Abstract
Piwi family proteins are essential for germline development and bind piwi-interacting RNAs (piRNAs)1,2,3. The grandchildless gene aub of Drosophila melanogaster encodes the piRNA-binding protein Aubergine (Aub), which is essential for formation of primordial germ cells (PGCs)4. Here we report that Piwi family proteins of mouse, Xenopus laevis and Drosophila contain symmetrical dimethylarginines (sDMAs). We found that Piwi proteins are expressed in Xenopus oocytes and we identified numerous Xenopus piRNAs. We report that the Drosophila homologue of protein methyltransferase 5 (dPRMT5, csul/dart5), which is also the product of a grandchildless gene5,6, is required for arginine methylation of Drosophila Piwi, Ago3 and Aub proteins in vivo. Loss of dPRMT5 activity led to a reduction in the levels of piRNAs, Ago3 and Aub proteins, and accumulation of retrotransposons in the Drosophila ovary. Our studies explain the relationship between aub and dPRMT5 (csul/dart5) genes by demonstrating that dPRMT5 is the enzyme that methylates Aub. Our findings underscore the significance of sDMA modification of Piwi proteins in the germline and suggest an interacting pathway of genes that are required for piRNA function and PGC specification.
Similar content being viewed by others
Main
Piwi family proteins are expressed in the germline and bind piRNAs that consist of 25–31 nucleotides1,2,3. Drosophila expresses three Piwi proteins: Aub4, Piwi7 and Ago3 (Refs 8, 9). Mice also express three Piwi proteins termed Miwi, Mili/PiwiL2 and Miwi2/PiwiL4 (ref. 10). Tens of thousands of distinct piRNAs have been described and most of them are species-specific1,2,3. In Drosophila, Piwi proteins and piRNAs (also known as rasiRNAs-repeat associated small interfering RNAs11) silence transposons in the germline2,10,12,13. A similar function has been found for a subset of mouse14 and zebrafish15 piRNAs. An amplification loop of piRNAs has been described8,9 but how primary piRNAs are generated is unknown3. Arginine methylation is an important post-translational modification that is mediated by two types of protein methyltransferases (PRMTs): type I enzymes catalyse asymmetric dimethylation of arginine residues (aDMA) and type II enzymes catalyse symmetric arginine dimethylation (sDMA; Fig. 1d)16. sDMA modifications occur in sequence motifs composed of arginines flanked by glycine (GRG) or alanine (GRA or ARG), which are often found as repeats16,17. PRMT5 and its cofactors MEP50/WD45 and pICln form the methylosome that methylates Sm proteins18,19.
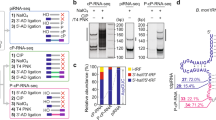
(a) Western blot analysis of Mili and Miwi in brain and testes. The Y12 monoclonal antibody (left panel) recognizes both Mili (green dot) and Miwi (red dot), whereas the specific anti-Mili antibody 17.8 (right panel) detected only Mili (green dot). 293T (Mili) refers to 293T cells with forced expression of Flag–Mili. (b) Protein immunoprecipitates from mouse testis (NMS, non-immune mouse serum). Mass spectrometry identified Miwi and Mili proteins in the Y12 and Mili protein in the anti-Mili immunoprecipitates (Supplementary Information, Table S1). (h.c) heavy, (l.c) light antibody chain. (c) RNA immunoprecipitation from mouse testis. (d) Arrangement of methyl groups in symmetric (sDMA) and asymmetric (aDMA) dimethylarginine. sDMA is produced by type II PRMT and is recognized by the SMY11 antibody. aDMA is produced by type I PRMT and is recognized by the ASYM24 antibody (e) Immunoprecipitates from mouse testis or purified recombinant proteins (Flag–Miwi, Flag–Mili) were probed on western bots with the indicated antibodies. The right panel shows a Coomassie-stained gel of purified recombinant proteins. (f) Full-length or truncated Flag–Miwi (68–862 or 1–212 amino acids) expressed in 293T cells were immunopurified with anti-Flag and probed with anti-Flag or SYM11 antibodies.
