Abstract
The tumour suppressor p53 induces apoptosis or cell-cycle arrest in response to genotoxic and other stresses1,2. In unstressed cells, the anti-proliferative effects of p53 are restrained by mouse double minute 2 (Mdm2), a ubiquitin ligase (E3) that promotes p53 ubiquitination and degradation3. Mdm2 also mediates its own degradation through auto-ubiquitination. It is unclear how the cis- and trans-E3 activities of Mdm2, which have opposing effects on cell fate, are differentially regulated. Here, we show that death domain-associated protein (Daxx)4 is required for Mdm2 stability. Downregulation of Daxx decreases Mdm2 levels, whereas overexpression of Daxx strongly stabilizes Mdm2. Daxx simultaneously binds to Mdm2 and the deubiquitinase Hausp, and it mediates the stabilizing effect of Hausp on Mdm2. In addition, Daxx enhances the intrinsic E3 activity of Mdm2 towards p53. On DNA damage, Daxx dissociates from Mdm2, which correlates with Mdm2 self-degradation. These findings reveal that Daxx modulates the function of Mdm2 at multiple levels and suggest that the disruption of the Mdm2–Daxx interaction may be important for p53 activation in response to DNA damage.
Similar content being viewed by others
Main
Daxx was initially identified as an adaptor protein for CD95 (Fas/Apo-1), linking the death receptor to the c-Jun amino-terminal kinase (JNK) pathway4,5, and it was subsequently implicated in other apoptotic scenarios6,7,8. Accumulating evidence suggests that Daxx has additional cellular functions. Mice deficient in Daxx die at early embryonic stages. These mice and the derived ES cells show enhanced spontaneous apoptosis9 indicating a critical role of Daxx in development that may be related to an anti-apoptotic function. Recently, an interaction between Daxx and the tumour suppressor p53 has been observed in overexpression experiments10,11,12. p53 induces cell-cycle arrest, apoptosis or senescence in response to stresses such as DNA damage and the activation of oncogenes1,2. In unstressed cells, p53 is a short-lived protein and its stability is mainly controlled by a RING domain-containing ubiquitin ligase (E3) Mdm2 (also known as Hdm2 in humans)3,13,14,15,16,17. As an E3, Mdm2 is capable of self-degradation18,19. However, the regulation of Mdm2 stability is not well understood.
The enhanced sensitivity for apoptosis in the Daxx deficient (Daxx−/−) ES cells, and the reported interaction of Daxx with p53, prompted us to examine whether Daxx affects the steady-state levels of p53 and its main regulator Mdm2. Interestingly, in the Daxx−/− ES cells, Mdm2 levels were markedly decreased whereas p53 levels were significantly elevated (Fig. 1a). These alterations were due to the lack of Daxx as shown by the restoration of Mdm2 and p53 expression by exogenous Daxx in the Daxx−/− cells (Fig. 1b). The generality of this effect of Daxx was confirmed by small interfering RNA (siRNA)-mediated downregulation in several human cell lines, including a pair of human colorectal carcinoma HCT116 cells that are wild type (p53+/+) and null (p53−/−) for p53, a pair of human osteosarcoma cells that are wild type (U2OS) and null (SaOS-2) for p53, and primary human fibroblast IMR90 cells (Fig. 1c and see Supplementary Information, Fig. S1a). Knocking down Daxx expression led to a decrease in Mdm2 levels in all of these cells and to an enhancement of p53 levels in the p53 wild-type cells. Conversely, overexpression of Daxx led to an increase in the steady-state levels of Mdm2 and a decrease in p53 levels (see Supplementary Information, Fig. S1b, c).
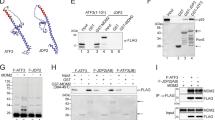
(a) The steady-state levels of Mdm2 and p53 are altered in the absence of Daxx. Lysates from wild-type (+/+) and Daxx-null (−/−) mouse ES cells were analysed by western blot. An uncropped full scan is shown in the Supplementary Information, Fig. S5a. (b) Introduction of HA–Daxx into Daxx−/− cells restores the expression of Mdm2 and p53. HA–Daxx and vector (V) transfected Daxx−/− ES cells (two independent transfectants each) were analysed for the expression of HA–Daxx, p53 and Mdm2 by western blot. (c) Effect of siRNA-mediated downregulation of Daxx on the steady-state levels of Mdm2 and p53 in human cells. Lysates from indicated cells were analysed by western blot. Protein bands were quantified by NIH Image software. Uncropped full scans for Mdm2, p53 and p21 of HCT116 cells are shown in the Supplementary Information, Fig. S5b. (d, f) Daxx modulates the half-life of Mdm2 and p53. Daxx+/+ and Daxx−/− ES cells (d) or U2OS cells transfected with siRNA (f) were treated with 25 (d) or 50 (f) μg ml−1 cycloheximide (CHX). To better compare the half-life of p53 and Mdm2 under different conditions, the blots on the left and their corresponding ones on the right were exposed for different times to achieve similar band intensity at time 0. An uncropped full scan of d is shown in the Supplementary Information, Fig. S5c. (e) Ubiquitination of Mdm2 and p53 is altered in Daxx−/− cells. Wild-type and Daxx−/− ES cells were treated with 20 μM MG-132 for 3 h. Lysates were denatured before immunoprecipitation (IP) with anti-Mdm2 or anti-p53 antibodies. The immunoprecipitates were analysed by western blotting using anti-ubiquitin and anti-Mdm2 or -p53 antibodies. (g) Overexpression of Daxx affects ubiquitination of endogenous Mdm2 and p53. U2OS cells were transfected with increasing amount of Daxx. Twenty-four hours later, cells were treated with 20 μM MG-132 for 4 h and were analysed for p53 and Mdm2 ubiquitination. An uncropped full scan of p53 in the lysates is shown in the Supplementary Information, Fig. S5d.
