 3120–3128 (1952)
Charton, M. Steric effects. II. Base-catalyzed ester hydrolysis. J. Am. Chem. Soc. 97, 3691–3693 (1975)
Charton, M. Steric effects. III. Bimolecular nucleophilic substitution. J. Am. Chem. Soc. 97, 3694–3697 (1975)
Charton, M. Steric effects. 7. Additional V constants. J. Org. Chem. 41, 2217–2220 (1976)
Charton, M. Steric effects. 8. Racemization of chiral biphenyls. J. Org. Chem. 42, 2528–2529 (1977)
Verloop, A. in Drug Design Vol. III (ed. Ariens, E. J. ) 133 (Academic, 1976)
Verloop, A. & Tipker, J. A comparative study of new steric parameters in drug design. Pharmacochem. Libr. 2, 63–81 (1977)
Verloop, A. in Pesticide Chemistry, Human Welfare and Environment Vol. 1 (eds Miyamoto, J. & Kearney, P. C. ) 339–344 (Pergamon, 1983)
Shahlaei, M. Descriptor selection methods in quantitative structure–activity relationship studies: a review study. Chem. Rev. 113, 8093–8103 (2013)
Dearden, J. C. & Cronin, M. T. D. in Smith and Williams’ Introduction to the Principles of Drug Design and Action (ed. Smith, H. J. & Williams, H. ). 185–209 (2006)
Pino, A., Giuliani, A. & Benigni, R. Toxicity mode-of-action: discrimination via infrared spectra and eigenvalues of the modified adjacency matrix. QSAR Comb. Sci. 22, 191–195 (2003)
Benigni, R., Giuliani, A. & Passerini, L. Infrared spectra as chemical descriptors for QSAR models. J. Chem. Inf. Model. 41, 727–730 (2001)
Benigni, R., Passerini, L., Livingstone, D. J., Johnson, M. A. & Giuliani, A. Infrared spectra information and their correlation with QSAR descriptors. J. Chem. Inf. Model. 39, 558–562 (1999)
Acknowledgements
This work was supported by the US National Science Foundation (CHE-0749506). We thank S. J. Miller and S. Yoganathan for discussions and for providing the peptide catalyst used in these studies. The support and resources of the Center for High Performance Computing at the University of Utah are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
A.M. and M.S.S. had the idea for the work; A.M. and E.N.B. performed the experiments; A.M. carried out computational and modelling analyses; A.M., E.N.B. and M.S.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Tables and Supplementary Figures. (PDF 6194 kb)
Rights and permissions
About this article
Cite this article
Milo, A., Bess, E. & Sigman, M. Interrogating selectivity in catalysis using molecular vibrations. Nature 507, 210–214 (2014). https://doi.org/10.1038/nature13019
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13019
This article is cited by
-
Analysis of vibronic coupling in a 4f molecular magnet with FIRMS
Nature Communications (2022)
-
Unravelling mechanistic features of organocatalysis with in situ modifications at the secondary sphere
Nature Chemistry (2019)
-
Comparing quantitative prediction methods for the discovery of small-molecule chiral catalysts
Nature Reviews Chemistry (2018)
-
Parameterization of phosphine ligands demonstrates enhancement of nickel catalysis via remote steric effects
Nature Chemistry (2017)
-
The evolution of drug design at Merck Research Laboratories
Journal of Computer-Aided Molecular Design (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.