Abstract
Persistent viral infections are characterized by the simultaneous presence of chronic inflammation and T cell dysfunction. In prototypic models of chronicity—infection with human immunodeficiency virus (HIV) or lymphocytic choriomeningitis virus (LCMV)—we used transcriptome-based modeling to reveal that CD4+ T cells were co-exposed not only to multiple inhibitory signals but also to tumor-necrosis factor (TNF). Blockade of TNF during chronic infection with LCMV abrogated the inhibitory gene-expression signature in CD4+ T cells, including reduced expression of the inhibitory receptor PD-1, and reconstituted virus-specific immunity, which led to control of infection. Preventing signaling via the TNF receptor selectively in T cells sufficed to induce these effects. Targeted immunological interventions to disrupt the TNF-mediated link between chronic inflammation and T cell dysfunction might therefore lead to therapies to overcome persistent viral infection.
Similar content being viewed by others
Main
Two independent hallmarks have been established for many chronic viral infections1. The first hallmark is a chronic inflammatory component characterized by elevated concentrations of cytokines known to have a role during acute inflammation, including type I interferons, interleukin 6 (IL-6) and tumor-necrosis factor (TNF)2,3,4. Often these chronic inflammatory reactions are also associated with additional pathological processes or alterations in the ensuing immune response1,3,5,6. The other important hallmark is a divergence from functional memory and effector T cell differentiation, which leads to the emergence of dysfunctional T cells, also called 'T cell exhaustion'7,8. Signaling via several extrinsic and cell-intrinsic negative immunological regulatory circuits results in specific dysfunction-associated gene-expression programs9,10.
While the link between chronic inflammation and T cell dysfunction in chronic viral infection remained vague for a long time11, published findings have now shed some light on potential candidates that link these conditions12,13. It has been demonstrated that type I interferons themselves are critically involved in T cell dysfunction and chronic inflammation12,13. This is of particular interest since type I interferons are classical pro-inflammatory cytokines critically involved in viral clearance during an acute viral infection2, yet they seem to have a completely different role once chronicity and persistence of the viral infection is initiated6. We reasoned that such dichotomous roles might be exerted by not simply a single class of molecules1,4 and therefore postulated that other factors known to be pro-inflammatory during acute infection might be involved in chronicity and would thereby link T cell dysfunction and chronic inflammatory processes to tissue protection.
Results
Inhibitory pathway activity in T cells from HIV-infected subjects
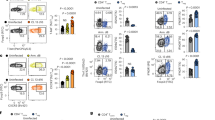
We assessed the complexity of inhibitory signaling on conventional CD4+ T cells in a cohort of subjects infected with human immunodeficiency virus (HIV). We stratified subjects in this cohort (n = 1,304) according to their viral titers (Supplementary Fig. 1a and Supplementary Table 1). Most subjects (n = 1,020) were successfully treated with combination antiretroviral therapy (cART), which resulted in a plasma viral load below the detection limit, while a smaller subset (n = 284) had high viral titers despite previous or ongoing cART (data not shown). Next we assessed surface expression of the inhibitory receptor PD-1 on CD4+ T cells from a subset of subjects. In subjects who controlled HIV or were successfully treated, we did not detect significant PD-1 expression (HIVloPD-1lo) (Fig. 1a,b and Supplementary Fig. 1b–d). However, in subjects with high viral load, we observed high expression of PD-1 (HIVhiPD-1hi) on CD4+ T cells (Fig. 1a,b and Supplementary Fig. 1b–d) and CD8+ T cells (Supplementary Fig. 1e–g), even on those from subjects treated with cART. PD-1 expression on CD4+ T cells from HIV-infected subjects correlated with a reduced number of CD4+ T cells and increased viral load, while the number of CD4+ T cells was inversely correlated with viral load (Supplementary Fig. 1h–j). Furthermore, expression of the immunomodulatory receptor CTLA-4 (CD152) was higher on CD4+ T cells and CD8+ T cells from HIVhiPD-1hi subjects than on those from HIVloPD-1lo subjects (Supplementary Fig. 1k–p).
(a) Flow cytometry analyzing PD-1 expression on CD4+ T cells from HIVloPD-1lo and HIVhiPD-1hi subjects. Numbers in quadrants indicate percent PD-1−CD4+ cells (bottom right) or PD-1+CD4+ cells (top right). (b) Cumulative results for analyses as in a, for HIVloPD-1lo subjects (n = 26) and HIVhiPD-1hi subjects (n = 37). (c) RNA fingerprint–enrichment analysis of PD-1, CTLA-4, prostaglandin E2 (PGE2), TGF-β and IL-10 for HIVhiPD-1hi subjects (n = 10) and HIVloPD-1lo HIV subjects (n = 10), showing probes with significantly different expression in a fingerprint (defined as a difference in expression (higher or lower) of 1.3-fold or more (P < 0.05 (unpaired Student's t-test)) in HIVhiPD-1hi and HIVloPD-1lo subjects compared with the expression of all other genes (one-sided Fisher's exact test). (d–i) Group prediction analysis via a 'support vector machine' approach; colors (d,f–i) indicate high (red) or low (blue) probability for the RNA fingerprint, and numbers above columns (d,f,g) indicate subject identifier. (d) Predicted probability for the classification of samples as in c as HIVhiPD-1hi on the basis of the inhibitory-pathway RNA fingerprint. (e) Mean prediction probabilities as in d. (f–h) Prediction probability for the classification of samples as HIV+ (keys) by the publicly available data sets GSE9927 (f), GSE23879 (g) and GSE18233 (h) comparing HIV-infected (HIV+) subjects and uninfected (HIV−) subjects (n = 11 and n = 9 (f), n = 14 and n = 9 (g), and n = 127 and n = 8 (h), respectively). (i) Predicted probability for the classification of samples as untreated (key) in a comparison (GSE18233) of HIV-infected subjects (n = 38) before cART (Untreated HIV+) and after cART (Treated HIV+). (j) Quantitative PCR analysis of DHRS3, CD79B, OAS1, TRADD and ALDOA mRNA of various RNA fingerprints (horizontal axes), in CD4+ T cells from HIVloPD-1lo subjects (n = 5) and HIVhiPD-1hi subjects (n = 5); results calculated by the change-in-cycling-threshold method were normalized to those of the control gene B2M and are presented relative to those of HIVloPD-1lo subjects, set as 1. (k) Frequency of cells positive for the integrin CD49d, the immunoglobulin β-chain CD79b, the cytokine receptor IL-4R or the chemokine receptor CCR4, of various RNA fingerprints (horizontal axes), among PD-1+CD4+ T cells from HIVloPD-1lo subjects (n = 10) or HIVhiPD-1hi subjects (n = 9). NS, not significant (P > 0.05); *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 (Mann-Whitney U-test (b,i (DHRS3, OAS1) and k (CD49d)), one-sided Fisher's exact test (c) or unpaired Student's t-test (e,j (CD79b, TRADD and ALDOA) and k (CD79b, IL4R and CCR4)). Data are from 63 (a,b), 10 (j) or 19 (k) experiments with one subject in each (mean and 25th and 75th percentiles (horizontal bars), 10th and 90th percentiles ('whiskers') and outliers (symbols) in b,k; mean + s.e.m. in j), one experiment with n = 20 subjects (c–f; mean + s.e.m. in e), n = 23 subjects (g) or n = 135 subjects (h), or two experiments with n = 38 subjects in each (i).
As HIV infection is associated with not only a reduced number of CD4+ T cells but also reduced function of these cells14, we investigated in an unbiased fashion which inhibitory pathways were active in CD4+ T cells during chronic infection. We analyzed the transcriptomes of CD4+ T cells from HIVhiPD-1hi subjects (n = 10) and HIVloPD-1lo subjects (n = 10) (data not shown). Next we established the RNA 'fingerprints' of CD4+ T cells from healthy donors, for five prominent co-inhibitory signaling pathways downstream of PD-1, CTLA-4, prostaglandin E2, IL-10, and transforming growth factor β (TGF-β), under defined in vitro conditions (Supplementary Fig. 1q–s and Supplementary Table 2). These RNA fingerprints allowed us to detect the presence of particular inhibitory pathways in the transcriptional profile of CD4+ T cells. RNA fingerprint–enrichment analysis using these RNA fingerprints, together with gene-set–enrichment analysis (GSEA)15, revealed that CD4+ T cells from HIVhiPD-1hi subjects showed significant enrichment for expression of genes encoding products associated with all inhibitory pathways, but those from HIVloPD-1lo subjects did not (Fig. 1c, Supplementary Fig. 1t,u and Supplementary Table 3). We next used group-prediction analysis based on each of the inhibitory-pathway RNA fingerprints to classify each sample as HIVhiPD-1hi or HIVloPD-1lo (Fig. 1d,e). This analysis led to accurate classification in most cases (Fig. 1d; individual mean prediction probabilities for each RNA fingerprint, Fig. 1e). Application of group-prediction analysis to published data sets (GEO accession codes GSE9927, GSE18233 and GSE23879)16,17,18 showed that the inhibitory-pathway RNA fingerprints were also present in CD4+ T cells from HIV-infected subjects in other studies (Fig. 1f–h and data not shown). Furthermore, using signature genes associated with each inhibitory pathway independently identified by alternative bioinformatics approaches19, we confirmed enrichment for inhibitory signaling in our data sets and the three publicly available data sets (data not shown). One of these data sets also allowed us to compare HIV-infected subjects before and after treatment; we found that treated subjects showed a reduced presence of the inhibitory-pathway RNA fingerprints (Fig. 1i and data not shown). We confirmed the expression of signature genes of the respective RNA fingerprints in CD4+ T cells from HIVhiPD-1hi subjects by quantitative PCR (Fig. 1j). Moreover, we found co-expression of markers of inhibitory-pathway RNA fingerprints in PD-1+CD4+ T cells from HIVhiPD-1hi subjects at the protein level, with the exception of the IL-10 RNA fingerprint, which did not include a marker accessible to analysis by flow cytometry (Fig. 1k). Together our data demonstrated that multiple co-inhibitory pathways were simultaneously active in CD4+ T cells from HIVhiPD-1hi subjects, which indicated that loss of function in these cells might not be associated with a single regulatory mechanism.
TNF receptor signaling is linked to co-inhibitory pathways
The simultaneous presence of multiple inhibitory pathways in CD4+ T cells from HIVhiPD-1hi subjects raised the question of whether they were independently induced or were facilitated by a common regulator. To identify molecules that potentially co-influenced gene expression, we developed a combinatorial algorithm based on gene-class testing, gene-network construction and gene-ontology analysis. We identified TNF as a central molecule in the resulting network (Fig. 2a), which made TNF the most likely candidate to be associated with the co-inhibitory pathways. In line with that result, assessment of upstream regulators by Ingenuity pathway analysis similarly revealed TNF as a potential molecule that influenced the observed changes in gene expression (Fig. 2b and Supplementary Table 4). These results suggested that chronic signaling via the TNF receptor (TNFR) might be an important determinant for the development of loss of function in CD4+ T cells. To confirm that hypothesis, we established an RNA fingerprint for TNF-stimulated CD4+ T cells isolated from healthy donors and observed substantial enrichment for that RNA fingerprint in CD4+ T cells from HIVhiPD-1hi subjects, using GSEA (Fig. 2c and Supplementary Table 3). Accordingly, application of group-prediction analysis based on the TNF RNA fingerprint classified HIVhiPD-1hi subjects correctly (Fig. 2d,e). Discrimination between CD4+ T cells from untreated chronically HIV-infected (HIV+) subjects and those from healthy (HIV−) donors was further validated in three additional publicly available data sets (GSE9927, GSE23879 and GSE18233; Fig. 2f–h and data not shown). Analysis of HIV+ subjects before and after cART showed that the expression of genes of the TNF signature was reduced after treatment (Fig. 2i and data not shown), which further supported the hypothesis that TNF might induce a permissive state that would allow the activity of co-inhibitory pathways.
(a) Contribution network derived from gene-ontology analysis of gene-expression data of CD4+ T cells from HIVhiPD-1hi and HIVloPD-1lo subjects (n = 10 per group), built on the basis of 177 pathways derived from gene-class testing, presented for genes in the 20 pathways with the most significant difference in expression. The number of shared gene-ontology terms was chosen as the maximal value to obtain a sufficiently connected network; lines between genes indicate affiliation with at least one shared gene-ontology term. (b) Mechanistic network of TNF from Ingenuity pathway analysis of upstream regulators, for data as in a. (c) GSEA with genes upregulated by TNF in CD4+ T cells (CD4 TNF up) as the gene set for data as in a: enrichment score = 0.5545, P = 0.0001 and FDR (false-discovery rate) = 0.0016 (enrichment was considered significant with a P value of < 0.05 and an FDR of < 0.25). (d) Group prediction analysis of the TNF RNA fingerprint, showing the prediction probability for the classification of samples as HIVhiPD-1hi on the basis of the TNF RNA fingerprint, on data as in a (presented as in Fig. 1d). (e) Mean prediction probabilities as in d. (f–h) Prediction probability for the classification of samples as HIV+ (keys) on the basis of the TNF RNA fingerprint, with the publicly available data sets GSE9927 (f), GSE23879 (g) and GSE18233 (h), comparing HIV+ subjects and HIV− subjects (n = 11 and n = 9 (f), n = 14 and n = 9 (g), and n = 127 and n = 8 (h), respectively), presented as in d. (i) Predicted probability for the classification of samples as untreated (key) in a comparison (GSE18233) of HIV-infected subjects (n = 38) before cART (Untreated HIV+) and after cART (Treated HIV+), presented as in d. *P < 0.0001 (unpaired Student's t-test). Data are from one experiment with n = 20 subjects (a–f; mean + s.e.m. in e), n = 23 subjects (g) or n = 135 subjects (h), or two experiments with n = 38 subjects in each (i).