We produced a highly specific monoclonal antibody (17.8) that recognizes Mili by western blotting (Fig. 1a, right panel), immunoprecipitation (Fig. 1b, c; Supplementary Information, Table S1) and immunofluorescence microscopy (Supplementary Information, Fig. S1). By serendipity we discovered that the widely used Y12 monoclonal antibody20 recognizes mouse Mili and Miwi proteins and their bound piRNAs (Fig. 1a–c). The Sm proteins of spliceosomal small nuclear ribonucleoproteins (snRNPs) constitute the main antigen for Y12 (Refs 20, 21). We did not identify piRNAs in immunoprecipitates of snRNPs, heterogeneous ribonucleoproteins (hnRNPs) or of the survival of motor neurons (SMN) complex using various antibodies (Supplementary Information, Fig. S2). By northern blot analysis we found that piR-1, but not miR-16, an abundant miRNA, was found in Y12 immunoprecipitates (Supplementary Information, Fig. S3), suggesting that Y12 recognizes Piwi but not Ago proteins.
The epitope that Y12 recognizes on Sm proteins consists of symmetrically dimethylated arginines in the glycine-arginine-rich regions of the proteins21. We reasoned that Y12 probably reacts with sDMA-containing epitopes in Mili and Miwi and we searched for arginine residues that could be symmetrically methylated. We found that most animal Piwi proteins contain sDMA motifs that are typically clustered close to the amino terminus (Supplementary Information, Table S2), whereas no animal Ago proteins contained such motifs (data not shown). However, we found that four of ten Arabidopsis Ago proteins contained sDMA motifs (Supplementary Information, Table S2).
To test whether Miwi and Mili contain sDMAs, we used SYM11 and ASYM24 antibodies, which specifically recognize proteins containing sDMA-glycine or aDMA-glycine repeats, respectively (Fig. 1d)22. SYM11 and Y12 reacted strongly with endogenous Miwi and Mili, but ASYM24 showed only faint reactivity towards endogenous Miwi (Fig. 1e). By contrast, recombinant Flag–Mili or Flag–Miwi, purified from baculovirus-infected Sf9 cells, were not recognized by Y12, SYM11 or ASYM24 (Fig. 1e) despite loading of large amounts of recombinant proteins on the gels (Fig. 1e, right panel). This is consistent with the finding that recombinant human Sm proteins expressed in Sf9 cells do not contain sDMAs because Sf9 cells do not express type II PRMTs and thus cannot produce sDMA modifications21. Our findings indicate that Mili and Miwi proteins contain sDMAs. The putative sDMA motifs of Miwi are concentrated very close to the N terminus, with the exception of one GRG triplet (Supplementary Information, Table S2). We transfected Flag-tagged full-length Miwi and two truncated forms of Miwi (amino acids 68–862 or 1–212) in 293T cells, purified the proteins by Flag immunoprecipitation and subjected them to western blotting with SYM11 antibody, which recognizes the N terminus of Miwi protein (Fig. 1f). This result suggests that sDMAs exist in the N terminus, probably as sDMA motifs (Supplementary Information, Table S2). pf Miwi proteins.
Next we asked whether the sDMA modification was conserved in Piwi family proteins from other species. A stumbling block in studying the molecular functions of Piwi proteins and piRNAs has been the lack of suitable cell culture systems. We reasoned that Xenopus oocytes might express Piwi proteins and piRNAs and thus prove useful not only to confirm that sDMAs of Piwi proteins are conserved but also as a model to study the function of Piwi proteins and piRNAs. By searching the Gurdon EST database at Xenbase23 we identified three Xenopus Piwi proteins, which we named Xili, Xiwi and Xiwi2 (Supplementary Information, Fig. S4). All three Xenopus Piwi proteins contain putative sDMA motifs (Supplementary Information, Table S2). Immunoprecipitation with Y12 from Xenopus oocytes (defolliculated, mixed Dumont stages I-VI), testis and liver revealed the presence of two proteins with relative molecular masses of about 95,000 and 110,000 in the Y12 immunoprecipitates from oocytes and testis (Fig. 2a).We identified these proteins by mass spectrometry as Xiwi and Xili, respectively (Supplementary Information, Table S3). Western blot analysis showed that Y12 recognized both Xiwi and Xili, whereas the anti-Mili (17.8) antibody reacted only with Xili (Fig. 2b). In addition, SYM11 recognized both Xiwi and Xili, indicating that Xiwi and Xili contain sDMAs.
