Abstract
Tauopathies, including frontotemporal dementia (FTD) and Alzheimer's disease (AD), are neurodegenerative diseases in which tau fibrils accumulate. Recent evidence supports soluble tau species as the major toxic species. How soluble tau accumulates and causes neurodegeneration remains unclear. Here we identify tau acetylation at Lys174 (K174) as an early change in AD brains and a critical determinant in tau homeostasis and toxicity in mice. The acetyl-mimicking mutant K174Q slows tau turnover and induces cognitive deficits in vivo. Acetyltransferase p300-induced tau acetylation is inhibited by salsalate and salicylate, which enhance tau turnover and reduce tau levels. In the PS19 transgenic mouse model of FTD, administration of salsalate after disease onset inhibited p300 activity, lowered levels of total tau and tau acetylated at K174, rescued tau-induced memory deficits and prevented hippocampal atrophy. The tau-lowering and protective effects of salsalate were diminished in neurons expressing K174Q tau. Targeting tau acetylation could be a new therapeutic strategy against human tauopathies.
Similar content being viewed by others
Main
Neurodegenerative tauopathies include brain disorders such as Pick's disease, FTD and progressive supranuclear palsy (primary tauopathies) and secondary tauopathies such as AD1,2. Tauopathies are characterized by neurofibrillary tangles (NFTs) of insoluble hyperphosphorylated tau fibrils. Although accumulation and distribution of NFTs has been used for diagnostic and staging purposes3, recent evidence supports a key pathogenic role of soluble tau oligomers4,5. Increased levels of tau oligomers have been detected in the frontal cortex of AD patients at early Braak stages4,6. Injection of recombinant tau oligomers, but not fibrils, impairs memory in non-transgenic mice7. In mouse models of tauopathy, levels of tau oligomers correlate with memory loss at various ages8. Moreover, suppression of soluble tau improves cognitive function without reducing levels of insoluble NFTs in inducible mouse models of tauopathy9,10.
Both autophagic and proteasomal degradation pathways have been implicated in the tau clearance11,12. In AD brains, tau oligomers appear to be ubiquitinated and to accumulate at synapses, where proteasome components are enriched13. Tau undergoes lysine acetylation14,15, which modulates diverse biological processes, including protein stability and protein-protein interactions16. Analysis of the soluble fraction of AD brain lysates shows that tau acetylation is enhanced in patients at early and moderate Braak stages, which is consistent with an early role for acetylation in modulating tau accumulation14. Acetylation, but not phosphorylation17, regulates aggregation of recombinant tau in vitro15,18. Moreover, inhibition of the deacetylase SIRT1 elevates tau acetylation, suppresses its polyubiquitination and slows its turnover in primary neurons14. However, it is unclear which lysine residue(s) are aberrantly acetylated in AD brains and whether they are key determinants of tau homeostasis in vivo.
Here we identify acetylation of tau at K174 (ac-K174) as an early critical pathological change to soluble tau in AD brains using mass spectroscopy and a newly developed antibody specific for ac-tau. We further establish a critical role of K174 in tau homeostasis and toxicity, and we test the effects of the prescription drug salsalate, which inhibits p300-induced tau acetylation, on tau-mediated behavioral deficits and neurodegeneration in PS19 transgenic mice, an FTD model.
Results
Tau is acetylated at K174 in human AD and tauopathy mice
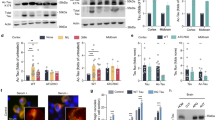
To identify acetylated lysines on soluble tau, we used mild detergents to extract lysates from human AD brains. Tau was immunoprecipitated from soluble AD lysates and subjected to proteolytic digestion and analysis by high-performance liquid chromatography–electrospray tandem mass spectrometry (HPLC-ESI-MS/MS). K174 on the native peptide 171-IPAKTPPAPK-180 was identified to be acetylated (Fig. 1a), and this was confirmed with a synthetic peptide of the same sequence (Fig. 1b). To further characterize ac-K174 in AD brains, we generated a rabbit polyclonal antibody, AC312, with an antigen containing ac-K163 and ac-K174 (ac-K163 was included on the basis of previous in vitro studies14,15). Incubation of a 441-residue recombinant human tau (hTau, variant 2N4R) with the acetyltransferase p300 led to strong AC312-positive signals in western blot analyses, which were nondetectable without p300 (Fig. 1c). A pan-tau antibody Ab707 (ref. 14) detected comparable levels of tau with or without p300, suggesting that AC312 is specific for ac-tau. To determine the specificity of AC312 for different ac-K residues in tau, we transfected human embryonic kidney (HEK293) cells with wild-type (WT) or non-acetylatable mutant (KR) human tau (hTau) constructs with or without p300 coexpression. AC312 detected a strong p300-induced ac-tau signal in cells expressing WT hTau and various KR mutants except for K174R, indicating that AC312 is specific for ac-K174, although the peptide antigen contains ac-K163 (Fig. 1d).
(a,b) ESI-MS/MS spectra of ac-K in peptide 171-IPAKTPPAPK-180 obtained after trypsin digestion of immunoprecipitated tau from AD brains (a) and acetyl-lysine containing synthetic peptide IPAKacTPPAPK (b). The corresponding Protein Pilot confidence scores were 99 (a,b); Kac*, marker ion at 126.1 for acetyllysine immonium ion identification. (c) Immunoblot analysis of recombinant tau in the presence or absence of p300 showing specificity of AC312. Ab707 detects total tau (ref. 14). (d) Immunoblot analysis of t-tau and ac-tau in lysates from HEK293 cells expressing tau mutants. (e,f) Analysis of AC312-positive ac-tau (K174) in AD brains (Braak stages 1–5). (e) Immunoblot analysis of tau-5 immunoprecipitates (IP) with AC312 and PHF-1 antibodies. (f) Levels of ac-K174 in AD brains at various Braak stages; n = 7 patients at Braak stage 0, n = 13 patients (Braak stages 1–2), n = 8 patients (Braak stages 3–5). *P < 0.05, one-way ANOVA, Tukey-Kramer post hoc analysis. Data are means ± s.e.m. See Supplementary Table 1 for the patient information. (g–j) AC312 immunoreactivity in the hippocampus of PS19 mice. (g) Merged image of AC312 and MC1 immunostaining at low magnification; scale bar, 100 μm. (h–j) High-magnification confocal images showing expression of AC312 (h) and MC1 (i) tau and their colocalization (j). Scale bars, 25 μm. Images are representative of two independent experiments.
We next assessed K174 acetylation in AD brains at different Braak stages (Supplementary Table 1). Braak staging is a well-established method that correlates cortical tau pathology progression with the worsening of clinical symptoms3. Human brain lysates were partially purified on a column with an antibody to tau (tau-5) to reduce nonspecific signal. Immunoblot analysis with the tau-5 antibody showed similar concentrations of tau in each sample (Fig. 1e). In contrast, AC312 immunoreactivity was weak or nondetectable in brain lysates at Braak stage 0 and stronger in lysates at early (stages 1–2) and later (3–5) Braak stages (Fig. 1e,f). Phosphorylated tau (p-tau) positive for PHF-1, which recognizes p-tau at S396 and S404, was more readily detected at later Braak stages. These findings suggest ac-K174 is among the early pathogenic alterations of tau in AD.