The effect of Daxx on Mdm2 and p53 steady-state levels is not due to changes in their transcription because Daxx does not alter the abundance of Mdm2 and p53 mRNAs in human U2OS and mouse ES cells (data not shown), but regulates the stability of these proteins. Compared with wild-type ES cells, the half-life of Mdm2 was significantly shortened, whereas the half-life of p53 was nearly doubled in the Daxx−/− ES cells (Fig. 1d), and these were accompanied by an increase in Mdm2 ubiquitination and a decrease in p53 ubiquitination (Fig. 1e). Treatment with the proteasome inhibitor MG-132 stabilized and eventually equalized the levels of Mdm2 (and p53) in both cell types (see Supplementary Information, Fig. S1d). Similarly, knocking down Daxx by siRNA accelerated Mdm2 degradation in several of the human cell lines, but increased the half-life of wild-type p53 (Fig. 1f and see Supplementary Information, Fig. S1e–g). Conversely, overexpression of Daxx prolonged the half-life of Mdm2 (see Supplementary Information, Fig. S1h), inhibited ubiquitination of endogenous Mdm2 and enhanced ubiquitination of endogenous p53 in a dose-dependent manner (Fig. 1g). However, the effect of Daxx was not observed when Mdm2 was stabilized by the proteasome inhibitor N-acetyl-Leu-Leu-Nle-Cho (ALLN; see Supplementary Information, Fig. S1c). Taken together, these results indicate that Daxx critically regulates the stability of both Mdm2 and p53 by modulating their ubiquitination and proteasomal degradation.
The stabilizing effect of Daxx on Mdm2 led us to test whether Daxx binds to Mdm2. Using a coimmunoprecipitation assay, the interaction between endogenous Daxx and Mdm2 was detected in several of the human cell lines examined, regardless of the status of p53 (Fig. 2a and see Supplementary Information, Fig. S2a). In addition, immunofluorescence microscopy analysis indicated that both endogenous Daxx and Mdm2 were found mainly in the nucleus, and their colocalization in subnuclear structures was evident when cells were treated with ALLN to stabilize Mdm2 (see Supplementary Information, Fig. S2b). The Daxx–Mdm2 interaction is likely to be direct, as shown by an in vitro pulldown assay with recombinant proteins (Fig. 2b). Deletion analyses revealed that the main binding domain for Mdm2 resides in a small region of Daxx (amino acids 157–260) that encompasses the second paired amphipathic helix domain (PAH2; see Supplementary Information, Fig. S2c, d).
(a) Association of Daxx and Mdm2 in vivo. Cell lysates from HCT116 cells and p53−/−Mdm2−/− MEFs were coimmunoprecipitated with anti-Mdm2 and an isotype-matching control antibody. An uncropped full scan is shown in the Supplementary Information, Fig. S5e. (b) Binding of Daxx and Mdm2 in vitro. Recombinant Flag–Daxx protein was mixed with bacterial lysates containing either GST or GST–Mdm2. The mixtures were incubated with glutathione beads for 4 h and the bound proteins were analysed for Daxx by western blotting (top), and for GST and GST–Mdm2 by Coomassie staining (middle and bottom panels). (c) The effect of Daxx on p53 steady-state level requires Mdm2. p53−/− Mdm2−/− MEFs were cotransfected with p53, Mdm2,and Daxx as indicated. Cell lysates were analysed by western blotting. Levels of GFP and actin were shown as controls for transfection efficiency and sample loading, respectively. An uncropped full scan is shown in the Supplementary Information, Fig. S5f. (d) In vitro ubiquitination of p53 by Mdm2 is enhanced by Daxx. p53 (10 ng) was incubated with 10 ng (+) and 25 ng (++) of immobilized GST–Mdm2 in the presence of Flag–Daxx (100 ng), E1, E2 and His–ubiquitin (His–Ub) as indicated. The reaction mixtures were analysed by western blotting. (e) Daxx enhances Mdm2-mediated p53 ubiquitination in vivo. Mdm2 and increasing amounts of Daxx were coexpressed with p53 in p53−/− Mdm2−/− MEF cells and the cells were treated with MG-132 for 4 h. Cell lysates were denatured. Anti-p53 immunoprecipitates and lysates were analysed by western blotting. (f) Ablation of Daxx sensitizes p53-mediated cell death. HCT116 cells were transfected with a control or Daxx siRNA. The cells were treated with 400 μM 5-FU for 20 h or left untreated. Percentages of sub-G1 cells are given. (g) Daxx does not affect the auto-ubiquitination of Mdm2 in vitro. Immobilized GST–Mdm2 protein (+, 12.5 ng; ++, 25 ng) was incubated with Daxx (100 ng), E1, E2 and Ub as indicated.