(a) Protein immunoprecipitates from Xenopus tissues. Xili (green dot) and Xiwi (red dot) were identified by mass spectrometry (Supplementary Information, Table S3). (b) Immunoprecipitates from Xenopus oocytes were probed on western blots with indicated antibodies. The asterisk indicates bovine IgG from tissue culture supernatant of anti-Mili hybridoma. (c) RNA immunoprecipitations from Xenopus. (d) Periodate oxidation and β-elimination of Xenopus piRNAs isolated from Y12 immunoprecipitates. (e) Nucleotide composition of Xenopus piRNAs. (f) Northern blot for XL-piR-3. (g) In situ hybridization for XL-piR-3 in Xenopus oocyte. Scale bar, 100 μm.
We isolated and analysed Xenopus piRNAs from Y12 immunoprecipitates and found that piRNAs consisting of 26–29 nucleotides were present in these immunoprecipitates (Fig. 2c). Their 3′-termini were not eliminated by periodate oxidation (Fig. 2d), indicating that they are probably 2′-O-methylated, as seen in piRNAs from other species15,24,25,26,27.
We next performed deep sequencing of Xenopus piRNAs from Y12 immunoprecipitates of oocytes and testis. The sequences and analysis are presented in the Supplementary Information Methods. The nucleotide composition of Xenopus piRNAs (Fig. 2e) shows enrichment of uridine in the first nucleotide position, and of adenine in the tenth nucleotide position. There is also enrichment for piRNAs whose first 10 nucleotides are complementary to the first 10 nucleotides of other piRNAs (Supplementary Information Methods). These features indicate that a fraction of Xenopus piRNAs target transposon transcripts and that they also participate in a piRNA amplification loop, as has been described for Drosophila and zebrafish piRNAs, and prepachytene mouse piRNAs8,9. Northern blotting of XL-piR-3, a representative piRNA, showed that it is expressed specifically in oocytes (Fig. 2f); in situ hybridization showed that it is localized predominantly in the cytoplasm of Xenopus oocytes and is expressed at higher levels in immature oocytes (Fig. 2g).
Genetic disruption of either Drosophila PRMT5 (dPRMT5, also know as capsuleenn (csul) or dart5) or its cofactor valois, (the Drosophila homologue of MEP50/WD45), results in complete loss of sDMA modifications of Sm proteins in ovaries5,6. However, in contrast to mammals18,19,28, the levels or function of Sm proteins are not affected by loss of sDMAs6,29.
Null or hypomorphic alleles of dPRMT5 (csul, dart5; Refs 5, 6) phenocopy aub-null alleles4 and we reasoned that dPRMT5 might be the methyltransferase that produces sDMAs in Aub, Piwi and Ago3, in vivo. To test this, we used ovaries from csul50/Df(2R)Jp7 females, which produce embryos that are genetically nulls for dPRMT5 (ref. 5), and w− as a wild-type control. Western blots of ovary lysates from wild-type and maternal null csul showed that there was near complete loss of SYM11 reactivity, indicating a marked reduction of sDMA-modified proteins in csul ovaries (Fig. 3a). There was no change in ASYM24 reactivity between wild-type and csul, indicating that aDMA-modified proteins were not affected (Fig. 3a). These findings confirm that dPRMT5 activity is required specifically for sDMA modification, as previously reported5,6. We immunoprecipitated Piwi and Aub proteins from wild-type and csul mutant ovaries (Fig. 3b) and probed the immunoprecipitates with SYM11 and ASYM24. SYM11 reacted very strongly with Aub and Piwi immunopurifed from wild-typet but not csul ovaries (Fig. 3c); ASYM24 reacted only weakly with Aub from wild-type ovaries. We also probed immunoprecipitates of Ago3 with SYM11 and ASYM24 and found that only Ago3 from wild-type ovaries reacted with SYM11 (Fig. 3d). These results indicate that, as seen for mouse and Xenopus Piwi family proteins, Drosophila Piwi, Aub and Ago3 contain sDMAs and that dPRMT5 is the methyltransferase that produces sDMAs of these proteins.
