Abstract
The Earth formed by accretion of Moon- to Mars-size embryos coming from various heliocentric distances. The isotopic nature of these bodies is unknown. However, taking meteorites as a guide, most models assume that the Earth must have formed from a heterogeneous assortment of embryos with distinct isotopic compositions1,2,3. High-precision measurements, however, show that the Earth, the Moon and enstatite meteorites have almost indistinguishable isotopic compositions4,5,6,7,8,9,10. Models have been proposed that reconcile the Earth–Moon similarity with the inferred heterogeneous nature of Earth-forming material, but these models either require specific geometries for the Moon-forming impact11,12 or can explain only one aspect of the Earth–Moon similarity (that is, 17O)1,2,3. Here I show that elements with distinct affinities for metal can be used to decipher the isotopic nature of the Earth’s accreting material through time. I find that the mantle signatures of lithophile O, Ca, Ti and Nd, moderately siderophile Cr, Ni and Mo, and highly siderophile Ru record different stages of the Earth’s accretion; yet all those elements point to material that was isotopically most similar to enstatite meteorites. This isotopic similarity indicates that the material accreted by the Earth always comprised a large fraction of enstatite-type impactors (about half were E-type in the first 60 per cent of the accretion and all of the impactors were E-type after that). Accordingly, the giant impactor that formed the Moon probably had an isotopic composition similar to that of the Earth, hence relaxing the constraints on models of lunar formation. Enstatite meteorites and the Earth were formed from the same isotopic reservoir but they diverged in their chemical evolution owing to subsequent fractionation by nebular and planetary processes13.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pahlevan, K. & Stevenson, D. J. Equilibration in the aftermath of the lunar-forming giant impact. Earth Planet. Sci. Lett. 262, 438–449 (2007)
Mastrobuono-Battisti, A., Perets, H. B. & Raymond, S. N. A primordial origin for the compositional similarity between the Earth and the Moon. Nature 520, 212–215 (2015)
Kaib, N. A. & Cowan, N. B. The feeding zones of terrestrial planets and insights into Moon formation. Icarus 252, 161–174 (2015)
Young, E. D. et al. Oxygen isotopic evidence for vigorous mixing during the Moon-forming giant impact. Science 351, 493–496 (2016)
Zhang, J., Dauphas, N., Davis, A. M., Leya, I. & Fedkin, A. The proto-Earth as a significant source of lunar material. Nat. Geosci. 5, 251–255 (2012)
Dauphas, N., Chen, J. & Papanastassiou, D. Testing Earth–Moon isotopic homogenization with calcium-48. Lunar Planet. Sci. Conf. XXXXVI, 2436 (2015)
Qin, L., Alexander, C. M. D., Carlson, R. W., Horan, M. F. & Yokoyama, T. Contributors to chromium isotope variation of meteorites. Geochim. Cosmochim. Acta 74, 1122–1145 (2010)
Javoy, M. et al. The chemical composition of the Earth: enstatite chondrite models. Earth Planet. Sci. Lett. 293, 259–268 (2010)
Warren, P. H. Stable-isotopic anomalies and the accretionary assemblage of the Earth and Mars: a subordinate role for carbonaceous chondrites. Earth Planet. Sci. Lett. 311, 93–100 (2011)
Dauphas, N. & Schauble, E. A. Mass fractionation laws, mass-independent effects, and isotopic anomalies. Annu. Rev. Earth Planet. Sci. 44, 709–783 (2016)
C´ uk, M. & Stewart, S. T. Making the Moon from a fast-spinning Earth: a giant impact followed by resonant despinning. Science 338, 1047–1052 (2012)
Canup, R. M. Forming a Moon with an Earth-like composition via a giant impact. Science 338, 1052–1055 (2012)
Dauphas, N., Poitrasson, F., Burkhardt, C., Kobayashi, H. & Kurosawa, K. Planetary and meteoritic Mg/Si and δ30Si variations inherited from solar nebula chemistry. Earth Planet. Sci. Lett. 427, 236–248 (2015)
Bottke, W. F., Walker, R. J., Day, J. M., Nesvorny, D. & Elkins-Tanton, L. Stochastic late accretion to Earth, the Moon, and Mars. Science 330, 1527–1530 (2010)
Rubie, D. C. et al. Highly siderophile elements were stripped from Earth’s mantle by iron sulfide segregation. Science 353, 1141–1144 (2016)
Rudge, J. F., Kleine, T. & Bourdon, B. Broad bounds on Earth’s accretion and core formation constrained by geochemical models. Nat. Geosci. 3, 439–443 (2010)
Nimmo, F., O’Brien, D. & Kleine, T. Tungsten isotopic evolution during late-stage accretion: constraints on Earth–Moon equilibration. Earth Planet. Sci. Lett. 292, 363–370 (2010)
Siebert, J., Corgne, A. & Ryerson, F. J. Systematics of metal–silicate partitioning for many siderophile elements applied to Earth’s core formation. Geochim. Cosmochim. Acta 75, 1451–1489 (2011)