To test whether Daxx regulates p53 degradation through Mdm2, a cotranfection assay was used in mouse embryonic fibroblasts (MEF) deficient in both p53 and Mdm2 (p53−/− Mdm2−/−). When Daxx was coexpressed with p53 in these cells it did not alter the expression levels of p53 (Fig. 2c, lanes 2–4). However, when Mdm2 was also expressed, the level of p53 was decreased in a Daxx dose-dependent manner, which correlated with the enhancement of Mdm2 expression (Fig. 2c, lanes 5–7). The possibility that, in addition to stabilizing Mdm2, Daxx may directly promote the intrinsic E3 activity of Mdm2 towards p53, was also considered. In an in vitro ubiquitination assay using purified recombinant proteins, Mdm2 ubiquitinated p53 as expected (Fig. 2d, lanes 2 and 5). Interestingly, when recombinant Daxx protein was included in the reaction p53 ubiquitination was strongly enhanced (Fig. 2d, lanes 3 and 6). To determine whether the effect of Daxx on the E3 activity of Mdm2 could be observed in vivo, Mdm2 and p53 were expressed with increasing amounts of Daxx in p53−/− Mdm2−/− MEF cells in the presence of MG-132, which prevented Mdm2 and p53 degradation and thus masked the effects of Daxx on their stability. Under these conditions, Daxx still significantly enhanced Mdm2-mediated p53 ubiquitination (Fig. 2e). Thus, Daxx is required not only for the stabilization of Mdm2 but also for its optimal E3 activity towards p53.
To confirm the function of Daxx in the p53 system, the effect of Daxx on p53-mediated transcription and apoptosis was examined. Downregulation of Daxx by siRNA led to enhanced expression of the p53 target p21 (WAF/Cip1; Fig. 1c). Treatment of p53 wild-type HCT116 cells with 5-fluorouracil (5-FU) has been shown to cause p53-dependent apoptosis20. Downregulation of Daxx by siRNA strongly potentiated 5-FU-induced apoptosis in HCT116 (p53+/+), but not in HCT116 (p53−/−) cells (Fig. 2f). These data show that Daxx inhibits p53 function.
To understand the mechanism whereby Daxx promotes the stability of Mdm2, an in vitro ubiquitination assay was performed but no inhibitory effect of Daxx on Mdm2 self-ubiquitination was found (Fig. 2g). As Daxx is not known to possess any enzymatic activity, we reasoned that it may function as an adaptor. To this end, we sought to identify new Daxx-interacting partners that might be functionally related to Mdm2. Using an affinity purification approach21, a protein with a relative molecular mass of approximately 135,000 (Mr(K) ∼135) that specifically bound to the recombinant Daxx protein was identified and mass spectrometry analysis revealed it as the deubiquitinase herpes virus-associated ubiquitin specific protease (Hausp22; Fig. 3a). Mdm2 and p53 were not detected, probably due to their relatively low abundance in Hela cells. Previous studies have implicated Hausp as a regulator of both p53 and Mdm2 (Refs 23–25). The interaction between endogenous Daxx and Hausp was verified using both a coimmunoprecipitation assay with anti-Daxx (Fig. 3b) and a reciprocal assay with anti-Hausp antibodies (see Supplementary Information, Fig. S2e). The Daxx–Hausp interaction was not dependent on either Mdm2 or p53, as it occurred in HCT116 (p53−/−) and Mdm2−/− p53−/− MEF cells (Fig. 3b). In the same experiment, the interaction between endogenous Daxx and p53 was also detected, confirming previous results using overexpression approaches10,11,12.