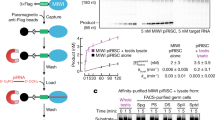
(a–c) Western blots from wild-type (WT) or csul (dPRMT5) mutant (−/−) ovary (a). Piwi or Aub immunoprecipitates from ovary lysates were probed on western blots with anti-Piwi and anti-Aub antibodies (b) or SYM11 and ASYM24 (c). (d) Ago3 immunoprecipitates from WT or csul mutant (−/−) ovary lysates were probed on western blots (WB) with the indicated antibodies. (e) Sequences of WT and mutant Aub (M), showing the four arginines that are substrates for methylation that were substituted by lysines. (f) Anti-Flag immunoprecipitates of S2 cells stably transfected with WT or M Flag–Aub were probed on western blots with the indicated antibodies. (g) Crosslinking of synthetic, radiolabelled piRNA to immunopurified WT or M Flag–Aub. (h) RNA immunoprecipitation. (i) Periodate oxidation and β-elimination of Drosophila piRNAs isolated from Piwi immunoprecipitates from WT or csul (−/−) mutant ovaries.
In Aub, the four arginines that are putative substrates for symmetric dimethylation are found in tandem very close to the N terminus (Fig. 3e). We used site-directed mutagenesis to change these arginines into lysines, which are not subjected to methylation by PRMTs. We stably transfected Flag-tagged wild-type or mutant Aub in Drosophila S2 cells (which express dPRMT5; ref. 6), purified the proteins by Flag immunoprecipitation and subjected them to western blotting with Flag, SYM11 and ASYM24 antibodies. SYM11 antibody reacted only with wild-type Aub (Fig. 3f). Next we assayed the binding of wild-type and mutant Aub to a synthetic piRNA. We incubated immunopurified wild-type or mutant Flag-Aub with a 5′-end radiolabelled synthetic piRNA containing 4-thio-uridine at the first position, then subjected them to crosslinking with ultraviolet light and NuPAGE analysis. Binding in the wild-type was similar to that of mutant Aub proteins (Fig. 3g), indicating that one or more of the four arginines in the N terminus of Aub are symmetrically dimethylated and arginine methylation does not affect piRNA binding.
Next, we isolated and analysed RNAs bound to Piwi and Aub from wild-type or csul ovaries and found that piRNAs remain bound to Piwi and Aub proteins in the csul ovaries (Fig. 3h). There was a mild reduction of Piwi piRNAs and marked reduction of Aub piRNAs in csul ovaries and corresponding reductions in protein levels of Piwi and Aub (Fig. 4a). Piwi-associated piRNAs were gel-purified and subjected to periodate oxidation followed by β-elimination. This procedure showed that Piwi-associated piRNAs purified from csul ovaries retain 2′-O-methylation of their 3' termini (Fig. 3i). These findings indicate that the lack of sDMA modifications of Piwi and Aub in csul ovaries does not impair the methylation of piRNAs or their binding to Piwi and Aub.
(a) Western blots of Drosophila ovary lysates from wild-type (WT), heterozygous (+/−) or homozygous (−/−) csul. (b) Northern blots of total RNA from ovary lysates. (c) Fold changes of HeT-A retrotransposon transcripts in ovaries was assessed with qRT–PCR. Values are mean ± s.d., n = 3. (d) Ago 3, Aub and Piwi localization in early stage egg chambers. Scale bar, 15 μm (e) Aub localization in csul oocytes. The arrow indicates pole (germ) plasm. Scale bar, 15 μm.
Next we compared protein levels of Piwi family proteins in wild-type, heterozygous and homozygous csul ovaries. Western blot analysis showed that there was a marked reduction in Aub and Ago3 protein levels and a lesser reduction in Piwi levels in csul ovaries, whereas the levels of the miRNA-binding protein Ago1 were not affected (Fig. 4a). mRNA levels of Aub, Piwi and Ago3 were the same in wild-type and csul ovaries (Supplementary Information, Fig. S5), indicating that dPRMT5 activity might be required to stabilize the Aub, Ago3 and Piwi proteins, most likely by symmetrically methylating their arginines. The level of a representative piRNA (roo-rasiRNA), was decreased in csul ovaries, consistent with a reduction of Piwi family proteins, whereas the level of a representative miRNA, miR-8, was not affected (Fig. 4b). The homozygous csul ovaries showed a 30-fold increase in the levels of the HeT-A retrotransposon transcript (Fig. 4c), whose expression is sensitive to mutations that disrupt piRNA-directed silencing in the female germline30. Collectively these results indicate that loss of dPRMT5 activity reduces the amounts of Piwi proteins and piRNAs, resulting in disruption of their function of transposon silencing.
