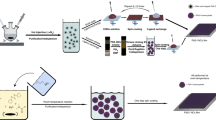
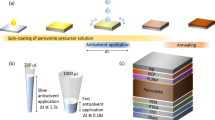
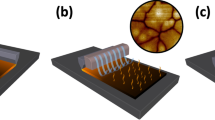
Abstract
Lead halide perovskite semiconductors have recently gained wide interest following their successful embodiment in solid-state photovoltaic devices with impressive power-conversion efficiencies, while offering a relatively simple and low-cost processability. Although the primary optoelectronic properties of these materials have already met the requirement for high-efficiency optoelectronic technologies, industrial scale-up requires more robust processing methods, as well as solvents that are less toxic than the ones that have been commonly used so successfully on the lab-scale. Here we report a fast, room-temperature synthesis of inks based on CsPbBr3 perovskite nanocrystals using short, low-boiling-point ligands and environmentally friendly solvents. Requiring no lengthy post-synthesis treatments, the inks are directly used to fabricate films of high optoelectronic quality, exhibiting photoluminescence quantum yields higher than 30% and an amplified spontaneous emission threshold as low as 1.5 μJ cm−2. Finally, we demonstrate the fabrication of perovskite nanocrystal-based solar cells, with open-circuit voltages as high as 1.5 V.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 July 2017
In the PDF version of this article previously published, the year of publication provided in the footer of each page and in the 'How to cite' section was erroneously given as 2017, it should have been 2016. This error has now been corrected. The HTML version of the article was not affected.
References
Boles, M. A., Ling, D., Hyeon, T. & Talapin, D. V. The surface science of nanocrystals. Nat. Mater. 15, 141–153 (2016).
Yang, J., Choi, M. K., Kim, D.-H. & Hyeon, T. Designed assembly and integration of colloidal nanocrystals for device applications. Adv. Mater. 28, 1176–1207 (2016).
Kamat, P. V. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C 112, 18737–18753 (2008).
Goodwin, E. D. et al. Effects of post-synthesis processing on CdSe nanocrystals and their solids: correlation between surface chemistry and optoelectronic properties. J. Phys. Chem. C 118, 27097–27105 (2014).
Burda, C., Chen, X., Narayanan, R. & El-Sayed, M. A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 105, 1025–1102 (2005).
Donega, C. d. M. Synthesis and properties of colloidal heteronanocrystals. Chem. Soc. Rev. 40, 1512–1546 (2011).
Peng, X. et al. Shape control of CdSe nanocrystals. Nature 404, 59–61 (2000).
Wang, R. et al. Colloidal quantum dot ligand engineering for high performance solar cells. Energy Environ. Sci. 9, 1130–1143 (2016).
McDonald, S. A. et al. Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nat. Mater. 4, 138–142 (2005).
Li, G. et al. Efficient light-emitting diodes based on nanocrystalline perovskite in a dielectric polymer matrix. Nano Lett. 15, 2640–2644 (2015).
Yoon, W. et al. Enhanced open-circuit voltage of PbS nanocrystal quantum dot solar cells. Sci. Rep. 3, 2225 (2013).
Oh, S. J. et al. Engineering charge injection and charge transport for high performance PbSe nanocrystal thin film devices and circuits. Nano Lett. 14, 6210–6216 (2014).
Koleilat, G. I. et al. Efficient, stable infrared photovoltaics based on solution-cast colloidal quantum dots. ACS Nano 2, 833–840 (2008).
Lan, X. et al. 10.6% certified colloidal quantum dot solar cells via solvent-polarity-engineered halide passivation. Nano Lett. 16, 4630–4634 (2016).
Zhang, H., Kurley, J. M., Russell, J. C., Jang, J. & Talapin, D. V. Solution-processed, ultrathin solar cells from CdCl3—capped CdTe nanocrystals: the multiple roles of CdCl3–ligands. J. Am. Chem. Soc. 138, 7464–7467 (2016).
Saliba, M. et al. A molecularly engineered hole-transporting material for efficient perovskite solar cells. Nat. Energy 1, 15017 (2016).
Saliba, M. et al. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 9, 1989–1997 (2016).
Yang, W. S. et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234–1237 (2015).
Cho, H. et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 350, 1222–1225 (2015).
Ke, X., Yan, J., Zhang, A., Zhang, B. & Chen, Y. Optical band gap transition from direct to indirect induced by organic content of CH3NH3PbI3 perovskite films. Appl. Phys. Lett. 107, 091904 (2015).
Miyata, A. et al. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 11, 582–587 (2015).
Stranks, S. D. et al. Electron–hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 342, 341–344 (2013).
Giorgi, G., Fujisawa, J.-I., Segawa, H. & Yamashita, K. Small photocarrier effective masses featuring ambipolar transport in methylammonium lead iodide perovskite: a density functional analysis. J. Phys. Chem. Lett. 4, 4213–4216 (2013).
