Abstract
The extracellular-regulated kinases ERK1 and ERK2 (commonly referred to as ERK1/2) have a crucial role in cardiac hypertrophy. ERK1/2 is activated by mitogen-activated protein kinase kinase-1 (MEK1) and MEK2 (commonly referred to as MEK1/2)-dependent phosphorylation in the TEY motif of the activation loop, but how ERK1/2 is targeted toward specific substrates is not well understood. Here we show that autophosphorylation of ERK1/2 on Thr188 directs ERK1/2 to phosphorylate nuclear targets known to cause cardiac hypertrophy. Thr188 autophosphorylation requires the activation and assembly of the entire Raf-MEK-ERK kinase cascade, phosphorylation of the TEY motif, dimerization of ERK1/2 and binding to G protein βγ subunits released from activated Gq. Thr188 phosphorylation of ERK1/2 was observed in isolated cardiomyocytes induced to undergo hypertrophic growth, in mice upon stimulation of Gq-coupled receptors or after aortic banding and in failing human hearts. Experiments using transgenic mouse models carrying mutations at the Thr188 phosphorylation site of ERK2 suggested a causal relationship to cardiac hypertrophy. We propose that specific phosphorylation events on ERK1/2 integrate differing upstream signals (Raf1-MEK1/2 or G protein–coupled receptor–Gq) to induce cardiac hypertrophy.
Similar content being viewed by others
Main
Cardiac hypertrophy occurs in response to increased mechanical load on the heart and through the action of several hormones and mediators1,2,3. It involves increased protein synthesis, cardiomyocyte growth and cytoskeletal reorganization and is often associated with interstitial fibrosis1,3. The mitogen-activated protein kinase (MAPK) cascade, consisting of Raf1, MEK1/2 and ERK1/2, has a prominent role in cardiac hypertrophy, even though ERK1/2 activation may not always be required1,3,4,5,6,7,8,9.
Activation of the cascade begins with membrane translocation and activation of Raf1, which leads to sequential phosphorylation and activation of MEK1/2 and then ERK1/2 (refs. 4,10,11). MEK1/2 activates ERK1/2 by dual phosphorylation of the threonine and tyrosine residues in the TEY motif of the activation loop (residues 183–185 in mouse Erk2). ERK2 can autophosphorylate at Tyr185, but phosphorylation of both Thr183 and Tyr185 by MEK1/2 is required for full activation4,10,11. Active ERK1/2 then phosphorylates cytosolic and nuclear substrates12,13. Scaffolding proteins such as SEF and β-arrestin have been suggested to retain active ERK1/2 in the cytoplasm14,15, but mechanisms that promote nuclear ERK1/2 translocation and activity and thereby lead to cardiac hypertrophy are largely unknown.
The observation that Raf1 binds to G protein βγ subunits (Gβγ)16,17 led us to investigate the potential of G proteins, some of which can also trigger cardiac hypertrophy2,18,19,20,21, to interact with proteins of the Raf-MEK-ERK1/2 cascade and to affect their activity and cellular localization.
Results
Gβγ induces autophosphorylation of Erk2 at Thr188
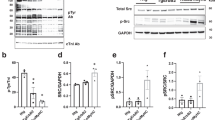
Purified active His6-tagged Mek1S218D,S222D,Δ32–51 mutant (simulating the Raf1-phosphorylated, active state22, termed Mek*) or His6-Erk2 (which is always partially active when purified10,11) strongly co-precipitated purified Gβγ, suggesting direct interactions between the proteins (Fig. 1a). Incubation of purified Erk2 with purified Gβγ caused increased phosphorylation of Erk2 to an extent similar to that caused by Mek* (Fig. 1b). However, this phosphorylation did not seem to occur in the classical TEY motif (as revealed by specific antibodies, Fig. 1b), nor did it significantly increase Erk2 activity (Supplementary Fig. 1a online). Because the Gβγ preparation contained no kinase activity toward Erk2 (Supplementary Fig. 1b), these results point to Gβγ-induced autophosphorylation of Erk2 at a site distinct from the TEY motif.
(a) Immunoblot showing co-immunoprecipitation of purified Mek* and purified Erk2 with purified Gβγ. Con, control (bovine serum albumin). (b) [32P]-autoradiography (top) and immunoblot analyses using phospho-specific antibodies directed against the phosphorylated TEY motif of Erk1/2 (pERK (TEY)) of purified Erk2 after incubation with purified Gβγ or Mek*. (c) Two-dimensional phosphopeptide mapping of Erk2 alone or after incubation of Erk2 with Mek* or Gβγ. Phosphopeptides resulting from trypsin digestion of Erk2 are designated P1, P2 and P3. (d) Phosphoaminoacid analysis of the phosphopeptides P1, P2 and P3 shown in c. (e) Two-dimensional phosphopeptide mapping of Erk2T188A after incubation with Gβγ showing induced phosphorylation of one phosphopeptide only (P1); incubation with Mek* resulted in the induction of two phosphopeptides (P1 and P2). (f) Immunoblot analyses for Thr188-phosphorylated Erk after incubation in the absence or presence of ATP, Gβγ or Mek*. (g) Co-immunoprecipitation of Gβ with Raf1 or with Erk1/2 in lysates of hypertrophied hearts. Immunoprecipitations (IPs) were performed with Raf1- or Erk1/2-specific antibodies (anti-Raf or anti-Erk1/2, respectively). n = 3 mice per group. (h) Immunoblot analyses for Thr188-phosphorylated Erk1/2 in lysates of hypertrophied hearts (TAC) compared to control lysates of healthy hearts. n = 3 mice per group. (i) Phosphorylation of Thr188 and the TEY motif of ERK1/2 in failing human hearts. The ratio of the pERK (Thr188) and pERK (TEY) to the ERK1/2 signal in the immunoblots was calculated for each individual and is quantified in the graphs. n = 10 individuals per group; *P < 0.001 versus nonfailing.
To identify this site, we analyzed the phosphorylation pattern of Erk2 by two-dimensional phosphopeptide mapping followed by phosphoaminoacid analyses (Fig. 1c,d). Incubation of purified Erk2 alone with [γ-32P]ATP resulted in a single phosphopeptide, P1, containing only phosphotyrosine, which we identified as the Tyr185-autophosphorylated peptide10 by mutating Tyr185 to Phe185 (data not shown). Incubation with Mek* yielded one additional phosphopeptide, P2, containing phosphotyrosine and phosphothreonine, representing the known phosphorylation sites Thr183 and Tyr185 (refs. 10,11).
A third phosphopeptide, P3, containing only phosphothreonine, was observed after incubation of Erk2 with Gβγ (Fig. 1c,d). Incubation of Erk2 with both Gβγ and Mek* generated all three phosphopeptides, indicating that P3 is distinct from P1 and P2 (Supplementary Fig. 1c). Mutation of Tyr185 to Phe185, which prevents autophosphorylation and autoactivation of Erk2 (ref. 10), abolished the appearance of phosphopeptide P3 after incubation with Gβγ (data not shown), indicating that P3 was dependent on active Erk2 and, thus, generated by autophosphorylation of Erk2.