Next we analysed by confocal microscopy the localization of Ago3, Aub and Piwi in wild-type and homozygous csul early-stage egg chambers. Previous studies have shown that Piwi is localized predominantly in the nuclei of follicle and germ cells8,9,31 whereas Ago3 and Aub are localized in the cytoplasm of germ cells8,9. In oocytes, Aub is concentrated in the germ (pole) plasm4. Confocal microscopy revealed that the level of Ago3 is markedly reduced in csul early-stage egg chambers, whereas there was only a mild reduction of Aub and Piwi protein levels (Fig. 4d).
PGC formation in Drosophila requires that cytoplasmic determinants are localized to the posterior pole of the embryo32 (Fig. 5, left panel). Genetic screens have identified grandchildless maternal genes that are required for PGC specification and invariably the protein or RNA products of these genes are concentrated in the pole plasm and are incorporated into the PGCs32. Among these genes are Aub4, dPRMT5 (csul, dart5; Refs 5, 6), and its cofactor valois33 and tudor34, whose protein product contains eleven tudor domains35. We examined the localization of Aub in csul oocytes by confocal microscopy and found that the levels of Aub in the pole plasm of stage 10 egg chambers were markedly reduced (Fig. 4e). Western blotting showed a marked reduction of Aub protein levels in csul ovaries (Fig. 4a); confocal microscopy showed that Aub levels are subtly reduced in early-stage egg chambers (Fig. 4d) but markedly reduced in later-stage egg chambers (Fig. 4e), suggesting that lack of sDMAs affects Aub levels at later stages in oogenesis.
These genes are classified according to the proteins they encode: A, proteins containing sDMAs, such as Aubergine (Aub); B, enzymes and cofactors that produce sDMAs, such as sPRMT5 (csul, dart5) and dMEP50 (Valois); C, proteins that contain Tudor domains, such as Tudor, which bind sDMAs. Proteins encoded by the grandchildless genes are concentrated in the pole plasm and are incorporated into the PGCs (shown in the red region).
Our studies show that sDMA modification of Piwi family proteins is a conserved post-translational modification and we have identified the methyltransferase PRMT5 (csul/dart5) as the enzyme that catalyses sDMAs of Piwi, Ago3 and Aub in Drosophila ovaries in vivo. Both Aub and dPRMT5 (csul/dart5) are grandchildless genes and our finding that Aub is a substrate for dPRMT5, indicates that an important function of dPRMT5 in pole plasm function and PGC specification involves methylation of Aub. Intriguingly, tudor domains bind to sDMAs36,37 and Tudor protein is also a product of a grandchildless gene that is required for pole plasm assembly and function. These findings suggest that pole plasm function may involve an interacting network of genes whose protein products contain sDMAs (Aub), the methyltransferase dPRMT5 and its cofactor valois/dMEP50, which produce sDMAs, and tudor domain (Tudor) proteins, which may bind to sDMA-containing proteins (Fig. 5). We note that both PRMT5 and genes that contain tudor domains are found in all species that express Piwi family proteins and that knockout of tudor-domain-containing 1/mouse tudor repeat 1 in mice leads to spermatogonial cell death and male sterility38. Furthermore, we note that other Drosophila proteins whose genes are required for piRNA accumulation or function, such as Spindle-E/homeless39,30 and Krimper40, contain tudor domains. It will be interesting to test whether proteins containing tudor domains interact with sDMA-modified Piwi family proteins and to elucidate their function.
Methods
Antibodies.
The anti-Mili monoclonal antibody 17.8 was generated by immunizing mice with recombinant GST–Mili (dilution for western blotting is 1:1000). Other antibodies used were anti-Flag (Sigma), anti-sDMA (SYM11; Millipore), anti-aDMA (ASYM24; Millipore), anti-TMG (Santa Cruz Biotech), anti-β-tubulin (Developmental Studies Hybridoma Bank); anti-hnRNPC (4F4), anti-SMN (2B1), anti-Gemin3 (12H12), anti-Gemin5 (10G11) and Y12 (gifts from G. Dreyfuss, University of Pennysylvania, PA); antibodies against Drosophila Ago1, Aub, Piwi and Ago3 (gifts from M.C. Siomi and H. Siomi, Keio University, Japan).