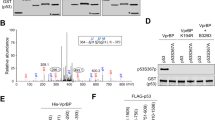
(a) Identification of Hausp as a Daxx-interacting protein. HeLa S3 lysates were incubated with M2 beads (control) or M2 beads coated with recombinant Flag–Daxx. The bound proteins were resolved on SDS–PAGE and visualized by Coomassie blue staining. The 135 K protein band in the Flag–Daxx lane was identified as Hausp by mass spectrometry. The peptide sequences obtained and their positions are shown on the right. (b) In vivo association of Daxx with Hausp. Lysates from wild-type p53 and p53-null HCT116 cells and p53−/− Mdm2−/− MEFs were immunoprecipitated with an anti-Daxx antibody and a control antibody. Precipitates and lysates were subjected to western blotting with the indicated antibodies. An uncropped full scan is shown in the Supplementary Information, Fig. S5g. (c) Colocalization of Hausp and Daxx. 293T cells were transfected with Hausp–GFP and HA–Daxx. The cells were immunostained with anti-Daxx antibody followed by Texas-red conjugated anti-rabbit IgG. The scale bar represents10 μm. (d) Direct binding of Hausp and Daxx. Purified recombinant Hausp and Daxx proteins (100 ng each) were incubated in 500 μl of lysis buffer for 2 h on ice. The mixtures were subjected to immunoprecipitation using an anti-Hausp antibodies or a control antibody. The precipitates were analysed by western blotting. (e) Ternary complex of Mdm2, Daxx and Hausp. HA–Hausp and GST–Mdm2 were cotransfected into H1299 in the presence or absence of Flag–Daxx. Cells were treated with 20 μM MG-132 for 4 h and the lysates were immunoprecipitated with M2 beads (lanes 3 and 4). Flag–Daxx and its binding complex were eluted using Flag peptide and the eluant was subject to a secondary immunoprecipitation with either anti-Mdm2 or a control antibody. An uncropped full scan is shown in the Supplementary Information, Fig. S5h.
Immunofluorescence microscopy analysis revealed that Hausp and Daxx colocalized in nucleoplasma with accumulation in speckled structures (Fig. 3c). Daxx binds directly to Hausp, as shown by an in vitro pulldown assay with purified recombinant proteins (Fig. 3d). Deletion analyses revealed two regions in Daxx that associate with Hausp: the N-terminal 160 amino acids, which contain the first paired amphipathic helix domain (PAH1), and amino acids 347–570, which encompass the acid-rich region (see Supplementary Information, Fig. S2c, f). Notably, these regions are distinct from the main interaction region for Mdm2.
The association of Daxx with Mdm2 and Hausp through non-overlapping regions and the similar effects of Daxx and Hausp on Mdm2 stability raised the intriguing possibility that Daxx might facilitate the Hausp–Mdm2 interaction in the cells, thus leading to Mdm2 stabilization. A sequential immunoprecipitation assay showed that Daxx, Mdm2 and Hausp were present in the same complex (Fig. 3e). Moreover, the Hausp–Mdm2 interaction was significantly enhanced by exogenous Daxx in a cotransfection assay (Fig. 4a), whereas this interaction was reduced in Daxx-deficient ES cells (Fig. 4b) and Daxx siRNA-treated U2OS cells (Fig. 4c). In contrast, the Mdm2–Daxx interaction was not affected when Hausp was ablated by siRNA (see Supplementary Information, Fig. S3a). These results, in combination with the Mdm2-independent Daxx–Hausp interaction (Fig. 3b), suggested that Daxx is the central component of the Mdm2–Daxx–Hausp complex and physically connects Hausp to Mdm2 in vivo.
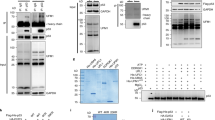
(a) Overexpression of Daxx enhances the interaction between Mdm2 and Daxx. p53−/− Mdm2−/− MEF cells were transfected with Flag–Hausp and HA–Mdm2, and increasing amounts of HA–Daxx as indicated. The cells were treated with 20 μM MG-132 for 4 h and lysates were immunoprecipitated with M2 antibodies. The immunoprecipitates and lysates were analysed by western blotting. (b) The Hausp–Mdm2 interaction is diminished in Daxx−/− ES cells. Wild-type and Daxx-deficient ES cells were treated with 5 μM MG-132 for 6 h. Cell lysates were immunoprecipitated with an anti-Hausp antibody and the control antibody. An uncropped full scan is shown in the Supplementary Information, Fig. S5i. (c) Interaction of endogenous Mdm2 and Hausp requires Daxx. U2OS cells treated with Daxx siRNA or control siRNA were incubated with 10 μM MG-132 for 4 h,and cell lysates were immunoprecipitated with anti-Hausp antibodies. (d) Hausp-mediated stabilization of Mdm2 is dependent on Daxx. U2OS cells treated with Daxx siRNA were transfected with increasing amounts of Hausp. The expression of the indicated proteins was examined by western blotting. An uncropped full scan of Mdm2 and p53 is shown in the Supplementary Information, Fig. S5j. (e) A role for Daxx in Hausp-mediated deubiquitination of endogenous Mdm2 and p53. U2OS cells were treated with Daxx siRNA and transfected with Hausp as in d. Cells were treated with 20 μM MG-132 for 4 h before harvest and the ubiquitination of Mdm2 and p53 was analysed.
