Abstract
Oriented mitosis is essential during tissue morphogenesis. The Wnt/planar cell polarity (Wnt/PCP) pathway orients mitosis in a number of developmental systems, including dorsal epiblast cell divisions along the animal–vegetal (A–V) axis during zebrafish gastrulation. How Wnt signalling orients the mitotic plane is, however, unknown. Here we show that, in dorsal epiblast cells, anthrax toxin receptor 2a (Antxr2a) accumulates in a polarized cortical cap, which is aligned with the embryonic A–V axis and forecasts the division plane. Filamentous actin (F-actin) also forms an A–V polarized cap, which depends on Wnt/PCP and its effectors RhoA and Rock2. Antxr2a is recruited to the cap by interacting with actin. Antxr2a also interacts with RhoA and together they activate the diaphanous-related formin zDia2. Mechanistically, Antxr2a functions as a Wnt-dependent polarized determinant, which, through the action of RhoA and zDia2, exerts torque on the spindle to align it with the A–V axis.
Similar content being viewed by others
Main
Cell division orientation during animal development can serve to correctly organize tissue architecture and to create cellular diversity. In a number of cases, oriented cell division relies on the orientation of the mitotic spindle, which can be controlled through extrinsic factors, cell intrinsic cues or physical constraints1,2,3. During zebrafish gastrulation, cells of the epiblast divide along the animal–vegetal (A–V) embryonic axis. The orientation of these divisions is influenced by positional information provided by the Wnt/planar cell polarity (Wnt/PCP) pathway4,5.
Rho GTPases have emerged as key mediators of the Wnt/PCP pathway6. Rho GTPases signal through Rho-associated kinases (Rock) and diaphanous-related formins (DRFs) to modulate cytoskeletal dynamics7. Rock can either promote actin polymerization through a series of phosphorylation events involving PAK1, LIM kinases and cofilin7, or promote actin contractility by phosphorylation of myosin light chain (MLC; ref. 8). DRFs are also known for their role in actin nucleation as well as in microtubule stabilization9,10,11, and have been involved in spindle positioning in a variety of systems, including mitosis in yeast12,13 and meiosis in mouse oocytes14,15. However, it is not known whether Rho GTPases and the actin cytoskeleton play a role during Wnt/PCP-mediated oriented division, whether Wnt/PCP polarizes the actin cytoskeleton and what is the machinery downstream of the Wnt/PCP pathway that controls positioning of the mitotic spindle.
Here we show that anthrax toxin receptor 2a plays a key role in oriented cell division of dorsal epiblast cells. Antxr2 was identified as one of the two cell surface toxin receptors used for intoxication by anthrax16. In vitro, Antxr2 promotes capillary-like network formation, suggesting a role in angiogenesis17, and mutations in the human ANTXR2 lead to the hyaline fibromatosis syndrome18. However, the precise physiological molecular function and cell biological role of Antxr2 are still unknown.
In this report, we show that the Wnt/PCP pathway, through RhoA and its effector Rock2, controls the formation of a cortical actin cap oriented along the embryonic A–V axis. Interaction of Antxr2a with actin triggers the accumulation of Antxr2a at the cap. Antxr2a also interacts with RhoA, preferentially in its GTP-bound form, and thereby contributes to activate the DRF zDia2. zDia2 is required to exert torque on the spindle, causing episodes of directed spindle rotation towards the A–V axis. Our data uncover the endogenous function of Antxr2a during embryogenesis and establish the molecular mechanism by which the Wnt/PCP pathway mediates oriented division of dorsal epiblast cells during gastrulation.
RESULTS
Antxr2a accumulates in a cap at the plasma membrane of dividing dorsal epiblast cells
Zebrafish Antxr2a is the closest homologue of the human ANTXR2 receptor19 (Supplementary Fig. S1a,b). antxr2a is ubiquitously transcribed during zebrafish gastrulation (Supplementary Fig. S1c). In dorsal epiblast cells, Antxr2a accumulates in endosomes (Supplementary Fig. S1d) and at the plasma membrane.
In dividing dorsal epiblast cells, cortical Antxr2a accumulates in a polarized cap (Fig. 1a, Supplementary Fig. S2a and Video S1; for controls see Supplementary Fig. S2e). By fitting a Gaussian distribution to the Antxr2a abundance profile along the perimeter of the cell (Fig. 1b and Supplementary Fig. S2b), we determined quantitative features of the Antxr2a cap by considering the mean (μ), standard deviation (σ), amplitude (A) and baseline (B) of the Gaussian curve. Thus, the Antxr2a cap occupies approximately half of the cell surface (2σ/perimeter of the cell; 56±4% (n = 16) for endogenous Antxr2a and 61±5% (n = 10) for Antxr2a–eYFP, enhanced yellow fluorescent protein). We defined the orientation of the cap in the cell as the position of the maximum of the Gaussian (μ). Antxr2a–eYFP accumulated by 4.4±0.5-fold (n = 27) at the ‘tip’ of the cap (that is, the ratio amplitude A/baseline B in Fig. 1b; 1.8±0.2-fold (n = 15) for Antxr2a immunostaining). We also quantified the polarization of the cap as the fold accumulation of Antxr2a when comparing the integral of fluorescence intensity in two opposite halves of the cell (see Methods for details): endogenous Antxr2a abundance is up to 1.50±0.08-fold (n = 37) higher in one of the halves. Similarly, Antxr2a–eYFP accumulated by up to 1.95±0.07 fold (n = 36).
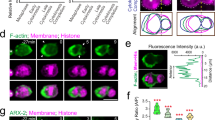
(a) Single confocal microscopy section of an epiblast cell expressing Antxr2a–eYFP (green) and histone H2A–mRFP (red) showing the Antxr2a cap (arrow). wt, wild type. (b) Gaussian curve fit to the cortical Antxr2a–eYFP fluorescence profile. (c) Confocal microscopy images from a movie of a dividing epiblast cell expressing Antxr2a–eYFP in an H2A::GFP transgenic embryo. Dotted lines illustrate the rotation of the spindle during mitosis (metaphase plate and, later, anaphase). (d) Fold accumulation of Antxr2a–eYFP and Lifeact–mRFP fluorescence as a function of time to analyse the dynamics of cap formation during mitosis. To determine the baseline level of biased cortical protein accumulation due to random fluctuation, the dynamics of non-polarized palmitoylated mRFP (Palm–RFP) accumulation was analysed. Asterisks in all graphs indicate statistical significance (Student’s t-test, asterisks, P<0.05). Mitotic phases and events related to the caps and spindle rotation are indicated. Error bars in all graphs are s.e.m. (e) Dynamics of Antxr2a cap in mitotic epiblast cells of an embryo expressing Antxr2a–eYFP (green) and H2A–mRFP (red). (f) Frames from a movie showing asymmetric localization of F-actin in a cap (Lifeact–mRFP, green; arrow at t = 0) in a dorsal epiblast cell of a transgenic H2A::GFP (red) embryo. F-actin cap dynamics during mitosis resemble Antxr2a cap dynamics (compare Fig. 1e,f, white arrows). Blue and yellow arrows show F-actin accumulation at the poles. (g) Quantification of cortical F-actin accumulation (fold accumulation in two cortical halves of the cells) in wild-type embryos, in antxr2a, rhoA, zdia2 and wnt5a morphants, in embryos injected with DNRock2 and in embryos treated with blebbistatin. To determine the symmetry baseline, embryos were injected with gap43–gfp messenger RNA. F-actin accumulates on one side of the cortex in wild-type, antxr2a and zdia2 morphants, but asymmetry is significantly reduced in embryos injected with rhoA and wnt5a morpholino oligonucleotide (MO), with DNRock2, and in embryos treated with blebbistatin. (h) Co-localization of Antxr2a (green) and F-actin (red) caps at the cortex in metaphase dorsal epiblast cells. Scale bars, 10 μm.