Drosophila stocks.
csul flies (csulRM: w–;csulRM50/CyO), were a gift from J. Anne (Deutsches Krebsforschungszentrum, Heidelberg, Germany) and a deletion that uncovers csul (wa Nfa–g; Df(2R)Jp7, was obtained from Bloomington Drosophila Stock Center (Indiana University).
Xenopus and piRNA sequencing.
Oocytes were isolated from ovaries and defolliculated. Testis and liver tissues were procured from euthanized animals. Y12-immunopurified Xenopus piRNAs from testis and oocytes were 5′-end labelled and gel-purified. Directional ligation of adaptors and cDNA generation was performed using the small RNA sample prep kit (Illumina). Deep sequencing was performed on an Illumina genome analyzer. For data and analysis for Xenopus piRNAs see Supplementary Information Methods and http://cbcsrv.watson.ibm.com/piwi_modification/.
Recombinant proteins and cDNA constructs.
Recombinant Flag–Miwi and Flag–Mili and GST–Mili were produced in baculovirus infected Sf9 cells. The cDNAs for Flag–Miwi and Flag–Mili production and Flag–Miwi truncations mutants (BC for 68–862 amino acids of Miwi and NP for 1–212 amino acids) were gifts from S. Kuramochi-Miyagawa and T. Nakano, Osaka University, Japan.
Recombinant wild-type and mutant Flag–Aub were produced in S2 cells. The pRH-Flag–Aub expression vector was a gift from M.C. Siomi and H. Siomi. To generate mutant Aub, arginine residues were changed to lysine (WT: 10A-R-G-R-G-R-G-R-K18 changed to mutant: 10A-K-G-K-G-K-G-K-K18), by site-directed mutagenesis (QuickChange XL, Stratagene) using the following primers: forward: 5′-CACCAAACCCTGTAATTGCTAAAGGAAAAGGTAAAGGAAAAAAGCCCAATAATGTAGAGGC 3′ and reverse: 5′-GCCTCTACATTATTGGGCTTTTTTCCTTTACCTTTTCCTTTAGCAATTACAGGGTTTGGTG 3′. Wild-type and mutant pRH-Flag-Aub expression vectors were co-transfected with pCoBlast (Invitrogen) at a ratio of 5:1 using cellfectin at 27 °C. Twenty-four hours after transfection, cells were grown in complete Schneider cell medium containing blasticidin (15 μg ml–1). Antibiotic resistant clones were expanded and propagated in the presence of blasticidin. Recombinant Aub expression was induced by growing S2 cells seeded at 2 × 106 cells ml–1 in complete Schneider cell medium containing 500 μM CuSO4 for 48 h, and the protein was purified by immunoprecipitation using anti-Flag M2 agarose (Sigma).
In situ hybridization and northern blotting.
Defolliculated oocytes were fixed with 10% neutral buffered formalin, paraffin embedded and sectioned at 5 μm; in situ hybridization was performed with a locked nucleic acid (LNA)-modified probe against XL-piR-3 (5′- UAAGUAGAAGAGCACCAAUGUCAUGUCC), an abundant piRNA sequenced from Xenopus oocytes. The sequence of the LNA probe was: 5′- ggAcatgaCattggtgcTcttctActta -3′-DIG (capital letters: LNA-modified nucleotides; DIG: digoxigenin). For northern blotting, total RNA was isolated from Xenopus mixed stage oocytes and from liver and probed with 5′-end radiolabelled DNA probes antisense to XL-piR-3 (probe: 5′- GGACATGACATTGGTGCTCTTCTACTTA) and antisense to miR-16 (probe: 5′- CACCAATATTTACGTGCTGCTA).
UV crosslinking.