We then investigated the role of Daxx in Hausp-mediated Mdm2 stabilization and deubiquitination. Increasing Hausp expression in U2OS cells stabilized endogenous Mdm2 in a dose-dependent manner (Fig. 4d, lanes 1–4), which was accompanied by a decrease in Mdm2 ubiquitination (Fig. 4e, lanes 1–3). However, when Daxx was knocked down using siRNA, the effect of Hausp on Mdm2 stability and ubiquitination was significantly reduced (Fig. 4d, lanes 5–8 and Fig. 4e, lanes 4–6). Similarly, the effect of Daxx on Mdm2 and p53 stability and ubiquitination was diminished when Hausp was knocked down by siRNA (see Supplementary Information, Fig. S3b, c). Additionally, Daxx mutants that failed to associate with either Hausp or Mdm2 were unable to stabilize Mdm2 (see Supplementary Information, Fig. S3d). Taken together, these results indicate that Daxx mediates the stabilizing effect of Hausp on Mdm2. In an in vitro assay, Daxx modestly increased Mdm2 deubiquitination by Hausp (see Supplementary Information, Fig. S3e). The robust effect of Daxx on the stability of endogenous Mdm2, and on the endogenous Mdm2–Hausp interaction, may be due to an additional level of regulation in physiological conditions.
Recent studies have revealed that accumulation of p53 in response to DNA damage is correlated with enhanced Mdm2 self-degradation and that this self-degradation is dependent on DNA damage-activated PI(3) kinase family members26,27. Consistent with these studies, treatment of U2OS cells with the DNA-damaging agent etoposide accelerated Mdm2 degradation (see Supplementary Information, Fig. S4a). The effect of Daxx on Mdm2 stability prompted us to examine whether the DNA-damage signal regulates the interactions of Daxx with Mdm2 and Hausp. Interestingly, treatment of U2OS cells with etoposide abolished the Mdm2–Daxx and Daxx–Hausp interactions and subsequently the Mdm2–Daxx–Hausp complex, and these events occurred even though Mdm2 was stabilized by MG-132 (Fig. 5a, left panels). The disruption of the binary Mdm2–Daxx interaction by DNA-damage signals seemed to be persistent and was not restored 4 h after treatment with etoposide (see Supplementary Information, Fig. S4b), whereas the binary Daxx–Hausp interaction was partially recovered by this time (see Supplementary Information, Fig. S4c). In contrast, the Daxx–p53 interaction was affected to a lesser extent, whereas the Hausp–p53 interaction was somewhat increased (see Supplementary Information, Fig. S4b, c). These data show that DNA-damage signals differentially and specifically affect the Daxx–Mdm2 and Daxx–Hausp interactions and disrupt the ternary complex. Importantly, the disruption of the Mdm2–Daxx–Hausp interactions occurred before the enhancement of Mdm2 ubiquitination (Fig. 5a, middle panels), probably contributing to Mdm2 degradation.
(a) DNA-damage dissociates Daxx and Hausp from Mdm2 and promotes Mdm2 ubiquitination. U2OS cells were treated with 20 μM MG-132 for 4 h and subsequently with 10 μM etoposide as indicated. The Mdm2–Daxx–Hausp interactions and in vivo Mdm2 ubiquitination were analysed. An uncropped full scan of Hausp and Daxx (lanes 1–5) is shown in the Supplementary Information, Fig. S5k. (b) ATM regulates the Mdm2–Daxx–Hausp complex and Mdm2 ubiquitination. U2OS cells were transfected with a control siRNA or an ATM-specific siRNA. The Mdm2–Daxx–Hausp interactions and in vivo Mdm2 ubiquitination were analysed as in a. An uncropped full scan of ATM is shown in the Supplementary Information, Fig. S5l. (c) A schematic representation of the role of Daxx in regulating Mdm2 and p53 stability. In unstressed cells, Mdm2 is stabilized in a complex with Daxx and Hausp leading to rapid ubiquitination and degradation of p53. On DNA damage, the Mdm2–Daxx–Hausp complex is disrupted in a manner that is at least partially dependent on ATM. Subsequently, Mdm2 undergoes self-ubiquitination and degradation, which allows accumulation of p53.
