Abstract
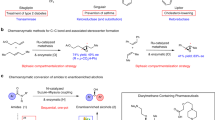
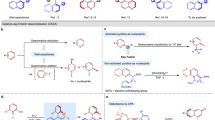
The chirality, or ‘handedness’, of a biologically active molecule can alter its physiological properties. Thus it is routine procedure in the drug discovery and development process to prepare and fully characterize all possible stereoisomers of a drug candidate for biological evaluation1,2. Despite many advances in asymmetric synthesis, developing general and practical strategies for obtaining all possible stereoisomers of an organic compound that has multiple contiguous stereocentres remains a challenge3. Here, we report a stereodivergent copper-based approach for the expeditious construction of amino alcohols with high levels of chemo-, regio-, diastereo- and enantioselectivity. Specifically, we synthesized these amino-alcohol products using sequential, copper-hydride-catalysed hydrosilylation and hydroamination of readily available enals and enones. This strategy provides a route to all possible stereoisomers of the amino-alcohol products, which contain up to three contiguous stereocentres. We leveraged catalyst control and stereospecificity simultaneously to attain exceptional control of the product stereochemistry. Beyond the immediate utility of this protocol, our strategy could inspire the development of methods that provide complete sets of stereoisomers for other valuable synthetic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
23 May 2018
A small modification to the procedure for handling dimethoxymethylsilane (DMMS)—rather than evaporation of volatiles before workup, the excess DMMS is quenched with ammonium fluoride—is described in the Supplementary Information to the accompanying Addendum (http://doi.org/10.1038/s41586-018-0112-4).
References
Jozwiak, K., Lough, W. J. & Wainer, I. W. Drug Stereochemistry: Analytical Methods and Pharmacology 3rd edn (Informa, 2012)
Wermuth, C. G. The Practice of Medicinal Chemistry 3rd edn (Elsevier, 2008)
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (eds) Comprehensive Asymmetric Catalysis Vol. I–III, Suppl. I–II (Springer, 1999)
Tian, X. et al. Diastereodivergent asymmetric sulfa-Michael additions of α-branched enones using a single chiral organic catalyst. J. Am. Chem. Soc. 133, 17934–17941 (2011)
McInturff, E. L., Yamaguchi, E. & Krische, M. J. Chiral-anion-dependent inversion of diastereo- and enantioselectivity in carbonyl crotylation via ruthenium-catalyzed butadiene hydrohydroxyalkylation. J. Am. Chem. Soc. 134, 20628–20631 (2012)
Wang, B., Wu, F., Wang, Y., Liu, X. & Deng, L. Control of diastereoselectivity in tandem asymmetric reactions generating nonadjacent stereocenters with bifunctional catalysis by cinchona alkaloids. J. Am. Chem. Soc. 129, 768–769 (2007)
Yan, X.-X. et al. Highly diastereoselective switchable enantioselective Mannich reaction of glycine derivatives with imines. J. Am. Chem. Soc. 130, 14362–14363 (2008)
Luparia, M. et al. Catalytic asymmetric diastereodivergent deracemization. Angew. Chem. Int. Edn 50, 12631–12635 (2011)
Lu, G., Yoshino, T., Morimoto, H., Matsunaga, S. & Shibasaki, M. Stereodivergent direct catalytic asymmetric mannich-type reactions of α-isothiocyanato ester with ketimines. Angew. Chem. Int. Edn 50, 4382–4385 (2011)
Mechler, M. & Peters, R. Diastereodivergent asymmetric 1,4-addition of oxindoles to nitroolefins by using polyfunctional nickel-hydrogen-bond-azolium catalysts. Angew. Chem. Int. Edn 54, 10303–10307 (2015)
Lee, E. C., Hodous, B. L., Bergin, E., Shih, C. & Fu, G. C. Catalytic asymmetric Staudinger reactions to form β-lactams: an unanticipated dependence of diastereoselectivity on the choice of the nitrogen substituent. J. Am. Chem. Soc. 127, 11586–11587 (2005)
Huber, J. D. & Leighton, J. L. Highly enantioselective imine cinnamylation with a remarkable diastereochemical switch. J. Am. Chem. Soc. 129, 14552–14553 (2007)
Huang, Y., Walji, A. M., Larsen, C. H. & MacMillan, D. W. C. Enantioselective organo-cascade catalysis. J. Am. Chem. Soc. 127, 15051–15053 (2005)
Simmons, B., Walji, A. M. & MacMillan, D. W. C. Cycle-specific organocascade catalysis: application to olefin hydroamination, hydro-oxidation, and amino-oxidation, and to natural product synthesis. Angew. Chem. Int. Edn 48, 4349–4353 (2009)