A synthetic piRNA containing a photo-reactive residue, 4-thio-uridine (s4U), at position 1 (5′-(s4U)GACAUGAACACAGGUGCUCAGAUAGCUUU-3′) was 5′-end labelled using γ-32P-ATP and T4 polynucleotide kinase (T4 PNK, New England Biolabs). The 32P-labelled RNAs (∼20,000 cpm) were incubated with wild-type or mutant Flag-Aub beads at 28 °C for 60 min in a buffer containing 20 mM Tris-HCl pH 7.5, 200 mM NaCl, 2.5 mM MgCl2, 0.05% NP-40 and complete EDTA-free protease inhibitors (Roche). Crosslinking was performed on ice by irradiation for 30 min with a 365 nm hand-held lamp (EL series UV lamp, UVP). Cross-linked proteins were separated by NuPAGE and detected by storage-phosphor autoradiography.
Note: Supplementary Information is available on the Nature Cell Biology website.
References
Kim, V. N. Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev. 20, 1993–1997 (2006).
O'Donnell K, A. & Boeke, J. D. Mighty Piwis Defend the Germline against Genome Intruders. Cell 129, 37–44 (2007).
Hartig, J. V., Tomari, Y. & Forstemann, K. piRNAs--the ancient hunters of genome invaders. Genes Dev. 21, 1707–1713 (2007).
Harris, A. N. & Macdonald, P. M. Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C. Development 128, 2823–32 (2001).
Anne, J., Ollo, R., Ephrussi, A. & Mechler, B. M. Arginine methyltransferase Capsuleen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development 134, 137–146 (2007).
Gonsalvez, G. B., Rajendra, T. K., Tian, L. & Matera, A. G. The Sm-protein methyltransferase, dart5, is essential for germ-cell specification and maintenance. Curr. Biol. 16, 1077–1089 (2006).
Cox, D. N. et al. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 12, 3715–3727 (1998).
Gunawardane, L. S. et al. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315, 1587–1590 (2007).
Brennecke, J. et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103 (2007).
Girard, A. & Hannon, G. J. Conserved themes in small-RNA-mediated transposon control. Trends Cell Biol. 18, 136–148 (2008).
Aravin, A. A. et al. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5, 337–350 (2003).
Sarot, E., Payen-Groschene, G., Bucheton, A. & Pelisson, A. Evidence for a piwi-dependent RNA silencing of the gypsy endogenous retrovirus by the Drosophila melanogaster flamenco gene. Genetics 166, 1313–1321 (2004).
Siomi, M. C., Saito, K. & Siomi, H. How selfish retrotransposons are silenced in Drosophila germline and somatic cells. FEBS Lett. 582, 2473–2478 (2008).
Aravin, A. A., Sachidanandam, R., Girard, A., Fejes-Toth, K. & Hannon, G. J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316, 744–747 (2007).
Houwing, S. et al. A Role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell 129, 69–82 (2007).
Krause, C. D. et al. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacol. Ther. 113, 50–87 (2007).
Bedford, M. T. & Richard, S. Arginine methylation an emerging regulator of protein function. Mol. Cell 18, 263–272 (2005).
Friesen, W. J. et al. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol. Cell Biol. 21, 8289–300 (2001).
Meister, G. et al. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 11, 1990–1994 (2001).
Lerner, E. A., Lerner, M. R., Janeway, C. A., Jr & Steitz, J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc. Natl Acad. Sci. USA 78, 2737–2741 (1981).
Brahms, H. et al. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J. Biol. Chem. 275, 17122–17129 (2000).
Boisvert, F. M., Cote, J., Boulanger, M. C. & Richard, S. A proteomic analysis of arginine-methylated protein complexes. Mol. Cell Proteomics 2, 1319–1330 (2003).
Bowes, J. B. et al. Xenbase: a Xenopus biology and genomics resource. Nucleic Acids Res. 36, 761–767 (2008).
Kirino, Y. & Mourelatos, Z. Mouse Piwi-interacting RNAs are 2′-O-methylated at their 3′ termini. Nature Struct. Mol. Biol. 14, 347–348 (2007).
Ohara, T. et al. The 3′ termini of mouse Piwi-interacting RNAs are 2′-O-methylated. Nature Struct. Mol. Biol. 14, 349–350 (2007).
Saito, K. et al. Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi- interacting RNAs at their 3′ ends. Genes Dev. 21, 1603–1608 (2007).
Horwich, M. D. et al. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr. Biol. 17, 1265–1272 (2007).