The presence of the TNF RNA fingerprint was accompanied by significantly higher concentrations of TNF in serum from HIVhiPD-1hi subjects than in that from HIVloPD-1lo subjects (Fig. 3a), while the expression of TNFRI and TNFRII was similar in both groups (Supplementary Fig. 2a,b). We detected less of the inhibitory cytoplasmic transcription factor NF-κB chaperone IκBα in HIVhiPD-1hi subjects than in HIVloPD-1lo subjects (Fig. 3b), indicative of the facilitation of NF-κB transcriptional activity via proteasomal degradation of IκBα after TNF-induced phosphorylation of IκBα20. Accordingly, we observed higher expression of mRNA from genes that are targets of TNF, such as TNFRSF4 (which encodes the receptor OX40), CD74 (which encodes the invariant chain CD74) and TNFAIP3 (which encodes the deubiquitinase A20) in HIVhiPD-1hi subjects than in HIVloPD-1lo subjects (Fig. 3c). At the protein level, we observed co-expression of PD-1 and OX40 or of PD-1 and CD74 on CD4+ T cells from HIVhiPD-1hi subjects (Fig. 3d). Furthermore, serum concentrations of TNF correlated with PD-1 expression on CD4+ T cells (Fig. 3e). Together these findings led us to perform bioinformatics in silico prediction to detect potential NF-κB-binding regions at the locus encoding human PD-1 (PDCD1); we identified three such regions (Supplementary Fig. 2c). To further investigate this, we transfected HEK293T human embryonic kidney cells with the relevant luciferase reporter constructs, stimulated the cells with TNF and measured luciferase activity. We found that a region 5.0 kilobases upstream of the transcription start site was responsive to stimulation with TNF, while a region 0.5 kilobases upstream of the transcription start site had high constitutive activity and a pre-described regulatory region in intron 4 (ref. 21) had low constitutive activity (Fig. 3f and Supplementary Fig. 2d), which indicated that PD-1 expression could be increased by TNF-induced activation of NF-κB. To directly assess the effect of TNF on PD-1 expression on CD4+ T cells, we pre-incubated CD4+ T cells from healthy donors for 3 d with TNF and then stimulated the cells with TNF alone or with a combination of antibody to the invariant signaling protein CD3 (anti-CD3), IL-2 and TNF. We detected higher PD-1 expression on memory CD4+CD45RA− T cells from healthy donors than in cells not pre-incubated with TNF (Supplementary Fig. 2e,f). Next, we pre-incubated peripheral blood mononuclear cells (PBMCs) from HIVloPD-1lo subjects for 3 d with TNF and then stimulated the cells as described above; this resulted in higher PD-1 expression on memory CD4+ T cells than in cells not pre-incubated with TNF (Fig. 3g,h), in support of the proposal that TNF could induce PD-1 expression on memory CD4+ T cells. To further substantiate those findings, we incubated PBMCs from healthy control subjects and HIV-infected subjects with anti-CD3 and blocked TNF with neutralizing antibody to TNF. Stimulation with anti-CD3 resulted in an increase in PD-1 expression on CD4+ T cells in all three cohorts, with HIVhiPD-1hi subjects showing the greatest frequency of PD-1+ cells (Fig. 3i,j). However, when neutralizing TNF, we were able to identify significant downregulation of PD-1 expression only on CD4+ T cells from HIVhiPD-1hi subjects (Fig. 3i,j), which suggested that PD-1 expression was under the additional control of signaling via TNFR only in this group. Efficient blockade of TNFR signaling in all three cohorts was demonstrated by the prevention of augmented expression of OX40, a true target of TNF, in T cells following activation (Fig. 3k). This effect of TNF was independent of the differentiation of CD4+ T cells or expression of the lymphoid marker CD7 in memory CD4+ T cells (Supplementary Fig. 2g). Together our results suggested a key role for TNFR signaling in the induction of co-inhibitory pathways in CD4+ T cells from HIVhiPD-1hi subjects.
(a) Concentration of TNF in serum from HIVloPD-1lo subjects (n = 20) and HIVhiPD-1hi subjects (n = 31). (b) IκBα expression in CD4+ T cells from HIVloPD-1lo subjects (n = 5) and HIVhiPD-1hi subjects (n = 6) (left), and cumulative results of such analyses (right). Numbers in top left and right corners (left plot) indicate mean fluorescence intensity of IκBα in HIVloPD-1lo subjects (right) or HIVhiPD-1hi subjects (left). (c) Quantitative PCR analysis of TNFRSF4, CD74 and TNFAIP3 mRNA in CD4+ T cells from HIVloPD-1lo subjects (n = 5) and HIVhiPD-1hi subjects (n = 5) (results presented as in Fig. 1j). (d) Frequency of cells expressing OX40 (left) or CD74 (right) among PD-1+CD4+ T cells from HIVloPD-1lo subjects (n = 10) and HIVhiPD-1hi subjects (n = 9). (e) Correlation between PD-1 expression on CD4+ T cells and serum concentration of TNF in subjects with HIV (n = 51): ρ = 0.549 and P < 0.0001 (Spearman's rank correlation test). (f) Luciferase activity (in arbitrary units (AU)) of HEK293T cells transfected with a luciferase reporter construct for a region 5.0 kilobases upstream of the transcription start site of the predicted human PDCD1 promoter (−5.0 kb) or a control construct (Control), then left unstimulated (US) or stimulated with TNF (key). (g,h) PD-1 expression on CD4+ T cells obtained from HIVloPD-1lo subjects (n = 12) and incubated with medium alone (US) or pre-incubated with TNF and restimulated with TNF (TNF) (g), or pre-incubated with medium (Med) or TNF and restimulated with anti-CD3, IL-2 plus TNF (anti-CD3–IL-2–TNF) (h) (left), and cumulative results of such analyses (right). (i) Flow cytometry of PBMCs obtained from healthy donors or HIVloPD-1lo or HIVhiPD-1hi subjects (left margin) and left unstimulated (US) or stimulated for 2 d with anti-CD3 with (left) or without (middle) anti-TNF. Numbers adjacent to outlined areas indicate PD-1+CD4+ T cells. (j,k) Expression of PD-1 (j) and OX40 (k) on CD4+ T cells obtained from healthy donors (n = 6), HIVloPD-1lo subjects (n = 14) or HIVhiPD-1hi subjects (n = 13) and treated as in i, analyzed by flow cytometry. Each symbol (b,e,g,h,j,k) represents an individual donor; small horizontal lines indicate the mean (±s.e.m.). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.000 (Mann-Whitney U-test (a,c (TNFRSF4) and d (CD74)), unpaired Student's t-test (b,c (CD74 and TNFAIP3), d (OX40) and f), paired Student's t-test (g,h) or one-way analysis of variance (ANOVA) with Bonferroni's FDR correction (j,k)). Data are from 51 (a,e), 11 (b), 10 (c), 19 (d), 12 (g,h) or 33 (i–k) experiments with one subject in each (presented as in Fig. 1b (a,d) or mean + s.e.m. (c)) or are representative of three independent experiments (f; mean ± s.d. of biological triplicates).
Association of TNF with chronic LCMV strain WE infection
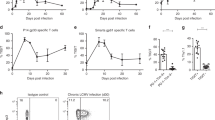
To gain mechanistic insight into the relevance of TNF in loss of function in CD4+ T cells during chronic viral infection, we searched for a relevant preclinical model characterized by elevated serum TNF concentrations. As previously reported22, infection of wild-type mice with lymphocytic choriomeningitis virus (LCMV) clone 13 resulted in persistent infection (Supplementary Fig. 3a) but was not accompanied by detectable concentrations of TNF in serum (Fig. 4a). In contrast, infection of newborn or infant mice with LCMV strain WE results in viral persistence, while such infection is cleared within 10 d in adult mice23,24. Chronic infection of neonatal mice with LCMV strain WE led to high viral titers (Fig. 4b) and persistent elevation of TNF concentrations in serum (Fig. 4c), which revealed LCMV clone–specific differences in the induction of persistently high concentrations of TNF in serum. Consistent with the presence and activity of virus-specific T cells during chronic infection, we detected liver damage in adult mice infected neonatally and chronically with LCMV strain WE (cnLCMV-WE mice) (Supplementary Fig. 3b). Notably, chronic infection of neonatal mice with LCMV strain WE did not lead to deletional tolerance of LCMV-specific CD4+ or CD8+ T cells, as these cells were readily detectable in the spleen and peripheral blood (Fig. 4d). LCMV-specific T cells detected in cnLCMV-WE mice showed higher PD-1 expression than that of T cells from mice that had cleared the acute infection (Fig. 4e). Furthermore, co-expression of other inhibitory molecules was observed only on subpopulations of PD-1-expressing T cells (Supplementary Fig. 3c,d). To assess similarities between CD4+ T cells in chronic HIV infection and those in cnLCMV-WE mice, we analyzed the transcriptomes of sorted PD-1−CD4+ and PD-1+CD4+ T cells from mice with chronic or acute infection with LCMV strain WE (Supplementary Fig. 3e). First, we identified a set of 556 genes expressed differentially in PD-1+CD4+ T cells isolated from mice with chronic infection with LCMV strain WE relative to their expression in PD-1−CD4+ T cells from those mice (data not shown). From that set of genes, we removed those genes that were expressed differentially in PD-1+CD4+ T cells relative to their expression in PD-1−CD4+ T cells during acute infection with LCMV strain WE (data not shown). This analysis established a set of 201 genes specific for CD4+ T cells during chronic infection with LCMV strain WE (Fig. 4f and Supplementary Table 5) that partially overlapped the reported signatures of exhausted CD4+ T cells during chronic infection with LCMV clone 13 (ref. 25) (Supplementary Fig. 3f).
(a) Concentration of TNF in serum from uninfected wild-type mice (Control) and mice chronically infected with LCMV clone 13 (key). (b) LCMV titer (b) and concentration of TNF (c) in serum from cnLCMV-WE mice. PFU, plaque-forming units. (d) Proportion of CD4+ T cells specific for the gp66 tetramer (an epitope consisting of amino acids 66–77 of LCMV glycoprotein) (left) and CD8+ T cells specific for the gp33 tetramer (right) among cells from uninfected wild-type mice (Control) and mice with acute (Acute WE) or chronic (cnLCMV-WE) infection with LCMV WE. (e) Frequency of gp66+ (left) or gp33+ (right) PD-1-expressing T cells from mice as in d. (f) Genes expressed differentially in PD-1+CD4+ T cells versus PD-1− CD4+ T cells in mice with acute or chronic infection with LCMV strain WE (above columns), showing the ten genes (left margins) with the greatest upregulation (left) or downregulation (right) in PD-1+CD4+ T cells relative to their expression in PD-1− CD4+ T cells. (g) GSEA with genes from the RNA fingerprint of CD4+ T cells from cnLCMV-WE mice (above plot) as the gene set for analysis of CD4+ T cells from HIVhiPD-1hi or HIVloPD-1lo subjects (enrichment defined as in Fig. 2c; enrichment score = 0.4743, P = 0.0025, and FDR = 0.0025). (h) Group prediction analysis of the RNA fingerprint in g, showing the prediction probability for the classification of samples as HIVhiPD-1hi or HIVloPD-1lo (presented as in Fig. 1d). (i) Mean prediction probabilities as in h. *P < 0.0001 (unpaired Student's t-test). Data are representative of two independent experiments with n = 4 mice per group (a,d,e; mean + s.e.m. (a) or presented as in Fig. 1b (d,e)), n = 4–5 mice per group (b; mean + s.e.m.) or n = 3 mice per group (c; mean + s.e.m.) or one experiment with n = 3–4 mice per group (f) or n = 10 subjects per group (g–i; mean + s.e.m. in i).
Use of the mouse CD4+ T cell RNA fingerprint of cnLCMV-WE mice for GSEA of HIV-infected subjects clearly showed enrichment for expression of the aforementioned gene set in CD4+ T cells from HIVhiPD-1hi subjects compared with expression of these genes in HIVloPD-1lo subjects (Fig. 4g). Furthermore, the mouse RNA fingerprint classified HIV-infected subjects correctly into HIVhiPD-1hi and HIVloPD-1lo groups (Fig. 4h,i). It further allowed discrimination between HIV+ subjects and HIV− subjects (Supplementary Fig. 3g). Collectively, the elevated concentrations of TNF and similarities in transcriptional reprogramming suggested that cnLCMV-WE mice would be a valid model for further study of the role of TNF in chronic viral infection.