The cap builds up before metaphase and decays within cytokinesis (Fig. 1c,d). At the onset of cytokinesis, Antxr2a accumulation at the plasma membrane shifts to the cleavage furrow (Fig. 1e, t = 7.6 min; arrow), where it subsequently concentrates at the contractile ring, at the midbody (Fig. 1e, t = 9.4–10.6 min; arrows) and, finally, at the intercellular bridge during abscission (Fig. 1e, t = 21.6 min; arrow). After abscission, remnants of Antxr2a from the midbody can still be seen in the two daughter cells (Fig. 1e, t = 25.5 min; arrows).
The Antxr2a cap co-localizes with an F-actin cap in dividing cells
The localization of Antxr2a during cytokinesis (Fig. 1e) is reminiscent of that of F-actin20,21. Using the Lifeact–mRFP (monomeric red fluorescent protein) biosensor22, we investigated whether F-actin accumulates at the Antxr2a cap before cytokinesis. Similarly to Antxr2a, F-actin is enriched 1.43±0.04-fold in a cap on one side of the cell from early mitosis to the beginning of anaphase (Fig. 1d,f,g and Supplementary Videos S3 and S4). Later, during anaphase, a second F-actin patch forms de novo at the other pole (Fig. 1f; yellow arrow). During cytokinesis, F-actin also accumulates at the contractile furrow and the midbody, as previously reported21. Indeed, both Antxr2a and F-actin co-localize at the polarized cap in metaphase–anaphase and at the contractile ring and midbody during cytokinesis (n = 33; Fig. 1h and Supplementary Video S5).
The Antxr2a cap is aligned with the A–V axis and the plane of division and controls oriented mitosis
It has previously been shown that dorsal epiblast cells preferentially divide along the A–V axis of the embryo4 (Fig. 2a,c). We indeed found that the polarized distribution of Antxr2a and F-actin at the cortex in metaphase correlates with the A–V axis of the embryo (Fig. 2e–h) and with the mitotic cleavage plane at the onset of cytokinesis (Fig. 2i–k and Supplementary Fig. S2c,d).
(a–d) Orientation of the mitotic plane of epiblast cells with respect to the A–V axis in H2A::GFP transgenic control embryos (a,c) and embryos injected with antxr2a morpholino oligonucleotide (b,d). (a,b) Confocal microscopy images of H2A–GFP expressing epiblast cells in control (a) and antxr2a morphant (b) embryos. Green (control) and red (morphant) lines represent division axes. (c,d) Polar graphs showing the frequency distribution of α (angle between the division axis and the A–V embryonic axis) in control (c) and morphant (d) embryos. Divisions are aligned along the A–V axis (bidirectional arrow on the left) in control embryos, but randomized in morphant embryos. (e–h) Alignment of Antxr2a (e,f) and F-actin (g,h) caps with respect to the embryonic A–V axis. (e,g) Confocal microscopy images of epiblast cells expressing either Antxr2a–eYFP (e) or Lifeact–mRFP (g) to show the orientation of the caps (arrows) with respect to the embryonic A–V axis (bidirectional arrow on the right). Arrows point to the peak of the cap, which is defined by μ when the intensity of fluorescence was fitted to a Gaussian distribution. wt, wild type. (f,h) Frequency distribution of the angle of Antxr2a (f) and F-actin (h) caps (determined by using the μ of the Gaussian fit; Methods) with respect to the embryonic A–V axis. Both Antxr2a and F-actin caps align with respect to the A–V axis. (i) Schematic representation for the definition of γ, the angle between the mitotic plane and the plane determined by the Gaussian μ and the centre of the cell, for the Antxr2a–eYFP cap. (j,k) Polar graphs showing the frequency distribution of γ for Antxr2a (j) and F-actin (k) caps with respect to the mitotic plane. Both the Antxr2a and F-actin caps align with the division plane. Diagrams shown in the left corner of all the polar graphs in this report indicate the parameter measured (it is the angle γ for the Antxr2a cap in j and for the F-actin cap in k). cc, centre of the cell. Scale bars, 10 μm. n, sample size (number of cells).
Furthermore, the Antxr2a cap is essential for oriented mitosis: in antxr2a morphants the preferential mitotic orientation is lost (Fig. 2b,d; for a summary of the phenotypes described in this report see Fig. 8a and for morpholino specificity controls Supplementary Fig. S3). These results indicate that the polarized distribution of Antxr2a provides spatial cues that determine the position of the plane of mitosis during oriented cell division of dorsal epiblast cells.
Episodes of directed rotation align the spindle with the A–V axis
To investigate how the plane of mitosis aligns with the cap and the A–V axis, we carried out time-lapse imaging of a doublecortin–green fluorescent fusion protein (GFP–DCX; ref. 23), which localizes to the centrosomes and the mitotic spindle (Fig. 3a and Supplementary Video S6). When centrosomes duplicate and migrate to opposite poles, their initial orientation is random with respect to the A–V axis (Fig. 3b). Later, and simultaneously with the presence of the Antxr2a/F-actin cap (Fig. 1d), the spindle rotates for a period of around 20 min until reaching the final position at anaphase B, which is aligned along the A–V axis of the embryo (Figs 3c and 2a,c).
(a) Confocal microscopy images from a movie of an epiblast cell expressing GFP–DCX. The mitotic spindle rotates from the onset of centrosome separation until it aligns with the A–V axis (arrow on the right) at anaphase B. Scale bar, 10 μm. (b,c) Frequency distribution of the mitotic spindle/A–V axis angle monitored by GFP–DCX. Mitotic spindles are randomly oriented initially (b) and aligned at the end (c). In this and other frequency distributions in this report, ‘random’ refers to P>0.05 (χ2 test), ‘aligned’ to P<0.05; Supplementary Table S1. (d) dmi calculated for spindle rotation in wild-type embryos (n = 21; Supplementary Note). The dmi was calculated for L = 1 to 9. For L = 2, 4, 5, 8 and 9 the dmi was significantly larger than in the random simulations. In dmi plots, error bars indicate the 1 σ spread in the dmi of 1,000 simulated data sets (Supplementary Note). (e) Example of a trace of spindle orientation with respect to the embryonic axis (wild type). Episodes of directed motion (red line) can be detected in wild-type cells, as defined by clusters of dai = 1 (for detection algorithm, Supplementary Note). The episode of directed motion ends at the A–V axis (green line). dai values are given for L = 4 and are shown colour coded in the graph. ‘Border’ corresponds to excluded time points at the beginning and end of the sequence (Supplementary Note). (f) Spindle orientation of 11 cells where directed episodes were detected, showing pre-episodes (black), episodes of directed motion (red) and post-episodes (blue). Traces were registered in time according to the end of the directed episode (t = 0). After t = 0, the mitotic spindles hardly rotate and maintain the orientation aligned with the A–V axis until the cells are committed to the mitotic plane at anaphase B (green line). (g) dmi for wt, antxr2a, rhoA, zdia2 (L = 4) and wnt5a morphants (L = 6) with their corresponding simulations of random rotation. For wild-type and wnt5a morphants, the dmi is significantly higher when compared with random rotation.