Several lines of evidence indicated that this autophosphorylation occurred on Thr188. First, we individually mutated all threonine residues of Erk2 to alanine and analyzed the ability of these Erk2 mutants to autophosphorylate using phosphopeptide mapping analysis. Only the Erk2T188A mutant showed a loss of Gβγ-induced phosphopeptide P3, whereas Mek*-induced phosphorylation and autophosphorylation remained unaffected (Fig. 1e). An Erk2T188S mutant with a more conservative amino acid substitution also showed almost no P3 (Supplementary Fig. 1d). Second, we generated phospho-Erk (Thr188)-specific antibodies, which gave a signal only after incubation of Erk2 with Gβγ and not with Mek* (Fig. 1f); these antibodies also did not give a signal when Erk2T188A was incubated with Gβγ or when they were preincubated with phospho-Erk (Thr188) peptide (Supplementary Fig. 1e,f). We termed this phosphorylation, which occurs in the corresponding regions of Erk1/2 in other species (Supplementary Fig. 1g), Thr188 phosphorylation.
Thr188 phosphorylation in hypertrophy and heart failure
In order to examine whether Gβγ-induced Thr188 phosphorylation plays a part in cardiac hypertrophy, we analyzed mouse hearts 3 weeks after inducing pressure overload by transverse aortic constriction (TAC)23. TAC induced a stable interaction of Raf1 and of Erk1/2 with Gβγ in the hearts, as shown by co-immunoprecipitation, as well as strong Thr188-phosphorylation of Erk1/2 (Fig. 1g,h).
An approximately fivefold increase in Thr188 phosphorylation was also observed in failing human hearts, comparable in extent to canonical ERK1/2 phosphorylation at Thr183 and Tyr185 (Fig. 1i). These data suggest that Gβγ-dependent Thr188 phosphorylation of ERK1/2 might have a role in cardiac hypertrophy and failure.
Upstream signals for Thr188 phosphorylation
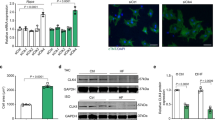
To investigate the specificity of upstream stimuli that initiate Gβγ-dependent Thr188 phosphorylation, we treated neonatal rat cardiomyocytes (NRCMs) either with angiotensin II, which triggers hypertrophy via Gq-coupled AT1 angiotensin receptors24, or with carbachol, a nonhypertrophic stimulus activating cardiac Gi-coupled M2 muscarinic receptors25 (Fig. 2). Stimulation with angiotensin II, but not with carbachol, markedly enhanced co-immunoprecipitation of Gβγ with both Raf1 and Erk1/2 (Fig. 2a,c). This angiotensin II–induced interaction was quite strong, as depletion of Gβ from angiotensin II–stimulated neonatal cardiomyocyte lysates by immunoprecipitation reduced the Raf1 and Erk1/2 content by approximately 70–80%, whereas Gβ depletion did not affect Raf1 or Erk1/2 content in nonstimulated or carbachol-stimulated lysates (Fig. 2b,d and Supplementary Fig. 2 online).
(a–d) Co-immunoprecipitation assays of Raf1 and Erk1/2 with Gβγ. Immunoblot analyses for Gβ, Raf1 and Erk1/2 in NRCM lysates after immunoprecipitation with anti-Raf1 or anti-Erk1/2. Co-immunoprecipitation of Gβ was observed after treatment of NRCM with angiotensin II (Ang, 100 nM, 5 min; a) but not after carbachol treatment (CCH, 300 μM, 10 min; c). Quantification of immunoblot detection of Raf1 and Erk1/2 before and after depletion of Gβ by Gβ-specific antibodies from unstimulated (basal) or from either angiotensin II–stimulated (b) or carbachol-stimulated (d) NRCM lysates. n = 7–9 experiments per group; *P < 0.001 compared with total Raf1 or Erk1/2 expression. (e,f) Immunoblot detection of Erk1/2 phosphorylation with pErk (Thr188)- and pErk (TEY)-specific antibodies in adult cardiac myocytes after short-term stimulation (e) and in NRCMs after long-term stimulation (f) with carbachol (300 μM, 10 min or 36 h), angiotensin II (100 nM, 5 min or 24 h), neuregulin-1-β1 (NRG, 20 ng ml−1, 10 min or 36 h) and phenylephrine (PE, 35 μM, 5 min or 48 h). All individual experiments were reproduced at least three times with similar results.
Investigation of other stimuli in transfected cell lines revealed that Gq-coupled receptors (M1 muscarinic and α1 adrenergic) caused direct interaction of Erk1/2 with Gβγ, whereas Gi-coupled receptors (M2 muscarinic and α2A adrenergic) did not (Supplementary Fig. 2).
Similarly, stimulation in cardiomyocytes of the hypertrophic AT1 and α1 adrenergic receptors1,2,3,4,24,25,26,27, but not of the nonhypertrophic M2 muscarinic receptor25, induced Thr188 phosphorylation of Erk1/2, whereas stimulation of each of the three types of receptors caused canonical Erk1/2 phosphorylation in the TEY motif (Fig. 2e,f). This was the case for both short-term stimulation in adult cardiomyocytes and for long-term, hypertrophic stimulation in neonatal cardiomyocytes (Fig. 2e,f and Supplementary Fig. 3a–c online).
Notably, neuregulin1-β1, a hypertrophic agonist that activates the receptor tyrosine kinase ErbB28,29, also induced Thr188 phosphorylation of Erk1/2 in cardiomyocytes (Fig. 2e,f). Although a full analysis of this effect is beyond the scope of our investigation, preliminary experiments suggest that Gβγ is also involved in this effect (Supplementary Fig. 3c–h). First, transfection of the GRK2 C-terminus, a Gβγ scavenger30, inhibited not only angiotensin II–mediated but also neuregulin-mediated protein synthesis and cytoskeletal rearrangement in neonatal cardiomyocytes (Supplementary Fig. 3a–c). Second, neuregulin stimulation of neonatal cardiomyocytes permitted immunoprecipitation of Gβγ with Raf1 or Erk1/2 (Supplementary Fig. 3d). Third, neuregulin-stimulation of cardiomyocytes triggered phosphorylation of Gαq/11 at Tyr356, which enhances Gαq/11 activity31, and mutation of Tyr356 to phenylalanine blocked neuregulin-induced hypertrophic protein synthesis (Supplementary Fig. 3e,f). Fourth, neuregulin-induced protein synthesis was impaired by the Erk2T188A and Erk2T188S mutants (Supplementary Fig. 3g,h). Because an inhibitor of Src kinase (PP2) blocked these effects (data not shown), the interaction between ErbB and Thr188 phosphorylation is presumably indirect. In summary, these data suggest that Thr188 phosphorylation may mediate cross-talk between receptor tyrosine kinases and G proteins.