Control of chronic viral infection by neutralization of TNF
To investigate the relevance of TNF, we neutralized TNF by administering anti-TNF to cnLCMV-WE mice (Supplementary Fig. 4a). On day 10, we observed an increase in LCMV-specific CD4+ or CD8+ T cells concomitant with a reduction in PD-1 expression on LCMV-specific as well as total CD4+ or CD8+ T cells (Fig. 5a–f and Supplementary Fig. 4b-f). In addition, large amounts of phosphorylated kinases IKKα and IKKβ, as a result of strong signaling via TNFR, were detected in CD4+ or CD8+ T cells in cnLCMV-WE mice; this was diminished more prominently in CD4+ T cells after neutralization of TNF (Fig. 5g). In contrast, during acute infection with LCMV strain WE, no phosphorylation of IKKα and IKKβ was detectable (Supplementary Fig. 4g,h), and PD-1 expression on total CD4+ or CD8+ T cells or LCMV-specific CD8+ T cells was not reduced after neutralization of TNF (Supplementary Fig. 4i–k).
(a) Flow cytometry of splenic T cells obtained from cnLCMV-WE mice after treatment with vehicle (Control) or neutralization of TNF (Anti-TNF) (left), and cumulative results of such analyses (right). Numbers in quadrants (left) indicate percent gp66− CD4+ T cells (bottom right) or gp66+ CD4+ T cells (top right). (b,c) Total splenic gp66+ CD4+ T cells from mice as in a (b) and PD-1 expression on those cells (c). (d) Flow cytometry of splenic T cells from mice as in a (left), and cumulative results of such analyses (right). Numbers in quadrants (left) indicate percent gp66− CD8+ T cells (bottom right) or gp66+ CD8+ T cells (top right). (e,f) Total splenic gp33+ CD8+ T cells from mice as in a (e) and PD-1 expression on those cells (f). (g) Immunoblot analysis of IKKα and IKKβ phosphorylated at Ser176 and Ser180 (p-IKKα/β) (top) and β-actin (bottom; loading control) in CD4+ or CD8+ T cells from mice as in a. (h) In vivo lysis of cells labeled with the cytosolic dye CFSE and pulsed with gp33, then injected into mice as in a. (i,j) Concentration of alanine aminotransferase (ALT) (i) and titer of LCMV (j) in serum from mice as in a (upward arrows indicate times of anti-TNF treatment). (k,l) Titer of LCMV in the liver (k) and spleen (l) of mice as in a. Each symbol (a–f,h) represents an individual mouse; small horizontal lines indicate the mean (±s.e.m.). *P < 0.05, **P < 0.01 and ****P < 0.0001 (unpaired Student's t-test). Data are representative of two independent experiments with n = 5 mice (a–f,h) or n = 3 mice per group (i–l; mean ± s.e.m. (i,j) or presented as in Fig. 1b (k,l)) or one experiment with n = 3 mice per group (g).
Since we observed a stronger effect of TNF on CD4+ T cells, we assessed global changes by transcriptome analysis of these cells (Supplementary Fig. 4l,m). Neutralization of TNF influenced mainly the transcriptome of PD-1+CD4+ T cells and reversed the expression of several members of the gene-expression signatures of the co-inhibitory signaling pathways identified in human CD4+ T cells (Supplementary Fig. 4l,m and Supplementary Table 6). Furthermore, PD-1+CD4+ T cells showed more transcriptome changes than did PD-1−CD4+ T cells after neutralization of TNF (Supplementary Fig. 4n). For Ly6c1 and Klrd1, two genes shown to have low expression in T cells during chronic viral infection25,26, their expression was restored after neutralization of TNF in PD-1+CD4+ T cells (Supplementary Fig. 4o). Moreover, the PD-1+CD4+ T cell signature of cnLCMV-WE mice was lost after neutralization of TNF (Supplementary Fig. 4p), as was the expression of genes encoding major transcription factors, such as Ikzf2 (which encodes Helios) and Eomes (which encodes Eomes), associated with chronic infection with LCMV clone 13 (ref. 25) (Supplementary Fig. 4q); this indicated that signaling via TNFR caused this gene-expression signature. Moreover, enrichment for the expression of genes associated with TNFR signaling in PD-1+CD4+ T cells was lost after neutralization of TNF (Supplementary Fig. 4r). Furthermore, genes downregulated in human CD4+ T cells in response to TNF had higher expression in mouse PD-1+CD4+ T cells after neutralization of TNF (Supplementary Fig. 4s), indicative of similar regulation of genes that are targets of TNF.
Next we investigated whether antigen-specific CD8+ T cell–mediated cytotoxicity was altered after neutralization of TNF. Indeed, we observed a significant increase in the CD8+ T cell–mediated lysis of target cells pulsed with an epitope consisting of amino acids 33–41 of LCMV glycoprotein (gp33) in vivo (Fig. 5h), which suggested that neutralization of TNF might have increased the number of LCMV-specific CD8+ T cells with cytotoxic function in cnLCMV-WE mice. The elevated serum concentrations of alanine aminotransferase found after neutralization of TNF due to immune system–mediated hepatitis (Fig. 5i) were indicative of increased virus-specific T cell immunity. Most notably, neutralization of TNF was followed by control of chronic viral infection, as shown by the clearance of LCMV from serum (Fig. 5j), liver (Fig. 5k) and spleen (Fig. 5l) within 10 d. In contrast, blockade of TNF during infection with LCMV clone 13 did not induce immune system–mediated control of chronic viral infection (Supplementary Fig. 5a–e). Together these data suggested that antiviral immunity to chronic viral infections associated with elevated concentrations of TNF might benefit from neutralization of TNF.
Furthermore, neutralization of TNF in cnLCMV-WE mice significantly enhanced the frequency and total number of IL-2- or interferon-γ (IFN-γ)-producing LCMV-specific CD8+ T cells (Fig. 6a,b and Supplementary Fig. 6a,b), consistent with a gain of T cell functionality. The expanded population of LCMV-specific CD4+ T cells had enhanced helper function, as indicated by an increase in both the frequency and number of LCMV-specific CD4+ T cells expressing IL-2, IFN-γ, IL-21 or CD40L (the ligand for the costimulatory receptor CD40) (Fig. 6c–f and Supplementary Fig. 6c–f). In addition, the number of TNF-expressing LCMV-specific CD4+ T cells was increased, while the frequency of these cells remained unaltered (Supplementary Fig. 6g,h). Moreover, assessment of the polyfunctionality of CD4+ T cells after neutralization of TNF showed an increase in the frequency of LCMV-specific CD4+ T cells expressing more than one cytokine (Fig. 6g), a result particularly evident in the population of cells expressing both IFN-γ and TNF (Fig. 6g,h). Together these data indicated that neutralization of TNF improved the functionality of both virus-specific CD4+ T cells and virus-specific CD8+ T cells.
(a,b) Flow cytometry analyzing the expression of IL-2 (a) and IFN-γ (b) in splenic gp33+ CD8+ T cells from cnLCMV-WE mice treated with vehicle (Control) or anti-TNF (left), and cumulative results of such analyses (right). (c–f) Flow cytometry analyzing the expression of IL-2 (c) IFN-γ (d), IL-21 (e) and CD40L (f) on splenic gp66+ CD4+ T cells from mice as in a (left), and cumulative results of such analyses (right). (g) Polyfunctional cytokine production by LCMV-specific CD4+ T cells as in a, showing average frequency of cells producing every possible combination of IL-2, IFN-γ, IL-21 and TNF (segments (left key); size correlates to frequency of that population); arcs around circumference indicate cytokine (right key) produced by cells under that arc (multiple arcs indicate polyfunctional cells). (h) Frequency of LCMV-specific CD4+ T cells expressing IFN-γ and TNF as in a. Numbers in outlined areas (a–f) indicate percent IL-2+ CD8+ T cells (a), IFN-γ+ CD8+ T cells (b), IL-2+ CD4+ T cells (c), IFN-γ+ CD4+ T cells (d), IL-21+ CD4+ T cells or CD40L+ CD4+ T cells (e). Each symbol (a–f,h) represents an individual mouse; horizontal lines and error bars indicate the mean (±s.e.m.). *P < 0.05 and **P < 0.001 (unpaired Student's t-test). Data are representative of two independent experiments with n = 5 mice per group.
Curtailment of antiviral immunity by CD4+ T cell TNFR signaling
To better understand the contribution of CD4+ and CD8+ T cells to the beneficial effect of the neutralization of TNF, we investigated the effect of signaling via the TNFR on both T cell populations. Notably, only PD-1+CD4+ T cells, not PD-1+CD8+ T cells, from cnLCMV-WE mice showed significant enrichment for expressed genes in a published mouse TNF signature27, compared with the expression of such genes in PD-1− cells, from cnLCMV-WE mice (Fig. 7a). To address the relevance of TNFR signaling to T cell immunity in vivo, we isolated CD8+ T cells from wild-type or TNFRI- and TNFRII-deficient Thy-1.2+ donor mice on day 8 after acute infection with LCMV strain WE and adoptively transferred the cells into Thy-1.1+ cnLCMV-WE host mice (Supplementary Fig. 7a–c). We observed no difference in the frequency of virus-specific TNFRI- and TNFRII-deficient donor CD8+ T cells and that of virus-specific wild-type donor CD8+ T cells (Supplementary Fig. 7d), while the frequency of TNFRI- and TNFRII-deficient PD-1-expressing donor cells was reduced (Supplementary Fig. 7e). Despite the immune system–mediated hepatitis and an initial drop in viral titers in the host mice, TNFRI- and TNFRII-deficient donor CD8+ T cells failed to control viremia (Supplementary Fig. 7f,g), which suggested critical involvement of CD4+ T cells in the beneficial effects of the neutralization of TNF.
(a) GSEA with a mouse TNF RNA fingerprint as gene set for the analysis of PD-1+ or PD-1− CD4+ T cells (left) and PD-1+ or PD-1− CD8+ T cells (right) (enrichment defined as in Fig. 2c; enrichment score = 0.4787 (left) or 0.2757 (right), P = 0.0099 (left) or 0.4065 (right), and FDR = 0.0237 (left) or 0.5083 (right)). (b) Flow cytometry analyzing the expression of gp66 on Thy-1.2+ (donor) CD4+ T cells from host mice given wild-type (WT) or TNFRI- and TNFRII-deficient (TNFRI-II-KO) donor CD4+ T cells (left), and cumulative results of such analyses (right). Numbers in outlined areas (left) indicate percent gp66+ CD4+ T cells. (c) Expression of PD-1 on Thy-1.2+ gp66+CD4+ T cells as in b. (d) Flow cytometry analyzing the expression of gp33 on Thy-1.2+ (donor) CD8+ T cells from host mice as in b (left), and cumulative results of such analyses (right). Numbers in outlined areas indicate percent gp33+ CD8+ T cells. (e) Expression of PD-1 on Thy-1.2+ gp33+CD8+ T cells as in d. (f,g) Flow cytometry of cells from mice given no cell transfer or transfer of wild-type or TNFRI- and TNFRII-deficient CD4+ and CD8+ T cells, showing the cumulative frequency (f) and cumulative number (g) of splenic gp33+Thy-1.1+CD8+ T cells. (h) Expression of PD-1 on splenic gp33+Thy-1.1+CD8+ T cells as in f,g. (i–k) Concentration of alanine aminotransferase (i), titer of LCMV (j) and concentration of TNF (k) in serum from mice as in f. (l) Quantification of serum LCMV-specific immunoglobulin G (IgG) titers of mice as in f. Each symbol (b–h,l) represents an individual mouse; small horizontal lines indicate the mean (±s.e.m.). *P < 0.05, **P < 0.01 and ****P < 0.0001 (unpaired Student's t-test (b–e) or one-way ANOVA with Bonferroni FDR correction (f–h,l)). Data are representative of one experiment (a) or two independent experiments with n = 4 mice (b–h,l) or n = 3-4 mice per group (i–k; mean ± s.e.m.).
To address the contribution of CD4+ T cells, we obtained wild-type or TNFRI- and TNFRII-deficient CD4+ T cells and CD8+ T cells from Thy-1.2+ donor mice at day 8 after acute infection with LCMV strain WE and transferred the CD4+ T cells together with genotype-matched CD8+ T cells into Thy-1.1+ cnLCMV-WE host mice (Supplementary Fig. 7h). At day 10 after transfer, we detected more virus-specific TNFRI- and TNFRII-deficient donor CD4+ T cells than wild-type donor CD4+ T cells (Fig. 7b). The virus-specific TNFRI- and TNFRII-deficient donor CD4+ T cells had lower expression of PD-1 than that of their wild-type counterparts (Fig. 7c). Notably, the co-transferred virus-specific TNFRI- and TNFRII-deficient CD8+ T cells were greater in number than were their wild-type counterparts and had lower PD-1 expression than that of their wild-type counterparts (Fig. 7d,e). We also observed a greater abundance of virus-specific endogenous CD8+ T cells with lower PD-1 expression after co-transfer of TNFRI- and TNFRII-deficient cells than after transfer of wild-type cells (Fig. 7f–h), which suggested that the TNFRI- and TNFRII-deficient donor T cells helped to sustain the antiviral immunity of TNFRI- and TNFRII-sufficient endogenous CD8+ T cells.