To investigate whether the rotation of the spindle is composed of transient episodes of directed movement, we estimated the direction autocorrelation index (dai, adapted from ref. 24) and the directed motion index (dmi). The dai is designed to capture transient episodes of processive directed rotation that persist at least during a particular time lag L. In the spindle trajectory, a time with apparent persisting direction has a dai value that approaches unity. The dmi is the average percentage of all points in a data set in which d a i = 1. By comparing the dmi in the experimental GFP–DCX trajectories with the dmi of random trajectories generated by Monte Carlo simulations, we determined the incidence of directed rotation episodes and their statistical significance (Supplementary Note). Figure 3d shows that, within a range of lag times from 1.8 to 7.3 min, the spindle rotates with average dmi values that are well above those expected in a scenario of random rotation. This indicates that the spindle is indeed subjected to discrete, transient episodes of directed rotation.
Three spindle rotation phases: random fluctuations, directed rotation and final anchoring along the A–V axis
Using this approach, we have identified three distinct, successive phases in the spindle trajectory: the directed episode (red line in Fig. 3e), the preceding period (pre-episode) and the following period (post-episode). During the pre-episode, the spindle fluctuates with a random, diffusive rotation, with a diffusion constant of D = 1.16±0.02 degree2 s−1 (Supplementary Note). These pre-episodes show no bias in their orientation with respect to the A–V axis (Fig. 3e,f).
In contrast, the detected directed episodes are oriented specifically towards the A–V axis (Fig. 3e,f). These episodes last 9.8±1.3 min and the spindle is displaced by 86±12 degrees on average, implying a mean spindle pole rotation velocity of 5.7 nm s−1, akin to a previously reported spindle rotation event mediated by actin/microtubules in HeLa cells25. Interestingly, the directed episodes terminate at the A–V axis (5.1±1.4 degrees; Fig. 3e,f), indicating that the directed motion proceeds until the A–V target is reached.
In the post-episodes, the spindle fluctuates randomly again, but fluctuations are 3.4 times smaller (D = 0.34±0.01 degree2 s−1) than in the pre-episodes (Fig. 3f), indicating that, once the target is reached, the spindle is anchored and the cell can divide with the proper A–V orientation.
The directed rotation of the spindle, the existence of the Antxr2a/F-actin cap and the functional role of Antxr2a in oriented mitosis suggest the following model. Embryonic spatial cues cause Antxr2a/F-actin to accumulate in a cap aligned with the A–V axis and this in turn mediates directed rotation of the spindle until it is anchored in alignment with the cap. This will ultimately determine the alignment of the mitotic spindle and the orientation of the division with respect to the A–V axis.
We next investigated the machinery that exerts torque and anchors the spindle and the underlying mechanism of Antxr2a cap formation and alignment.
RhoA and diaphanous control spindle rotation
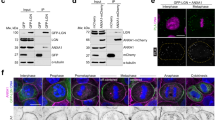
Wnt/PCP controls oriented mitosis in dorsal epiblast cells4. Moreover, RhoA has been shown to mediate Wnt/PCP signalling26 and to regulate actin dynamics at the cell cortex27, prompting us to look at a possible role of RhoA during spindle rotation (Fig. 4). We found that RhoA immunoprecipitates with Antxr2a (Fig. 4a), suggesting that RhoA is implicated in the Antxr2a-mediated oriented mitosis. In rhoA morphants, as opposed to wild-type embryos, the dmi value of the spindle trajectories is not significantly different from that of random fluctuations (Fig. 3g and Supplementary Fig. S4d). The spindle fluctuates randomly (Fig. 4b; D = 0.75±0.01 degree2 s−1), it is not aligned with the Antxr2a cap (Fig. 4c,d), and it is not anchored with respect to the A–V axis (Fig. 4e and Supplementary Fig. S4g and Video S7). This correlates with a randomization of the plane of division of dorsal epiblast cells in rhoA morphants (Fig. 4f,g). Therefore, Antxr2a, which is required for oriented mitosis, is a binding partner of RhoA, and RhoA in turn is implicated in spindle rotation.
(a) Co-immunoprecipitation of zRhoA and Antxr2a. HeLa cells were transfected with zRhoA as well as with human Antxr2a–V5 (hAntxr2a–V5), zebrafish Antxr2a–V5 (zAntxr2a–V5) or an empty vector (Ctrl). Immunoprecipitation (IP) was carried out using an anti-V5 antibody. Antxr2a and co-immunoprecipitated zRhoA were detected using anti-V5 and anti-RhoA antibodies, respectively. Both human and zebrafish Antxr2a co-immunoprecipitate zRhoA. TCE, total cell extraction. (b) Spindle rotation with respect to the A–V embryonic axis of a representative rhoA morphant dorsal epiblast cell. In rhoA morphants, the dmi is not significantly different from the dmi for random motion (Fig. 3g and Supplementary Fig. S4d). dai values for L = 4 are shown (colour coded). (c) Example of the Antxr2a cap in metaphase of a rhoA morphant dorsal epiblast cell (top) and the subsequent cytokinesis (bottom) to show that in these cells the Antxr2a cap is randomized and can be perpendicular to the mitotic plane. (d,e,g) Frequency distribution of γ (d), orientation of the Antxr2a cap (e) and the mitotic plane (g) with respect to the A–V axis in rhoA morphant cells. Note that the positions of Antxr2a caps with respect to the mitotic plane and the A–V axis as well as the orientation of the mitotic plane with respect to the A–V axis are randomized in these embryos. (f) Confocal microscopy image of dorsal epiblast cells from H2A::GFP transgenic embryos injected with rhoA morpholino oligonucleotide. Division axes are marked by magenta lines. Note the randomized division planes. Scale bars, 10 μm. (h) Frequency distribution of γ for the F-actin cap in rhoA morphants. The F-actin cap is not aligned with respect to the A–V axis in these embryos. Uncropped images of blots/gels are shown in Supplementary Fig. S8a.
It has previously been shown that the zebrafish DRF zDia2 acts downstream of RhoA (ref. 28). DRFs are known to nucleate actin cables as well as to bind and stabilize microtubules, and have been implicated in spindle positioning in other systems9,10,11,12,13,14,15. Interestingly, zdia2 morphants show randomized orientation of the mitotic plane (Fig. 5a and Supplementary Fig. S5k). However, as in control embryos, F-actin and Antxr2a form caps (Fig. 1g and Supplementary Fig. S5a,c) that co-localize (Supplementary Fig. S5j) and are aligned along the A–V axis (Fig. 5b,c, and Supplementary Fig. S5f,h), indicating that zDia2 might act downstream of (or in parallel with) F-actin and Antxr2a.
(a–c,e,f,h,i) Frequency distribution of the orientation of the mitotic plane (a), the Antxr2a cap (b,e) and the F-actin cap (c,f,h,i) with respect to the A–V axis (a–c,i) or the mitotic plane (e,f,h) in zdia2 (a–c,e,f) and antxr2a (h,i) morphants. In zdia2 embryos, the plane of mitosis is randomized (a), and the Antxr2a (b) and F-actin (c) caps are aligned with respect to the A–V axis, but not with respect to the mitotic plane (e,f). In antxr2a morphants, the F-actin cap is aligned with respect to the A–V axis (i), but not with respect to the mitotic plane (h). (d,g) Spindle rotation of representative zdia2 (d) or antxr2a (g) morphant dorsal epiblast cells. In zdia2 and antxr2a morphants, the rotation of the spindle is not significantly different from random (dmi; Fig. 3g and Supplementary Fig. S4e, f). dai values for L = 4 are shown (colour coded).