Thr188 phosphorylation is causally related to hypertrophy
Thr188 phosphorylation of Erk1/2 occurred in response to TAC or various hypertrophic stimuli. Several lines of experimentation suggest that Thr188 phosphorylation has a causal role in cardiac hypertrophy. First, in neonatal cardiomyocytes, overexpression of the phosphorylation-deficient Erk2T188S mutant effectively inhibited angiotensin II–induced as well as neuregulin-induced protein synthesis (Fig. 3a and Supplementary Fig. 3g,h). Second, Erk2T188D, which mimics Thr188 phosphorylation, showed a gain-of-function phenotype in neonatal cardiomyocytes: it conferred to the nonhypertrophic M2 receptor stimulus carbachol the ability to increase protein synthesis and induce cardiomyocyte growth and cytoskeletal reorganization (Fig. 3b,c). These results suggest that Thr188 phosphorylation may be causal in activating the prohypertrophic functions of Erk1/2 in cardiomyocytes.
(a–c) [3H]isoleucine incorporation (a,b) and Alexa–Fluor 488–labeled phalloidin staining (c) of NRCMs transfected with control plasmid (mock), wild-type Erk2T188T, Erk2T188S or Erk2T188D incubated with or without angiotensin II (a) or carbachol (b,c). n = 6–10 experiments per condition; *P < 0.001 versus all other conditions. Inset in b, Erk2 immunoblot. Scale bar in c, 20 μm. (d) Representative longitudinal heart sections of wild-type (WT) mice and transgenic mice overexpressing Erk2T188D, Erk2T188T and Erk2T188S 6 weeks after TAC versus controls. Scale bar, 2 mm. (e) Heart weight (HW) to body weight (BW) ratio of WT mice and transgenic mice overexpressing Erk2T188D, Erk2T188S, Erk2T188T or Erk2T188A. n = 10–14 mice per group; *P < 0.001 versus WT (+ TAC) and Erk2T188T (+ TAC). (f) Histological sections of left ventricular myocardium from WT mice and Erk2T188D, ERK2T188S, Erk2T188T or Erk2T188A transgenic mice. Sections were stained with H&E or Sirius Red. Scale bars, 50 μm. (g) M-mode echocardiography. Scale bars represent 500 ms (x axes) or 1 mm (y axes). (h,i) Dose-response curves for dp / dtmax (h) and dp / dtmin (i) after dobutamine infusion, determined by measurements of left ventricular pressures in WT and Erk2T188D-mutant mice after TAC. n = 8–12 mice per group; P < 0.05 versus WT. (j) HW to tibia length ratios of WT and Erk2T188S mice without or with angiotensin II treatment (left) and WT, Erk2T188D and Erk2T188T mice without or with carbachol treatment (right) for 14 d. n = 5–10 mice per group; *P < 0.001 versus WT and Erk2T188T.
To assure the specificity of all Erk2T188 mutants used in this study, we verified that they were not altered in their kinase abilities, their sensitivity to upstream stimulation or their Gβγ-binding properties (Supplementary Fig. 4 online). The amounts of co-precipitated Gβγ and kinase activity (as determined by myelin basic protein phosphorylation assays) were indistinguishable between hemagglutinin (HA)-tagged wild-type Erk2 and the various tagged Erk2T188 mutants precipitated from transfected HEK293 cells, and increased to similar extents after stimulation of endogenous M3 receptors32 (Supplementary Fig. 4a,b). Gβγ-dependent inositol phosphate production (a readout of Gβγ function) was similarly suppressed by the various Erk2T188 mutants (Supplementary Fig. 4c). These data indicate that, with the exception of their phosphorylation status at Thr188, the Erk2 mutants behaved normally.
The importance of Thr188 phosphorylation of Erk1/2 for the development of cardiac hypertrophy in vivo was then tested in several transgenic mouse lines with cardiac-specific expression of the following proteins: wild-type Erk2 (Erk2T188T), Erk2T188A and Erk2T188S (phosphorylation-deficient Erks), and Erk2T188D (gain-of-function Erk); wild-type FVB/N mice served as an additional control (Supplementary Fig. 5a–c online). TAC was again used to induce hypertrophy (Supplementary Fig. 5d), and mice were then analyzed by gross cardiac morphology and histology and by echocardiography and cardiac catheterization (Fig. 3d–i and Supplementary Figs. 5 and 6 online). No abnormalities were detected without TAC (Fig. 3d,e and Supplementary Figs. 5e–n and 6). TAC led to an increase in morphological and echocardiographic wall thickness, heart weight (Fig. 3d–g and Supplementary Fig. 5e,f,l) and cardiomyocyte size (Fig. 3f and Supplementary Fig. 5j). These changes were significantly more pronounced in Erk2T188D-expressing lines and less pronounced in Erk2T188A-expressing and Erk2T188S-expressing lines than in control (wild-type and Erk2T188T-expressing) lines. In addition, the extent of hypertrophy correlated with induction of the marker proteins atrial natriuretic factor and brain natriuretic peptide (Supplementary Fig. 5n–p) and with interstitial fibrosis (Fig. 3f and Supplementary Fig. 5k,m).
Fractional shortening and indices of left ventricular contractility (dp / dtmax) and relaxation (dp / dtmin) were reduced by TAC only in Erk2T188D-expressing mice, indicating cardiac dysfunction (Fig. 3g–i and Supplementary Figs. 5g and 6a). These effects became more prominent when mouse hearts were stimulated with dobutamine (Fig. 3h,i and Supplementary Fig. 6a). Of note, cardiac function was not depressed in Erk2T188A-expressing and Erk2T188S-expressing mice (Supplementary Figs. 5g and 6b,d). In addition, Erk2T188S expression attenuated the hypertrophic response to application of angiotensin II for 14 d (Fig. 3j and Supplementary Fig. 7a–e online).
Furthermore, as in cardiomyocytes (Fig. 3b,c), the Erk2T188D mutation converted carbachol-induced M2 receptor stimulation for 14 d into a hypertrophic stimulus in Erk2T188D-expressing mice (Fig. 3j, Supplementary Fig. 7f–l online). Although carbachol treatment induced no hypertrophy in control mice (wild-type or Erk2T188T-expressing mice), it increased heart weight, wall thickness and atrial natriuretic factor and brain natriuretic peptide expression in Erk2T188D-expressing mice (Fig. 3j and Supplementary Fig. 7f,g,k,l).
Notably, under basal conditions and under TAC conditions, Erk1/2 phosphorylation at the TEY motif, Erk1/2 activities toward MBP and Gβγ co-immunoprecipitation with Erk1/2 were similar in the hearts of all mice tested (whether overexpressing wild-type Erk2 or Erk2T188 mutants) (Supplementary Fig. 8a–e online). Only when Erk1/2 in lysates was maximally phosphorylated with purified Mek* before the MBP activity assays were performed did Erk2-transgenic mice show higher Erk activities correlating with the degree of Erk2 overexpression (Supplementary Fig. 8f). These results suggest that total Erk1/2 activity in the heart might be limited by upstream signals rather than by the Erk1/2 expression level. Furthermore, these results indicate that the phenotype of ErkT188D-expressing mice is not due to altered kinase activity or Gβγ binding.