In accordance with the improved antiviral activity, we detected enhanced immune system–mediated viral hepatitis after transfer of TNFRI- and TNFRII-deficient CD4+ T cells and CD8+ T cells as described above (Fig. 7i). Notably, transfer of TNFRI- and TNFRII-deficient T cells was followed by a sustained reduction in serum titers of LCMV (Fig. 7j) and serum concentrations of TNF (Fig. 7k), as well as higher titers of LCMV-specific antibodies in serum than such titers in host mice that received wild-type cells (Fig. 7l). The lower TNF concentrations in cells from host mice that received TNFRI- and TNFRII-deficient T cells might also explain the greater frequency and number of virus-specific endogenous CD4+ T cells (Supplementary Fig. 7i,j) that also had lower PD-1 expression (Supplementary Fig. 7k) than that of such cells in host mice that received wild-type cells. Along those lines, PD-1 expression was lower on transferred and endogenous CD4+ T cells (Supplementary Fig. 7l-n) and CD8+ T cells (Supplementary Fig. 7o–q) in mice that received TNFRI- and TNFRII-deficient T cells than in mice that received wild-type T cells. Together these results demonstrated that prevention of TNFR signaling in T cells restored the impaired antiviral adaptive immunity during chronic viral infection, an effect accompanied by reduced systemic concentrations of TNF.
Induction of T cell dysfunction by persistent NF-κB activity
To link the activation of NF-κB following TNFR signaling to T cell dysfunction, we characterized the functionality of CD4+ T cells with persistent activation of NF-κB using a transgenic mouse line expressing constitutively active IKKβ. We crossed R26StopFLIkk2ca mice (with a loxP-flanked neomycin-resistance–stop cassette cloned into the ubiquitously expressed Rosa26 gene (R26StopFL), plus sequence encoding a Flag-tagged constitutively active form of mouse IKKβ (Ikk2ca) followed by a cassette for the expression of green fluorescent protein (GFP))28 to Cd4-Cre-ERT2 mice (with tamoxifen-inducible expression of Cre recombinase (Cre-ERT2) from the T cell–specific Cd4 locus)29 to generate Ikk2E/E mice (homozygous for the expression of constitutively active ('E') IKKβ); application of tamoxifen to the resultant Ikk2E/E mice allowed us to induce continuous activity of NF-κB and GFP selectively in CD4+ T cells (Supplementary Fig. 8a,b). Analysis of GFP expression revealed that approximately 50–70% of CD4+ T cells in the tamoxifen-treated Ikk2E/E mice had undergone Cre-mediated recombination (Supplementary Fig. 8b). On day 15 after acute infection of Ikk2E/E mice with LCMV strain WE, we observed significantly fewer total CD4+ T cells when continuous NF-κB activity was induced in CD4+ T cells (in tamoxifen-treated Ikk2E/E mice) than in Ikk2E/E mice without tamoxifen treatment (Fig. 8a). In line with that, the frequency of LCMV-specific CD4+ T cells was lower among CD4+ T cells with continuous NF-κB activity (from tamoxifen-treated Ikk2E/E mice) than among those from Ikk2E/E mice without tamoxifen treatment (Supplementary Fig. 8c). Notably, PD-1 expression was higher in LCMV-specific CD4+ T cells with continuous activation of NF-κB (in tamoxifen-treated Ikk2E/E mice) than in those from Ikk2E/E mice without tamoxifen treatment (Fig. 8b), consistent with our finding of NF-κB-responsive binding sites at the PDCD1 locus (Fig. 3). In confirmation of the finding of TNF-mediated control of CD4+ T cell function (Fig. 5), CD4+ T cells with continuous activation of NF-κB (in tamoxifen-treated Ikk2E/E mice) also showed impaired helper function; i.e., lower expression of IL-21, IL-2, IFN-γ and CD40L than that of CD4+ T cells from Ikk2E/E mice without tamoxifen treatment (Fig. 8c). In addition, tamoxifen-treated Ikk2E/E mice, with continuous activation of NF-κB in CD4+ T cells, showed a substantially lower frequency of LCMV-specific CD8+ T cells (Supplementary Fig. 8d), higher PD-1 expression (Supplementary Fig. 8e) and a lower frequency of IL-2 and IFN-γ expressing cells than that of Ikk2E/E mice without tamoxifen treatment (Supplementary Fig. 8f), in further support of the proposal of impaired helper function of CD4+ T cells characterized by continuous NF-κB activity. Collectively, these data supported the proposal that continuous NF-κB signaling in CD4+ T cells caused by chronically elevated TNF expression is an important link between chronic inflammation and T cell dysfunction during chronic viral infection.
(a) Cumulative number of splenic CD4+ T cells in Ikk2E/E mice treated with oil (Control) or tamoxifen (TAM). (b,c) Frequency of PD-1+ cells (b) and IL-21+, IL-2+, IFN-γ+ or CD40L+ cells (c) among splenic gp66+ CD4+ T cells from mice as in a, analyzed by flow cytometry. Each symbol represents an individual mouse; small horizontal lines indicate mean (±s.e.m.). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 (unpaired Student's t-test (a–c (IL-21, IL-2 and IFN-γ)) or Mann-Whitney U-test (c (CD40L)). Data are representative of two independent experiments with n = 6 mice per group.
Discussion
Most persistent viral infections are accompanied by T cell dysfunction and chronic inflammation5,7. Here we demonstrated that elevated concentrations of TNF, a cytokine with pleiotropic functions that is involved in acute as well as chronic inflammatory processes, could be directly linked to T cell dysfunction during chronic viral infection. Blockade of TNFR signaling in CD4+ T cells was sufficient to reverse T cell dysfunction, while CD8+ T cells alone were unable to restore sustained antiviral immunity. The low activation of NF-κB observed in CD8+ T cells during infection with LCMV indicated that TNF was not critically involved in the well-described exhaustion of CD8+ T cells30 and instead suggested that chronic exposure to TNF affected mainly the function of CD4+ T cells. Our results identified a role for prolonged TNFR signaling and downstream activation of NF-κB in restricting the expression of helper molecules such as IL-2, IL-21, IFN-γ and CD40L that are essential for antiviral T cell immunity31,32,33. Therefore, TNF might be a complementary therapeutic target for certain chronic viral infections, in combination with targeting of other immunoregulatory pathways.
In several chronic viral infections, increased TNF concentrations have been reported to be directly correlated to viral load and inversely correlated to the number of CD4+ T cells34. Successful treatment with anti-TNF results in reduced concentrations of TNF35,36. At later stages of HIV infection, prolonged TNFR signaling has been linked to chronic inflammation37,38, persistence of opportunistic infections and enhanced suppressive activity of myeloid cells39. Our results indicate it is likely that TNF-induced activation of NF-κB can mediate the upregulation of PD-1 expression during chronic viral infection beyond the PD-1 expression triggered by signaling via the T cell antigen receptor as a consequence of chronic antigen exposure40,41. Conceptually, we postulate that TNF renders T cells receptive to inhibitory signals and might thus cause broad immunological dysfunction. For example, by triggering both increased expression of the PD-1 ligand PD-L1 on cells of the innate immune system and tissue cells42 and increased expression of PD-1 on T cells, TNF might strongly enforce inhibitory PD-1 signaling and thereby directly link T cell dysfunction and inflammation.
Despite the identification of a link between TNF and T cell dysfunction in two chronic viral infections, we recognize that other factors might function in a fashion similar to that of TNF in other settings of chronic infection. Elevated serum concentrations of type I interferons have been shown to be critical for loss of T cell function during chronic infection with LCMV clone 13 (refs. 12,13). Our data support the proposal that the persistent presence of both type I interferons and TNF regulates T cell function, which suggests a link between cytokine-mediated induction of chronic inflammation and attenuation of T cell responses that probably act to prevent immunopathology. Notwithstanding the complexity of TNF-induced co-inhibitory signals, prevention of TNFR signaling selectively in T cells sufficed to control chronic infection. Therefore, therapeutic approaches would ideally use targeted disruption of TNFR signaling in T cells. So far, in initial reports of only limited numbers of subjects, neutralization of TNF has been shown to be safe in patients infected with hepatitis C virus or HIV who have rheumatoid arthritis or chronic inflammatory bowel disease43,44,45,46. Further clinical confirmation of that observation in randomized clinical studies is warranted to substantiate the proposal that TNF might represent a promising pharmacological target. Collectively, our results introduce TNF as a link between T cell dysfunction and chronic inflammation during chronic viral infection. These findings open new avenues for combination immunotherapy that directly targets regulatory pathways so far associated with T cell dysfunction, with blockade of TNF for patients with chronic TNF-associated viral infection.
Methods
Recruitment of subjects.
HIV-positive subjects were recruited from our outpatient cohort (n = 1304). 37 subjects with increased CD4+ T cell PD-1 expression (HIVhiPD-1hi) were identified by flow cytometry. For a subject to be defined as HIVhiPD-1hi, the cut off was set at 15% of PD-1+ cells in the CD4+ T cell population with HIV copies > 100/ml despite previous or ongoing cART. 36 HIV-infected subjects with PD-1 expression on CD4+ T cells below 3% and HIV copies < 50/ml were used as controls (HIVloPD-1lo). In 20 of all subjects assessed (10 HIVhiPD-1hi and 10 HIVloPD-1lo), we were able to collect enough RNA for whole-genome transcriptome analysis (Supplementary Fig. 1a and Supplementary Table 1).
To define RNA fingerprints for inhibitory pathways in healthy CD4+ T cells, blood samples in form of standard buffy coat preparations were collected from four different healthy blood donors at the Center for Transfusion Medicine and processed immediately as described below. All blood samples from healthy donors and HIV+ subjects were collected after informed consent was obtained following Institutional Review Board approval (University of Cologne, Ethics Commission of the Faculty of Medicine: 03-143 and 07-093).
Isolation of CD4+ T cells from healthy donors and HIV-infected subjects.
CD4+ T cells from buffy coat samples were isolated as described previously47. PBMCs from HIV-infected subjects were prepared using Ficoll gradient (Amersham Biosciences). CD4+ T cells were isolated by positive selection (130-045-101; Miltenyi Biotech) using magnetic cell sorting columns after depletion of CD14+ monocytes (130-050-201; Miltenyi Biotech).
Antibodies and flow cytometry.
Analysis of human CD4+ and CD8+ T cells by flow cytometry was performed using the following antibodies: allophycocyanin-conjugated, phycoerythrin–cyanine 7–conjugated, or phycoerythrin-conjugated anti-PD-1 (1:25 dilution; EH12.2H7; BioLegend), fluorescein isothiocyanate-conjugated anti-OX40 (1:25 dilution; ACT35; eBioscience), phycoerythrin-conjugated anti-TNFRI (1:25 dilution; H398; AbSerotec), allophycocyanin-conjugated anti-TNFRII (1:25 dilution; 3G7A02; BioLegend), allophycocyanin-conjugated anti-CTLA-4 (1:25 dilution; BNI3; BD Biosciences), peridinin chlorophyll protein-cyanine 5.5– or phycoerythrin-conjugated anti-CD4 (1:50 dilution; RPA-T4; BioLegend), fluorescein isothiocyanate– or V450-conjugated anti-CD3 (1:50 dilution; UCHT1; BD), peridinin chlorophyll protein–cyanine 5.5– or V500-conjugated anti-CD8 (1:50 dilution; RPA-T8; BD), phycoerythrin–eFluor 610–conjugated anti-CD45RA (1:100 dilution; HI100; eBioscience) or allophycocyanin–cyanine 7–conjugated anti-CD45RA (1:100 dilution; HI1006; BioLegend), eFluor 450–conjugated anti-CD7 (1:50 dilution; eBio124-1D1; eBioscience), phycoerythrin-conjugated anti-CD62L (1:100 dilution; DREG-56; BD), fluorescein isothiocyanate–conjugated anti-CD74 (1:25 dilution; 5-329; eBioscience), fluorescein isothiocyanate–conjugated anti-CD49d (1:25 dilution; 9F10; BD), fluorescein isothiocyanate-conjugated CD79b (eBioscience, CB3-1, 1:25), phycoerythrin-conjugated anti-IL4-R (BD, hIL4R-M57, 1:25 dilution) and phycoerythrin-conjugated CCR4 (BD, 1G, 1:25 dilution).