Gonsalvez, G. B. et al. Two distinct arginine methyltransferases are required for biogenesis of Sm-class ribonucleoproteins. J. Cell Biol. 178, 733–740 (2007).
Gonsalvez, G. B., Praveen, K., Hicks, A. J., Tian, L. & Matera, A. G. Sm protein methylation is dispensable for snRNP assembly in Drosophila melanogaster. RNA 14, 878–887 (2008).
Vagin, V. V. et al. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313, 320–324 (2006).
Cox, D. N., Chao, A. & Lin, H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127, 503–514 (2000).
Strome, S. & Lehmann, R. Germ versus soma decisions: lessons from flies and worms. Science 316, 392–393 (2007).
Anne, J. & Mechler, B. M. Valois, a component of the nuage and pole plasm, is involved in assembly of these structures, and binds to Tudor and the methyltransferase Capsuleen. Development 132, 2167–2177 (2005).
Boswell, R. E. & Mahowald, A. P. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell 43, 97–104 (1985).
Arkov, A. L., Wang, J. Y., Ramos, A. & Lehmann, R. The role of Tudor domains in germline development and polar granule architecture. Development 133, 4053–4062 (2006).
Selenko, P. et al. SMN tudor domain structure and its interaction with the Sm proteins. Nature Struct. Biol. 8, 27–31 (2001).
Cote, J. & Richard, S. Tudor domains bind symmetrical dimethylated arginines. J. Biol. Chem. 280, 28476–28483 (2005).
Chuma, S. et al. Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc. Natl Acad. Sci. USA 103, 15894–15899 (2006).
Savitsky, M., Kwon, D., Georgiev, P., Kalmykova, A. & Gvozdev, V. Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes Dev. 20, 345–354 (2006).
Lim, A. K. & Kai, T. Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 104, 6714–6719 (2007).
Acknowledgements
We are grateful to M.C. Siomi, H. Siomi, K. Saito, G. Dreyfuss for antibodies; to J. Anne for csul flies; to S. Kuramochi-Miyagawa and T. Nakano for Miwi and Mili cDNA constructs; to Rebecca Beerman for help with fly methodology; to Y. Kawamura for immunofluorescence protocols and to members of the Mourelatos lab for discussions. Mass spectrometry was performed at the Proteomics Core Facility (University of Pennsylvania) and at the Proteomics Resource of the Keck Foundation (Yale University). Illumina sequencing was performed at the Functional Genomics Core of the University of Pennsylvania. Protein production was at the Protein Expression Core of the Wistar Institute. We apologize to colleagues whose studies were not cited because of space limitations. This work was supported by a Human Frontier Science Program Long Term Fellowship to Y.K., and NIH grants to T.A.J. (NS046573), P.S.K. (GM76621) and Z.M.(GM0720777, NS056070, UL1RR024134). Z.M. also received ITMAT-PENN and URF-PENN.
Author information
Authors and Affiliations
Contributions
Y.K. and Z.M. conceived and designed the experiments; Y.K., N.K., M.P-S, E.K., S.C., P.S.K. and I.R. performed the experiments and analysis and generated all the figures; T.A.J. guided the Drosophila methodology and experiments; I.R. performed the bioinformatics analysis; T.A.J. and I.R. provided substantial input into the writing of the manuscript; Y.K. and Z.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1116 kb)
Rights and permissions
About this article
Cite this article
Kirino, Y., Kim, N., de Planell-Saguer, M. et al. Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol 11, 652–658 (2009). https://doi.org/10.1038/ncb1872
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1872
This article is cited by
-
Arginine methylation promotes siRNA-binding specificity for a spermatogenesis-specific isoform of the Argonaute protein CSR-1
Nature Communications (2021)
-
RNase κ promotes robust piRNA production by generating 2′,3′-cyclic phosphate-containing precursors
Nature Communications (2021)
-
Binding of guide piRNA triggers methylation of the unstructured N-terminal region of Aub leading to assembly of the piRNA amplification complex
Nature Communications (2021)
-
Comparative Proteomics Reveal Me31B’s Interactome Dynamics, Expression Regulation, and Assembly Mechanism into Germ Granules during Drosophila Germline Development
Scientific Reports (2020)
-
PRMT5 promotes DNA repair through methylation of 53BP1 and is regulated by Src-mediated phosphorylation
Communications Biology (2020)