We examined AC312 immunoreactivity in PS19 transgenic mice, which express hTau with the P301S mutation under the mouse prion promoter and develop cognitive and synaptic deficits related to hippocampal function19. The specificity of AC312 immunoreactivity was confirmed in nontransgenic (Mapt+/+) and tau-deficient (Mapt−/−) mice (Supplementary Fig. 1a). In PS19 mice at age 6 months, when early pathological changes start to appear19, pathological hTau was detected with MC1, an antibody specific for a pathogenic conformation of tau that precedes formation of tangles20,21. AC312 and MC1 immunoreactivity was colocalized along the mossy fiber of the hippocampus (Fig. 1g–j and Supplementary Fig. 1b).
K174Q slows tau turnover and promotes accumulation
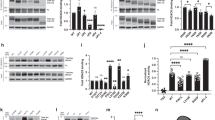
Augmentation of tau acetylation via SIRT1 inhibition slows tau degradation in cultured neurons in vitro14. To determine whether ac-K174 is important in slowing tau turnover, we replaced K174 with glutamine (K174Q), which neutralizes the positive charge and mimics the acetylated state. We used a K174R mutant to mimic deacetylated tau. Both mutants are commonly used to assess the effects of acetylation22,23,24. To measure tau half-life, we infected rat primary neurons with lentiviral vectors expressing WT, K174Q or K174R hTau, and quantified hTau levels in the presence of the translation inhibitor cycloheximide. K174Q hTau had a longer half-life than K174R or WT hTau (Fig. 2a). As polyubiquitination on K174 should be blocked by both K174Q and K174R, acetylation of K174, not blockage of polyubiquitation alone, seems to slow tau degradation.
(a) Primary neurons were infected with lenti-WT, K174Q or K174R hTau for 4 d before cycloheximide (CHX) treatment. Left, representative immunoblot of WT, K174Q or K174R hTau in primary neurons treated with CHX for 0–32 h. Right, quantification of WT, K174Q or K174R hTau relative to GAPDH. n = 15 (WT), n = 8 (K174Q), n = 8 (K174R) from four independent experiments. *P < 0.05; ***P < 0.001, one-way ANOVA, Tukey-Kramer post hoc analysis. Values are mean ± s.e.m. (b,d) Representative images from 8–15 mice showing HT7 immunostaining of hippocampus 10 d after injection of AAV-WT and K174Q hTau (b) or AAV-K174Q and AAV-K174R hTau (d); scale bars, 500 μm. (c,e) Quantification of hTau mRNA 3 months after infection with AAV1 vectors. GAPDH was used as internal control. n = 10 (WT) and 12 (K174Q) mice, Mann-Whitney nonparametric test (c); n = 9 (for K174R and K174Q), unpaired Student's t-test (e); values are mean ± s.e.m. (f,g) Representative immunoblot from 11–12 mice with HT7 (f) and quantification (g) of tau monomers and putative dimers in hippocampus injected with AAV-WT or AAV-K174Q hTau. n = 11 (WT), n = 12 (K174Q). ***P < 0.001, unpaired Student's t-test; values are mean ± s.e.m. (h,i) Representative immunoblot with HT7 (h) and quantification (i) of monomeric tau and putative tau dimers in hippocampus injected with AAV-K174Q or AAV-K174R hTau. n = 9 mice per group. *P < 0.05, unpaired Student's t-test; values are mean ± s.e.m. (j) Left, tau aggregation kinetics of recombinant hTau assayed by thioflavin T fluorescence following the addition of heparin. r.f.u., relative fluorescence units. Right, amplitude of curve obtained from parameters fit to the Gompertz equation showing higher propensity of aggregation of K174Q and K174R tau than WT tau. **P < 0.01, one-way ANOVA, Tukey-Kramer post hoc analysis from three independent experiments. KQ, K174Q tau; KR, K174R tau. Values are mean ± s.e.m.
We next determined the effects of ac-K174 on tau accumulation in vivo by injecting adeno-associated virus type 1 (AAV1) vectors expressing WT (AAV-WT), K174Q (AAV-K174Q) and K174R (AAV-K174R) hTau constructs into the hippocampi of 2- to 3-month-old non-transgenic (NTG) C57BL/6 mice. Tau overexpression is known to induce toxicity in the hippocampus, which can be reliably targeted with AAV1 vectors. We compared K174Q to WT and K174R. Immunostaining with an anti-hTau antibody, HT7, showed similar distributions of hTau throughout the hippocampus 10 d after injection for all three constructs (Fig. 2b,d). Similar levels of hTau transduction were maintained 3 months after injection for all three constructs, as determined by quantitative reverse-transcription PCR (qRT-PCR) (Fig. 2c,e). However, western blot analysis of hippocampal lysates revealed that concentrations of monomeric K174Q tau were higher than those of WT (Fig. 2f,g) or K174R tau (Fig. 2h,i). In agreement with the effects of acetylation on the half-life of tau in vitro, these results indicate that ac-K174 acetylation, not the blockage of polyubiquitination, leads to monomeric tau accumulation in vivo. In addition, AAV-K174Q expression led to a marked increase in tau signal at ∼140 kD, which is probably dimeric tau (Fig. 2f,g and Supplementary Fig. 2). Notably, infection with AAV-K174R tau led to an increase of tau dimers similar to that seen with AAV-K174Q (Fig. 2h,i), supporting the importance of K174, but not its acetylation, in tau aggregation. Indeed, recombinant K174Q and K174R tau showed a higher propensity for aggregation than WT tau in vitro, as indicated by the time-dependent increase of thioflavin T signal, which identifies tau aggregates with β-sheet structures (Fig. 2j).
Acetylation of the KXGS motif suppresses tau phosphorylation at S262 and S356 (p-S262/S356) of KIGS18. K174Q expression via AAV infection in hippocampus did not significantly affect acetylation of KIGS motif (Supplementary Fig. 3) or levels of p-S262/S356 (unpaired Student's t-test) (Supplementary Fig. 4a,b). Levels of other p-tau species, including p-T231, p-T181 or p-S396 were also not significantly affected by K174Q expression (Supplementary Fig. 4c–e). Notably, K174Q expression led to higher levels of AT8-positive p-tau (S202/T205) than did WT or K174R tau expression in hippocampus (Supplementary Fig. 4f–i).
K174Q tau worsens neurodegeneration and behavioral deficits
To determine how ac-K174 affects toxicity of overexpressed hTau, we examined mouse hippocampal volumes 3 months after injection of AAV-WT, AAV-K174Q and AAV-K174R hTau. As reported previously, AAV-induced expression of WT hTau led to a loss of hippocampal volume25. K174Q hTau induced more severe hippocampal atrophy than did WT and K174R hTau (Fig. 3a,b), suggesting that ac-K174 induces more severe neurodegeneration. To assess the functional outcome of high levels of ac-K174 in the hippocampus, we first used an open-field assay, as hippocampal lesions induce hyperlocomotion26. Additionally, we used the small Y-maze spontaneous-alternation task to evaluate hippocampal-dependent spatial working memory and the Morris water maze (MWM) to assess spatial learning and memory, which are known to be impaired in tauopathy mouse models27.