For mouse samples the following antibodies were used: Pacific blue–conjugated, fluorescein isothiocyanate– or phycoerythrin-conjugated anti-PD-1 (1:50 dilution; J43; eBioscience), phycoerythrin–cyanine 7–conjugated anti-CD8 (1:100 dilution; 53-6.7; eBioscience), allophycocyanin–cyanine 7–conjugated anti-CD4 (1:100 dilution; RM 4-5; eBioscience), peridinin chlorophyll protein–cyanine 5.5–conjugated anti-Thy-1.2 (1:200 dilution; 30-H12; eBioscience), V450-conjugated anti-Thy-1.1 (1:200 dilution; OX-7; BD), phycoerythrin–cyanine 7–conjugated IL-2 (1:50 dilution; JES6-5H4; eBioscience), phycoerythrin-conjugated anti-IL-21 (1:50 dilution; FFA21; eBioscience), peridinin chlorophyll protein–cyanine 5.5–conjugated anti-IFN-γ (1:50 dilution; XMG1.2; eBioscience), phycoerythrin-conjugated anti-CD40L (1:50 dilution; MR1; eBioscience), phycoerythrin–cyanine 7–conjugated anti-TIM-3 (1:50 dilution; RMT3-23; eBioscience), phycoerythrin-conjugated anti-LILRB4 (1:50 dilution; H1.1; BioLegend), fluorescein isothiocyanate–conjugated anti-2B4 (1:100 dilution; m2B4(B6)458.1; BioLegend), phycoerythrin-conjugated anti-CTLA-4 (1:100 dilution; UC10-4B9; eBioscience), fluorescein isothiocyanate–conjugated anti-LAG3 (1:100 dilution; eBioC9B7W; eBioscience), phycoerythrin-conjugated PIR-B (1:50 dilution; 6C1; BioLegend), peridinin chlorophyll protein–eFluor 710–conjugated anti-BTLA (1:100 dilution; 6F7; eBioscience), peridinin chlorophyll protein–cyanine 5.5–conjugated anti-CD160 (1:100 dilution; 7H1; BioLegend,), peridinin chlorophyll protein–eFluor 710–conjugated anti-CD200 (1:100 dilution; OX90; eBioscience), allophycocyanin-conjugated dextramers specific for gp33 (H-2Db–KAVYNFATC; Immudex) and np396 (H-2Db–FQPQNGQFI; Immudex) and allophycocyanin-conjugated tetramers specific for gp66 (I-Ab–DIYKGVYQFKSV; NIH Tetramer core facility) were used.
For intracellular staining of IκBα, PBMC were fixed with BD Cytofix Buffer (BD) and permeabilized with BD Phosflow Perm Buffer II chilled to −20 °C (BD). Cells were stained overnight with phycoerythrin-conjugated anti-IκBα (1:25 dilution; 25/IkBa/MAD-3; BD). For mouse samples, antibody staining was done in presence of Fc receptor blockade (monoclonal antibody 2.4G2 to mouse CD16-CD32 (10 μg/ml); prepared in-house) in PBS. Quantification of cell s was done with fluorochrome-labeled microbeads (CountBright absolute counting beads; Life Technologies). Human samples were stained in PBS. A FACSCalibur, FACSCanto II or LSR II (all BD Biosciences) and FlowJo software (TreeStar) were used for acquisition and data analysis. Flow cytometry data for patient stratification presented in Figure 1 were acquired on a FACSCalibur.
For sorting of CD4+ and CD8+ T cells by flow cytometry for adoptive transfer or gene expression analysis of PD-1+CD4+, PD-1−CD4+, gp33-dextramer+CD8+, and or gp33-dextramer−CD8+ T cells, splenocytes were isolated from mice and enriched for T cells using the Pan T Cell Isolation Kit II (130-095-130; Miltenyi Biotech), followed by staining with the respective antibodies and filtering through a 100 μm mesh. Next, cells were sorted with a FACSAria III (BD Biosciences).
For dextramer or tetramer and intracellular cytokine staining samples were incubated with gp33 or np396 dextramer for 15 min or gp66 tetramer for 1 h at 37 °C. Next, the respective antibodies were added and analyzed by flow cytometry. LIVE/DEAD Fixable Stain kit (Life Technologies) was used to exclude dead cells. For intracellular cytokine staining, splenocytes were incubated with PMA (phorbol 12-myristate 13-acetate), ionomycin, brefeldin A and monensin (eBioscience) for 4 h at 37 °C. After surface staining with the respective antibodies, cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% saponin and stained with the respective intracellular antibodies for 30 min at 4 °C.
CD4+ T cell stimulation for the generation of human RNA fingerprints.
Pathway-specific genome-wide transcriptional changes (RNA fingerprints) were determined following short-term stimulation of primary CD4+ T cells from human buffy coat samples concomitant with signaling via these inhibitory pathways (details, Supplementary Fig. 1). Genes comprising the different RNA fingerprints are listed in Supplementary Table 2. For the CTLA-4 and PD-1 fingerprints, pure CD4+ T cells from buffy coat samples were stimulated by mixture with artificial antigen-presenting cells (aAPC) composed of magnetic beads (14013, Life Technologies) coated with the following antibodies: 5% anti-CD3 (OKT3; Orthoclone; Janssen-Cilag), 10% anti-CD28 (9.3; a gift from J.L. Riley), and 85% anti-PD-1 (PD-1-17; Wyeth Research), 85% anti-CTLA-4 (ER5.3D6; a gift from J.G. Gribben) or 85% anti–MHC class I (W6/32; prepared in-house), as previously described47. For defining the prostaglandin E2, TGF-β and IL-10 fingerprints, CD4+ T cells were stimulated with beads coated with anti-CD3, anti-CD28 and anti–MHC class I (identified above), and prostaglandin E2, TGF-β, or IL-10 were added at a concentration of 1 μM, 30 ng/ml or 50 ng/ml, respectively.
For the generation of a TNF RNA fingerprint of CD4+ T cells, isolated CD4+ T cells were either left unstimulated or incubated with 100 ng/ml TNF (Sigma-Aldrich) for 5 d.
Assessment of CD4+ T cell function for the generation of RNA fingerprints.
T cell proliferation was analyzed by flow cytometry using CFSE (carboxyfluorescein diacetate succinimidyl ester) staining, as previously described47. The concentration of IFN-γ in cell culture supernatants was measured using the human Th1/Th2 Cytokine kit II (551809; BD Biosciences) following the manufacturer's instructions.
RNA preparation, microarray hybridization and primary data analysis.
To define RNA fingerprints CD4+ T cells of four different healthy donors were either left unstimulated or stimulated via inhibitory receptors respectively soluble factors as described above. After 8 h, magnetic beads were removed and cells were lysed in TRIzol reagent (15596018; Life Technologies). CD4+ T cells of HIV-infected subjects were lysed in TRIzol immediately after isolation to retrieve the in vivo state of transcriptional regulation in these cells. RNA isolation, cDNA and cRNA transcription, as well as microarray hybridization and analysis of Illumina Bead array expression data were performed as described previously47. All statistical and bioinformatics analysis was performed using R language (https://www.r-project.org) and Bioconductor packages. All microarray samples are listed in Supplementary Table 7.
Establishment of RNA fingerprints for inhibitory pathways.
Specific RNA fingerprints for each inhibitory molecule were identified by a two-step process. First, gene expression data for each inhibitory molecule were compared against the gene expression data of resting and activated CD4+ T cells47. All genes differentially expressed in one of the two comparisons, defined by a change in expression (upregulated or downregulated) of twofold or more with a P value of <0.05 (Student's t-test), were identified and a global RNA fingerprint for each inhibitory pathway was thereby generated, reflecting the transcriptional changes downstream of receptor activation. Transcripts present in more than one RNA fingerprint, for example, shared between CTLA-4 and PD-1, were excluded by subtraction of all transcripts differentially expressed in any of the other comparisons from the global RNA fingerprint for each inhibitory pathway. By this subtraction approach, we ended up with transcripts unique for each inhibitory pathway. For further analysis, transcripts were cross-annotated between different microarray platforms and species using gene symbols.
Establishment of the TNF RNA fingerprint.
A specific human TNF RNA fingerprint was established by comparison of the gene expression of unstimulated CD4+ T cells and TNF-stimulated CD4+ T cells after 5 d of culture. All genes differentially expressed in this comparison, defined by a change in expression (upregulated or downregulated) of twofold or more with a P value of <0.05 (Student's t-test), were identified and a global RNA fingerprint was thereby generated, reflecting the transcriptional changes downstream of TNFR activation. For further analysis, transcripts were cross-annotated between different microarray platforms and species using gene symbols.
Application of RNA fingerprints to the in vivo signature of HIV-infected subjects.
To access evidence for an in vivo contribution of RNA fingerprints to gene expression in CD4+ T cells, three different methods were used47. First, association between significantly altered RNA fingerprint transcripts of Illumina gene expression data from HIVloPD-1lo and HIVhiPD-1hi subjects were modeled and tested via χ2-statistic (RNA fingerprint–enrichment analysis). Therefore, the proportion of significantly altered RNA fingerprint transcripts (a change in expression (upregulated or downregulated) of 1.3-fold or more with a P value of <0.05 (Student's t-test)) in the fingerprint were compared to the overall proportion and tested in a contingency table using Fisher's exact test. Furthermore, 1,000 random fingerprints comprising the same number of transcripts as each individual RNA fingerprint were generated and a P value for each random RNA fingerprint was calculated by a sampling strategy. Second, 'leave-one-out' supervised classification using support vector machines was performed (group prediction analysis). Prediction probability for the classification of a single sample as either HIVloPD-1lo or HIVhiPD-1hi is reported for each individual RNA fingerprint. Third, a method to test enrichment of predefined gene sets (GSEA) was used to determine whether differences in RNA fingerprints were statistically significant in HIVloPD-1lo subjects versus HIVhiPD-1hi subjects. Enrichment of an RNA fingerprint in these analysis would reflect that signaling via the inhibitory receptors was active at one point in vivo and resulted in corresponding transcriptional changes, but would not require differences in expression of the respective receptor or molecule.
Three additional publicly available data set reporting HIV infections in human CD4+ T cells were obtained from the GEO database (GSE9927, GSE18233 and GSE23879)16,17,18 and RNA fingerprint activation was tested as described above.
Ontology-based gene identification analysis.
We developed an algorithm based on gene-ontology (GO) analysis, which takes the approach of gene class testing one step further to detect key players within the classes identified (Life and Medical Sciences Bonn: https://github.com/LIMES-immunogenomics/GOAna). Here, we used it to find genes encoding products with a central role in the differentiation of HIVloPD-1lo and HIVhiPD-1hi samples. In a first step, gene classes based on GO terms are used to calculate pairwise centroid distances (Euclidean distances) between the two groups. To obtain a statistically significant measure of the calculated distances, class permutations followed by recalculation of the distances are performed. This first step of finding significant GO terms can be substituted by any other gene class–testing approach, such as GSEA. In a second step, the list of significant GO terms is further analyzed by construction of a network of contributing genes. Here, all genes composed within the significant GO terms are extracted and an edge (line) is drawn between gene x and gene y if x and y belong to at least one of the GO terms. By increasing the number of shared GO terms, the network gets more and more specific toward genes that can be found in several GO terms from the list and are therefore key players within the classes identified. For network visualization, Cytoscape 2.2 was used. The number of shared GO terms was chosen as the maximal value to obtain a sufficiently connected network. For the analysis presented in the manuscript, we used R 2.9.2 (https://www.r-project.org), Bioconductor 2.2 (https://bioconductor.org/packages/2.2/BiocViews.html) including the software package 'annotate' and the annotation packages 'GO', 'WG6' and 'WG6v2', which were available in 2009, and have recapitulated our findings with current versions of R and analysis packages with similar results.
Upstream regulator analysis.
To confirm the results of our novel algorithm to identify potentially associated molecules, we performed upstream regulator analysis using Ingenuity pathway analysis (IPA; Qiagen). Differentially expressed genes between HIVloPD-1lo and HIVhiPD-1hi subjects were calculated with a change in expression (upregulated or downregulated) of twofold or more and a P value of <0.05 (Student's t-test) as the cutoff criteria to identify upstream regulators. Expression changes ('fold' values) and P values of the respective genes were uploaded to IPA and the upstream regulator analysis was performed. Details on the method and the visualization are provided at http://www.ingenuity.com.
Quantitative PCR od human samples.
Total RNA extracted using TRIzol from CD4+ T cells was used to generate cDNA, along with the Transcriptor First Strand cDNA synthesis kit (04897030001, Roche Diagnostics). Quantitative PCR was performed using the LightCycler Taqman master kit and the Universal Probe Library assay specific for CD74, TRADD, AOS1, ALDOA, CD79B, DHRS3, TNF, TNFAIP3, TNFRSF4, PDCD1 and B2M (encoding β2-microglobulin; Roche Diagnostics) on a LightCycler 480 II (Roche Diagnostics). Results were normalized to B2M expression. All oligonucleotides are listed in Supplementary Table 8.
Serum TNF concentrations in HIV+ subjects.
Serum samples from HIV-infected subjects were analyzed for TNF concentrations by enzyme-linked immunosorbent assay (88-7346; eBioscience) according to the manufacturer's instructions.
Assessment of TNF-dependent PD-1 expression.
