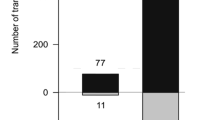
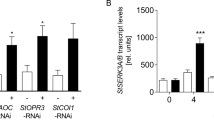
Abstract
Plant immune responses are usually accompanied by the production of extracellular superoxide at and surrounding infection sites1,2,3. Extracellular reactive oxygen intermediates (ROIs) in plants were proposed to drive programmed cell death correlated with disease resistance (the hypersensitive response). ROIs derived from this oxidative burst are generated by plasma membrane NADPH oxidases, anchored by gp91phox proteins related to those responsible for the respiratory oxidative burst activated in mammalian neutrophils during infection4,5. Mutation of Arabidopsis thaliana respiratory burst oxidase (Atrboh) genes eliminated pathogen-induced ROI production but had only a modest effect on the hypersensitive response4. We show that Atrboh function can be activated by exogenous ROIs. Unexpectedly, the subsequent oxidative burst can suppress cell death in cells surrounding sites of NADPH oxidase activation. This cell death requires salicylic acid, a plant immune system activator6. Thus, ROIs generated by Atrboh proteins can antagonize salicylic acid–dependent pro-death signals. These results have implications for understanding how salicylic acid activates defense signaling in cells spatially removed from infection sites without causing cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 23, 345–357 (1983).
Bestwick, C.S., Brown, I.R., Bennett, M.H.R. & Mansfield, J.W. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv. phaseolicola. Plant Cell 9, 209–221 (1997).
Apel, K. & Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 (2004).
Torres, M.A., Dangl, J.L. & Jones, J.D.G. Arabidopsis gp91-phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99, 523–528 (2002).
Cross, A.R. & Segal, A.W. The NADPH oxidase of professional phagocytes–prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta 1657, 1–22 (2004).
Durrant, W.E. & Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209 (2004).
Torres, M.A. & Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8, 397–403 (2005).
Levine, A., Tenhaken, R., Dixon, R. & Lamb, C.J. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593 (1994).
Kwak, J.M. et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633 (2003).
Foreman, J. et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446 (2003).
Dietrich, R.A., Richberg, M.H., Schmidt, R., Dean, C. & Dangl, J.L. A novel zinc-finger protein is encoded by the Arabidopsis lsd1 gene and functions as a negative regulator of plant cell death. Cell 88, 685–694 (1997).
Jabs, T., Dietrich, R.A. & Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273, 1853–1856 (1996).
Rustérucci, C., Aviv, D.H., Holt, B.F. III, Dangl, J.L. & Parker, J.E. The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13, 2211–2224 (2001).
Aviv, D.H. et al. Runaway cell death, but not basal disease resistance, in lsd1 is SA- and NIM1/NPR1-dependent. Plant J. 29, 381–391 (2002).
Joo, J.H., Wang, S., Chen, J.G., Jones, A.M. & Fedoroff, N.V. Different signaling and cell death roles of heterotrimeric G protein {alpha} and {beta} subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17, 957–970 (2005).
Govrin, E. & Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 10, 751–757 (2000).
Enyedi, A.J., Yalpani, N., Silverman, P. & Raskin, I. Localization, conjugation and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc. Natl. Acad. Sci. USA 89, 2480–2484 (1992).
Dorey, S. et al. Spatial and temporal induction of cell death, defense genes, and accumulation of salicylic acid in tobacco leaves reacting hypersensitively to a fungal glycoprotein elicitor. Mol. Plant Microbe Interact. 10, 646–655 (1997).
Shirasu, K., Nakajima, H., Rajasekhar, V.K., Dixon, R.A. & Lamb, C.J. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9, 261–270 (1997).
Draper, J. Salicylate, superoxide synthesis and cell suicide in plant defense. Trends Plant Sci. 2, 162–165 (1997).
Wildermuth, M.C., Dewdney, J., Wu, G. & Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565 (2001).
Reeves, E.P. et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416, 291–297 (2002).
Delledonne, M., Zeier, J., Marocco, A. & Lamb, C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. USA 98, 13454–13459 (2001).
Wendehenne, D., Durner, J. & Klessig, D.F. Nitric oxide: a new player in plant signalling and defence responses. Curr. Opin. Plant Biol. 7, 449–455 (2004).
Auh, C.-K. & Murphy, T.M. Plama membrane redox enzyme is involved in the synthesis of O2- and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 107, 1241–1247 (1995).
Guo, F.Q., Okamoto, M. & Crawford, N.M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302, 100–103 (2003).
Allan, A.C. & Fluhr, R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9, 1559–1572 (1997).
Levine, A., Pennell, R., Palmer, R. & Lamb, C.J. Calcium-mediated apoptosis in a plant hypersensitive response. Curr. Biol. 6, 427–437 (1996).
Davletova, S. et al. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17, 268–281 (2005).
Kliebenstein, D.J., Dietrich, R.A., Martin, A.C., Last, R.L. & Dangl, J.L. LSD1 regulates salicylic acid induction of copper-zinc superoxide dismutase in Arabidopsis thaliana. Mol. Plant Microbe Interact. 12, 1022–1026 (1999).
Acknowledgements
We thank K. Overmyer and J. McDowell for comments on the manuscript. This work was supported by grants from the US National Science Foundation and the US National Institutes of Health to J.L.D. The Sainsbury Laboratory is supported by the Gatsby Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Torres, M., Jones, J. & Dangl, J. Pathogen-induced, NADPH oxidase–derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37, 1130–1134 (2005). https://doi.org/10.1038/ng1639
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1639
This article is cited by
-
ROS accumulation-induced tapetal PCD timing changes leads to microspore abortion in cotton CMS lines
BMC Plant Biology (2023)
-
Is localized acquired resistance the mechanism for effector-triggered disease resistance in plants?
Nature Plants (2023)
-
Black scurf of potato: Insights into biology, diagnosis, detection, host-pathogen interaction, and management strategies
Tropical Plant Pathology (2023)
-
Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes
BMC Plant Biology (2022)
-
Metabolomic and Transcriptomic Changes Induced by Potassium Deficiency During Sarocladium oryzae Infection Reveal Insights into Rice Sheath Rot Disease Resistance
Rice (2021)