In zdia2 morphants, the misalignment of the division plane with respect to the cap and the A–V axis (Fig. 5e,f) is due to the failure of the cap to torque the spindle: the spindle fluctuates randomly (Figs 3g, 5d and Supplementary Fig. S4e; D = 0.43±0.02 degree2 s−1) and remains randomly oriented (Supplementary Fig. S4h). This indicates that zDia2 is not required either for the formation of the Antxr2a/F-actin cap, or for the alignment of the cap with respect to the A–V axis. Instead, zDia2 is essential to align the spindle pole with respect to the Antxr2a/F-actin cap.
Antxr2a interacts with GTP–RhoA and mediates spindle rotation
Our finding that Antxr2a interacts with RhoA (Fig. 4a) and that, in the absence of Antxr2a, cell division orientation is randomized (Fig. 2b,d) prompted us to explore whether Antxr2a itself plays a role during spindle rotation. antxr2a morphants show a phenotype identical to that of zdia2 morphants: the spindle fluctuates randomly (Figs 3g, 5g and Supplementary Fig. S4f,i; D = 0.59±0.01 degree2 s−1) and the F-actin cap forms (Fig. 1g and Supplementary Fig. S5d), but it is not aligned with respect to the mitotic plane (Fig. 5h), although it is still aligned with respect to the embryonic A–V axis (Fig. 5i). Therefore, F-actin cap formation and alignment depend neither on Antxr2a nor on zDia2.
Instead, our data suggest that Antxr2a acts as an effector of RhoA to activate zDia2, which in turn exerts the torque on the spindle necessary for oriented division. Activation of diaphanous is mediated by its binding with GTP-bound RhoA, which releases diaphanous auto-inhibition29,30. We investigated whether Antxr2a also interacts preferentially with GTP-bound RhoA. Indeed, we found that Antxr2a immunoprecipitates preferentially a mutant form of RhoA (RhoA G14V; ref. 31), which is locked in the GTP-bound state (Supplementary Fig. S6a), supporting a role of Antxr2a as an effector of RhoA. Moreover, a random mitosis phenotype caused by reduction in the zDia2 levels is restored by overexpression of Antxr2a (Supplementary Fig. S6b), suggesting that zDia2 activity can indeed be modulated by the levels of Antxr2a.
RhoA and Rock2 are required for cap formation
In contrast to zdia2 and antxr2a morphants, where the caps are properly formed and aligned with respect to the A–V axis (Fig. 5), in rhoA morphants the relative accumulation of F-actin is significantly diminished (Fig. 1g), and the Antxr2a cap and this defective F-actin cap are not properly oriented with respect to the A–V axis (Fig. 4e,h). This indicates that RhoA is essential for the formation and alignment of the caps.
Rock is a downstream target of RhoA that could mediate the formation of the actin cap in a zDia2-independent manner26,32,33. Rock can promote actin contractility by MLC phosphorylation8, which drives local F-actin accumulation and could then lead to cap formation. Indeed, we found that embryos injected with a dominant negative form of Rock2 (DNRock2; ref. 33) showed randomized divisions (Fig. 6a). In these embryos, the F-actin accumulation at the cap is also impaired (Fig. 1g). In addition, both the actin and the Antxr2a caps are misaligned with respect to the embryonic axis (Fig. 6b,c). Downstream of Rock2, myosin inhibition by blebbistatin treatment led to a decrease in actin cap formation (Fig. 1g) and misalignment of the Antxr2a and F-actin caps (Fig. 6d,e). These results indicate that the F-actin cap forms through local myosin activation by Rock2 downstream of RhoA.
(a–e) Frequency distribution of the orientation of the mitotic plane (a), the Antxr2a cap (b,d) and the F-actin cap (c,e) with respect to the A–V axis in embryos injected with DNRock2 (a–c) and embryos treated with blebbistatin (d,e). Embryos injected with DNRock show randomized division (a). In embryos injected with DNRock2 and embryos treated with blebbistatin, both the Antxr2a (b,d) and F-actin (c,e) caps are not aligned with respect to the A–V axis. (f) Immunoprecipitation of actin and Antxr2a. HeLa cells were transfected with either wild-type (wt) Antxr2a–V5 or with a mutant form of Antxr2a lacking the actin interacting domain (Antxr2aΔAID-V5). Immunoprecipitation (IP) was carried out using an anti-actin antibody. Actin and co-immunoprecipitated Antxr2a were detected using anti-actin and anti-V5 antibodies, respectively. Actin could immunoprecipitate wild-type Antxr2a, but failed to pull down Antxr2aΔAID-V5. (g) Quantification of the actin interacting assay in f (n = 3 experiments). Antxr2aΔAID-V5 interacts with actin 70% less than does wild-type Antxr2a. (h) Confocal microscopy images of dorsal epiblast cells expressing wild-type Antxr2a–eYFP (left) or Antxr2aΔAID-V5–eYFP (right). Whereas wild-type Antxr2a–eYFP forms a cap in one side of the cell (blue arrow), Antxr2aΔAID-V5–eYFP shows a lower cortical localization and is unable to form the cap. Scale bars, 10 μm. Uncropped images of blots/gels are shown in Supplementary Fig. S8b.
Both in rhoA morphants and in embryos injected with the DNRock2, the Antxr2a and the F-actin caps are misaligned with respect to the A–V axis, raising the possibility that the F-actin cap is required for the formation and alignment of the Antxr2a cap. We found that Antxr2a interacts with actin through a 51 amino acid domain in its cytosolic tail (actin interacting domain, AID; Fig. 6f). This AID is remarkably conserved among all the anthrax toxin receptors (Supplementary Fig. S1a). A mutant Antxr2a lacking this domain (Antxr2aΔAID) shows impaired actin interaction (Fig. 6f,g). YFP–Antxr2aΔAID is less efficiently retained at the cell cortex and is unable to form a cap (Fig. 6h; for quantification see Supplementary Fig. S6c,d), indicating that the formation of the Antxr2a cap requires the interaction with actin.
Our results indicate that RhoA, through Rock and myosin-mediated actin contractility, is required for the formation of an F-actin cap that is aligned with the A–V axis, and that interaction of Antxr2a with actin leads to the accumulation of Antxr2a at the F-actin cap. We next investigated the machinery that determines the orientation of the RhoA-dependent F-actin cap with respect to the the embryonic A–V axis.
Wnt/PCP signalling controls the alignment of the F-actin cap
The Wnt/PCP pathway mediates planar cell polarity by the RhoA-dependent regulation of actin dynamics26, controls oriented mitosis of dorsal epiblast cells4 and has been implicated in convergence–extension during gastrulation34. Consistent with this, we found that in rhoA morphants the A–V orientation of the Antxr2a/F-actin cap is defective (Fig. 4e,h). Moreover, we observed a convergence–extension phenotype in antxr2a, rhoA and zdia2 morphants similar to that seen under conditions of defective Wnt/PCP signalling 26,28 (Fig. 7a and Supplementary Fig. S7).