The PI(3) kinase family member ataxia telangiectasia mutated (ATM) has a prominent role in the initiation of DNA-damage responses. When cells were treated with the PI(3) kinase inhibitor wortamannin before the addition of etoposide, the Mdm2–Daxx–Hausp complex was stabilized (see Supplementary Information, Fig. S4d). Also, when the expression of ATM was decreased by siRNA, the interactions of Daxx with both Mdm2 and Hausp were less affected by etoposide, and, consistently, the ubiquitination of Mdm2 was not significantly enhanced in these cells (Fig. 5b). Therefore, DNA-damage signals disrupt the Mdm2–Daxx–Hausp complex, at least in part, through the activation of ATM. Mdm2 is phosphorylated by ATM at Ser 395 in vitro and this phosphorylation interferes with the inhibitory effect of Mdm2 on p53 (ref. 28). However, mutations that either prevent or mimic this phosphorylation exhibited similar binding to Daxx compared with wild-type Mdm2 (see Supplementary Information, Fig. S4e). Therefore, the Mdm2–Daxx–Hausp complex may be regulated by ATM through an additional phosphorylation event.
The E3 activity of Mdm2 not only regulates p53, but also drives Mdm2 self-degradation. A longstanding issue pertaining to p53 regulation is how the cis- and trans-E3 activities of Mdm2 are differentially regulated. This study reveals that Daxx is an integral part of this differential regulation through its ability to prevent Mdm2 self-degradation and to promote p53 degradation. In unstressed cells, Daxx interacts simultaneously with Mdm2 and Hausp and mediates the stabilizing effect of Hausp on Mdm2, thereby preventing p53 activation (Fig. 5c). Daxx also enhances the intrinsic E3 activity of Mdm2 towards p53. Previous studies suggested that Daxx could also directly inhibit p53 transcriptional activity10,11,12. Thus, Daxx may regulate p53 function at multiple levels. In response to DNA damage, Mdm2 dissociates from Daxx and Hausp in a manner that is at least partially dependent on ATM. This is likely to make Mdm2 unstable due to accelerated self-ubiquitination and subsequently leads to the accumulation of p53 (Fig. 5c). Therefore, the regulation of the Mdm2–Daxx–Hausp ternary complex may represent a novel pathway for p53 activation in response to DNA damage. Mutations in p53 have been associated with approximately 50% of all human tumours. Increasing evidence indicates that in tumours that retain wild-type p53, its activation by stresses is often compromised, for example, by the overexpression of Mdm2 (ref. 29). Given the robust effects of Daxx on Mdm2, it may be a valuable therapeutic target for the activation of p53.
Methods
Cell culture and generation of Daxx stable cell lines.
Cells were maintained in standard culture conditions. To reconstitute Daxx in Daxx−/− ES cells, the ES cells were transfected with either HA–mDaxx or the parental vector pBabe–puro using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Stable transfectants were pooled after culturing for 2–3 weeks in medium containing 3 μg ml−1 puromycin. L929 cells stably transfected with pEBB (L–EBB) and HA–Daxx–pEBB (L–Daxx) were previously described4.
Plasmids.
Plasmids for expressing Daxx, Mdm2, p53 and Hausp in mammalian cells were constructed in pRK5 with Flag, HA or GST tag as indicated. Deletion and point mutations were generated by PCR. GFP–Hausp was constructed in pEGFP–C1 (Clontech, Mountain View, CA) and GST–Mdm2 in pGEX-1ZT.
Immunoprecipitation and western blotting.
Whole cell lysates were made in lysis buffer (50 mM HEPES at pH 8.0, 150 mM NaCl, 0.5% Triton X-100, 0.5% NP-40, 100 mM NaF, 1 mM PMSF, 1 mM DTT, 1× complete protease cocktail and 10% glycerol) and pre-cleared with protein A–G-coupled Sepharose beads for 2 h. The lysates were then immunoprecipitated with the indicated antibodies and isotype-matched control antibodies plus protein A–G Sepharose for at least 4 h or overnight. Beads were washed four times with lysis buffer, once with ice-cold PBS and boiled in 2× loading buffer. Protein samples were resolved by SDS–PAGE and transferred onto nitrocellulose membrane, which was blocked in 5% skim milk in PBST and probed with the indicated antibodies. The following antibodies were used for immunoprecipitation and western blotting: Human Mdm2 immunoprecipitation, Ab-1 and Ab-4 (Oncogene, San Diego, CA); human Mdm2 western blotting, Ab-1, Ab-4 and SMP-14 (Santa Cruz Biotechnology, Santa Cruz, CA); mouse Mdm2 western blotting, Ab-3 (Oncogene), Ab-4 and SMP-14; p53 western blotting and immunoprecipitation, DO-1 or FL-393 (Santa Cruz); Daxx immunoprecipitation and western blotting, M-112 (Santa Cruz); Hausp western blotting and immunoprecipitation, BL-851 (Bethyl Laboratories, Montgomery, TX). Anti-ATM (Ab-1) was from Calbiochem (San Diego, CA) and anti-p21 (C-19, Santa Cruz). Antibodies against phospho-Ser 15 of p53 and phospho-Ser 1981 of ATM were purchased from Cell Signaling (Danvers, MA).