Koh, T. M. et al. Formamidinium-containing metal-halide: an alternative material for near-IR absorption perovskite solar cells. J. Phys. Chem. C 118, 16458–16462 (2014).
Zhang, W. et al. Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells. Nat. Commun. 6, 10030 (2015).
He, H. et al. Exciton localization in solution-processed organolead trihalide perovskites. Nat. Commun. 7, 10896 (2016).
Nie, W. et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 347, 522–525 (2015).
Deschler, F. et al. High photoluminescence efficiency and optically pumped lasing in solution-processed mixed halide perovskite semiconductors. J. Phys. Chem. Lett. 5, 1421–1426 (2014).
Yantara, N. et al. Inorganic halide perovskites for efficient light-emitting diodes. J. Phys. Chem. Lett. 6, 4360–4364 (2015).
Schmidt, L. C. et al. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 136, 850–853 (2014).
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, Xx = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015).
Tyagi, P., Arveson, S. M. & Tisdale, W. A. Colloidal organohalide perovskite nanoplatelets exhibiting quantum confinement. J. Phys. Chem. Lett. 6, 1911–1916 (2015).
Sichert, J. A. et al. Quantum size effect in organometal halide perovskite nanoplatelets. Nano Lett. 15, 6521–6527 (2015).
Hu, F. et al. Superior optical properties of perovskite nanocrystals as single photon emitters. ACS Nano 9, 12410–12416 (2015).
Bekenstein, Y., Koscher, B. A., Eaton, S. W., Yang, P. & Alivisatos, A. P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc. 137, 16008–16011 (2015).
Akkerman, Q. A. et al. Solution synthesis approach to colloidal cesium lead halide perovskite nanoplatelets with monolayer-level thickness control. J. Am. Chem. Soc. 138, 1010–1016 (2016).
Sun, S., Yuan, D., Xu, Y., Wang, A. & Deng, Z. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano 10, 3648–3657 (2016).
Bai, S., Yuan, Z. & Gao, F. Colloidal metal halide perovskite nanocrystals: synthesis, characterization, and applications. J. Mater. Chem. C 4, 3898–3904 (2016).
De Roo, J. et al. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano 10, 2071–2081 (2016).
Swarnkar, A. et al. Quantum dot–induced phase stabilization of 𝛼-CsPbI3 perovskite for high-efficiency photovoltaics. Science 354, 92–95 (2016).
Stoumpos, C. C. et al. Crystal growth of the perovskite semiconductor CsPbBr3: a new material for high-energy radiation detection. Cryst. Growth Des. 13, 2722–2727 (2013).
Sebastian, M. et al. Excitonic emissions and above-band-gap luminescence in the single-crystal perovskite semiconductors CsPbBr3 and CsPbCl3 . Phys. Rev. B 92, 235210 (2015).
Akkerman, Q. A. et al. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 137, 10276–10281 (2015).
Zorn, G., Dave, S. R., Gao, X. & Castner, D. G. Method for determining the elemental composition and distribution in semiconductor core-shell quantum dots. Anal. Chem. 83, 866–873 (2011).
Yakunin, S. et al. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 6, 8056 (2015).
Dou, L. et al. Atomically thin two-dimensional organic–inorganic hybrid perovskites. Science 349, 1518–1521 (2015).
D’ Innocenzo, V., Srimath Kandada, A. R., De Bastiani, M., Gandini, M. & Petrozza, A. Tuning the light emission properties by band gap engineering in hybrid lead halide perovskite. J. Am. Chem. Soc. 136, 17730–17733 (2014).
Arora, N. et al. Photovoltaic and amplified spontaneous emission studies of high-quality formamidinium lead bromide perovskite films. Adv. Funct. Mater. 26, 2846–2854 (2016).
Yakunin, S. et al. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 6, 8056 (2015).
Xu, Y. et al. Two-photon-pumped perovskite semiconductor nanocrystal lasers. J. Am. Chem. Soc. 138, 3761–3768 (2016).
Vybornyi, O., Yakunin, S. & Kovalenko, M. V. Polar-solvent-free colloidal synthesis of highly luminescent alkylammonium lead halide perovskite nanocrystals. Nanoscale 8, 6278–6283 (2016).
Park, Y.-S., Bae, W. K., Baker, T., Lim, J. & Klimov, V. I. Effect of auger recombination on lasing in heterostructured quantum dots with engineered core/shell interfaces. Nano Lett. 15, 7319–7328 (2015).
Gollner, C. et al. Random lasing with systematic threshold behavior in films of CdSe/CdS core/thick-shell colloidal quantum dots. ACS Nano 9, 9792–9801 (2015).
Grim, J. Q. et al. Continuous-wave biexciton lasing at room temperature using solution-processed quantum wells. Nat. Nanotech. 9, 891–895 (2014).
Moreels, I. et al. Nearly temperature-independent threshold for amplified spontaneous emission in colloidal CdSe/CdS quantum dot-in-rods. Adv. Mater. 24, OP231–OP235 (2012).