(a,b) Representative Nissl staining (left) and volume (right) of hippocampus from NI mice (n = 7) or mice injected with AAV-WT hTau (WT, n = 7) or AAV-K174Q hTau (KQ, n = 7) (a) or NI mice (n = 10) or mice injected with KQ (n = 9) or AAV-K174R hTau (KR, n = 9) (b). ***P < 0.001, *P < 0.05, one-way ANOVA, Tukey-Kramer post hoc analysis. Scale bars, 500 μm; values are mean ± s.e.m. (c,e) Hippocampal expression of K174Q hTau leads to hyperactivity in the open-field (OF) test (c) and fewer spontaneous alternations in the Y-maze (e). n = 9 (NI), 9 (WT), 9 (KQ); n = 10 (NI), 8 (KQ) and 9 mice (KR). *P < 0.05, one-way ANOVA, Dunnett's post hoc analysis. Values are mean ± s.e.m. (d) Pearson correlation analysis of the extent of hyperactivity in the OF and the levels of tau monomers in mice injected with AAV-K174Q hTau (KQ, n = 18). (f–i) Effects of hippocampal expression of K174Q, WT and K174R hTau on spatial learning as assessed by MWM (f,h) and probe trials (g,i). R1–4: reversal training day 1–4; n = 9 mice per condition (f,g) or 10 (NI), 9 (KQ) or 9 (KR) mice (h,i). *P < 0.05, **P < 0.01, multilevel mixed-effects linear regression model (the naive and reversal phases were analyzed separately) (f,h). **P < 0.01, ***P < 0.001, two-way ANOVA, Bonferroni post-test (g). Values are mean ± s.e.m. AU, arbitrary units; avg, average.
In the open-field test, mice injected with AAV-K174Q showed significantly higher total activity than noninjected (NI) mice, whereas mice expressing comparable levels of WT or K174R hTau behaved similarly to NI mice (Fig. 3c). Notably, the levels of K174Q tau monomers were positively correlated with total activity in the open field (Fig. 3d). In small Y-maze tests, hippocampal expression of K174Q but not WT or K174R hTau significantly reduced the percentage of spontaneous alternations (Fig. 3e), suggesting that ac-K174 tau impairs spatial working memory. In MWM tests, mice expressing K174Q hTau learned more slowly than NI mice and mice expressing WT and K174R hTau in the first phase of spatial learning (Fig. 3f,h). After all groups were trained to criterion, we performed reversal training by moving the hidden platform to a new location. Mice expressing K174Q took longer to find the platform in its new location than did NI controls and mice expressing WT or K174R hTau (Fig. 3f,h). These findings demonstrate that ac-K174 impairs spatial learning. In probe trials, in which the platform is removed from the MWM to measure memory retention, mice expressing K174Q hTau but not WT hTau crossed the target platform location significantly fewer times than did NI control mice, suggesting that K174Q expression impairs memory retention (Fig. 3g). A direct comparison of the effects of K174R and K174Q expression on memory retention was inconclusive, as the NI mice in this cohort showed no clear memory retention (Fig. 3i).
Inhibiting p300 reduces ac-K174 and total tau
The main acetyltransferases responsible for tau acetylation are p300 and its close homolog CBP14,15. To further examine the role of p300, we deleted its encoding gene Ep300 by infecting primary mouse neurons expressing loxP-flanked Ep300 (p300F/F) with lentivirus expressing Cre recombinase in culture (Fig. 4a). Deletion of p300 lowered levels of total tau (t-tau), ac-K174 and p-tau (S202/T205) (Fig. 4b). Levels (relative to t-tau) of ac-K174 and AT8-positive p-tau were also reduced by p300 deletion (Fig. 4c). Pharmacological inhibition of p300 with C646 reduces levels of p-tau and ac-tau in primary neurons14; however, owing to its poor brain permeability and pharmacokinetic (PK) properties (data not shown), we tested other small molecules that inhibit p300, including salicylate, a nonsteroidal anti-inflammatory drugs (NSAIDs) (K.S. and E.V., unpublished data). In HEK293 cells, salicylate reduced acetylation of histone H2A at K5 (H2AK5), a well-established substrate of p300, in a dose-dependent manner (Supplementary Fig. 5a). In rat primary neurons infected with a lentiviral vector carrying WT hTau (lenti-WT), salicylate reduced levels of p300 (Fig. 4d). Levels of ac-K174 (Fig. 4d) and AT8-positive p-tau (Fig. 4d) were also reduced, relative to t-tau levels, by salicylate treatment. Consistent with the idea that ac-K174 inhibits tau degradation, reduction of ac-K174 with salicylate enhanced tau turnover in primary neurons (Supplementary Fig. 5b).
(a–c) Immunoblot analysis (a) and quantification (b,c) of p300, ac-tau, p-tau and t-tau expression in lysates from primary mouse neurons derived from p300F/F pups and infected with lentivirus expressing WT hTau (lenti-WT), lenti-control (empty vector) or lenti-Cre virus. For quantification, levels in nontreated cells were set to 1. n = 11 samples per group from three independent experiments; ***P < 0.001, **P < 0.01, unpaired Student's t-test (ac-tau) or Kruskal-Wallis test (p-tau). Values are mean ± s.e.m. (d) Immunoblot analysis and quantification of p300, ac-tau, p-tau and t-tau in rat primary cortical neurons at 12 d in vitro (DIV 12) infected with lenti-WT tau and treated with 0, 5 or 10 mM salicylate for 24 h. Levels in nontreated cells were set to 1. n = 4 samples from four independent experiments. *P < 0.05, **P < 0.01, one-way ANOVA, Tukey-Kramer post hoc analysis. Values are mean ± s.e.m. (e) Immunoblot analysis and quantification of ac-H3K18 and H3 in cortical histone extract from 10- to 11-month-old NTG and PS19 littermates. Levels in NTG group were set to 1. n = 9 mice per group; **P < 0.01, unpaired Student's t-test. Values are mean ± s.e.m. (f–h) Immunoblot analysis and quantification of ac-H3K18 and H3 (f; n = 11 mice (male) per group), ac-H2AK5 and H2A (g; n = 8 mice (female) per group) or ac-H2BK12K15 and H2B (h; n = 11 mice (male) per group) in mice treated with vehicle or SSA. Levels in vehicle-treated group were set to 1. **P < 0.01, *P < 0.05, unpaired Student's t-test. Values are mean ± s.e.m. (i) ELISA quantification of t-tau protein (left) and qRT-PCR analysis of t-tau mRNA (right) in mouse cortex after treatment with SSA or vehicle. Levels in vehicle-treated group were set to 1. n = 11 (10- to 11-month-old, male) mice per condition; *P < 0.05, unpaired Student's t-test. ns, not significant. Values are mean ± s.e.m. (j) Left, immunoblot analysis of ac-tau (AC312), p-tau (AT8) and t-tau (tau-5) in PS19 mice treated with SSA or vehicle; vehicle-treated NTG and tau-knockout (KO) mice were included as controls. Right, quantification of ac-tau (left) and p-tau relative to t-tau (middle) or GAPDH (right) in the soluble hippocampal lysates from PS19 mice treated with vehicle or SSA. Levels in vehicle-treated group (veh) were set to 1. n = 8 (10- to 11-month-old, female) mice per condition, **P < 0.01, *P < 0.05, unpaired Student's t-test. Values are mean ± s.e.m.