Taken together, the data obtained in the transgenic mice indicate that Thr188 phosphorylation has a key role in Erk1/2-mediated cardiac hypertrophy. Blockade of Thr188 phosphorylation inhibited cardiac hypertrophy, whereas simulation of Thr188 phosphorylation enhanced TAC-induced hypertrophy or caused hypertrophy when combined with a normally nonhypertrophic M2 receptor stimulus.
Gβγ binding requires the entire activated MAPK cascade
Because upstream activation of the MAPK cascade by a hypertrophic stimulus was essential for Gβγ interactions with Raf1 or Erk1/2, for Thr188 phosphorylation and for the prohypertrophic phenotype of Erk2T188D transgenic mice, we further analyzed the role of the upstream kinases in regulating the interaction of Gβγ and Erk1/2.
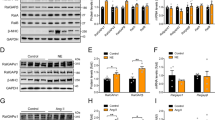
Immunoprecipitation of HA-tagged Raf1 from HEK293 cells co-precipitated not only Gβγ but also Erk1/2 after stimulation of Gq-coupled M3 receptors32 (Fig. 4a). Further experiments revealed that Gβγ binding to proteins of the MAPK cascade depends on the formation of a complex that includes all the members of the MAPK cascade. Silencing endogenous Raf1 expression by RNA interference or inhibition of MEK1/2 markedly reduced immunoprecipitation of Gβγ with Erk1/2 (Fig. 4b,c). In line with these observations, inhibition of the MAPK cascade by silencing Raf1, applying a MEK inhibitor, PD98059, or overexpressing dominant-negative versions of Mek1 and Erk2 (Mek1K97M (refs. 33,34) and Erk2T183A,Y185A (ref. 33), respectively) all prevented Gβγ binding to the MAPK cascade, as reflected by increased Gβγ-dependent inositol phosphate formation after carbachol stimulation (Fig. 4d–f). These results suggest that Gβγ acts as a scaffold for the entire MAPK cascade.
(a) Immunoblot of Gβ, ERK1/2 and HA-Raf1 (Raf1) after Raf1 was immunoprecipitated with HA-specific antibodies (anti-HA) from cell lysates of HEK293 cells treated with carbachol for 5 min. (b) Immunoblot showing carbachol-stimulated (5 min) interaction of Erk1/2 with Gβ by co-immunoprecipitation of Gβ with anti-ERK1/2 before and after gene silencing of Raf1. (c) Immunoblot showing carbachol-stimulated (5 min) interaction of HA-Raf1 with Gβ assessed by co-immunoprecipitation before and after inhibition of Mek1/2 activity by PD98059 (50 μM). (d–f) Carbachol-stimulated (50 μM, 15 min) inositol phosphate signaling after downregulation of endogenous Raf1 by RNA interference (d), inhibition of endogenous Mek activity by PD98059 (left) or by expression of the dominant-negative Mek1K97M (right) (e) or expression of the dominant-negative Erk2T183A,Y185A mutant (dn-Erk2) (f) compared to control cells. The decrease in Raf1 protein level by small interfering RNA to Raf1 (siRaf1) was confirmed by immunoblotting with Raf1-specific antibodies (d, inset, anti-Raf). (g,h) Interactions of HA-tagged wild-type Erk2 (AA1–358, where AA stands for 'amino acids'), truncated Erk2 mutants (AA1–318, AA22–358 and AA22–318) and dimerization-deficient Erk2Δ174–177 with Gβγ as assessed by co-immunoprecipitation (g) and carbachol-stimulated inositol phosphate signaling (h) in HEK293 cells. Inset, Erk2 immunoblot. (i) Immunoprecipitation of HA-tagged Erk2T188T followed by analyses of co-immunoprecipitates with Flag-tagged Erk2T188T, Erk2T188D, Erk2T188S or Erk2Δ174–177 after carbachol stimulation. (j) Two-dimensional phosphopeptide mapping of Erk2Δ174–177 after incubation with either Gβγ or Mek*. Phosphopeptides resulting from trypsin digestion of Erk2Δ174–177 are designated P1 and P2. n = 3–10 experiments per group; *P < 0.01 versus the respective controls (d,e,f,h). All individual experiments were reproduced at least three times with similar results.
Gβγ binding and Thr188 phosphorylation require ERK dimers
To further delineate the mechanisms of Gβγ binding and Thr188 phosphorylation, we analyzed the Gβγ-Erk2 interface by investigating the binding of Erk2 truncation mutants to Gβγ in co-immunoprecipitation studies and by measuring Gβγ-dependent inositol phosphate formation. These experiments showed that the Erk2 kinase domain (amino acids 22–318) is essential for the interaction of Erk2 with Gβγ (Fig. 4g,h).
The kinase domain is also crucial for homodimerization of Erk1/2 (ref. 33). To test whether Erk1/2 dimerization might have a role in Gβγ binding and Thr188 phosphorylation, we examined dimer formation by using HA-tagged wild-type Erk2 and Flag-tagged Erk2 constructs (wild-type ERK2T188T, Erk2Δ174–177, Erk2T188S and Erk2T188D). Co-precipitation assays showed that stimulation of Gq-coupled M1 muscarinic receptors (Fig. 4i) and Gi-coupled M2 muscarinic receptors (data not shown) induced the formation of dimers of Erk2 with the Erk2T188 mutants but not with the known dimerization-deficient Erk2Δ174–177 mutant.
Thr188 phosphorylation might occur as an intermolecular event: incubation of Erk2 with Gβγ yielded phosphopeptides with either Thr188 or Tyr185 phosphorylation but not with phosphorylation on both sites (Fig. 1d). This result is compatible with intermolecular phosphorylation in an Erk2 dimer of an inactive Erk2 molecule by a Tyr185-phosphorylated Erk2 molecule. Indeed, incubation of Erk2Δ174–177 with Mek* produced phosphopeptides P1 and P2, but incubation of Erk2Δ174–177 with Gβγ failed to produce P3, indicating a lack of Thr188 phosphorylation (Fig. 4j). These findings suggest that Erk2 dimerization might be necessary for Gβγ binding and for Thr188 phosphorylation.
Thr188 phosphorylation promotes nuclear Erk localization
To investigate the downstream effects of Thr188 phosphorylation that might cause cardiac hypertrophy, we determined the phosphorylation of Erk1/2 targets in the Erk2T188-mutant mouse lines (Fig. 5a,b). Elk1, mitogen- and stress-activated protein kinase-1 (MSK1), c-Myc and p90 ribosomal S6 kinase (p90RSK) are relevant Erk1/2 targets in cardiac myocytes35,36,37,38,39. The basal level of phosphorylation of the Erk1/2 targets (p90RSK at Ser380, p70S6K at Thr421 and Ser424, Elk1 at Ser383, MSK1 at Thr581, c-Myc at Thr58 and Ser62) was similar for all Erk2T188-mutant mouse lines (data not shown). TAC increased the level of phosphorylation of the cytosolic proteins p90RSK (at Ser380) and p70S6K (at Thr421 and Ser424)36,39,40 similarly in all lines (Fig. 5b), suggesting that Thr188 phosphorylation did not affect the phosphorylation of cytosolic Erk1/2 substrates.