Badro, J., Brodholt, J. P., Piet, H., Siebert, J. & Ryerson, F. J. Core formation and core composition from coupled geochemical and geophysical constraints. Proc. Natl Acad. Sci. USA 112, 12310–12314 (2015)
Schönbächler, M., Carlson, R., Horan, M., Mock, T. & Hauri, E. Heterogeneous accretion and the moderately volatile element budget of Earth. Science 328, 884–887 (2010)
Rubie, D. C. et al. Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89–108 (2015)
Boyet, M. & Carlson, R. W. 142Nd evidence for early (>4.53 Ga) global differentiation of the silicate Earth. Science 309, 576–581 (2005)
Burkhardt, C. et al. A nucleosynthetic origin for the Earth’s anomalous 142Nd composition. Nature 537, 394–398 (2016)
Render, J., Fischer-Gödde, C., Burkhardt, C. & Kleine, T. Molybdenum isotopes and the building blocks of the Earth. Lunar Planet. Sci. Conf. XXXXVII, 2639 (2016)
Dauphas, N. & Chaussidon, M. A perspective from extinct radionuclides on a young stellar object: the Sun and its accretion disk. Annu. Rev. Earth Planet. Sci. 39, 351–386 (2011)
Tang, H. & Dauphas, N. 60Fe-60Ni chronology of core formation in Mars. Earth Planet. Sci. Lett. 390, 264–274 (2014)
Nittler, L. R. et al. The major-element composition of Mercury’s surface from Messenger X-ray spectrometry. Science 333, 1847–1850 (2011)
Fitoussi, C. & Bourdon, B. Silicon isotope evidence against an enstatite chondrite Earth. Science 335, 1477–1480 (2012)
Canup, R. M. & Asphaug, E. Origin of the Moon in a giant impact near the end of the Earth’s formation. Nature 412, 708–712 (2001)
Kruijer, T. S., Kleine, T., Fischer-Gödde, M. & Sprung, P. Lunar tungsten isotopic evidence for the late veneer. Nature 520, 534–537 (2015)
Touboul, M., Puchtel, I. S. & Walker, R. J. Tungsten isotopic evidence for disproportional late accretion to the Earth and Moon. Nature 520, 530–533 (2015)
Dauphas, N., Burkhardt, C., Warren, P. H. & Fang-Zhen, T. Geochemical arguments for an Earth-like Moon-forming impactor. Phil. Trans. R. Soc. Lond. A 372, 20130244 (2014)
Acknowledgements
S. Jacobson provided the PDFs for the Rubie et al. model15 and J. Siebert and J. Badro provided assistance during development of the code to reproduce their model19. Discussions with A. Morbidelli, D. Rubie, A. Campbell, F. Ciesla, R. Yokochi, M. Roskosz, N. Greber and C. Burkhardt were greatly appreciated. J. Hu double-checked all the derivations and codes used in this contribution. This work was supported by NSF (CSEDI, grant EAR1502591; Petrology and Geochemistry, grant EAR1444951) and NASA (LARS, grant NNX14AK09G).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Additional information
Reviewer Information Nature thanks W. Bottke, R. Carlson and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
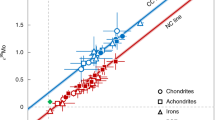
Extended Data Figure 1 Isotopic anomalies for Δ17O, ε48Ca, ε50Ti, ε54Cr, ε64Ni, ε92Mo, ε100Ru and μ142Nd in bulk meteorites and planetary materials.
See Supplementary Table 1. Enstatite meteorites (EH and EL chondrites, aubrite achondrites) and lunar samples have isotopic compositions (yellow shading) that are identical or very similar to the terrestrial mantle composition. Other meteorites and planetary materials display isotopic anomalies in one or several elements. The terrestrial mantle composition is, by definition, at 0 (blue horizontal lines).
Extended Data Figure 2 CDFs of lithophile O, Ca, Ti and Nd, moderately siderophile Cr, Ni and Mo and siderophile Ru atoms in the present terrestrial mantle.
See equation (2) and Fig. 1 for the corresponding PDFs and details. For each element, the horizontal dashed line at CDF = 0.05 gives the fraction of the Earth’s final mass after which 95% of an element at present in the mantle was delivered (the red triangles mark x0.95 as given by equation (3)).
Extended Data Figure 3 Example of terrestrial accretion and core partitioning for a scenario from ref. 19 of near-constant mantle redox evolution.
The blue and red lines correspond to model outputs from ref. 19 (updated from their supplementary figure 2) while the dashed black lines are from a Mathematica code developed for this study (available in the Supplementary Information) that makes the same assumptions as ref. 19 (see Supplementary Equations). The simulations use a final equilibration pressure of 60 GPa, a FeO mol% concentration in the magma ocean that decreases linearly with the accreted fraction from 8% to 5.8% (see the oxygen fugacity path 6 in figure 1 of ref. 19) and an equilibration temperature that follows the ‘hot liquidus’ of ref. 19. As shown, there is excellent agreement between ref. 19 and the present study. ‘D Core/Mantle’ refers to the ratio of the concentrations in the core and mantle.
Extended Data Figure 4 PDFs of Ca (lithophile), Cr, and Ni (moderately siderophile) for the accretion of elements in the Earth’s mantle in the model of ref. 19.