To examine the potential protective effects of p300 inhibition against tauopathy in vivo, we first sought to determine whether p300 is aberrantly activated in the brains of PS19 mice. H3K18 acetylation has been used as an activity marker for p300 and its close homolog, CREB-binding protein (CBP), as deletion of p300 and CBP leads to a complete loss of ac-H3K18 (refs. 28,29,30). Levels of ac-H3K18 in the brain were significantly higher in PS19 mice than in age-matched nontransgenic (NTG) controls (Fig. 4e). To inhibit p300 and CBP in the brain, we treated PS19 mice with salsalate (SSA), a prodrug of salicylate used to treat rheumatoid arthritis. The results of PK analysis showed that, upon absorption, SSA penetrates the brain and gives rise to relatively stable salicylate levels over 8 h (Supplementary Fig. 6).
We investigated the effects of SSA treatment in PS19 mice after the onset of tau-mediated deficits in spatial memory. We tested two independent cohorts: one cohort of 8- to 9-month-old female PS19 mice and NTG controls and one of 7- to 8-month-old male PS19 mice and NTG controls. The male and female mice were tested separately to avoid confounding effects of sex hormones on the other gender in behavior tests. Mice were given SSA (225 mg per kg of body weight (mg/kg)) or vehicle by oral gavage. The length of the treatment was 60 d for the female cohort and 84 d for the male cohort. The dose of 225 mg/kg was well tolerated; no weight difference was observed in vehicle- and SSA-treated PS19 mice during treatment (data not shown). Inhibition of brain p300 was assessed by measurement of acetylation of well-established substrates for p300 and CBP29,30,31, including ac-H3K18, ac-H2AK5 and ac-H2BK12K15, all of which were reduced significantly by SSA treatment (Fig. 4f–h). These results demonstrated clear target engagement of SSA in the brain when administered at 225 mg/kg.
SSA treatment also reduced the levels of t-tau, as quantified with ELISA, but did not affect levels of tau mRNA (Fig. 4i). SSA did not affect acetylation or phosphorylation of the KXGS motif (Supplementary Fig. 7), consistent with a lack of effect induced by K174Q. Notably, SSA induced a reduction in ac-K174 relative to t-tau and a trend of reduction in AT8-positive p-tau, which did not reach significance when normalized to t-tau (Fig. 4j).
SSA ameliorates pathology and memory deficits in PS19 mice
We next determined whether inhibition of ac-K174 tau ameliorates tau-mediated pathological and behavioral deficits. PS19 mice show hippocampal atrophy at advanced ages32. In the female cohort, hippocampal volume was similar in PS19 and NTG controls mice at age 8 months (Fig. 5a,b). At 10 months of age, vehicle-treated PS19 mice showed significant hippocampal atrophy, as compared to NTG controls; SSA treatment restored hippocampal volumes of 10-month-old PS19 mice to those of 8-month-old NTG and PS19 mice (Fig. 5a,b). Similarly, male SSA-treated PS19 mice also showed significantly (P = 0.0038) less hippocampal atrophy at 10 months of age (Supplementary Fig. 8a,b). Prominent NFTs and dystrophic neuritis, positive for Gallyas silver staining, were detected throughout the forebrains in PS19 mice (Fig. 5c). SSA reduced the number of Gallyas silver–positive neurons and neurites in the cortex and hippocampus of PS19 mice, in both the female (Fig. 5c,d), and the male cohorts (Supplementary Fig. 8c,d).
(a,b) Nissl staining (a) and volume (b) of hippocampus from 8-month-old untreated (NT) NTG and PS19 mice,10 month-old NTG mice treated with vehicle (veh) and 10-month-old PS19 mice treated with vehicle or SSA. Scale bars, 500 μm. n = 6 NTG NT and 9 PS19 NT (8 months old); n = 9 NTG veh, 6 PS19 veh, and 6 PS19 SSA mice (10–11 months old); **P < 0.01, one-way ANOVA, Tukey-Kramer post hoc analysis. Values are mean ± s.e.m. (c,d) Analysis of NFTs by Gallyas silver staining (c) and quantification of silver-positive cells in neuritis from neocortex and hippocampal regions CA1 and CA3 (d) in vehicle-treated NTG mice and PS19 mice treated with vehicle or SSA. Scale bar, 250 μm (c, top); 25 μm (c, middle and bottom); n = 8 mice (age 10–11 months) per genotype per treatment; *P < 0.05, unpaired Student's t-test. Values are mean ± s.e.m. (e–h) Effects of SSA treatment on spatial memory loss in NTG and PS19 mice as assessed by fixed-location dry maze (e,f) and MWM (g,h). Visible, training with visible hole (days 1–2); hidden, learning with hidden hole (days 3–5). n = 10 (NTG veh), 8 (PS19 veh) or 8 (PS19 SSA) 10- to 11-month-old mice (e,f); n = 13 (NTG veh), 15 (NTG SSA) 11 (PS19 veh) or 10 (PS19 SSA) 9- to 10-month-old mice (g,h). The multilevel mixed-effects linear regression model was used to assess the learning curve (e,g). ***P < 0.001, **P < 0.01, *P < 0.05, paired Student's t-test; values are mean ± s.e.m.
To assess SSA's effects on tau-mediated deficits in spatial learning and memory, we used the MWM or a fixed-location dry maze, a modified Barnes maze that measures spatial learning and memory but is less physically demanding than the MWM33. The male cohort was tested in the MWM. The female cohort was tested in the dry maze, as female mice were slightly older and appeared physically weaker than those in the male cohort. Mice in the fixed-location dry maze learned to locate the escape hole using spatial cues in the spatial learning segment. We observed no significant differences in learning rates among vehicle-treated NTG mice and vehicle- or SSA-treated PS19 mice (Fig. 5e). In the probe trial, vehicle-treated NTG mice spent more time in the target quadrant than in the non-target quadrant, whereas vehicle-treated PS19 mice had no preference for the target quadrant, showing impaired memory retention and retrieval (Fig. 5f). SSA-treated PS19 mice spent more time in the target quadrant, showing improved spatial memory. Similarly, in the MWM, no spatial learning deficits were observed in the four groups (Fig. 5g). In the probe trial, vehicle-treated PS19 mice had no preference for the target quadrant (Fig. 5h). In contrast, SSA-treated PS19 mice showed preferences for the target quadrant similar to that of vehicle- or SSA-treated NTG mice, confirming SSA's protective effects against tau-mediated memory impairment.
Ac-K174 inhibition mediates effects of salicylate or SSA
To investigate the mechanism underlying the effects of salicylate on tau, we examined primary rat neurons infected with AAV-WT or AAV-K174Q hTau and treated with salicylate. In neurons expressing WT (Fig. 6a,b) or K174Q hTau (Fig. 6d,e), levels of p300 and mouse t-tau were similarly reduced, suggesting that salicylate induced a similar extent of p300 inhibition. However, although salicylate lowered levels of WT hTau (Fig. 6c), it did not reduce those of K174Q hTau (Fig. 6f), suggesting that salicylate's tau-lowering effect requires inhibition of ac-K174 in primary neurons.