(a,b) Immunoblot analyses of heart lysates of WT mice and Erk2T188-transgenic mouse lines for measuring phosphorylation of the nuclear Erk1/2 targets Elk1, MSK1 and cMyc (a) and of cytosolic p90RSK and p70S6K (b) after 6 weeks of TAC compared to age-matched WT mice without TAC. n = 4 mice per group. (c) Immunohistochemical detection of phospho-Elk1 in heart sections of control and TAC-operated Erk2T188D-, Erk2T188T- and Erk2T188S-transgenic mice with phospho-Elk1–specific antibodies and subsequent DAB staining (brown). The nuclei were counterstained with hematoxylin (violet). Some cardiomyocyte nuclei are marked with arrowheads. (d,e) Ratios of nuclear to cytosolic distribution (d) and fluorescence imaging (e) of YFP-tagged Erk2T188T, Erk2T188D and Erk2T188S without and with carbachol stimulation. COS-7 cells were co-transfected with either M1 receptors (Gq-coupled; d) or M2 receptors (Gi-coupled; d,e). n = 7–39; *P < 0.001. (f) Localization of Erk2 in heart sections of T188D (line 6), T188T (line 12) and T188A (line 14) transgenic mice (see Supplementary Fig. 5a–c) before and 6 weeks after TAC using Erk2-specific antibodies followed by DAB staining. Some cardiomyocyte nuclei are marked with arrowheads. (g) Immunohistochemical localization of Thr188-phosphorylated (pErk (Thr188)) and canonically phosphorylated (pErk (TEY)) Erk1/2. Some cardiomyocyte nuclei are marked with arrowheads. All scale bars, 20 μm.
In contrast, simulation of Thr188 phosphorylation had pronounced effects on nuclear targets of Erk1/2 (Elk1 at Ser383, MSK1 at Thr581 and c-Myc at Thr58 and Ser62) (Fig. 5a,c). TAC increased the level of phosphorylation of these ERK1/2 targets modestly in control hearts (wild-type and Erk2T188T-expressing mice) and massively in Erk2T188D-expressing hearts; however, TAC barely altered the phosphorylation of these targets in Erk2T188S-expressing and Erk2T188A-expressing hearts (Fig. 5a,c). These data parallel the hypertrophic effects of the ErkT188 mutants (Fig. 3) and suggest that Thr188 phosphorylation specifically increases phosphorylation of nuclear Erk1/2 targets.
Confocal microscopy of the subcellular distribution of yellow fluorescence protein (YFP)-tagged Erk2T188 mutants in COS1 cells strengthened this hypothesis (Fig. 5d,e). Basal nuclear-to-cytosolic ratios of YFP fluorescence were similar for all Erk2T188 mutants(Fig. 5d,e). However, stimulation of co-transfected Gq-coupled M1 receptors induced nuclear localization of wild-type Erk2 and Erk2T188D, whereas the nuclear-to-cytosolic distribution of Erk2T188S was unaffected (Fig. 5d). In contrast, after stimulation of Gi-coupled receptors, only Erk2T188D localized to the nucleus, whereas both wild-type Erk2 and Erk2T188S remained unaffected (Fig. 5d,e).
Likewise, immunohistochemistry in sections of Erk2T188-expressing hearts showed that TAC caused pronounced nuclear Erk2T188D localization, whereas this change in localization was modest in wild-type hearts and nearly absent in Erk2T188A-expressing hearts (Fig. 5f). In parallel, nuclear Elk1 phosphorylation after TAC was marked in sections of Erk2T188D-expressing hearts, modest in wild-type hearts and weak in Erk2T188S-expressing hearts (Fig. 5c). Finally, immunohistochemical detection of phosphorylated Erk1/2 forms showed essentially nuclear localization of Thr188-phosphorylated Erk1/2, whereas TEY-phosphorylated Erk1/2 was both cytosolic and nuclear (Fig. 5g).
Taken together, these data show that Thr188 phosphorylation (in the presence of a stimulus that induces phosphorylation of the TEY motif) triggers nuclear localization of Erk1/2 and phosphorylation of nuclear targets such as Elk1 that are known to initiate cardiac hypertrophy35,36.
Thr188 phosphorylation of Erk1/2 is a sustained process
If Thr188 phosphorylation is important for cardiac hypertrophy, it should persist over a long period of time. Indeed, Thr188 phosphorylation occurred more slowly and was sustained for a longer period of time than was phosphorylation at the TEY motif, as seen in HEK293 cells treated with a M3 receptor agonist for up to 60 min (Fig. 6a), in mouse hearts for up to 4 months after TAC (Fig. 6b) or in mouse hearts up to 14 d of angiotensin II treatment (data not shown). In all three cases, TEY phosphorylation reached a maximum and then declined (Fig. 6a,b) or oscillated (data not shown). In contrast, Thr188 phosphorylation showed a steady, continuous increase over a long period of time (Fig. 6a,b and data not shown), consistent with a role in the long-term process of cardiac hypertrophy.
(a,b) Time courses of Thr188 (pErk1/2 (Thr188)) versus canonical (pErk (TEY)) Erk1/2 phosphorylation. HEK293 cells were transfected with HA-tagged Erk2 and stimulated with carbachol (100 μM). n = 3 experiments; *P < 0.05 compared to unstimulated control (a); or WT mice were subjected to TAC. n = 5 mice per group; *P < 0.05 compared to control mice (b). (c) Activation of the MAPK cascade induces phosphorylation of ERK1/2 within the TEY motif. Subsequent dimerization of ERK1/2 in conjunction with a Gβγ-releasing stimulus (specifically, activation of G protein–coupled receptors (GPCRs) coupled to Gq proteins) leads to direct interaction of Gβγ with ERK1/2 and causes intermolecular autophosphorylation of ERK1/2 at Thr188. Gβγ binding and Thr188 phosphorylation of ERK1/2 is also mediated via receptor-tyrosine kinases (RTK) via a mechanism that might also involve Gq proteins. Phosphorylation of Thr188 stimulates nuclear localization of ERK1/2, nuclear ERK1/2 target phosphorylation and ERK1/2-mediated hypertrophy. Activation of the MAPK cascade by GPCRs coupled to Gi proteins does not lead to direct Gβγ binding to ERK1/2 and, therefore, does not induce Thr188 phosphorylation.
Discussion
The MAPK cascade integrates various signaling components to elicit multiple biological responses11,41. Canonical activation of ERK1/2 requires dual phosphorylation at the TEY motif by MEK1/2. The signaling specificity of ERK1/2 is thought to be achieved primarily via protein-protein interactions, which direct its activity toward specific targets. To our knowledge, modifications of ERK1/2 other than TEY phosphorylation have not previously been shown to produce specific biological responses.