(a) Tail bud stage embryos injected with antxr2a, rhoA or zdia2 morpholino oligonucleotide showing shorter A–V axis (red, magenta and blue lines, respectively) when compared with control embryos (wt, green line). Lateral view, dorsal to the right (for further details see Supplementary Fig. S7 and Note). (b,c) Frequency distribution of the orientation of Antxr2a (b) and F-actin (c) caps with respect to the A–V axis in wnt5a morphant embryos. The orientation of Antxr2a and F-actin caps with respect to the A–V axis of the embryo is random. (d) Co-localization of Antxr2a–YFP (green) and F-actin (Lifeact–mRFP; red) caps in wnt5a morphant embryos. (e) dmi measured for spindle rotation in wnt5a morpholino oligonucleotide embryos (n = 14). The dmi is measured for a window size Lranging from 1 to 10. For L = 3, 4, 6, 7, 8 and 9 the dmi is significantly larger (P<0.05) than the dmi for random rotation. (f) Spindle rotation of wnt5a morphant dorsal epiblast cells (n = 12) showing episodes of directed motion (red), pre-episodes (black) and post-episodes (blue) as in Fig. 3f. Time registration of the traces is with respect to the end of the directed episode (t = 0). Note that, in these cells, directed episodes can be detected, but, unlike wild-type cells, the spindle does not align with the A–V axis at the end of the directed rotation episode (t = 0). In addition, as in wild-type cells, the mitotic spindles hardly rotate and maintain the orientation until the cells divide after the episode of oriented rotation. (g) Spindle rotation of a representative wnt5a morphant dorsal epiblast cell. Similarly to wild-type cells, wnt5a morphant cells show episodes of directed motion (red line). However, unlike the case in wild-type cells, these episodes may not be directed towards the A–V axis, and therefore the final position is often not aligned with the axis (90°; green line). dai values for L = 6 are shown (colour coded). (h,i) Antxr2a (h) and F-actin (i) caps are aligned with respect to the A–V axis of the embryo in wnt5a morphants. Scale bar, 10 μm.
In morphants defective for the Wnt/PCP ligand Wnt5a, both the F-actin and the Antxr2a caps are indeed misaligned with respect to the embryonic A–V axis (Fig. 7b,c; see also Supplementary Fig. S5g,i). Consistent with the role of F-actin in the formation of the Antxr2a cap, in wnt5a morphant embryos, the Antxr2a cap does co-localize with the F-actin cap (Fig. 7d). Therefore, Wnt/PCP signalling, through its effector RhoA, does indeed control the alignment of both the F-actin and the Antxr2a cap.
Interestingly, in wnt5a morphants, the spindle still maintains its proper orientation with respect to the cap (Fig. 7h,i and Supplementary Fig. S5b). Consistently, in wnt5a morphants, as in wild-type embryos, the spindle does show episodes of directed rotation (Figs 3g, 7e–g), the pre-episodes show large fluctuations of the spindle (D = 0.63±0.01 degree2 s−1) and the post-episodes show small random fluctuations (D = 0.16±0.01 degree2 s−1). The spindle is anchored at the end of the directed episodes, but, in contrast to wild type, this occurs randomly with respect to the A–V axis (Fig. 7f,g and Supplementary Fig. S4j), consistent with the misalignment of the Antxr2a/F-actin cap with respect to the A–V axis (Fig. 7b,c). Therefore, the Wnt/PCP pathway is required to align the F-actin cap (and, therefore, the Antxr2a cap) with the A–V axis, which, in turn, serves as a spatial cue to guide the rotation of the mitotic spindle.
DISCUSSION
Our data uncover the endogenous function of the Antxr2a receptor in oriented mitosis and provide a molecular mechanism by which the Wnt/PCP pathway controls the polarity of cells during oriented cell division of epiblast cells during zebrafish gastrulation. We propose a model (Fig. 8b) in which Wnt/PCP signalling promotes the polarized activity of RhoA with respect to the embryonic A–V axis. RhoA in turn activates the downstream effectors Rock2–myosin, which are required for the polarized cortical accumulation of F-actin in a cap. Antxr2a interacts with actin, leading to its recruitment to the F-actin cap. At the cap, Antxr2a interacts with GTP-RhoA, contributing to the activation of a second RhoA effector, zDia2. zDia2 mediates spindle rotation to align it with the cap, ensuring oriented mitosis, which is involved in body extension during zebrafish gastrulation.
(a) Diagram representing the phenotypes for the Antxr2a and F-actin cap positions with respect to the embryonic A–V axis and the mitotic plane as well as directed spindle rotation for the specified genetic backgrounds. In wild type, both Antxr2a and F-actin caps form and are aligned with respect to the embryonic axis and the mitotic plane. The spindle shows oriented rotation towards the A–V axis. In rhoA morphants, the F-actin cap is not properly formed, and the remnants are not aligned to the A–V axis; the spindle fluctuates without oriented rotation and therefore the positions of the caps do not correlate with the mitotic plane. In zdia2 morphants, both caps are formed and aligned along the A–V axis. The spindle fluctuates randomly and therefore the positions of the caps do not correlate with the mitotic plane. In antxr2a morphants, the Antxr2a cap is absent, but the F-actin cap forms and it is aligned along the A–V axis. The spindle fluctuates randomly and therefore the positions of the caps do not correlate with the mitotic plane. In wnt5a morphants, both caps are formed, but are not aligned with respect to the A–V axis. Here, the spindle shows directed rotation and the final position of the spindle aligns with the caps. (b) Model. (1) The Wnt/PCP pathway acts as an extrinsic cue that polarizes RhoA activity with respect to the embryonic A–V axis. (2) Local RhoA activity leads to Rock2 and myosin activation, which is essential for the formation and A–V alignment of the F-actin cap. (3) Antxr2a interacts with actin and is therefore recruited to the actin cap. (4) Antxr2a interacts with RhoA and together they activate zDia2; zDia2 may act by nucleating actin cables or by interacting with microtubules at the spindle pole, thereby connecting the poles to the actin cap (see discussion). (5) zDia2 activity exerts torque and aligns the spindle with respect to the embryonic A–V axis. (6) Spindle is A–V aligned. (7) As a result, divisions are A–V oriented.
We found therefore two distinct functions for RhoA during division of epiblast cells. First, RhoA, through activation of Rock2, is involved in F-actin cap formation and alignment. Rock2 could form the F-actin cap by locally promoting actin polymerization7 or by promoting actin contractility through activation of MLC (ref. 8). Supporting the latter, we found that incubating embryos with blebbistatin, an MLC inhibitor, led to a decrease in F-actin accumulation at the cap. It is therefore possible that a myosin activity gradient forms, which in turn drives an actin cortical flow, as has been observed in other systems35,36,37. This would generate an accumulation of actin aligned with the A–V axis.
RhoA is also required for the activation of zDia2, which mediates torquing of the spindle to align it with the cap. Antxr2a contributes to the activation of zDia2 by RhoA. This is consistent with the fact that Antxr2a interacts with RhoA in the GTP-bound form (Supplementary Fig. S6a), the phenotypes of antxr2a and zdia2 morphants are identical (Fig. 5) and zDia2 activity can be modulated by the levels of Antxr2a expression (Supplementary Fig. S6b).
Activated zDia2 could exert its role in spindle torquing by nucleating polarized actin cables emanating from the Antxr2a/F-actin cap. Motor proteins would then interact with the Antxr2a/F-actin cap and walk on the actin filaments, thus exerting torque on the spindle to align it with the cap. Indeed, several studies have correlated the actin cable nucleation activity of formins with rotation and positioning of the mitotic spindle38,39,40,41.
An alternative scenario is that zDia2, independent of its nucleation activity, acts as a physical link between RhoA–Antxr2a at the cap and the microtubules. This interaction would thus couple the tip of astral microtubules to an actin–myosin flow to form the cap, generating a torque on the spindle. Indeed, it has been shown that DRFs can bind microtubules in vitro and in vivo 42. Further experiments will be required to unravel the possible molecular link between the Antxr2a/F-actin cap and microtubules.