(a–f) Immunoblot analysis (a,d) and quantification (b,c,e,f) of p300 and t-tau in cultured rat primary cortical neurons infected with AAV-WT or AAV-K174Q tau and treated with 5 mM salicylate (SS) or vehicle (veh) for 24 h (DIV 12). Levels in veh-treated cells were set to 1. n = 4 samples from two independent experiments. ***P < 0.001, **P < 0.01, unpaired Student's t-test; values are mean ± s.e.m. (g) Left, immunoblot analysis of ac-H2AK5, H2A, p-tau (AT8 and PHF-1), t-tau (HT7) and GAPDH in mice infected with AAV-K174Q and treated with SSA or vehicle; right, quantification of ac-H2AK5 and H2A. n = 11 (vehicle) or 10 (SSA) mice; *P < 0.05, unpaired Student's t-test; values are mean ± s.e.m. (h) ELISA analysis of K174Q tau after SSA treatment. n = 11 (vehicle) or 10 (SSA) mice; ns, not significant; P = 0.3647; unpaired Student's t-test; values are mean ± s.e.m. (i) Levels of PHF-1–positive p-tau normalized to HT7 (left) or AT8-positive p-tau normalized to HT7 (middle) or GAPDH (right) after SSA treatment. n = 11 (vehicle) or 10 (SSA) mice; ns, not significant, unpaired Student's t-test. Values are mean ± s.e.m. (j) Nissl staining (left) and hippocampal volume (right) in mice expressing K174Q tau after SSA treatment. Scale bars, 500 μm; n = 11 (vehicle) or 10 (SSA) mice; ns, not significant, unpaired Student's t-test. Values are mean ± s.e.m.
We next determined whether SSA's beneficial effects involve inhibition of ac-K174 in vivo. NTG C57BL/6 mice were injected with AAV-K174Q; beginning two months after injection, mice were treated daily with SSA for one month. Ac-H2AK5 levels were reduced in the SSA-treated group compared to vehicle-treated controls, confirming SSA's inhibitory effect on p300 (Fig. 6g). However, in contrast to our results in PS19 mice, SSA treatment failed to reduce levels of t-tau in AAV-K174Q–injected mice, as compared to vehicle-treated controls (Fig. 6h). Levels of PHF-1–positive p-tau relative to t-tau or of AT8-positive p-tau normalized to that of t-tau or GAPDH, were also not affected by SSA treatment (Fig. 6i). Moreover, SSA did not prevent or reduce hippocampal atrophy in AAV-K174Q–injected mice (Fig. 6j), supporting the importance of ac-K174 inhibition in SSA's protective effects.
Discussion
Here we report a critical role for ac-K174, an early pathological modification of soluble tau in AD brains, in tau accumulation and toxicity. The acetyl-mimic tau mutant K174Q degrades more slowly than WT tau or K174R tau, which mimics the non-acetylatable form. Hippocampal expression of K174Q, but not WT or K174R tau, leads to accumulation of tau monomers that are associated with profound cognitive deficits related to AD. SSA inhibits p300, lowers ac-K174 levels in the brain and protects against behavioral deficits, hippocampal atrophy and accumulation of NFTs in aged PS19 mice. The tau-lowering and protective effects of salicylate and SSA were diminished in neurons and mice expressing K174Q tau. Our findings point to inhibitors of tau acetylation as a novel therapeutic modality to treat tauopathies.
Levels of ac-K174 were elevated at early Braak stages in AD brains, consistent with its role in early pathogenic alteration. In PS19 mice, ac-K174 colocalized with MC1, which is enriched in soluble tau in AD lysates and represents one of the earliest pathological tau conformations20,21. In addition to K174, other tau acetylation sites have been reported, including K274, K280 and K259, K290, K321 and K353 (KXGS motifs)15,18,34,35,36. Both ac-K274 and ac-K280 have been found to be specifically associated with insoluble, thioflavin-positive tau aggregates in AD and other tauopathies34,35,36. Although ac-K280 has been implicated in deficient tau-microtubule interactions and pathological tau aggregation in vitro15, its pathogenicity in vivo has not been established. Acetylation of the KIGS motif, which appears to be reduced in AD brains, suppresses tau aggregation and promotes microtubule binding in vitro18. However, we show here that ac-K174 has few effects on acetylation or phosphorylation of KXGS motifs, suggesting that different tau acetylation sites may exert distinct effects on tau pathogenecity.
Our findings establish the pathogenicity of ac-tau in vivo. Behavioral deficits, including hyperactivity and impairments in working memory and spatial learning, were observed in mice expressing K174Q tau, but not in mice expressing WT or K174R tau at similar levels. The positive correlation of cognitive deficits with monomeric K174Q tau levels provides evidence supporting the pathogenicity of ac-K174 monomers. Tau-mediated neurodegeneration is exacerbated by ac-K174, but hippocampal atrophy is not abolished in mice expressing K174R, indicating that other mechanisms could be involved. Indeed, K174Q and K174R mutants enhance the formation of dimeric tau, which could be neurotoxic. Aberrant cell-cycle reentry induced by high levels of hTau25 could also contribute to neurodegeneration in mice injected with AAV-K174Q or AAV-K174R. Our results also support a role of ac-K174 in tau homeostasis. Expression of K174Q, but not K174R, leads to longer half-life in primary neurons and aberrant accumulation in vivo, showing that ac-K174 acetylation, not the blockage of K174 polyubiquitination, impairs tau clearance. Notably, a recent mass-spectrometry study provided clear evidence that acetylation and ubiquitination occur on the same lysine residues, suggesting a close relationship between these two modifications37.
One of the main enzymes that acetylates tau is p300 (ref. 14), which can be inhibited by salicylate or SSA, an ancient drug commonly used as an NSAID. Pharmacokinetically, SSA is quickly metabolized into its active component, salicylate. Unlike salicylate, aspirin (acetylsalicylate) leads to higher levels of ac-tau in cultured neurons (data not shown). SSA and aspirin have been widely used to treat rheumatoid arthritis and related illnesses in the past decades, and work presumably via inhibition of cyclo-oxygenase (COX)38. Interestingly, patients taking NSAIDs, including salicylate and derivatives, have a reduced risk of AD39. However, later trials with more specific COX-2 inhibitors did not establish a protective effect against AD. One possible explanation is that the benefits of salicylate might involve mechanisms independent of COX-2 inhibition, such as partial attenuation of p300 activity, which was identified recently as one of the most prominently dysregulated networks in AD brains in a system proteomic study40.
The resistance of K174Q to the tau-lowering and protective effects of salicylate and SSA strongly supports inhibition of ac-K174 as a key underlying mechanism. Treatment with salicylate lowered levels of ac-tau and t-tau in primary neurons expressing WT tau, but not in those expressing K174Q tau. SSA lowered ac-K174 and t-tau and protected against hippocampal atrophy in PS19 mice but failed to reduce tau or restore hippocampal volume in AAV-K174Q–injected mice. Other mechanisms are likely to be involved as well. For example, salicylate or SSA may exert protection by enhancing autophagy via activation of adenosine monophosphate–activated protein kinase (AMPK)41. The marked reduction of NFTs induced by SSA could be due to activation of autophagy. However, we did not observe significant difference in autophagy activity, as measured by abundance of LC3-II, in brains from PS19 mice treated with SSA or vehicle (Supplementary Fig. 9). More comprehensive analyses are needed to fully address the involvement of autophagy. SSA-induced AMPK activation could also lead indirectly to p300 inhibition and degradation42,43. p300 is critical for nuclear factor-κB activation and glial inflammatory responses44,45. We examined microgliosis or astrogliosis and observed no effect from SSA treatment, which suggests that anti-inflammation is not one of the main protective mechanisms (Supplementary Fig. 10).