Di Stasio, F. et al. Single-mode lasing from colloidal water-soluble CdSe/CdS quantum dot-in-rods. Small 11, 1328–1334 (2015).
Edri, E., Kirmayer, S., Kulbak, M., Hodes, G. & Cahen, D. Chloride inclusion and hole transport material doping to improve methyl ammonium lead bromide perovskite-based high open-circuit voltage solar cells. J. Phys. Chem. Lett. 5, 429–433 (2014).
Giesbrecht, N. et al. Synthesis of perfectly oriented and micrometer-sized MAPbBr3 perovskite crystals for thin-film photovoltaic applications. ACS Energy Lett. 1, 150–154 (2016).
Arora, N. et al. High open-circuit voltage: fabrication of formamidinium lead bromide perovskite solar cells using fluorene–dithiophene derivatives as hole-transporting materials. ACS Energy Lett. 1, 107–112 (2016).
Kulbak, M. et al. Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 7, 167–172 (2016).
Sutton, R. J. et al. Bandgap-tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv. Energy Mater. 6, 1502458 (2016).
Gao, P., Gratzel, M. & Nazeeruddin, M. K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 7, 2448–2463 (2014).
Shockley, W. & Queisser, H. J. Detailed balance limit of efficiency of p–n junction solar cells. J. Appl. Phys. 32, 510–519 (1961).
Kulbak, M., Cahen, D. & Hodes, G. How important is the organic part of lead halide perovskite photovoltaic cells? Efficient CsPbBr3 cells. J. Phys. Chem. Lett. 6, 2452–2456 (2015).
Ryu, S. et al. Voltage output of efficient perovskite solar cells with high open-circuit voltage and fill factor. Energy Environ. Sci. 7, 2614–2618 (2014).
Ball, J. M., Lee, M. M., Hey, A. & Snaith, H. J. Low-temperature processed meso-superstructured to thin-film perovskite solar cells. Energy Environ. Sci. 6, 1739–1743 (2013).
Helander, M. G., Greiner, M. T., Wang, Z. B. & Lu, Z. H. Pitfalls in measuring work function using photoelectron spectroscopy. Appl. Surf. Sci. 256, 2602–2605 (2010).
Calloni, A. et al. Stability of organic cations in solution-processed CH3NH3PbI3 perovskites: formation of modified surface layers. J. Phys. Chem. C 119, 21329–21335 (2015).
Schulz, P. et al. Interface energetics in organo-metal halide perovskite-based photovoltaic cells. Energy Environ. Sci. 7, 1377–1381 (2014).
de Mello, J. C., Wittmann, H. F. & Friend, R. H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 9, 230–232 (1997).
Acknowledgements
The research leading to these results has received funding from the European Union 7th Framework Programme under Grant Agreement No. 614897 (ERC Consolidator Grant ‘TRANS-NANO’) from the Cariplo Foundation through grant agreement no. 2013-0656 (‘Green nanomaterials for next-generation photovoltaics, GREENS)’.
Author information
Authors and Affiliations
Contributions
Q.A.A., L.M., M.P. and A.P. conceived the experiments. Q.A.A. developed the synthesis of the nanocrystals inks, M.G. and J.M.B. fabricated and tested the solar cells, F.D.S. and P.R. studied the optical properties of the films and F.D.S. performed the ASE measurements, P.R. performed the cross-section, F.P. performed the XPS measurements, G.B. performed the HRTEM measurements and M.P. performed the UPS measurements. Q.A.A., M.G., J.M.B, A.P. and L.M. wrote the manuscript, with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–16, Supplementary Tables 12 and Supplementary References. (PDF 4403 kb)
Supplementary Video 1
Video of CsPbBr3 nanocrystal synthesis. (MP4 3877 kb)
Rights and permissions
About this article
Cite this article
Akkerman, Q., Gandini, M., Di Stasio, F. et al. Strongly emissive perovskite nanocrystal inks for high-voltage solar cells. Nat Energy 2, 16194 (2017). https://doi.org/10.1038/nenergy.2016.194
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.194
This article is cited by
-
Extremely long-lived charge separation and related carrier spin excitation in CsPbBr3 perovskite quantum dots with an electron acceptor benzoquinone
Nano Research (2024)
-
First-principles calculations to investigate structural, optoelectronic and thermoelectric properties of Sn-based halide perovskites: CsSnCl3 and CH3NH3SnCl3
Journal of the Korean Ceramic Society (2024)
-
A Rising 2D Star: Novel MBenes with Excellent Performance in Energy Conversion and Storage
Nano-Micro Letters (2023)
-
Photoinduced large polaron transport and dynamics in organic–inorganic hybrid lead halide perovskite with terahertz probes
Light: Science & Applications (2022)
-
CsPbBr3 deposited by laser ablation: effects of post-growth aging, oxygen adsorption and annealing on film properties
Applied Physics A (2022)