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science 340, 1065–1068 (2013)
Schindler, C. S. & Jacobsen, E. N. A new twist on cooperative catalysis. Science 340, 1052–1053 (2013)
Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley VCH, 2010)
Zhu, S., Niljianskul, N. & Buchwald, S. L. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J. Am. Chem. Soc. 135, 15746–15749 (2013)
Shi, S.-L. & Buchwald, S. L. Copper-catalyzed selective hydroamination reactions of alkynes. Nature Chem. 7, 38–44 (2015)
Zhu, S. & Buchwald, S. L. Enantioselective CuH-catalyzed anti-Markovnikov hydroamination of 1, 1-disubstituted alkenes. J. Am. Chem. Soc. 136, 15913–15916 (2014)
Yang, Y., Shi, S.-L., Niu, D., Liu, P. & Buchwald, S. L. Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science 349, 62–66 (2015)
Niu, D. & Buchwald, S. L. Design of modified amine transfer reagents allows the synthesis of α-chiral secondary amines via CuH-catalyzed hydroamination. J. Am. Chem. Soc. 137, 9716–9721 (2015)
Bandar, J. S., Pirnot, M. T. & Buchwald, S. L. Mechanistic studies lead to dramatically improved reaction conditions for the Cu-catalyzed asymmetric hydroamination of olefins. J. Am. Chem. Soc. 137, 14812–14818 (2015)
Miki, Y., Hirano, K., Satoh, T. & Miura, M. Copper-catalyzed intermolecular regioselective hydroamination of styrenes with polymethylhydrosiloxane and hydroxylamines. Angew. Chem. Int. Edn 52, 10830–10834 (2013)
Hannedouche, J. & Schulz, E. Asymmetric hydroamination: a survey of the most recent developments. Chemistry 19, 4972–4985 (2013)
Deutsch, C., Krause, N. & Lipshutz, B. H. CuH-catalyzed reactions. Chem. Rev. 108, 2916–2927 (2008)
Barker, T. J. & Jarvo, E. R. Developments in transition-metal-catalyzed reactions using electrophilic nitrogen sources. Synthesis 24, 3954–3964 (2011)
Lipshutz, B. H., Noson, K., Chrisman, W. & Lower, A. Asymmetric hydrosilylation of aryl ketones catalyzed by copper hydride complexed by nonracemic biphenyl bis-phosphine ligands. J. Am. Chem. Soc. 125, 8779–8789 (2003)
Chen, J.-X. et al. Highly chemoselective catalytic hydrogenation of unsaturated ketones and aldehydes to unsaturated alcohols using phosphine-stabilized copper(I) hydride complexes. Tetrahedron 56, 2153–2166 (2000)
Moser, R., Boskovic, Z. V., Crowe, C. S. & Lipshutz, B. H. CuH-catalyzed enantioselective 1,2-reductions of α,β-unsaturated ketones. J. Am. Chem. Soc. 132, 7852–7853 (2010)
Acknowledgements
We thank the National Institutes of Health (grant GM-58160 to S.L.B.). The content of this paper is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health. We thank Y.-M. Wang and M. T. Pirnot for help preparing the manuscript. The departmental X-ray diffraction instrumentation was purchased with the help of funding from the National Science Foundation (CHE-0946721). We thank P. Müller for X-ray crystallographic analysis of compound 5a.
Author information
Authors and Affiliations
Contributions
S.-L.S. and S.L.B. conceived the idea and designed the research. S.-L.S. and Z.L.W. performed the experiments. S.-L.S. and S.L.B. wrote the manuscript. All authors commented on the final draft of the manuscript and contributed to the analysis and interpretation of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see contents page for details. (PDF 11324 kb)
Rights and permissions
About this article
Cite this article
Shi, SL., Wong, Z. & Buchwald, S. Copper-catalysed enantioselective stereodivergent synthesis of amino alcohols. Nature 532, 353–356 (2016). https://doi.org/10.1038/nature17191
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17191
This article is cited by
-
Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines
Nature (2023)
-
Asymmetric α-allylic allenylation of β-ketocarbonyls and aldehydes by synergistic Pd/chiral primary amine catalysis
Nature Communications (2023)
-
Observation of Chiral-selective room-temperature phosphorescence enhancement via chirality-dependent energy transfer
Nature Communications (2023)
-
Combining nickel and squaramide catalysis for the stereodivergent α-propargylation of oxindoles
Nature Synthesis (2022)
-
Synthetic approaches to 1,4-dicarbonyl compounds
Nature Synthesis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.