Our study provides proof-of-principle evidence that partial p300 inhibition could be used to treat tauopathies. Because p300 is important for many biological functions, completely inhibiting it could cause serious side effects. However, a recent study showed that p300 activity is aberrantly high in AD brains40. Identified as one of the most prominently dysregulated pathways in AD, aberrantly activated p300 could underlie hyperacetylation of tau in tauopathies40. In cases of moderate and severe AD, p300 activity has been found to be significantly higher than in non-demented controls in the CA1 region of the hippocampus, where activated p300 and p-tau are colocalized40. p300 activity was also elevated in aged PS19 brains. Thus, partial inhibition of p300 could normalize its aberrant activation and suppress hyperacetylation of its substrates, including tau. Indeed, the protection of SSA against tau-mediated cognitive deficits could have important clinical implications. Even after disease onset in PS19 mice, SSA treatment improved spatial memory, reduced NFT formation and abolished hippocampal atrophy, which is associated with increased risk for conversion from mild cognitive impairment to AD46,47,48. Notably, the dosage (225 mg/kg) used in our study is equivalent to ∼1,350 mg per day for a 75-kg person49, lower than the dose typically prescribed (3,000 mg per day). Our study supports clinical evaluation of SSA and its derivatives as therapies in human tauopathies.
Methods
Primary antibodies and other reagents.
Monoclonal antibodies were as follows: tau-5 (AHB0042, Life Technologies, 1:10,000), HT7 (MN1000, Thermo Scientific, 1:1,000), Tau12 (MAB2241, EMD Millipore), 12E8 (Prothena Biosciences), PHF-1 (a kind gift from P. Davies), MC-1 (a kind gift from P. Davies, 1:250), AT-8 (MN1020, Thermo Scientific), anti-GAPDH (MAB374, Millipore, 1:500), anti-GFAP (MAB3402, Millipore, 1:500), anti-CD68 (MCA1957GA, Serotec, 1:500), anti-LC3 (M152-3, MBL, 1:1,000), anti-histone H2B (#2934, Cell Signaling, 1:1,000). Polyclonal antibodies were as follows: anti-HA (#3724, Cell Signaling, 1:1,000), K48-linkage specific polyubiquitin antibody (#4289, Cell Signaling, 1:500), anti-KIGS antibody (a kind gift from L. Petrucelli, 1:1,000), anti-histone H3 (acetyl K18) antibody (ab1191, Abcam, 1:20,000), anti-histone H3 antibody (ab1791, Abcam, 1:100,000), anti-histone H2B (acetyl K12/K15, 1:500) antibody (ab1759, Abcam, 1:500), anti-histone H2A (acetyl K5) (ab1764, Abcam, 1:500), anti-histone H2A (ab18255, Abcam).
Expression plasmids. For expression in HEK293T cells, cDNA encoding WT, K163R, K174R, K274R, K280R, K369R, K174Q, or K274Q hTau was cloned into pcDNA3.1 vector (Invitrogen). For expression in primary neurons, cDNA encoding WT, K174Q or K174R hTau was cloned into lentiviral FUGW vectors.
Chemicals. MG-132, cycloheximide, and DMSO were from Sigma; IU1-47 was a kind gift from D. Finley. Salicylate (sodium salicylate) was purchased from Sigma-Aldrich. Salsalate tablet (75% purity) was from Amneal Pharmaceuticals (Glasgow, KY).
Human brain samples.
De-identified human brain samples were obtained from V. Haroutunian (The Icahn School of Medicine at Mount Sinai, NY, USA) or from W.W. Seeley (UCSF). Human brain samples were lysed with RIPA buffer and subject to immunoprecipitation, mass spectrometry and western blot. All studies on human samples were carried out according to the guidelines of the Committee on Human Research (CHR) at the University of California, San Francisco.
Immunoprecipitation for HPLC-ESI-MS/MS and western blots.
Immunoprecipitation was performed using Pierce Direct IP Kit (Thermo Scientific), essentially according to the manufacturer's protocol. Briefly, antibody (monoclonal anti-Tau; ab80579, Abcam) was immobilized onto agarose gel with sodium cyanoborohydride. Brain homogenates were then lysed in RIPA buffer, applied to antibody-conjugated column and rotated overnight at 4 °C. Samples were washed four times. Elution was performed in low pH elution buffer (pH 2.8), with neutralizing TRIS-HCl buffer (pH 9.5) in the collection tubes.
Electrospray ionization–quadrupole-quadrupole-time-of-ight (ESI-QqTOF) mass spectrometric analysis.
Gel bands were manually excised. Then, the gel spots were destained and dehydrated with acetonitrile. Subsequently, the proteins were reduced with 10 mM DTT at 60 °C for 30 min, alkylated with 100 mM iodoacetamide (37 °C, 45 min) and incubated with 250 ng sequencing grade trypsin (Promega) at 37 °C overnight. The proteolytic peptide mixtures obtained after tryptic digestion were analyzed by reversed-phase nano-HPLC-ESI-MS/MS with an Eksigent nano-LC 2D HPLC system (Eksigent), which was directly connected to a quadrupole time-of-flight (QqTOF) QSTAR Elite mass spectrometer (AB SCIEX). Briefly, peptides were applied to a guard column (Dionex) and washed with the aqueous loading solvent (2% solvent B in A, flow rate, 20 μL/min) for 10 min. Subsequently, samples were transferred onto the analytical C18-nanocapillary HPLC column (Dionex) and eluted at a flow rate of 300 nL/min for most samples with a linear gradient 2–80% solvent B in A over 85 min. Solvents were prepared as follows: mobile phase A, 2% acetonitrile + 98% 0.1% formic acid (v/v) in water; mobile phase B, 98% acetonitrile + 2% 0.1% formic acid (v/v) in water. Electrospray-mass spectra (ESI-MS) and tandem mass spectra (ESI-MS/MS) were recorded in positive-ion mode with a resolution of 12,000–15,000 full width at half maximum (FWHM). For collision-induced dissociation tandem mass spectrometry (CID-MS/MS), the mass window for precursor ion selection of the quadrupole mass analyzer was set to ±1 m/z. The synthetic acetylated peptide IPAKacTPPAPK (from ThermoFisher Scientific/Pierce Biotechnology, Rockford, IL) for mass spectrometric comparison was analyzed by reverse-phase HPLC-ESI-MS/MS with anEksigent Ultra Plus nano-LC 2D HPLC system connected to a quadrupole time-of-flight TripleTOF 5600 mass spectrometer (AB SCIEX) as described50. Database searches were performed using Protein Pilot Software 4.5 revision 1656 (AB SCIEX) using the Paragon Algorithm 4.5.0.0.1654 developed by AB SCIEX51 or an in-house Mascot server version 2.3.02 (ref. 52). Peptides containing posttranslational modifications were manually inspected based on an adaptation of previously published criteria.
Western blotting.
Cells or human or mouse brain tissues were homogenized in RIPA buffer containing protease inhibitor cocktail (Sigma), 1 mM phenylmethyl sulfonyl fluoride, phosphatase inhibitor cocktail (Sigma), 5 mM nicotinamide (Sigma), and 1 μM trichostatin A (Sigma). After sonication, lysates from human or mouse brain were centrifuged at 170,000g at 4 °C for 15 min and 18,000g at 4 °C for 15 min. Supernatants were collected, and protein concentrations were determined by the Bradford assay (Bio-Rad). The same amount of proteins were resolved on a 4–12% SDS-PAGE gel (Invitrogen), transferred to nitrocellulose membrane (GE Healthcare), and probed with appropriate antibodies. Bands in immunoblots were visualized by enhanced chemiluminescence (Pierce) and quantified by densitometry and ImageJ software (NIH).