A single force exerted from a polarized cap would not rotate the spindle, but would decentre it. However, we did observe rotation of the spindle to be aligned with the polarized cap and we did not observe decentring of the spindle, indicating that a single cap can indeed generate the torque of the spindle. A recent report shows that spindle centring is mediated by a different, independent machinery, involving cortical dynein localization, Polo-like kinase 1 (Plk1) and Ran–GTP (ref. 43). A single force from one pole (the cap) could then torque the spindle if the centre of the spindle (its centre of rotation) is independently fixed by the dynein/Plk1/Ran–GTP system.
In humans Antxr2 promotes formation of capillary-like networks in vitro17, and in zebrafish embryos deficient for Antxr2a show defects during vasculature morphogenesis (our unpublished results). Vasculogenesis also requires oriented endothelial cell division44 and it is also regulated by the Wnt/PCP pathway45, opening the possibility that the role for the anthrax toxin receptor as a key Wnt/PCP determinant controlling the orientation of cell division may be universal and not restricted to early stages of development.
METHODS
Staging and maintenance of embryos.
All zebrafish strains were maintained as described in ref. 46. Injections were carried out in wild-type (AB and TL), slb/wnt11tx226 mutant or Tg(H2A::GFP) embryos. Embryos were staged as previously described47.
Plasmids, cDNAs and antibodies.
The antxr2a complementary DNA was amplified from a full length cDNA clone (BC139636; Open Biosystems) using the primers P1 (5′-CGGGATCCATGACAAAGGAAAATCTTTGGAGC- 3′) and P2 (5′-GGAATTCCGGTAATGCTGCGTGCGACTGAAATT- 3′) and cloned into either pCS2–eYFP or pCS2–eCFP (enhanced cyan fluorescent protein) as BamHI–EcoRI, carboxy-terminal constructs. All the constructs used for injections were cloned into pCS2+.
For immunoprecipitations and Western blots, human CMG2 (isoform a)–V5 cloned into pcDNA3.1/V5-HIS-TOPO (ref. 48) and Antxr2a and RhoA cloned into pCS2+ were used. Antxr2aAID was synthesized by GeneArt, amino-terminal tagged with Myc and cloned into pIRES vector.
For antibody staining, embryos were fixed in 4% paraformaldehyde, stored in methanol at 4 °C for 24 h and processed as described in ref. 49. For Antxr2a endogenous level detection, a polyclonal antibody serum against zebrafish Antxr2a was generated (Eurogentec) and used at 1:50.
mRNA and morpholino injections
For the visualization of Antxr2a and F-actin, 75 pg of antxr2a–eYFP either alone or together with 25 pg of h2a–mRFP or 25 pg of Lifeact–mRFP mRNAs were injected into one cell of 16-cell stage, wild-type, transgenic or morphant embryos and monitored by three-dimensional confocal microscopy imaging at shield stage (6 hours post-fertilization).
For the co-localization experiments of Antxr2a to early and recycling endosomes, 100 pg of eCFP–rab5c (ref. 50), eCFP–da-rab5c (ref. 51) or eYFP–rab11a mRNAs were injected together with 75 pg of either antxr2a–eYFP or antxr2a–eCFP mRNA into one-cell stage, wild-type embryos.
For Antxr2a overexpression experiments, 150 pg of mRNA was injected into one-cell stage, wild-type embryos.
For controls, 25 pg of gap43–GFP, 25 pg of palmitoylated–mRFP and 100 pg of frizzled7–eYFP mRNAs were injected into one cell of 16-cell stage, wild-type or H2A:GFP transgenic embryos.
For the spindle rotation experiments, 50 pg GFP–doublecortin (GFP–dcx) mRNA was injected into one-cell stage, wild-type embryos.
For capped mRNA synthesis, all the constructs were linearized by NotI restriction digestion and transcribed by SP6 polymerase using the mMESSAGE mMACHINE Kit (Ambion).
Three non-overlapping morpholinos, one against the 5’UTR (untranslated region) sequence of the zebrafish antxr2a gene (UTR morpholino oligonucleotide, 5′-CGCGGATCAAAAATAATAAGAGAGG- 3′), another against the coding sequence (ATG morpholino oligonucleotide, 5′-GCTCCAAAGATTTTCCTTTGTCATG- 3′, Gene Tools) and another splicing morpholino oligonucleotide52 were used to knock down Antxr2a activity in zebrafish embryos. All the experiments carried out in this work were carried out with either 1.5 ng of the antxr2a UTR morpholino oligonucleotide or 4 ng of the splicing morpholino oligonucleotide, with the exception of the genetic interaction experiments, where we used 1 ng of the UTR morpholino oligonucleotide to make the phenotype weaker. The controls for morpholino oligonucleotide specificity were done with 2 ng of the ATG morpholino oligonucleotide and with 4 ng of the UTR and splicing morpholino oligonucleotides.
To knock down RhoA and zDia2 function during gastrulation, one-cell stage embryos were injected with 4 ng of rhoA morpholino oligonucleotide26 or 4 ng of zdia2 morpholino oligonucleotide28. For the rescue experiment of zdia2 morphant phenotype by the overexpression of Antxr2a, embryos were injected with 2,ng of zdia2 morpholino oligonucleotide to obtain a hypomorphic condition. To interfere with Rock2 activity, embryos were injected with different mRNA concentrations of a dominant negative form of Rock2 (DNRock2), and epiblast cell divisions were monitored. 75,pg of DNRock2 mRNA was sufficient to randomize cell divisions of epiblast cells. This concentration of DNRock2 together with Lifeact–mRFP or antxr2a–eYFP mRNAs were injected in one cell of 16-cell stage embryos and used for the analysis of the F-actin and Antx2a caps.
For the genetic experiments, morpholino oligonucleotides against wnt5a (ref. 53) and knypek (refs 54, 55) were also used.
Blebbistatin treatment.
16-cell stage embryos were injected either with 75 pg of antxr2a–eYFP or with 25 pg of Lifeact–mRFP mRNAs. Embryos at 50% epiboly were manually dechorionated and incubated with 50 μM blebbistatin (Sigma). Antxr2a/F-actin caps were analysed in epiblast cells after 1–2 h of blebbistatin treatment.
RNA in situ hybridization
Whole mount in situ staining of zebrafish embryos was carried out as previously described49. RNA in situ probes no tail (ntl, ref. 56), distalex3 (dlx3, ref. 57) and hatching gland gene-1 (hgg, ref. 58) were synthesized using a DIG RNA labelling kit (Roche). For antxr2a in situ, an antisense RNA probe was synthesized from a BamHI-linearized, pBluescript–antxr2a plasmid by using T3 RNA polymerase. The sense RNA probe synthesized from the XhoI-linearized plasmid using T7 RNA polymerase was used as a control.
Western blot and immunoprecipitation.
The HeLa cell line was grown in complete modified Eagle’s medium (Gibco) supplemented with 10% fetal calf serum, 2 mM L-glutamine, penicillin and streptomycin.