Immunofluorescence microscopy.
Cells cultured on coverslips were either treated with ALLN for 4 h or left untreated and washed twice with cold PBS. Cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 10 min, blocked with 5% BSA and incubated with anti-Daxx (M-112) and anti-Mdm2 (SMP-14) antibodies as indicated, followed by a Texas-red conjugated anti-mouse IgG and a FITC-conjugated anti-rabbit IgG antibody. The cells were mounted with DAPI-containing medium (Vector Laboratories, Burlingame, CA) and the images were acquired with a confocal microscope.
siRNA treatment.
Daxx siRNA sequences were CAGAAACATTAATAAACAATA (Qiagen, Valencia, CA) and AAGGAGTTGGATCTCTCAGAA (Dharmacon, Lafayette, CA). The Hausp siRNA sequence was cccaaattattccgcggcaaa (Qiagen) and ATM siRNA sequence was AATGGTGCTATTTACGGAGCT (Qiagen). Cells were seeded in 6-cm plates and transfected with ∼100 pmol siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction.
Identification of Hausp as a Daxx-interacting protein.
Flag–Daxx–pRK5 and Flag–pRK5 were transfected into 293T cells. Twenty-four hours later, the cells were lysed in immunoprecipitaiton lysis buffer (20 mM Tris–HCl at pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 2 mM DTT and freshly added protease inhibitors) and cell lysates were incubated with M2 beads (Sigma) for 3 h at 4 °C. After extensive washing, the beads were incubated with extracts of HeLa S3 cells for 3 h21. Beads were washed extensively and proteins were eluted with 3× Flag peptide (Sigma). Proteins specifically associated with Flag–Daxx were analysed by mass spectrometry.
Apoptosis assay.
HCT116 cells transfected with siRNA were treated with 5-FU (Sigma) for 20 h. The cells were fixed with 70% ethanol and stained with propidium iodine. The percentages of apoptotic cells were determined using Facscan Flow Cytometer (Becton Dickinson, San Jose, CA).
In vivo ubiquitination.
Cells were treated with MG-132 for 3–6 h and lysed in 1% SDS. After boiling for 5 min, lysates were diluted 10 times with cold lysis buffer supplemented with 1× complete inhibitor and 10 mM N-ethylmaleimide (NEM; Sigma). After immunoprecipitation with the indicated antibodies, the immunoprecipitates were resolved by 7% SDS–PAGE and transferred onto nitrocellulose membrane. The blot was blocked in 5% BSA and probed with an anti-ubiquitin antibody (Pierce, Holmdel, NJ) according to the manufacturer's instruction.
In vitro ubiquitination and deubiquitination assay.
Flag-tagged Hausp, Daxx and p53 were expressed in 293T cells and purified using M2 beads as previously described21. The non-specific binding proteins were removed by sequential washes with lysis buffers containing 0.25, 0.5 and 1 M KCl. Recombinant proteins were eluted using Flag peptide. GST–Mdm2 was expressed in BL21(DE3) and then purified using glutathione beads. GST–Mdm2 bound to beads were mixed with 100 nM E1, 1 μM E2 and 2 μg His6–Ub (Boston Biochem, Cambridge, MA) in a final volume of 20 μl reaction buffer (40 mM Tris–HCl at pH 7.6, 2.5 mM Mg2+–ATP, 2 mM DTT). The reaction was carried out at 37 °C for 1 h.
For the deubiquitination assay, Flag–Mdm2 was ubiquitinated in vitro and separated from free ATP by centrifugation through Microcon (Millipore, Billerica, MA). Ubiquitinated Mdm2 was treated with recombinant Flag–Hausp and Flag–Daxx in deubiquitination buffer (50 mM Tris–HCl at pH 7.4, 150 mM NaCl, 10 mM DTT and 5 mM MgCl2). The reactions were stopped by adding SDS (final concentration 1%) and boiling for 5 min. The mixtures were diluted 10 times with buffer containing 10 mM NEM. After immunoprecipitated with an anti-Mdm2 antibody, Mdm2 was analysed by western blot with anti-ubiquitin and anti-Mdm2 antibodies.
Note: Supplementary Information is available on the Nature Cell Biology website.
References
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Vousden, K. H. & Lu, X. Live or let die: the cell's response to p53. Nature Rev. Cancer 2, 594–604 (2002).
Michael, D. & Oren, M. The p53–Mdm2 module and the ubiquitin system. Semin. Cancer Biol. 13, 49–58 (2003).