We have discovered a mechanism by which a protein-protein interaction between ERK1/2 and Gβγ results in a modification of ERK1/2—Thr188 phosphorylation—that directs ERK1/2 toward nuclear targets. This mechanism (Fig. 6c) depends on upstream signals—specifically activation of Gq-coupled receptors, which release Gβγ, activation of the entire Raf-MEK-ERK cascade and subsequent phosphorylation of ERK1/2 within the TEY motif, and ERK dimerization. The integration of these signaling events leads to autophosphorylation of ERK2 at Thr188 (Thr208 in ERK1). This autophosphorylation is of a sustained nature, suggesting that it is resistant to dephosphorylation.
The precise roles of ERK1 and ERK2 in cardiac hypertrophy are still unclear6,9,35,36,40. We found that Thr188 phosphorylation is causally and specifically involved in the induction of cardiac hypertrophy in vitro and in vivo, directing ERK1/2 to the nucleus and leading to the phosphorylation of substrates such as Elk1, MSK1 and c-Myc. These proteins have all been shown to be involved in cardiomyocyte hypertrophy36,37,38. Notably, Thr188 phosphorylation does not affect the overall kinase activity of ERK1/2. Thus, ERK1/2 integrates upstream signals by which phosphorylation at different sites has differing effects on ERK1/2 (phosphorylation on Thr183 and Tyr185 induces its kinase activity; phosphorylation on Thr188 targets it to the nucleus). Only when both types of phosphorylation occur do ERK1 and ERK2 cause pronounced cardiac hypertrophy. A distinct role of Thr188 phosphorylation in cardiac hypertrophy is suggested by the findings that Mek1S217E,S221E-transgenic mice develop compensated hypertrophy with no pathological consequences6, whereas Erk2T188D-transgenic mice showed both hypertrophy and pathological changes associated with contractile dysfunction under stress in our study.
Spatio-temporal regulation of ERK1/2 is a crucial determinant for triggering specific biological responses to extracellular signals42,43, and translocation of activated ERK1/2 to the nucleus is essential for growth factor–induced gene expression and cell cycle entry41,42,43,44. Our data suggest that the integration of canonical ERK1/2 activation signals with Gq-mediated activation of ERK1/2 can lead to its accumulation in the nucleus. However, it is not yet clear whether this nuclear accumulation of ERK1/2 is due to increased nuclear translocation or to nuclear retention of ERK1/2. In line with earlier work33,45, dimerization of ERK1/2 is a prerequisite for direct Gβγ-ERK1/2 interaction and subsequent Thr188 phosphorylation.
Appropriate upstream signals are crucial for Gβγ recruitment and subsequent Thr188 phosphorylation. Many G protein–coupled receptors can activate the MAPK cascade, but only Gq-coupled receptors cause cardiac hypertrophy24,25,26,27,40. Likewise, Gβγ binding and Thr188 phosphorylation can be triggered by Gq- but not Gi-coupled receptors. Given that the different Gβγ subunits are biochemically quite similar46, this specificity may stem from compartmentalization of signaling molecules or from different activation modes, as has been postulated for the specificity of Gβγ-regulated ion channels47. Of note, neuregulin and the mechanical stress caused by TAC can also recruit Gβγ to the MAP kinase cascade and thereby initiate Thr188 phosphorylation, suggesting a new and important link between different signaling cascades.
Our results show that Thr188 phosphorylation initiates hypertrophy in cardiomyocytes and is present in hypertrophied mouse hearts and in failing human hearts. Transgenic mice expressing Erk2T188D, which mimics constitutive Thr188 phosphorylation, develop excessive cardiac hypertrophy after TAC and even after treatment with the nonhypertrophic stimulus carbachol, whereas mice expressing Erk2 lacking this phosphorylation site show an attenuated hypertrophic response. It will be useful to test whether Thr188 phosphorylation also occurs in other proliferative responses that involve ERK1/2. This mechanism, which seems crucial for maladaptive cardiac hypertrophy, may also be fundamental to many other hypertrophic and proliferative disease states.
Methods
Myelin basic protein phosphorylation assay in vitro.
We determined Erk2 kinase activity by incubating purified His6-Erk2 in 20 mM HEPES, pH 7.2, 2 mM EDTA, 100 μM ATP and 10 mM MgCl2 with 1 μM Erk2 in the presence or absence of purified Gβγ (500 nM)30,45 or His6-Mek1* (ref. 22) (2.5 nM). After 5 min of preincubation, we added [γ-32P]ATP (and 7.5 μg MBP per 50 μl) and let the reaction proceed for another 5 min at 30 °C.
Two-dimensional phosphopeptide mapping of recombinant Erk2.
We incubated purified His6-Erk2, His6-Erk2T188A, His6-Erk2T188S, His6-Erk2Δ174–177 or His6-Erk2Y185F (1 μM) with Gβγ subunits (500 nM)30 in the presence or absence of His6-Mek1* (5 nM) in 20 mM HEPES, pH 7.2, 2 mM EDTA, 10 μM ATP, 10 mM MgCl2 and 100 μM [γ-32P]ATP for 20 min at 30 °C. We separated proteins by SDS-PAGE, transferred them to a polyvinylidene fluoride membrane and visualized them with Ponceau S (Sigma). We then digested His6-Erk2, His6-Erk2T188A, His6-Erk2T188S, His6-Erk2Δ174–177 or His6-Erk2Y185F with trypsin (Promega) before separating the phosphopeptides by two-dimensional phosphopeptide mapping via electrophoresis (first dimension) and thin-layer chromatography (second dimension) as previously described30.
Antibodies used for immunodetection of proteins.
We used the following antibodies for immunoprecipitation (IP), immunoblotting (IB) or immunohistochemistry (IHC): antibodies to Flag (F3165, Sigma; IP, IB), HA (clone 12CA5, Roche; IP), HA (Hiss Diagnostics; IB), phospho-MSK1 (Thr581) (9595, Cell Signaling; IB), MSK1 (sc-25417, Santa Cruz; IB), phospho-cMyc (Thr58 and Ser62) (9401, Cell Signaling; IB), cMyc (9402, Cell Signaling; IB), phospho-p90RSK (RSK1) (Ser380) (9341, Cell Signaling; IB), ribosomal S6 kinases RSK1, RSK2 and RSK3 (9347, Cell Signaling; IB), phospho-Elk1 (Ser383) (9186, Cell Signaling (IB); 90121-1, Imgenex (IHC)), Elk1 (9182, Cell Signaling; IB), p70S6K (Thr421 and Ser424) (9204, Cell Signaling; IB), p70S6K (9202, Cell Signaling, IB), Gβ (sc-378, Santa Cruz; IP, IB), Raf1 (R19120, BD Biosciences; IP, IB), MEK1 (sc-18, Santa Cruz; IP, IB), ERK1/2 (9102, Cell Signaling; IP, IB), phospho-ERK1/2 (9101, Cell Signaling; IB, IHC), Erk2 antibodies raised against recombinant His6-Erk2 (Biogenes; IB, IHC) and phospho-ERK (Thr188) antibodies. We raised phospho-ERK1/2 (Thr188)-specific antibodies against a synthetic peptide corresponding to residues 181–195 of mouse Erk2. After removing peptide-specific antibodies using a CNBr-sepharose column containing unphosphorylated peptide, we isolated phosphospecific antibodies with a column containing the immunogenic, phosphorylated peptide (FLTEYVA-(p)T-RWYRAPE-amide conjugated to Limulus polyphemus hemocyanine (Biogenes)). In experiments using this phospho-ERK1/2 (Thr188)-specific antibody, we always included unphosphorylated peptide (∼7.5 μg ml−1) to ensure elimination of residual nonphosphospecicific antibodies. We prepared cell lysates and heart lysates from transgenic Erk2 mice as described in the Supplementary Methods online.