Purification of GST fusion proteins and in vitro acetylation.
For purification of GST fusion proteins, h-tau 2N4R coding sequence was subcloned into pGEX-4T-1 bacterial expression vector (Sigma) and transformed in BL21 (DE3) strain. After induction with 100 μM isopropyl β–D-1-thiogalactopyranoside, cells were harvested and sonicated in PBS with 1 mM EDTA, 0.5% Triton X-100 and protease inhibitor cocktail (Sigma). GST-tagged tau or p300 proteins were purified by glutathione-agarose beads (Genscript). In vitro acetylation reactions were performed essentially as described53 with minor modifications. In brief, 1 μg of recombinant hTau, 2 nM acetyl-CoA (Sigma), and 100 ng of purified GST-p300 in acetylation buffer (50 mM HEPES, pH 8.0, 10% glycerol, 1 mM dithiothreitol (DTT), and 10 mM Na butyrate) were incubated for 2 h at 30 °C with constant shaking. Reactions were stopped by an addition of 2 × LDS sampling buffer (Invitrogen), followed by SDS-PAGE and western blot analysis.
Primary neuronal cultures and viral infections.
Mouse primary neurons were cultured from genetically modified pups. To generate p300F/F neurons, cortices of neonatal pups from p300F/F homozygotes breeding (p300F/F mice were a kind gift of P. Brindle) on postnatal day 0 or 1, and dissociated and plated as described previously52. All other experiments using primary neuronal cultures were derived from neonatal rats due to the higher yield. Briefly, cortices of Sprague-Dawley rat pups (Charles River Laboratories) were dissociated on postnatal day 0 or 1. Purified mouse or rat cells were plated at 160,000 cells/ml in neurobasal medium supplemented with B27 (Invitrogen) on poly-ornithine-coated plates. Lentivirus was generated, purified, and used for infection as described54. Recombinant lentivirus was produced by cotransfection of the shuttle vector (pRRL), two helper plasmids, delta8.9 packaging vector, and VSV-G envelope vector into 293T cells and purified by ultracentrifugation52. Viral titers were measured by p24 enzyme-linked immunosorbent assays at the Gladstone-UCSF Laboratory of Clinical Virology. AAV1-WT, AAV1-K174Q and AAV1-K174R tau with CMV–β-actin promoter were generated and purified by GENEDETECT (Auckland 0600, New Zealand). Primary neuronal cultures were infected with either equal amounts (∼3 × 107 – 5 × 107 PFU) of lentiviral vectors or AAV1 (∼5 × 108 genomic particles) expressing tau WT and mutants at 3–5 days in vitro (DIV 3–5). Once available, tau-expressing AAV1 vectors were used in later experiments as they exhibited less toxicity than lentiviral vectors. If necessary, viruses were diluted with conditioned medium for infection. All drug treatments and analyses were performed at DIV 8–11.
RNA isolation and quantitative reverse-transcription PCR.
Hippocampi from AAV-tau injected mice were dissected and homogenized with a 21G needle in RLT buffer with 1% β-mercaptoethanol. RNA was isolated with the RNeasy Mini-Kit (Qiagen), and the remaining DNA was removed by incubation with RNase-free DNase (Ambion). Purified mRNA was then converted to cDNA by the TaqMan reverse transcription (RT) kit (Applied Biosystems). Quantitative RT-PCR was performed on the ABI 7900 HT sequence detector (Applied Biosystems) with SYBR Green PCR master mix (Applied Biosystems). The average value of three replicates of each sample was expressed as the threshold cycle (Ct), at which the fluorescence signal starts to increase rapidly. Then, the difference (ΔCt) between the Ct value for h-tau and the Ct value for mouse GAPDH (ΔCt = Ct(human tau) – Ct(GAPDH)) was calculated for each sample. The relative levels of gene expression for each sample was determined by 2−ΔCt and expressed as fold change. The following primers were used for quantitative RT-PCR: h-tau (forward, 5′-GTTGGGGGACAGGAAAGATCAG-3′; reverse, 5′-CCGGGAGCTCCCTCATC-3′), mouse GAPDH (forward, 5′-GGGAAGCCCATCACCATCTT-3′; reverse, 5′-GCCTTCTCCATGGTGGTGAA-3′).
In vitro tau aggregation assay.
Site-directed mutagenesis was used to generate K174Q and K174R mutants of the 0N4R tau isoform in a pET28a vector. WT and mutant proteins were expressed in Escherichia coli and purified by heat denaturation followed by cation exchange chromatography55. For aggregation assays, tau proteins (10 μM) were incubated in assay buffer: D-PBS, pH 7.2 (Invitrogen), 2 mM MgCl2, 1 mM DTT. Heparin (Santa Cruz, MW 6,000–25,000) was reconstituted in assay buffer then added at a final concentration of 44 μg/ml to induce tau aggregation. The aggregation assay was carried out at 37 °C with agitation. When monitoring aggregation kinetics, thioflavin T (ThT, Sigma) was dissolved in assay buffer and included in the reaction at a final concentration of 10 μM. The thioflavin T fluorescence intensity was measured in a Spectramax M5 reader (Molecular Devices) set to 444 nm/485 nm (excitation/emission) with an emission cutoff of 480 nm. Samples were read in a 384-well plate format, in quadruplicate, at 37 °C, with reads every 5 min and agitation between reads. Control reactions containing WT or mutant tau without heparin were included as baseline readings in each experiment. Baseline-subtracted kinetic data were fit to the Gompertz equation56,57

where y = the fluorescence at time t, A is the maximum signal/amplitude of curve, ti is the inflection point, ti – b = lag time, and b =1/kapp where kapp is the apparent elongation rate constant. Amplitude values are represented relative to WT controls for each experiment to adjust for day-to-day variations in absolute ThT signal intensity.
Mice.
Mice were assigned into gender- and age-matched treatment groups in a randomized manner. The sample size for each experiment was determined on the basis of previous experience with each of the animal models used. For AAV-tau injection experiments, male (C57BL/6) mice at 2–3 months of age were purchased from the Jackson Laboratory and housed in a pathogen-free barrier facility at the University of California, San Francisco (UCSF) with a 12-h light/12-h dark cycle and ad libitum access to food and water. All behavior experiments were performed during daylight hours. All animal procedures were carried out under UCSF Institutional Animal Care and Use Committee–approved guidelines. For SSA treatment experiments, female (first cohort) and male (second cohort) PS19 mice were purchased from the Jackson Laboratory. The mice were housed in a conventional facility at Stanford University with a reverse 12-h light/12-h dark cycle and ad libitum access to food and water. All behavior experiments with PS19 mice were performed during daylight hours and carried out under Stanford University School of Medicine Institutional Animal Care and Use Committee–approved guidelines.
Stereotaxic injections.
Mice were anesthetized with avertin (250 mg/kg) by intraperitoneal injection, and secured on a stereotaxic frame (Kopf Instruments). Equal amount (∼3 × 109 genomic particles) of AAV1 expressing tau WT, tau K174Q and tauK174R mutant were injected into dentate gyrus of 3-month-old mice by stereotaxic injection at a rate of 0.5 μl/min. The following coordinates were used (anterior-posterior -2.1, medial-lateral ±1.7, dorsal-ventral -2.1).