PBMCs from HIVloPD-1lo or HIVhiPD-1hi subjects were stimulated with plate-bound anti-CD3 (0.5 μg/ml; OKT3; Orthoclone; Janssen-Cilag). To analyze TNF-dependent PD-1 expression, samples were incubated including 10 μg/ml infliximab (Remicade; MSD) for 48 h before determination of PD-1 expression on CD4+ T cells by flow cytometry. Additionally, OX40 expression was analyzed and served as a known TNF-dependent control.
In addition, PBMCs from HIVloPD-1lo subjects were either left unstimulated or incubated with 500 ng/ml TNF (Sigma-Aldrich) in medium for 3 d. Next, cells were either left unstimulated or stimulated with 500 ng/ml TNF or with 500 ng/ml TNF, 2.0 μg/ml anti-CD3 and 100 IU/ml IL-2 (Proleukin; Chiron) for an additional 2 d before flow cytometry.
CD4+ T cells from healthy donors were isolated as previously described48 and were either left unstimulated or incubated with 500 ng/ml TNF (Sigma-Aldrich) in medium for 3 d. Next, CD4+ T cells were either left unstimulated or stimulated with 500 ng/ml TNF or with 500 ng/ml TNF, 2.0 μg/ml anti-CD3 and 100 IU/ml IL-2 for an additional 2 d before flow cytometry.
Cloning of PDCD1 constructs with potential NF-κB-binding regions.
The corresponding PDCD1 genomic regions were amplified by PCR using human genomic DNA as source material. The full-length NF-κB-binding site constructs were amplified with oligonucleotides listed in Supplementary Table 9. After digestion with Acc65 I and HindIII, the fragments were cloned into the pGL4.24 vector with a minP element upstream of the potential binding motif and a destabilized downstream Firefly luciferase (PRE8421, Promega).
Luciferase assay.
HEK293T human embryonic kidney cells (CRL-11268; ATCC) were maintained in DMEM containing 10% heat-inactivated FBS. To assess regulation of PDCD1 expression by signaling via TNF mediated by binding of NF-κB to the genomic PDCD1 locus, constructs containing the three potential NF-κB binding regions were transfected separately into HEK293T cells in 96-well plates, together with a plasmid encoding renilla luciferase, for normalization. To assess TNFR signaling, transfected cells were stimulated with TNF. Lysis and analysis were performed 24 h after transfection using the Promega Dual Luciferase Kit (PR-E1980, Promega) as described previously48. Luciferase activity was counted on a MicrolumatPlus LB 96V plate reader (Berthold).
Mice.
C57BL/6J mice, Thy-1.1+ mice, R26StopFLIkk2ca mice28, CD4-CreERt2 mice29 and Tnfrsf1a−/−Tnfrsf1b−/− (TNFRI- and TNFRII-deficient) mice were maintained under specific pathogen–free conditions in single ventilated cages in the animal facility of the University of Bonn, Munich or Düsseldorf. All mice used in this study were maintained on the C57BL/6 genetic background. For tamoxifen application, tamoxifen (Sigma-Aldrich) was dissolved in olive oil to 40 mg/ml, vortexed, and mixed with olive oil to a final concentration of 40 mg/ml, and 8 mg was applied by oral gavage both on day −3 and day 0 before LCMV infection. The health status of animals was checked daily, and after observation of severe disease, animals were sacrificed and 'counted as' dead. All animal experiments were done in accordance with the Principles of Laboratory Animal Care guidelines (US National Institutes of Health publication 85-23, 1996 revision) and were approved by the Local Animal Care Commission of North Rhine-Westphalia. Experiments were conducted with sex-matched female or male mice, 6–12 weeks of age at the start of each experiment.
Chronic and acute LCMV infection.
Chronic infection with LCMV was induced by infection of newborn mice with 1 × 106 plaque-forming units (PFU) of LCMV (WE strain). At 8 weeks after infection, mice were used for experiments. Acute infection in 6- to 8-week-old mice was established by inoculation of mice with 2 × 104 PFU of LCMV strain WE. For adoptive transfer of CD4+ and CD8+ T cells into animals chronically infected with LCMV, T cells were obtained from spleens at day 8 after acute infection of 6- to 8-week-old mice with LCMV strain WE (2 × 104 PFU). For infection with LCMV clone 13, 6-week-old mice were given intravenous injection of 2 × 106 PFU of LCMV clone 13 strain.
Titers of virus for the chronic LCMV model were determined by plaque assay on Vero cells49 or using a plaque assay as described previously50. Organs were harvested into HBSS and were homogenized using a Tissue Lyzer (Qiagen). MC57 mouse fibrosarcoma cells (0.8 × 106/ml) were added to tenfold serial dilutions of virus samples on 24-well plates. After 3 h, overlay medium containing 1% methylcellulose was added. After 48 h, plates were fixed (4% formalin), permeabilized (1% Triton X HBSS), and stained with antibody specific for LCMV nucleoprotein (VL-4; prepared in-house) and peroxidase-conjugated anti-rat (A9542; Sigma-Aldrich). Plaques were developed using color reaction solution containing OPD (o-phenlyenediamine dihydrochloride; Sigma-Aldrich), 50 mM Na2HPO4 and 25 mM citric acid.
Neutralization of TNF in mice chronically infected with LCMV.
To block TNFR signaling in mice chronically infected with LCMV, TNF-neutralizing antibody (25 mg/kg; infliximab; Remicade; MSD) or PBS was administered intraperitoneally each second day for 10 d for LCMV strain WE or 15 d for LCMV clone 13 (starting on day 10 after infection). Mice were sacrificed on day 10 of treatment for LCMV strain WE and day 15 of treatment for LCMV clone 13, and CD4+ and CD8+ T cells were assessed.
In vivo analysis of LCMV-specific CD8+ T cell function.
At day 14 after initiation of anti-TNF treatment, spleen cells from uninfected wild-type animals were isolated and loaded for 30 min with 1 μM gp33 peptide or np118 peptide (as a negative control) in phosphate-buffered saline. gp33 peptide–loaded spleen cells were labeled with 1 μM CFSE (carboxyfluorescein diacetate succinimidyl ester) and np118 peptide–loaded cells were labeled with 0.1 μM CFSE, for 10 min at 37 °C. CFSEhi and CFSElo spleen cells were mixed at a ratio of 1:1, and 2 × 107 total cells were injected intravenously. After 16 h, spleens were harvested. The ratio of surviving CFSEhi spleen cells to surviving CFSElo spleen cells was assessed by flow cytometry. 'In vivo kill' was calculated as follows: % specific kill: 100 − [100 × (CFSEhi / CFSElo)exp mice / (CFSEhi / CFSElo)control mice (where 'exp mice' means mice treated with neutralizing anti-TNF, and 'control mice' means mice given PBS).
Adoptive transfer of CD4+ and CD8+ T cells.
To assess the role of TNFR signaling on the induction of PD-1 expression on gp66-specific CD4+ T cells and gp33-specific CD8+ T cells, 2 × 106 CD4+ and 2 × 106 CD8+ T cells were isolated as described above from Thy-1.1+ congenic wild-type or TNFRI- and TNFRII-deficient mice 8 d after acute infection with LCMV and were transferred into Thy-1.2+ congenic mice chronically infected with LCMV. After 10 d, host mice were sacrificed and assessed.
Immunoblot analysis.
Lysates of purified CD4+ and CD8+ T cells from control- or infliximab-treated LCMV-infected mice were prepared as previously described48, followed by immunoblot analysis and detection with antibody specific for IKKα and IKKβ phosphorylated at Ser176 and Ser180 (16A6; Cell Signaling), as well as polyclonal antibody to β-tubulin (P/N 926-42211; LI-COR Biosciences) as loading control.
Serum alanine aminotransferase determination.
Serum alanine aminotransferase (ALT) was analyzed from mouse whole blood using ALT strips from Roche Diagnostics according to the manufacturer's instructions. Measurement was performed in a Reflovet machine from SCIL animal care. Values above assay range were diluted before measurement.
TNF analysis in mouse serum.
Serum concentrations of TNF were analyzed by enzyme-linked immunosorbent assay (88-7324; eBioscience) according to the manufacturer's instructions.
Analysis of LCMV-specific antibodies in mouse serum.
For analysis of antibodies specific for LCMV glycoprotein, plates were coated overnight with anti–human Fc (109-001-008; Jackson). After blockade, plates were incubated with 100 μl/well LCMV glycoprotein gp1–Fc supernatant (prepared in-house) overnight. Serial dilutions of serum were added, followed by incubation for 90 min. After washing, diluted anti–mouse IgG (A3673; Sigma-Aldrich) was added for 60 min. Plates were washed and stained with 0.1 M NaH2PO4 and ABTS (10102946001; Roche Diagnostics) and were read at 405 nm (Infinite M200; Tekan).
Whole-genome gene expression in murine cells and bioinformatics analysis.
Prior to array-based gene-expression profiling, isolated total RNA was further purified using the MinElute Reaction Cleanup Kit (28206; Qiagen). Biotin labeled cRNA was generated using the TargetAmp Nano-g Biotin-aRNA Labeling Kit for the Illumina System (TAN091096; Epicentre). Biotin-labeled cRNA (1.5 μg) was hybridized to MouseWG-6 v2.0 Beadchips (Illumina) and was scanned on an Illumina iScan system. Raw intensity data were processed and exported with BeadStudio 3.1.1.0 (Illumina). Subsequent analyses were performed using Partek Genomics Suite V6.6 (PGS). Non-normalized data were imported from BeadStudio using the default PGS report builder. Data were then quantile-normalized, and transcripts with variable expression within the data set, as well as genes expressed differentially between the different conditions, were calculated using two- and three-way ANOVA models, including batch correction. If not stated otherwise, genes expressed differentially were defined by a change in expression (upregulated or downregulated) of twofold or more and an unadjusted P value of <0.05.
GSEA of mouse cells.
GSEA (Broad Institute: http://www.broadinstitute.org/gsea/index.jsp) was used to determine whether the expression of a defined set of genes was significantly altered in two different states. Gene sets for comparison of mouse cells was based on a published TNF RNA fingerprint27 and association with exhaustion on profiles of murine CD4+ T cells25. Cross-annotation of the different data sets on different microarray platforms was performed based on gene symbols.
Quantitative PCR analysis of mouse T cells.
Total RNA was extracted with TRIzol reagent (15596018; Life Technologies) from T cells sorted by flow cytometry. cDNA was synthesized with the Transcriptor First Strand cDNA synthesis kit (04897030001; Roche Diagnostics). Quantitative PCR was performed for Ly6c1, Klrd1 and Actb using the LightCycler Taqman master kit and the Universal Probe Library assay (Roche Diagnostics) on a LightCycler 480 II (Roche Diagnostics). Results were normalized to Actb expression. All oligonucleotides are listed in Supplementary Table 10.
Code availability.
The scripts necessary to run the GOAna algorithm in current versions of the R language (R 3.2.0 and greater) with current Bioconductor packages (Bioconductor 3.1 and greater) are provided at a website of Life and Medical Sciences Bonn (https://github.com/LIMES-immunogenomics/GOAna). Additional computer codes for bioinformatics analysis of gene expression data are available on request.
Statistical analysis.
All statistical analysis except analysis of gene-expression data were performed using GraphPad Prism software version 5.0 (GraphPad Software) or SPSS 21.0 (IBM SPSS). Analysis of more than two groups were performed by analysis of variance with the post-hoc Bonferroni multiple comparison test. To determine the significance of differences between two groups, the Student's t-test for normally distributed data with equal variances was used; otherwise, the Mann-Whitney U-test was used. The monotonic relationship between variables was evaluated with Spearman's rank-correlation test. No statistical method was used to predetermine sample size. Experimental group allocation was determined by genotype or HIV titer and PD-1 expression, and investigators were not blinded to allocation during experiments or further analytical assessment, except analysis of serum ALT, serum TNF, serum LCMV-specific antibodies, and LCMV titers.
Accession codes.
GEO: microarray data, GSE52185.
References
Rouse, B.T. & Sehrawat, S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 10, 514–526 (2010).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O'Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Hunter, C.A. & Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
McFadden, G., Mohamed, M.R., Rahman, M.M. & Bartee, E. Cytokine determinants of viral tropism. Nat. Rev. Immunol. 9, 645–655 (2009).
Deeks, S.G., Tracy, R. & Douek, D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity 39, 633–645 (2013).
Crouse, J., Kalinke, U. & Oxenius, A. Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 15, 231–242 (2015).
Wherry, E.J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Kim, P.S. & Ahmed, R. Features of responding T cells in cancer and chronic infection. Curr. Opin. Immunol. 22, 223–230 (2010).
Doering, T.A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).
Wherry, E.J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Youngblood, B., Wherry, E.J. & Ahmed, R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr. Opin. HIV AIDS 7, 50–57 (2012).
Teijaro, J.R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013).
Wilson, E.B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013).
Porichis, F. & Kaufmann, D.E. HIV-specific CD4 T cells and immune control of viral replication. Curr. Opin. HIV AIDS 6, 174–180 (2011).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Sedaghat, A.R. et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J. Virol. 82, 1870–1883 (2008).