Immunoblot detection of phospho-Thr188 in vitro.
For detection of phospho-Erk2 (Thr188) in vitro, we incubated His6-Erk2 or His6-Erk2T188A without or with ATP (10 μM) and Gβγ (500 nM)30 in the presence or absence of His6-Mek1* (10 nM)33,34 in 20 mM HEPES, pH 7.2, 2 mM EDTA and 10 mM MgCl2 for 20 min at 30 °C. We separated proteins by urea SDS-PAGE followed by immunoblot analysis.
Human heart samples.
In accordance with the Declaration of Helsinki, written informed consent had been obtained from all participants or the family of prospective heart donors before cardiectomy. The Ethical Committee of the Würzburg Medical Faculty, Germany, approved the experiments.
Transgenic mice.
Care of the mice was in accordance with the Committee on Animal Research of the regional government (Regierung von Unterfranken, Germany) reviewed and approved all experimental protocols (reference number Az 54-2531.01-62/06). See the Supplementary Methods for details.
Osmotic minipumps.
We implanted osmotic minipumps (Alzet) containing angiotensin II (200 ng g−1 h−1; Bachem) or carbachol (1 μg g−1 d−1; Sigma) subcutaneously in wild-type (FVB/N background) mice (Harlan) and transgenic ERK2T188-mutant mice (T188S, T188D, T188T) for a period of 14 d. We used ketamine (150 μg g−1) plus xylazine (0.5 μg g−1) for anesthesia during surgical implantation of the minipumps. For assessment of the angiotensin II- or carbachol-induced development of cardiac hypertrophy, we performed echocardiography before and 14 d after implantation and measured heart weights and tibia lengths 14 d after implantation.
Echocardiography.
We anesthetized mice intraperitoneally with pentobarbital (35 mg g−1) and placed them on a heated platform (42 °C) to maintain body temperature. We obtained transthoracic echocardiograms with a Vevo 770 high-resolution imaging system (VisualSonics) equipped with a 30-MHz probe (RMV-707B). We obtained two-dimensional M-mode images in the short axis view at the proximal level of the papillary muscles to determine the end-diastolic and end-systolic internal diameters (LVIDED and LVIDES) and end-diastolic septal and posterior wall thicknesses. We calculated fractional shortening (FS) by VisualSonics Cardiac Measurements software as follows: FS (%) = [(LVIDED – LVIDES) / LVIDED] × 100. We measured peak blood flow velocities at the site of TAC (Vmax [mm s−1]) by Doppler ultrasound, and we calculated aortic pressure gradients (PG in mm Hg) by the formula PG = 4 × (Vmax / 1,000)2.
Immunohistochemistry.
We dewaxed and rehydrated 2-μm sections, incubated them in methanol containing 0.3% H2O2 for 60 min and subsequently microwaved them in 10 mM citric acid (pH 6.0) for 20 min at 700 W. After washing in 0.1 M phosphate buffer with 0.25% Triton X-100 (TPBS), we blocked the sections in TPBS supplemented with 5% normal goat serum for 2 h at 25 °C. We then applied primary antibodies in TPBS supplemented with 5% normal goat serum in dilutions (antibodies to Erk2, phospho-Erk (Thr188), phospho-Erk (TEY) or phospho-Elk1 (Ser383)) overnight at 4 °C. We detected the primary antibodies with biotinylated goat antibody to rabbit IgG followed by incubation with Vectastain Elite ABC Reagent (Vector ABC Elite kit). We then rinsed the tissue and stained it with diaminobenzidine (DAB)–glucose oxidase. We performed counterstaining of the nuclei with hematoxylin. As a control, we omitted the primary antibodies.
Statistical analyses.
We evaluated statistical significance by analysis of variance and used Bonferroni's test as post-hoc test, with P < 0.05 regarded as significant. We show all data as means ± s.e.m. We reproduced individual experiments at least three times. All histological, echocardiographic and confocal evaluations were done in a blinded fashion.
Additional methods.
Description of plasmids and detailed methodology are provided in Supplementary Methods.
Note: Supplementary information is available on the Nature Medicine website.
References
Sugden, P.H. & Clerk, A. Cellular mechanisms of cardiac hypertrophy. J. Mol. Med. 76, 725–746 (1998).
Wettschureck, N. et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat. Med. 7, 1236–1240 (2001).
Lips, D.J., deWindt, L.J. & van Kraaij, D.J.W. & Doevendans, P.A. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur. Heart J. 24, 883–896 (2003).
Muslin, A.J. Role of Raf proteins in cardiac hypertrophy and cardiomyocyte survival. Trends Cardiovasc. Med. 15, 225–229 (2005).
Glennon, P.E. et al. Depletion of mitogen-activated protein kinase using an antisense oligodeoxynucleotide approach downregulates the phenylephrine-induced hypertrophic response in rat cardiac myocytes. Circ. Res. 78, 954–961 (1996).
Bueno, O.F. et al. The MEK1–ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 19, 6341–6350 (2000).
Takeishi, Y. et al. Src and multiple MAP kinase activation in cardiac hypertrophy and congestive heart failure under chronic pressure-overload: comparison with acute mechanical stress. J. Mol. Cell. Cardiol. 33, 1637–1648 (2001).
Haq, S. et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 103, 670–677 (2001).
Purcell, N.H. et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 104, 14074–14079 (2007).
Rossomando, A.J. et al. Identification of Tyr-185 as the site of tyrosine autophosphorylation of recombinant mitogen-activated protein kinase p42mapk. Proc. Natl. Acad. Sci. USA 89, 5779–5783 (1992).
Seger, R. & Krebs, E.G. The MAPK signalling cascade. FASEB J. 9, 726–735 (1995).
Luttrell, L.M. 'Location, location, location': activation and targeting of MAP kinases by G protein–coupled receptors. J. Mol. Endocrinol. 30, 117–126 (2003).
Caunt, C.J., Finch, A.R., Sedgley, K.R. & McArdle, C.A. Seven-transmembrane receptor signalling and ERK compartmentalization. Trends Endocrinol. Metab. 17, 276–283 (2006).
Torii, S., Nakayama, K., Yamamoto, T. & Nishida, E. Regulatory mechanisms and function of ERK MAP kinases. J. Biochem. 136, 557–561 (2004).
Tohgo, A. et al. The stability of the G protein–coupled receptor–β-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 278, 6258–6267 (2003).