See Supplementary Equations for details. The blue and red curves correspond to two endmember accretion scenarios that can reproduce geophysical and geochemical constraints for the Earth’s core and mantle. The red curves correspond to a scenario wherein the mantle FeO concentration evolves from about 34 mol% to 5.9 mol%; the blue curves correspond to a scenario wherein the mantle FeO evolves from 8 mol% to 5.9 mol% (bottom right panel; FeO path numbers 13 and 6 in ref. 19). The black dashed curves are the predictions from equation (1). The non-flat density of Ca is an artefact due to the fact that the FeO concentration of the mantle is arbitrarily prescribed, such that in the scenarios investigated, the CaO concentration of the accreted material would need to increase as the FeO decreases. This model assumes full impactor core-target mantle equilibration and considers small impactors (1/1,000th the size of the present Earth). The metal–silicate distribution coefficients evolve as a function of the evolving pressure–temperature–composition–oxygen fugacity conditions. The values of x0.95 for Cr and Ni in the near-constant FeO model of Badro et al.19 are 0.81 and 0.23, respectively (compared to 0.85 and 0.39 for equation (3)).
Extended Data Figure 5 PDFs of Ca (lithophile), Cr, Ni (moderately siderophile), and Ru (highly siderophile) for the accretion of elements in the Earth’s mantle in the model of ref. 15.
The red curves are from the model15; see Supplementary Equations for details. The black dashed curves are the predictions from equation (1). The model of ref. 15 uses a dynamical simulation (Grand Tack 4:1-0.5-8) as input that gives the mass and heliocentric distance of each embryo or planetesimal that is accreted by the Earth as a function of time. Embryos and planetesimals closer to the Sun are assumed to be more reduced while those further away are assumed to be more oxidized. Following each impact, the impactor mantle is assumed to merge with the target mantle without metal equilibration, a fraction of the impactor core merges with the target core without silicate equilibration, and fractions of the impactor core and target mantle are allowed to equilibrate before the equilibrated metal sinks to the Earth’s core and the equilibrated silicate mixes with the rest of the Earth’s mantle. The metal–silicate distribution coefficients evolve as a function of the evolving pressure–temperature–composition–oxygen fugacity conditions. The values of x0.95 for Cr and Ni in the model of Rubie et al.15 are 0.88 and 0.41, respectively (compared to 0.85 and 0.39 for equation (3)). Note that according to this model, half of the Ru in the mantle could have been delivered before the late veneer; x0.95 = 0.14.
Extended Data Figure 6 Proportions of the E component in a three-stage accretion model of the Earth.
See Supplementary Equations and Fig. 2 legend for details. Unlike in Fig. 2, μ142Nd variations were taken into account in the minimization. Stages I, II and III comprise 0%–60%, 60%–99.5%, and 99.5%–100% of the Earth’s accretion. The accreted material is assumed to be mixtures of four components with the isotopic compositions of enstatite (E), ordinary (O), and carbonaceous chondrites (CI and CO/CV). The five free parameters of the model are the fractions of E in stages I, II and III, and the proportions of CI and CO/CV in the non-enstatite component. These parameters are estimated using a χ2-minimization to reproduce the isotopic composition of the Earth’s mantle for Δ17O, ε48Ca + ε50Ti, ε54Cr, ε64Ni, ε92Mo, ε100Ru and μ142Nd (seven independent constraints). The best-fitting model corresponds to about 100% E during all three stages, with up to 6% of O during stage II. The calculated isotopic anomalies in the Earth’s mantle are those of E-chondrites: Δ17O = −0.01 ± 0.06, ε48Ca = −0.37 ± 0.24, ε50Ti = −0.19 ± 0.27, ε54Cr = +0.06 ± 0.04, ε64Ni = +0.01 ± 0.19, ε92Mo = +0.34 ± 0.37, ε100Ru = −0.11 ± 0.08, and μ142Nd = −7 ± 3 (χ2 = 53). These match approximately the terrestrial composition (that is, no anomaly by definition). Note that the fit is much worse than when μ142Nd is not included (χ2 = 11), in part because all chondrite groups (including E) display resolvable negative μ142Nd anomalies. This can be explained if a component not sampled in chondrites contributed to making the Earth23 or if the accessible Earth has a slightly superchondritic Sm/Nd ratio22. Existing data on μ145Nd, μ148Nd and μ150Nd yield a μ142Nd value corrected for the presence of nucleosynthetic anomalies of −5 ± 2, suggesting that the shift in μ142Nd between E-chondrites and the Earth’s mantle of −7 ± 3 could be due in part to 146Sm decay23.
Supplementary information
Supplementary Information
This file contains derivations of the equations used in the main text and Mathematica code used to calculate the partitioning of moderately siderophile elements in the model of ref. 19. (PDF 575 kb)
Supplementary Table 1
This table contains a compilation of isotopic anomalies in planetary materials. (XLS 363 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Dauphas, N. The isotopic nature of the Earth’s accreting material through time. Nature 541, 521–524 (2017). https://doi.org/10.1038/nature20830
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20830
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.