Rotger, M. et al. Swiss HIV Cohort Study; Center for HIV/AIDS Vaccine Immunology. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 6, e1000781 (2010).
Vigneault, F. et al. Transcriptional profiling of CD4 T cells identifies distinct subgroups of HIV-1 elite controllers. J. Virol. 85, 3015–3019 (2011).
McKinney, E.F., Lee, J.C., Jayne, D.R., Lyons, P.A. & Smith, K.G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Oeckinghaus, A., Hayden, M.S. & Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 (2011).
Prokunina, L. et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat. Genet. 32, 666–669 (2002).
Salvato, M., Borrow, P., Shimomaye, E. & Oldstone, M.B. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J. Virol. 65, 1863–1869 (1991).
Belnoue, E., Fontannaz-Bozzotti, P., Grillet, S., Lambert, P.H. & Siegrist, C.A. Protracted course of lymphocytic choriomeningitis virus WE infection in early life: induction but limited expansion of CD8+ effector T cells and absence of memory CD8+ T cells. J. Virol. 81, 7338–7350 (2007).
Doyle, L.B., Doyle, M.V. & Oldstone, M.B. Susceptibility of newborn mice with H-2k backgrounds to lymphocytic choriomeningitis virus infection. Immunology 40, 589–596 (1980).
Crawford, A. et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Graham, C.M., Christensen, J.R. & Thomas, D.B. Differential induction of CD94 and NKG2 in CD4 helper T cells. A consequence of influenza virus infection and interferon-gamma? Immunology 121, 238–247 (2007).
Ruan, H., Pownall, H.J. & Lodish, H.F. Troglitazone antagonizes tumor necrosis factor-α-induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-κB. J. Biol. Chem. 278, 28181–28192 (2003).
Sasaki, Y. et al. Canonical NF-κB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity 24, 729–739 (2006).
Sledzinń´ska, A. et al. TGF-β signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol. 11, e1001674 (2013).
Speiser, D.E. et al. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat. Rev. Immunol. 14, 768–774 (2014).
Yi, J.S., Du, M. & Zajac, A.J. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324, 1572–1576 (2009).
Fröhlich, A. et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324, 1576–1580 (2009).
Elsaesser, H., Sauer, K. & Brooks, D.G. IL-21 is required to control chronic viral infection. Science 324, 1569–1572 (2009).
Nokta, M., Rossero, R., Loesch, K. & Pollard, R.B. Kinetics of tumor necrosis factor α and soluble TNFRII in HIV-infected patients treated with a triple combination of stavudine, didanosine, and hydroxyurea. AIDS Res. Hum. Retroviruses 13, 1633–1638 (1997).
De Pablo-Bernal, R.S. et al. TNF-α levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J. Antimicrob. Chemother. 69, 3041–3046 (2014).
Aukrust, P. et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J. Infect. Dis. 179, 74–82 (1999).
Aukrust, P. et al. Serum levels of tumor necrosis factor-α (TNF α) and soluble TNF receptors in human immunodeficiency virus type 1 infection--correlations to clinical, immunologic, and virologic parameters. J. Infect. Dis. 169, 420–424 (1994).
Aukrust, P. et al. Effects of intravenous immunoglobulin in vivo on abnormally increased tumor necrosis factor-α activity in human immunodeficiency virus type 1 infection. J. Infect. Dis. 176, 913–923 (1997).
Sade-Feldman, M. et al. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 38, 541–554 (2013).
Keir, M.E., Butte, M.J., Freeman, G.J. & Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Han, S., Asoyan, A., Rabenstein, H., Nakano, N. & Obst, R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc. Natl. Acad. Sci. USA 107, 20453–20458 (2010).
Planès, R. et al. HIV-1 Tat protein induces PD-L1 (B7-H1) expression on dendritic cells through tumor necrosis factor α- and toll-like receptor 4-mediated mechanisms. J. Virol. 88, 6672–6689 (2014).
Cepeda, E.J., Williams, F.M., Ishimori, M.L., Weisman, M.H. & Reveille, J.D. The use of anti-tumour necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann. Rheum. Dis. 67, 710–712 (2008).
Brunasso, A.M.G., Puntoni, M., Gulia, A. & Massone, C. Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology 50, 1700–1711 (2011).
Kim, S.Y. & Solomon, D.H. Tumor necrosis factor blockade and the risk of viral infection. Nat. Rev. Rheumatol. 6, 165–174 (2010).
Habib, S.F., Hasan, M.Z. & Salam, I. Infliximab therapy for HIV positive Crohn's disease: A case report. J. Crohns Colitis 3, 302–304 (2009).
Chemnitz, J.M. et al. RNA fingerprints provide direct evidence for the inhibitory role of TGFβ and PD-1 on CD4+ T cells in Hodgkin lymphoma. Blood 110, 3226–3233 (2007).
Beyer, M. et al. Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat. Immunol. 12, 898–907 (2011).
Ahmed, R., Salmi, A., Butler, L.D., Chiller, J.M. & Oldstone, M.B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984).
Lang, P.A. et al. Reactive oxygen species delay control of lymphocytic choriomeningitis virus. Cell Death Differ. 20, 649–658 (2013).
Acknowledgements
We thank J.L. Riley (University of Pennsylvania) for anti-CD28; J.G. Gribben (Queen Mary University of London) for anti-CTLA-4; M. Schell, M. Kraut, C. Nabakowski, S. Winter and N. Koch for technical assistance; colleagues at the Division of Transfusion Medicine (University Hospital Bonn) for technical support; A. Sharpe for discussions; and the US National Institutes of Health Tetramer Core Facility (contract HHSN272201300006C) for gp66 tetramers. Supported by the Köln Fortune Program of the Faculty of Medicine of the University of Cologne (J.M.C.), the German Research Foundation (SFB 832, SFB 704, INST 217/575-1, INST 217/576-1 and INST 217/577-1 to J.L.S. and M.B.; SFB TRR57 and SFB TRR36 to Z.A., P.A.K. and C.K.; SFB TRR57 to J.T.; and LA2558/3-1, SFB974 and TRR60 to P.A.L. and K.S.L.), the German Research Foundation excellence cluster ImmunoSensation (M.B., Z.A., J.L.S., C.K. and P.A.K.), the German Federal Ministry of Research and Education (01KI0771 and 01KI1017 to C.L., G.F. and P.H.), the German Center for Infection Research (Z.A., P.A.K. and C.K.; partner site Bonn, J.T.), the H. J. & W. Hector Foundation (J.T.), the Alexander von Humboldt Foundation (SKA2008 and SKA2010) and the Jürgen Manchot Foundation (MOI II).
Author information
Authors and Affiliations
Contributions
M.B., Z.A., J.M.C. and P.H. designed, performed and supervised experiments, analyzed data and wrote the manuscript; D.M., J.S. and A.H. analyzed data; C.L., Y.T., P.V.S., L.S., M.K., J.T., R.S., A.P. and P.A.L. performed experiments; K.S.L., C.K. and G.F. discussed the results; A.O., T.B. and M.H. provided analytical tools; P.A.K. and J.L.S. designed, supervised and analyzed experiments and wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.B., Z.A., J.M.C., P.H., P.A.K. and J.L.S. have applied for patents for the usage of anti-TNF therapy in chronic viral infection.
Integrated supplementary information
Supplementary Figure 1 Analysis of inhibitory signaling in T cells from human HIV-infected subjects.
(a) Workflow for screening of HIV-infected subjects. Boxes in grey indicate the subject groups chosen for further studies. Samples from subjects with at least 106/ml CD4+ T cells were used for further studies (*). Subjects were excluded when RNA amount and quality did not reach necessary quality standards for genomic analysis (**). (b) Flow cytometric analysis of PD-1 expression on CD4+ T cells from HIVloPD-1lo or HIVhiPD-1hi subjects. Mean PD-1 expression of CD4+ T cells from HIVloPD-1lo (n = 26) or HIVhiPD-1hi subjects (n = 37). (c) Relative PDCD1 mRNA expression of CD4+ T cells from HIVloPD-1lo (n = 5) or HIVhiPD-1hi subjects (n = 7) by qPCR. (d) Representative flow cytometry dot plots from one HIVloPD-1lo and one HIVhiPD-1hi subject using current state-of-the-art methodology. (e-g) Flow cytometric analysis of PD-1 expression on CD8+ T cells from HIVloPD-1lo or HIVhiPD-1hi subjects. (e) Representative flow cytometry dot plots from one HIVloPD-1lo and one HIVhiPD-1hi patient. (f) Proportion of PD-1-expressing CD8+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 8). (g) Mean PD-1 expression of CD8+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 8). (h-j) Correlation between CD4+ T cell count of each subject and HIV-RNA (h), CD4+ T cell count and CD4+ T cell PD-1 expression (i), and HIV RNA and CD4+ T cell PD-1 expression (j). White circles: HIVloPD-1lo subjects; grey circles: HIVhiPD-1hi subjects. (k) Representative flow cytometry dot plots from one HIVloPD-1lo or one HIVhiPD-1hi subject for CTLA-4 expression on CD4+ T cells. (l) Proportion of CTLA-4-expressing CD4+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 8). (m) Mean CTLA-4 expression of CD4+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 8). (n) Representative flow cytometry dot plots from one HIVloPD-1lo or one HIVhiPD-1hi subject for CTLA-4 expression on CD8+ T cells. (o) Proportion of CTLA-4-expressing CD8+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 8). (p) Mean CTLA-4 expression of CD8+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 8). (q-s) Generation of RNA fingerprints. (q) Prior to assessment of transcriptional changes the functional impact of all components on purified CD4+ T cells was analyzed. Freshly isolated primary human CD4+ T cells were labeled with CFSE and left unstimulated or were stimulated as indicated. After 4 days, CFSE dilution was analyzed by flow cytometry. The overall percentage of dividing cells is displayed in the corresponding gate. For each condition at least four individual experiments were performed. Shown here are representative results. (r) CD4+ T cells were stimulated as above. After four days the concentration of IFN-γ was determined using flow cytometric bead assays. For each condition at least four individual experiments were performed. Mean ± s.d. (s) Visualization of fold changes and amount of genes significantly altered in CD4+ T cell transcription profiles after indicated stimulations of four different healthy blood donors defining the RNA fingerprint of the particular analyzed component. (t) Schematic overview of analysis of gene expression data for the contribution of RNA fingerprints to the differences between CD4+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 10). (u) Enrichment of inhibitory pathways in HIV-infected individuals. Gene set enrichment analysis (GSEA) using genes regulated by PD-1, CTLA-4, PGE2, TGF-β, and IL-10 as the gene set in CD4+ T cells from HIVhiPD-1hi and HIVloPD-1lo subjects. ES: enrichment score, FDR: false-discovery rate. (b,c,g,m,p) Mean ± s.e.m. (b,c,f,g,l,m,o,p) *P < 0.05 (Student’s t-test). (f,l,o) Bounds of boxes denote interquartile range; lines within boxes denote mean; whiskers indicate interdecile range. Dots represent outliers.
Supplementary Figure 2 TNF-dependent regulation of PD-1 expression.
(a) Relative mRNA expression of CD4+ T cells from HIVloPD-1lo (n = 10) or HIVhiPD-1hi subjects (n = 10) for TNFRI and TNFRII by microarray analysis. (b) Expression of TNFRI and TNFRII on CD4+ T cells from HIVloPD-1lo (n = 5) or HIVhiPD-1hi subjects (n = 6). (c) Schematic representation of the human PDCD1 locus. The PDCD1 promoter predicted by analysis using Genomatix is shown in violet. (d) Luciferase reporter constructs driven by the Genomatix-predicted human PDCD1 promoter (-5.0 kb, red), the region directly upstream of the transcriptional start site (-0.5 kb, green), and an intronic enhancer in intron 4 (intron 4, blue)21 were transfected into HEK293T cells and luciferase activity was assessed after 24 hours in unstimulated cells and cells stimulated with TNF. Control represents the empty pGL4.24 construct. Mean ± s.d. of triplicate cultures are shown. Data are representative of three independent experiments. (e,f) Expression of PD-1 on memory CD4+ T cells from healthy donors pre-stimulated for 3 days with TNF (TNF) or medium alone (US), restimulated with (e) TNF or (f) anti-CD3, IL-2, and TNF (each n = 6). Left, exemplified flow cytometry data, right, cumulative data. (g) Differentiation of CD4+ T cells from HIVloPD-1lo (n = 5) or HIVhiPD-1hi subjects (n = 6) in naïve and memory CD4+ T cells as well as CD7+ or CD7- memory CD4+ T cells. (e,f) *P < 0.05 (Student’s t-test). (a,b,e,f,g) Mean ± s.e.m. n.s. not significant.
Supplementary Figure 3 Analysis of mouse chronic neonatal LCMV infection as model for late-stage HIV-infection.