HeLa cells were transfected for 24 h with either hCMG2 (isoform a)/hAntxr2a and zRhoA, with zAntxr2a and zRhoA (or RhoA, G14V) or for control with zRhoA and empty vectors (3 μg cDNA total per 9 cm2) as previously described48. HeLa cells were harvested, washed with PBS and homogenized by passage through a 22G injection needle in HB (2.9 mM imidazole and 250 mM sucrose, pH 7.4) containing Roche Mini Tablet Protease Inhibitor Cocktail. Protein quantification was done with a Pierce BCA kit. Proteins were loaded at 40 mg protein per lane and separated on a 4–12% acrylamide precast Novex gel (Invitrogen) under reducing conditions and transferred to nitrocellulose membranes (Schleicher and Schuell) by semi-dry transfer.
For immunoprecipitation, cells were lysed for 30 min at 4 °C in immunoprecipitation buffer (0.5% NP40, 500 mM Tris-HCl at pH 7.4, 20 mM EDTA, 10 mM NaF, 2 mM benzamidine and a cocktail of protease inhibitors, Roche), centrifuged for 3 min at 2,000 g and supernatants were incubated for 16 h at 4 °C with antibodies and beads. The rabbit anti-hRhoA antibody (Cell Signalling) recognizes zRhoA and was used to detect zRhoA. Anti-Myc, anti-V5 and anti-tubulin monoclonal antibodies were obtained from Covance, Invitrogen and Sigma, respectively. HRP secondary antibodies were from Pierce and the anti-actin antibody (clone Calpha) from Millipore.
Embryo mounting and confocal imaging.
Live embryos at shield stage were manually dechorionated and mounted in 1% low-melting-point agarose in E3 fish medium. For Antxr2a, and F-actin visualization, four-dimensional confocal microscopy time-lapse images of dorsal epiblast cells were acquired at room temperature using an upright Zeiss 710 multiphoton confocal microscope with a ×20/1.0 water dipping objective. When embryos were injected with either h2A–mRFP or Lifeact–mRFP mRNA, Antxr2a–eYFP was imaged using the 488 nm laser. Between 17 and 30 data points were acquired of defined z-stacks with a step size of 1–1.5 μm over a time interval of 1.2–1.5 min (depending on the exact size of the z-stack).
For cell division orientation in dorsal epiblast cells, shield stage, transgenic Tg(H2A::GFP) embryos uninjected or injected with either 1.5 ng of anxtr2a morpholino oligonucleotide or 4 ng or rhoA morpholino oligonucleotide were mounted. z-stacks were collected at 2 min intervals. Embryos were imaged for approximately 4 h (until bud stage). The co-localization studies with Rab proteins were carried out on a Zeiss 510 Meta microscope with a ×60 water immersion objective. CFP/YFP channels were acquired by sequential scanning.
Image analysis and quantification.
All confocal microscopy images were analysed and quantified using the ImageJ (http://rbs.info.nih.gov/ij/) software. To analyse significance between two independent samples, P values were determined in Microsoft Excel (unpaired t-test, two-tailed distribution). For significance between more than two samples a one-way analysis of variance test was used.
For cell divisions, embryos were mounted so that the A–V axis corresponds to the y axis. For each division that occurred from the beginning to end of gastrulation, x, y coordinates were measured and the angle between the division axis and the A–V axis of the embryo calculated. In our measurements, α = 90°when the cells divide parallel to the y axis and α = 0°when cells divide perpendicular to the y axis.
The three-dimensional surface reconstruction movies for Antxr2a and F-actin were created acquiring z-stacks covering the entire cell. However, to allow for a better appreciation of the shape of the fluorescent signal at the surface of the central region, we removed the highest and lowest z-stacks. Three-dimensional reconstruction movies were created using the ImageJ three-dimensional viewer plug-in.
Fluorescence intensity profiles of Antxr2a or F-actin caps around the perimeter of dorsal epiblast cells were created using ImageJ ‘plot profile’. The intensity profile was then analysed and fitted to a Gaussian distribution in MATLAB R2008b using the following formula:

where A is the amplitude, μ is the mean, σis the standard deviation and O is the offset.
To calculate the enrichment of F-actin, Antxr2a and Palm–mRFP at the cortex, the cell cortex was segmented into two equal hemispheres and the fold of fluorescence accumulation in these two halves of the cell was measured. For each hemisphere, the integral of fluorescence intensity at the cortex was measured and compared with the integral of fluorescence intensity in the opposite hemisphere. Hemispheres were shifted along the cortex to find the maximum ratio (maximum asymmetry between two opposite halves).
For the quantitative dynamic analysis of Antxr2a, F-actin and Palm–mRFP accumulation in Fig. 1c, movies were set in register with respect to abscission (end of cytokinesis).
For the analysis of the F-actin caps, Gaussian fits were carried out. These Gaussian fits had an R2>0.68. These fits were used to calculate μ. The plane between μ and the centre of the cell is the plane of the cap. This plane was used to determine the position of the cap with respect to the A–V axis of the embryo. For this, embryos were always mounted with the animal pole (A) to the top and the vegetal pole (V) to the bottom. The plane of the cap was also used to determine the angle between the position of the Antxr2a/F-actin caps and the mitotic plane. For the F-actin cap, the fit to a Gaussian distribution were carried out only when the asymmetry of F-actin accumulation between the two halves of the cell was at least 1.1. In conditions where the accumulation of the cap was decreased (in rhoA morphants, embryos injected with DNRock2 or embryos treated with blebbistatin), Gaussian fits had R2<0.68. Therefore, the plane of the cap was calculated as the plane between the position of the maximum enrichment fluorescence intensity at the cortex of F-actin of Antxr2a and the cell centre.
References
Castanon, I. & Gonzalez-Gaitan, M. Oriented cell division in vertebrate embryogenesis. Curr. Opin. Cell Biol. 23, 697–704 (2011).
Neumuller, R. A. & Knoblich, J. A. Dividing cellular asymmetry: Asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 23, 2675–2699 (2009).
Gonczy, P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355–366 (2008).
Gong, Y., Mo, C. & Fraser, S. E. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689–693 (2004).
Quesada-Hernandez, E. et al. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr. Biol. 20, 1966–1972 (2010).
Schlessinger, K., Hall, A. & Tolwinski, N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 23, 265–277 (2009).
Ridley, A. J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 (2006).
Riento, K. & Ridley, A. J. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4, 446–456 (2003).
Bartolini, F. & Gundersen, G. G. Formins and microtubules. Biochim. Biophys. Acta 1803, 164–173 (2010).
Chesarone, M. A., DuPage, A. G. & Goode, B. L. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 11, 62–74 (2010).
Goode, B. L. & Eck, M. J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76, 593–627 (2007).
Lee, L., Klee, S. K., Evangelista, M., Boone, C. & Pellman, D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144, 947–961 (1999).
Miller, R. K., Matheos, D. & Rose, M. D. The cortical localization of the microtubule orientation protein, Kar9p, is dependent upon actin and proteins required for polarization. J. Cell Biol. 144, 963–975 (1999).
Azoury, J. et al. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 (2008).
Schuh, M. & Ellenberg, J. A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992 (2008).
Scobie, H. M., Rainey, G. J., Bradley, K. A. & Young, J. A. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl Acad. Sci. USA 100, 5170–5174 (2003).
Reeves, C. V., Dufraine, J., Young, J. A. & Kitajewski, J. Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene 29, 789–801 (2010).
Deuquet, J., Lausch, E., Superti-Furga, A. & van der Goot, F. G. The dark sides of capillary morphogenesis gene 2. EMBO J. 31, 3–13 (2012).
Macqueen, D. J. & Johnston, I. A. Evolution of follistatin in teleosts revealed through phylogenetic, genomic and expression analyses. Dev. Genes. Evol. 218, 1–14 (2008).