Crespo, P., Xu, N., Simonds, W.F. & Gutkind, J.S. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature 369, 418–420 (1994).
Slupsky, J.R. et al. Binding of Gβγ subunits to cRaf1 downregulates G protein-coupled receptor signalling. Curr. Biol. 9, 971–974 (1999).
Faure, M., Voyno-Yasenetskaya, T.A. & Bourne, H.R. cAMP and βγ subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J. Biol. Chem. 269, 7851–7854 (1994).
Schwindinger, W.F. & Robishaw, J.D. Heterotrimeric G protein βγ dimers in growth and differentiation. Oncogene 20, 1653–1660 (2001).
Tachibana, H., Naga Prasad, S.V., Lefkowitz, R.J., Koch, W.J. & Rockman, H.A. Level of β-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation 111, 591–597 (2005).
Li, Z. et al. Effects of two Gβγ-binding proteins—N-terminally truncated phosducin and β-adrenergic receptor kinase C terminus (βARKct)—in heart failure. Gene Ther. 10, 1354–1361 (2003).
Mansour, S.J. et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265, 966–970 (1994).
Schmitt, J.P. et al. Consequences of pressure overload on sarcomere protein mutation–induced hypertrophic cardiomyopathy. Circulation 108, 1133–1138 (2003).
Thomas, W.G. et al. Adenoviral-directed expression of the type 1A angiotensin receptor promotes cardiomyocyte hypertrophy via transactivation of the epidermal growth factor receptor. Circ. Res. 90, 135–142 (2002).
Brown, J.H. et al. Pathways and roadblocks in muscarinic receptor–mediated growth regulation. Life Sci. 60, 1077–1084 (1997).
Sakata, Y., Hoit, B.D., Liggett, S.B., Walsh, R.A. & Dorn, G.W. II. Decompensation of pressure-overload hypertrophy in Gαq-overexpressing mice. Circulation 97, 1488–1495 (1998).
Akhter, S.A. et al. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 280, 574–577 (1998).
Rohrbach, S. et al. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation 100, 407–412 (1999).
Baliga, R.R. et al. NRG-1–induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am. J. Physiol. 277, H2026–H2037 (1999).
Lorenz, K., Lohse, M.J. & Quitterer, U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature 426, 574–579 (2003).
Umemori, H. et al. Activation of the G protein Gq/11 through tyrosine phosphorylation of the α subunit. Science 276, 1878–1881 (1997).
Luo, J., Busillo, J.M. & Benovic, J.L. M3 muscarinic acetylcholine receptor–mediated signaling is regulated by distinct mechanisms. Mol. Pharmacol. 74, 338–347 (2008).
Khokhlatchev, A.V. et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93, 605–615 (1998).
Hartkamp, J., Troppmair, J. & Rapp, U.R. The JNK/SAPK activator mixed lineage kinase 3 (MLK3) transforms NIH 3T3 cells in a MEK-dependent fashion. Cancer. Res. 59, 2195–2202 (1999).
Bogoyevitch, M.A. & Sudgen, P.H. The role of protein kinases in adaptational growth of the heart. Int. J. Biochem. Cell. Biol. 28, 1–12 (1996).
Bueno, O.F. & Molkentin, J.D. Involvement of extracellular signal–regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ. Res. 91, 776–781 (2002).
Zhong, W. et al. Hypertrophic growth in cardiac myocytes is mediated by Myc through a cyclin D2–dependent pathway. EMBO J. 25, 3869–3879 (2006).
Markou, T., Hadzopoulou-Cladarasb, M. & Lazou, A. Phenylephrine induces activation of CREB in adult rat cardiac myocytes through MSK1 and PKA signaling pathways. J. Mol. Cell. Cardiol. 37, 1001–1011 (2004).
Valks, D.M. et al. Phenylephrine promotes phosphorylation of Bad in cardiac myocytes through the extracellular signal–regulated kinases 1/2 and protein kinase A. J. Mol. Cell. Cardiol. 34, 749–763 (2002).
Dorn, G.W. II & Force, T. Protein kinase cascades in regulation of cardiac hypertrophy. J. Clin. Invest. 115, 527–537 (2005).
Garrington, T.P. & Johnson, G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11, 211–218 (1999).
Chang, L. & Karin, M. Mammalian MAP kinase signalling cascades. Nature 410, 37–40 (2001).
Pouysségur, J., Volmat, V. & Lenormand, P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem. Pharmacol. 64, 755–763 (2002).
Brunet, A. et al. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor–induced gene expression and cell cycle entry. EMBO J. 18, 664–674 (1999).
Cobb, M.H. & Goldsmith, E.J. Dimerization in MAP kinase signaling. Trends Biochem. Sci. 25, 7–9 (2000).
Müller, S., Heckman, M. & Lohse, M.J. Specific enhancement of β-adrenergic receptor kinase activity by defined Gβ and Gγ subunits. Proc. Natl. Acad. Sci. USA 90, 10439–10443 (1993).
Frank, M., Thümer, L., Lohse, M.J. & Bünemann, M. G protein activation without subunit dissociation depends on Gαi-specific region. J. Biol. Chem. 280, 24584–24590 (2005).
Acknowledgements
We wish to thank U. Quitterer for stimulating discussions during the early phase of this work; M. Maier-Peuschel, M. Vidal, K. Deiß, D. Calebiro, K. Hadameck, M. Frank, K. von Hayn and A. Zürn for their help; M. Fischer, M. Babl, M. Hoffmann, N. Ziegler and C. Dees for excellent technical assistance; S. Jacobs for help with the immunohistochemistry; and U. Zabel and R. Schreck for helpful discussion. This work was supported by the Deutsche Forschungsgemeinschaft, the Leducq Foundation and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Contributions
K.L. performed all of the experiments and their analyses; J.P.S. performed the aortic banding surgery and provided advice for echocardiography; E.M.S. generated the transgenic mouse lines; and M.J.L. and K.L. conceived the study and wrote the manuscript. E.M.S. and particularly J.P.S. provided critical comments on the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–8 and Supplementary Methods (PDF 762 kb)
Rights and permissions
About this article
Cite this article
Lorenz, K., Schmitt, J., Schmitteckert, E. et al. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med 15, 75–83 (2009). https://doi.org/10.1038/nm.1893
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1893
This article is cited by
-
FGF23 and klotho at the intersection of kidney and cardiovascular disease
Nature Reviews Cardiology (2024)
-
HDAC5 inhibition attenuates ventricular remodeling and cardiac dysfunction
Orphanet Journal of Rare Diseases (2023)
-
Alteration in tyrosine phosphorylation of cardiac proteome and EGFR pathway contribute to hypertrophic cardiomyopathy
Communications Biology (2022)
-
The potential of remdesivir to affect function, metabolism and proliferation of cardiac and kidney cells in vitro
Archives of Toxicology (2022)
-
Dual Specific Phosphatase 7 Exacerbates Dilated Cardiomyopathy, Heart Failure, and Cardiac Death by Inactivating the ERK1/2 Signaling Pathway
Journal of Cardiovascular Translational Research (2022)