(a) LCMV serum titers in chronic clone 13 LCMV-infected mice (n = 5). (b) ALT serum concentration in control (n = 3) and chronic neonatal LCMV-WE infected mice (cnLCMV-WE, n = 3). (c,d) Flow cytometric analysis of TIM-3, LILRB4, 2B4, CTLA-4, LAG3, PIR-B, BTLA, CD160, and CD200 co-expression on (c) CD4+PD-1+ and (d) CD8+PD-1+ T cells from cnLCMV-WE mice (n = 5). (e) Left, sorting strategy to isolate PD-1 expressing CD4+ T cells from acute LCMV WE-infected and cnLCMV-WE mice for gene expression analysis. Right, analysis of purities of isolated cell populations. (f) GSEA using genes from the murine chronic clone 13 LCMV-infected gp66+CD4+ T cell RNA fingerprint25 as the gene set in CD4+PD-1+ and CD4+PD-1− T cells from cnLCMV-WE mice. ES: enrichment score, FDR: false-discovery rate. (g) Prediction probability for each sample being classified as HIV-positive (HIV+) or uninfected control (HIV−) based on group prediction analysis of the cnLCMV-WE T cell RNA fingerprint using an additional publicly available dataset comparing HIV-infected and uninfected individuals (GSE9927)16. Colors indicate high (red) and low (blue) probability for the cnLCMV-WE RNA fingerprint.
Supplementary Figure 4 Neutralization of TNF in mice with chronic neonatal LCMV strain WE infection restores immunity to LCMV and reverts CD4+PD-1+ T cell gene expression.
(a) Model for TNF neutralization in 8 week old cnLCMV-WE mice. (b) Left, representative, right, cumulative flow cytometric analysis of np396 expression on splenic CD8+ T cells after vehicle treatment or TNF neutralization (each n = 5). (c) Total numbers of splenic np396+CD8+ T cells (each n = 5). (d) PD-1 expression on splenic np396+CD8+ T cells (each n = 5). (e) Left, representative, right, cumulative flow cytometric analysis of PD-1 expression on splenic CD4+ T cells after vehicle treatment (n = 5) or TNF neutralization (n = 4). (f) Left, representative, right, cumulative flow cytometric analysis of PD-1 expression on splenic CD8+ T cells from animals after vehicle treatment (n = 6) or TNF neutralization (n = 5). (g-k) Role of TNFR-signaling in acute murine LCMV strain WE infection. Wild-type mice were acute infected with LCMV strain WE (2 × 104 pfu) and analyzed after 10 days. (g,h) Immunoblot analysis of pIkkα/β (Ser176/180) (top) and β-actin (bottom) in (g) CD4+ and (h) CD8+ T cells from animals after vehicle treatment or TNF neutralization during acute LCMV strain WE infection (n = 3). Data shown are representative of three mice each. (i-k) Left, representative, right, cumulative flow cytometric analysis of (i) PD-1 expression on CD4+ T cells, (j) PD-1 expression on CD8+ T cells, and (k) gp33-specific CD8+ T cells from animals after vehicle treatment or TNF neutralization during acute LCMV strain WE infection (n = 3). (l) Heatmap of z-transformed gene expression data for genes expressed in low amounts in at least one of the inhibitory conditions in human CD4+ T cells, up-regulated in CD4+PD-1+ T cells from mice after TNF neutralization. (m) Heatmap of z-transformed gene expression data for genes highly expressed under at least one of the inhibitory conditions in human CD4+ T cells, down-regulated in CD4+PD-1+ T cells from mice after TNF neutralization. (n) Fold-change-fold-change plot showing the influence of TNF-neutralization on gene expression in CD4+PD-1− and CD4+PD-1+ T cells. The y-axis compares the expression profiles between CD4+PD-1− T cells from mice after vehicle treatment or TNF neutralization, whereas the x-axis compares the expression profiles of CD4+PD-1+ T cells. Highlighted in red are genes assessed in o. (o) Relative mRNA expression of CD4+PD-1− and CD4+PD-1+ T cells from mice after vehicle treatment or TNF neutralization for Ly6c1 and Klrd1 by qPCR. Mean ± s.e.m. of at least triplicates, representative of two independent experiments. *P < 0.05 (Student’s t-test). (p) GSEA using the murine cnLCMV-WE CD4+ T-cell RNA fingerprint as the gene set in CD4+PD-1+ T cells from mice after vehicle treatment or TNF neutralization. (q) Heatmap of z-transformed gene expression data for transcription factors associated with CD4+ T cell exhaustion in LCMV clone 13 infection25 in CD4+PD-1+ T cells from mice after vehicle treatment or TNF neutralization. (r) GSEA using a murine TNF RNA fingerprint (GSE2504)27 as the gene set in CD4+PD-1+ and CD4+PD-1− T cells in mice after vehicle treatment (left) or TNF neutralization (right). (s) GSEA using the human TNF RNA fingerprint genes defined in CD4+ T cells as the gene set in CD4+PD-1+ T cells in mice after vehicle treatment or TNF neutralization. (b-f,i-k) Mean ± s.e.m.*P < 0.05 (Student’s t-test). Data are representative of two independent experiments. n.s. not significant. (p,r,s) ES: enrichment score, FDR: false-discovery rate.
Supplementary Figure 5 Neutralization of TNF in mice infected with LCMV clone 13 partially restores immunity to LCMV.
(a) Model for TNF neutralization of chronic LCMV clone 13-infected mice. (b) Quantification of LCMV titers in serum over time (Control n = 5, anti-TNF n = 9). (c-e) LCMV titers in the liver (c), kidney (d), and lung (e) after TNF neutralization (Control n = 6, anti-TNF n = 11). Box plots showing 25th, mean and 75th percentiles (horizontal bars), 10th and 90th percentage (whiskers), and outliers (dots). (c-e) *P < 0.05 (Student’s t-test). n.s. not significant. Data are representative of two independent experiments.
Supplementary Figure 6 Neutralization of TNF in mice with chronic neonatal LCMV strain WE infection restores cytokine production by CD4+ and CD8+ T cells.
(a) Numbers of IL-2 and (b) IFN-γ expressing splenic gp33+CD8+ T cells after TNF neutralization in cnLCMV-WE mice. Numbers of (c) IL-2, (d) IFN-γ, (e) IL-21, and (f) CD40L expressing splenic gp66+CD4+ T cells. (g) Cumulative flow cytometric analysis of TNF expression in splenic gp66+CD4+ T cells. (h) Numbers of TNF expressing splenic gp66+CD4+ T cells. (a-f,h) *P < 0.05 (Student’s t-test). (a-h) Mean ± s.e.m. Each n = 5. Data are representative of two independent experiments. n.s. not significant.
Supplementary Figure 7 Role of TNFR-signaling in T cells in mice with chronic neonatal LCMV strain WE infection.
(a) Protocol used to determine the effect of TNF on T cells in cnLCMV-WE mice. 2 × 106 CD8+ T cells from Thy1.2 congenic wild-type or TNFRI-TNFRII-deficient mice 8 days after acute infection with LCMV strain WEwere transferred to Thy1.1 mice with chronic neonatal LCMV infection and assessed after 10 days. (b,c) Left, sorting strategy to isolate Thy1.2+CD4+ and CD8+ T cells from acute LCMV strain WE-infected (b) wild-type and (c) TNFRI-TNFRII-deficient mice for adoptive transfer in cnLCMV-WE Thy1.1+ mice. Right, analysis of purities of isolated cell populations. (d,e) Flow cytometric analysis of gp33-specific Thy1.2+CD8+ T cells from mice receiving wild-type (n = 3) or TNFRI-TNFRII-deficient CD8+ T cells (n = 3). (d) Cumulative percentage of splenic gp33-specific CD8+ T cells. (e) Cumulative percentage of splenic PD-1+ gp33-specific CD8+ T cells. (f) ALT serum concentration in mice receiving no transfer (n = 4), wild-type (n = 3), or TNFRI-TNFRII-deficient CD8+ T cells (n = 3). (g) Quantification of LCMV titers in serum as in f. (h) Protocol used to determine the effect of TNF on T cells in cnLCMV-WE mice. 2 × 106 CD8+ T cells and 2 × 106 CD4+ from Thy1.2+ congenic wild-type or TNFRI-TNFRII-deficient mice 8 days after acute infection with LCMV strain WE were transferred to Thy1.1+ mice with chronic neonatal LCMV infection and assessed after 10 days. (i-k) Flow cytometric analysis of gp66-specific Thy1.1+CD4+ T cells from mice receiving no transfer (n = 4), wild-type (n = 4), or TNFRI-TNFRII-deficient CD4+ and CD8+ T cells (n = 4). (i) Cumulative percentage of splenic gp66-specific Thy1.1+CD4+ T cells. (j) Cumulative numbers of splenic gp66-specific Thy1.1+CD4+ T cells. (k) Cumulative percentage of splenic PD-1+ gp66-specific Thy1.1+ CD4+ T cells. (l) Left, representative flow cytometric analysis of PD-1 expression on Thy1.2+CD4+ T cells from mice receiving wild-type or TNFRI-TNFRII-deficient CD4+ and CD8+ T cells. Right, cumulative data. (m,n) Flow cytometric analysis of PD-1 expression on Thy1.1+CD4+ T cells as in i. (m) Cumulative percentage of splenic Thy1.1+CD4+PD-1+ T cells. (n) Cumulative numbers of splenic Thy1.1+CD4+PD-1+ T cells. (o) Left, representative flow cytometric analysis of PD-1 expression on Thy1.2+CD8+ T cells as in l. Right, cumulative data. (p,q) Flow cytometric analysis of PD-1 expression on Thy1.1+CD8+ T cells as in I. (p) Cumulative percentage of splenic Thy1.1+CD8+PD-1+ T cells. (q) Cumulative numbers of splenic Thy1.1+CD8+PD-1+ T cells. Data from 4 mice per group are shown. (d,e,i-q) Mean ± s.e.m. (d,e,l,o) *P < 0.05 (Student’s t-test). (i-k,m,n,p,q) *P < 0.05 vs. wild-type (one-way ANOVA with Bonferroni FDR correction). n.s. not significant. Data are representative of two independent experiments.
Supplementary Figure 8 Constitutive NF-κB activity in mice acutely infected with LCMV strain WE induces PD-1 expression and loss of T cell helper function.
(a) Model for induction of constitutive Ikk activity in oil- (Control) or tamoxifen-treated CD4-Cre-ERt2 × R26StopFLIkk2ca mice (IkkE/E) before acute WE LCMV infection. (b) Flow cytometric analysis of successful recombination of the transgenic allele in CD4+ T cells from oil (Control) or tamoxifen-treated mice (Tam) as evidenced by GFP expression 14 days after infection. Left, exemplary flow cytometric data, right, cumulative data from tamoxifen-treated mice (n = 6). (c) Flow cytometric analysis of gp66-specific CD4+ T cells from oil (Control) or tamoxifen-treated mice (Tam) (each n = 6). (d) Flow cytometric analysis of gp33-specific CD8+ T cells from oil (Control) or tamoxifen-treated mice (Tam) (each n = 6). (e) PD-1 expression on splenic gp33+CD8+ T cells from oil (Control) or tamoxifen-treated mice (Tam) (each n = 6). (f) IL-2 and IFN-γ expression on splenic gp33+CD8+ T cells from oil (Control) or tamoxifen-treated mice (Tam) (each n = 6). (b-f) *P < 0.05 (Student’s t-test). Mean ± s.e.m. Data are representative of two independent experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 3, 7–10 (PDF 1473 kb)
Supplementary Table 2
Genes comprising the different RNA fingerprints (XLSX 33 kb)
Supplementary Table 4
Tabular output from IPA upstream regulator analysis. The output is sorted by predicted "activity" of the analyzed molecule. (XLSX 81 kb)
Supplementary Table 5
Genes associated with PD-1 expression in CD4+ T cells from chronic neonatal WE LCMV-infected mice. (XLSX 16 kb)
Supplementary Table 6
Genes identified as differentially expressed between anti-CD3 and anti-CD28-stimulated CD4+ T cells and any of the five inhibitory molecules expression in human CD4+ T cells (FC | 2.0 |, p < 0.05, Diff > 100) which show a counter-regulation in CD4+ PD-1+ T cells from chronic neonatal WE LCMV-infected mice after TNF neutralization (XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Beyer, M., Abdullah, Z., Chemnitz, J. et al. Tumor-necrosis factor impairs CD4+ T cell–mediated immunological control in chronic viral infection. Nat Immunol 17, 593–603 (2016). https://doi.org/10.1038/ni.3399
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3399
This article is cited by
-
Development trends of immune activation during HIV infection in recent three decades: a bibliometric analysis based on CiteSpace
Archives of Microbiology (2023)
-
Pre-treatment levels of inflammatory markers and chemotherapy completion rates in patients with early-stage breast cancer
International Journal of Clinical Oncology (2023)
-
Breast cancer microenvironment and obesity: challenges for therapy
Cancer and Metastasis Reviews (2022)
-
TNF in the liver: targeting a central player in inflammation
Seminars in Immunopathology (2022)
-
Insights into the biology and therapeutic implications of TNF and regulatory T cells
Nature Reviews Rheumatology (2021)