Cao, L. G. & Wang, Y. L. Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. J. Cell Biol. 111, 1905–1911 (1990).
Murthy, K. & Wadsworth, P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 15, 724–731 (2005).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Distel, M., Hocking, J. C., Volkmann, K. & Koster, R. W. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 191, 875–890 (2010).
Bouzigues, C. & Dahan, M. Transient directed motions of GABA(A) receptors in growth cones detected by a speed correlation index. Biophys. J. 92, 654–660 (2007).
Fink, J. et al. External forces control mitotic spindle positioning. Nat. Cell Biol. 13, 771–778 (2011).
Zhu, S., Liu, L., Korzh, V., Gong, Z. & Low, B. C. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: use of zebrafish as an in vivo model for GTPase signaling. Cell Signal 18, 359–372 (2006).
Sit, S. T. & Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 124, 679–683 (2011).
Lai, S. L. et al. Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements. PLoS One 3, e3439 (2008).
Li, F. & Higgs, H. N. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340 (2003).
Liu, W. et al. Mechanism of activation of the Formin protein Daam1. Proc. Natl Acad. Sci. USA 105, 210–215 (2008).
Hancock, J. F. & Hall, A. A novel role for RhoGDI as an inhibitor of GAP proteins. EMBO J. 12, 1915–1921 (1993).
Matsui, T. et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 15, 2208–2216 (1996).
Marlow, F., Topczewski, J., Sepich, D. & Solnica-Krezel, L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 12, 876–884 (2002).
Rohde, L. A. & Heisenberg, C. P. Zebrafish gastrulation: cell movements, signals, and mechanisms. Int. Rev. Cytol. 261, 159–192 (2007).
Poincloux, R. et al. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc. Natl Acad. Sci. USA 108, 1943–1948 (2011).
Fukui, Y., Kitanishi-Yumura, T. & Yumura, S. Myosin II-independent F-actin flow contributes to cell locomotion in dictyostelium. J. Cell Sci. 112, 877–886 (1999).
Vallotton, P., Gupton, S. L., Waterman-Storer, C. M. & Danuser, G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc. Natl Acad. Sci. USA 101, 9660–9665 (2004).
Hwang, E., Kusch, J., Barral, Y. & Huffaker, T. C. Spindle orientation in Saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J. Cell Biol. 161, 483–488 (2003).
Liakopoulos, D., Kusch, J., Grava, S., Vogel, J. & Barral, Y. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell 112, 561–574 (2003).
Sagot, I., Klee, S. K. & Pellman, D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4, 42–50 (2002).
Yang, H. C. & Pon, L. A. Actin cable dynamics in budding yeast. Proc. Natl Acad. Sci. USA 99, 751–756 (2002).
Ishizaki, T. et al. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 3, 8–14 (2001).
Kiyomitsu, T. & Cheeseman, I. M. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat. Cell Biol. 14, 311–317 (2012).
Zeng, G. et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood 109, 1345–1352 (2007).
Cirone, P. et al. A role for planar cell polarity signaling in angiogenesis. Angiogenesis 11, 347–360 (2008).
Westerfield, M. The zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) (Univ. Oregon Press, 2000).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dynam. 203, 253–310 (1995).
Abrami, L. et al. Functional interactions between anthrax toxin receptors and the WNT signalling protein LRP6. Cell Microbiol. 10, 2509–2519 (2008).
Montero, J. A. et al. Shield formation at the onset of zebrafish gastrulation. Development 132, 1187–1198 (2005).
Kimmel, C. B., Warga, R. M. & Kane, D. A. Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development 120, 265–276 (1994).
Ulrich, F. et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell 9, 555–564 (2005).
O’Connor, M. N. et al. Functional genomics in zebrafish permits rapid characterization of novel platelet membrane proteins. Blood 113, 4754–4762 (2009).
Kilian, B. et al. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 120, 467–476 (2003).
Topczewski, J. et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell 1, 251–264 (2001).
Solnica-Krezel, L. et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 123, 67–80 (1996).
Schulte-Merker, S., van Eeden, F. J., Halpern, M. E., Kimmel, C. B. & Nusslein-Volhard, C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120, 1009–1015 (1994).
Akimenko, M. A., Ekker, M., Wegner, J., Lin, W. & Westerfield, M. Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J. Neurosci. 14, 3475–3486 (1994).
Thisse, C., Thisse, B., Halpern, M. E. & Postlethwait, J. H. Goosecoid expression in neurectoderm and mesendoderm is disrupted in zebrafish cyclops gastrulas. Dev. Biol. 164, 420–429 (1994).
Acknowledgements
We are grateful to B. Kunz and N. Zangger for generating the zAntxr2a construct for the pulldown, the phylogenetic tree and multiple sequence alignments, S. Abke, E. Paluch, C. Low, G. Weidinger, R. W. Köster, E. Kardash, L. Solnica-Krezel, A. J. Ridley, G. Davidson, S. Loubéry and O. Schaad, and the bioimaging and fish platforms for reagents and technical help and T. Hannich for critical reading. This work was supported by the SNSF, the Swiss SystemsX.ch initiative and LipidX-2008/011 (M.G-G. and F.G.v.d.G.), by the Fondation SANTE-Vaduz/Aide au Soutien des Nouvelles Thérapies (F.G.v.d.G.) and by the ERC, the NCCR Frontiers in Genetics and Chemical Biology programmes and the Polish–Swiss research program (M.G-G.).
Author information
Authors and Affiliations
Contributions
I.C. designed and carried out the experiments, generated and interpreted most of the data and prepared the manuscript. L.A. designed and carried out the immunoprecipitation experiments. L.H. designed the algorithm and simulation assays for the directed rotation experiments and C.P.H. supported experimentation and contributed to data interpretation. I.C., F.G.v.d.G. and M.G-G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2219 kb)
Supplementary Note
Supplementary Information (PDF 212 kb)
Supplementary Table
Supplementary Information (XLSX 58 kb)
Supplementary Movie 1
Supplementary Information (AVI 3482 kb)
Supplementary Movie 2
Supplementary Information (AVI 312 kb)
Supplementary Movie 3
Supplementary Information (AVI 2707 kb)
Supplementary Movie 4
Supplementary Information (AVI 452 kb)
Supplementary Movie 5
Supplementary Information (AVI 411 kb)
Supplementary Movie 6
Supplementary Information (AVI 920 kb)
Supplementary Movie 7
Supplementary Information (AVI 369 kb)
Rights and permissions
About this article
Cite this article
Castanon, I., Abrami, L., Holtzer, L. et al. Anthrax toxin receptor 2a controls mitotic spindle positioning. Nat Cell Biol 15, 28–39 (2013). https://doi.org/10.1038/ncb2632
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2632
This article is cited by
-
Capillary morphogenesis gene 2 (CMG2) mediates growth factor-induced angiogenesis by regulating endothelial cell chemotaxis
Angiogenesis (2022)
-
Wnt-controlled sphingolipids modulate Anthrax Toxin Receptor palmitoylation to regulate oriented mitosis in zebrafish
Nature Communications (2020)
-
Different mechanisms of two anti-anthrax protective antigen antibodies and function comparison between them
BMC Infectious Diseases (2019)
-
NDP52 tunes cortical actin interaction with astral microtubules for accurate spindle orientation
Cell Research (2019)
-
Up-regulated NRIP2 in colorectal cancer initiating cells modulates the Wnt pathway by targeting RORβ
Molecular Cancer (2017)