Yang, X., Khosravi-Far, R., Chang, H. Y. & Baltimore, D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89, 1067–1076 (1997).
Chang, H. Y., Nishitoh, H., Yang, X., Ichijo, H. & Baltimore, D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281, 1860–1863 (1998).
Perlman, R., Schiemann, W. P., Brooks, M. W., Lodish, H. F. & Weinberg, R. A. TGF-β-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nature Cell Biol. 3, 708–714 (2001).
Zhong, S. et al. Promyelocytic leukemia protein (PML) and Daxx participate in a novel nuclear pathway for apoptosis. J. Exp. Med. 191, 631–640 (2000).
Salomoni, P. & Khelifi, A. F. Daxx: death or survival protein? Trends Cell Biol. 16 97–104 (2006).
Michaelson, J. S., Bader, D., Kuo, F., Kozak, C. & Leder, P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 13, 1918–1923 (1999).
Zhao, L. Y. et al. Negative regulation of p53 functions by Daxx and the involvement of MDM2. J. Biol. Chem. 279, 50566–50579 (2004).
Gostissa, M. et al. The transcriptional repressor hDaxx potentiates p53-dependent apoptosis. J. Biol. Chem. 279, 48013–48023 (2004).
Kim, E. J., Park, J. S. & Um, S. J. Identification of Daxx interacting with p73, one of the p53 family, and its regulation of p53 activity by competitive interaction with PML. Nucleic Acids Res. 31, 5356–5367 (2003).
Fakharzadeh, S. S., Trusko, S. P. & George, D. L. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 10, 1565–1569 (1991).
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997).
Fuchs, S. Y., Adler, V., Buschmann, T., Wu, X. & Ronai, Z. Mdm2 association with p53 targets its ubiquitination. Oncogene 17, 2543–2547 (1998).
Honda, R., Tanaka, H. & Yasuda, H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 (1997).
Fang, S., Jensen, J. P., Ludwig, R. L., Vousden, K. H. & Weissman, A. M. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem. 275, 8945–8951 (2000).
Honda, R. & Yasuda, H. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene 19, 1473–1476 (2000).
Bunz, F. et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 104, 263–269 (1999).
Tang, J. et al. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 279, 20369–20377 (2004).
Everett, R. D. et al. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16, 1519–1530 (1997).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002).
Cummins, J. M. et al. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 428, doi: 10.1038/nature02501 (2004).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 13, 879–886 (2004).
Stommel, J. M. & Wahl, G. M. Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J. 23, 1547–1556 (2004).
Meulmeester, E. et al. Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol. Cell 18, 565–576 (2005).
Maya, R. et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 15, 1067–1077 (2001).
Momand, J., Jung, D., Wilczynski, S. & Niland, J. The MDM2 gene amplification database. Nucleic Acids Res. 26, 3453–3459 (1998).
Acknowledgements
We thank P. Leder for wild type and Daxx−/− ES cells, H. Wu and Y. Xiong for p53−/− Mdm2−/− MEF cells, W. Gu for Hausp cDNA, G. Maul and B. Vogelstein for reagents, and D. George and S. Fuchs for advice. We also thank the Proteomic Core Facility of the Abramson Cancer Center at the University of Pennsylvania for mass spectrometry analysis and the National Cell Culture Center for providing HeLa S3 cells. J.T. was a postdoctoral appointee of an National Cancer Institute training grant (T32CA09140). X.Y. is supported by National Institutes of Health (NIH) grants (CA88868 and GM60911) and a Leukemia & Lymphoma Society Scholar Award.
Author information
Authors and Affiliations
Contributions
J.T. L.Q. And X.Y conceived and designed the experiments, analysed data and wrote the paper. J.T. and L.Q. performed the experiments. J.Z. and J.M. performed ES cell culture. W.W. and W.E. contributed reagents and performed apoptosis analysis. Y.Y.D. performed real-time RT−PCR.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 1023 kb)
Rights and permissions
About this article
Cite this article
Tang, J., Qu, LK., Zhang, J. et al. Critical role for Daxx in regulating Mdm2. Nat Cell Biol 8, 855–862 (2006). https://doi.org/10.1038/ncb1442
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1442
This article is cited by
-
HIRA vs. DAXX: the two axes shaping the histone H3.3 landscape
Experimental & Molecular Medicine (2024)
-
S100A6 inhibits MDM2 to suppress breast cancer growth and enhance sensitivity to chemotherapy
Breast Cancer Research (2023)
-
DAXX-ATRX regulation of p53 chromatin binding and DNA damage response
Nature Communications (2022)
-
Reciprocal regulation of Daxx and PIK3CA promotes colorectal cancer cell growth
Cellular and Molecular Life Sciences (2022)
-
TRIM15 and CYLD regulate ERK activation via lysine-63-linked polyubiquitination
Nature Cell Biology (2021)