Oral gavage.
Oral gavage was performed either at the animal facility at Stanford University (for PS19 mice) or at Preclinical Therapeutics Core, UCSF Helen Diller Family Comprehensive Cancer Center (for AAV-injected WT mice). Each group was assigned a different treatment in a semi-random manner. The investigators who performed the dosing and behavioral tests were blinded to the genotypes of the mice and to which vectors were injected. In the first PS19 cohort, two groups of 8- to 9-month-old PS19 female mice and one group of NTG littermate controls were used. PS19 female mice were given vehicle (PEG400/Tween 20 (4:1)) or SSA (225 mg/kg body weight) daily via single oral gavage ingestion (100 μl solution per 10 g body weight). NTG littermates were administered vehicle. Dosing was administered for 5 weeks before the initiation of the behavioral tests, and continued until the mice were sacrificed (60 d in total). In the repeat cohort, two groups (vehicle- and SSA-treated) of 7- to 8-month-old PS19 male mice and two groups (vehicle and SSA) of NTG littermate controls were used. The vehicle used in cohort 2 contained PEG-400 (10%, v/v), Tween-80 (10%, v/v), Labrasol (10%, v/v), hydroxypropyl β-cyclodextrin (20%, w/v), sodium phosphate (mono-basic), sodium phosphate (dibasic) and sodium chloride. Dosing was administered for about 60 d before the initiation of the MWM test and continued until the mice were sacrificed (86 d in total). Body weights of animals were monitored once a week. In the AAV-K174Q–injected cohort, 3-month-old C57BL/6 mice were injected with AAV-K174Q tau. Two months after injection, mice were treated daily with either vehicle or SSA (225 mg/kg body weight) for 6 weeks until sacrificed.
Hippocampal volume quantification and immunostaining and Gallyas silver staining.
Investigators were blinded to the genotypes or treatment of the mice. For quantification of hippocampal volume, mice hemibrains were cut at 30 μm coronally, and all hippocampi, including sections, were collected. Brain sections were mounted on microscope slides (Fisher Scientific) in an anterior-to-posterior order, starting from the section where the hippocampal structure first becomes visible (first section) to the section where hippocampal structure just disappears (last section). Mice with missing sections were excluded from the analyses, a pre-established criterion. Mounted brain sections were dried at room temperature for 24 h and stained with cresyl violet (Nissl staining). After being defatted for 15 min in 100% xylene and 10 min in 100% ethanol then rehydrated, sections were stained in 0.1% cresyl violet solution and mounted in DePeX mounting medium (VWR). Images were acquired with a Keyence BZ-9000 microscope. Hippocampal volume was estimated using ImageJ (NIH) Volumest plugin (http://lepo.it.da.ut.ee/~markkom/volumest/). 10–12 hippocampal-containing sections were typically used for each analysis. For immunostaining, floating sections were permeabilized and incubated in blocking solution (10% NGS in 0.3% Triton X-100 TBST) at room temperature for 1 h. After incubation with anti-HT7 (MN1000, Thermo Scientific), immunoreactive structures were detected with either Alexa Fluor 488– or Alexa Fluor 555–conjugated secondary antibodies (Invitrogen). For AC312 immunostaining, to reduce the nonspecific nuclear staining, the antibody was pre-absorbed by acetone Tau-knockout (KO) mice brain powder. Antigen retrieval was first performed by heating the sections in 10 mM citric acid at 90 °C for 30 min. Sections were then incubated with brain powder pre-absorbed AC312 and AT8 (Fisher) or MC1. After overnight incubation, the sections were incubated with secondary antibodies including Cy3-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch) and fluorescein-labeled goat anti-mouse IgG (Vector Laboratories). All images were acquired by DM5000B microscope (Leica) or CSU-W1 spinning disk confocal microscope (Nikon) with 60× oil immersion objective lens and analyzed by either Micro-Manager software (UCSF) or ImageJ (NIH). For orthogonal images, images were collected as Z stacks with size of Z step as 0.15-μm and total 2.5-μm stacked images were rendered for orthogonal view with ImageJ. Gallyas silver staining was performed as described58. Briefly, sections were placed in 5% periodic acid followed by alkaline silver iodide solution and developer solution. After washing with acetic acid and water, they were placed in 0.1% gold chloride, followed by sodium thiosulphate solution, washed and counterstained in 0.1% nuclear fast red. For quantification, three sections were analyzed by bright light digital microscopy with an Olympus BX41 microscopy. Four fields per section and brain region were analyzed at 400× magnification, and silver-positive neuronal cell bodies and neurites were estimated and averaged per 0.1 mm2.
PK analyses of SSA and salicylate (SS) in brain and plasma.
SSA was administered to C57BL/6 mice (males and females) via oral garage as a single bolus dose. Mice were sacrificed at predetermined time points and plasma and brain tissue samples were harvested for analysis by PPL labs (Redwood City, CA). SSA and its metabolite SA were measured simultaneously using oxopropanoyloxy benzoic acid as an internal standard solution59. To extract SSA/SA from brain, 200 μl of 50% acetonitrile in 0.1% formic acid was added to 30–50 mg of cortical tissue, followed by homogenization and vortexing. After addition of 200 μl of the internal standard solution, the samples were centrifuged at approximately 15,000 rpm and the supernatants were transferred for injection. To measure SSA/SA in the plasma, 10 μl of plasma samples were mixed with 90 μl H2O and 100 μl of Internal Standard Solution, followed by centrifugation and injection. HPLC was performed in C18 column, following a gradient program (10–90% acetonitrile in 6 min) with a flow rate of 0.4 ml/min. The injection volume was ∼5 μl. The mass spectrometry analyses were perform on 3200 QTRAP system (AB SCIEX, Framingham, MA) using MQL algorithm.
Human tau ELISA.
High-binding black 96-well plates (Costar, Corning, NY) were coated with 2.5 μg/ml HT7 capture antibody diluted in TBS, pH 8. After incubating at 4 °C overnight, plates were blocked with 3% BSA and washed with TBST (TBS containing 0.05% Tween-20). Plates were washed with TBST before the addition of 50 μl per well diluted PS19 mice brain lysates and hTau441 (2N4R, 441 amino acids), diluted in a TBS assay buffer, pH 8. Assay plates containing samples and standards were incubated overnight at 4 °C while shaking. Alkaline phosphatase (AP)-conjugated tau-5 antibody was diluted into assay buffer before being added to the assay plate (50 μl per well) for incubation with PS19 mice brain lysates and hTau441 standards for 1 h at room temperature while shaking. Plates were washed with TBST before being developed using alkaline phosphatase substrate (T2214; Applied Biosystems, Foster City, CA). Luminescence counts were measured using SpectraMax M5 (Molecular Devices, Sunnyvale, CA). Log-transformed luminescence counts from individual samples were interpolated to concentration using a third-order polynomial fit to the respective standards (GraphPad Prism 5.00, GraphPad Software, San Diego, CA). PS19 mice tau levels were plotted after correction for dilution factor.
Morris water maze.
Experimenters were blind to the genotypes or treatments of the mice for all behavioral analyses. The water maze consisted of a pool (122 cm in diameter) containing opaque water (20 ± 1 °C) and a platform (14 cm in diameter) submerged 1.5 cm under the water. Hidden platform training (days 1–5) consisted of 10 sessions (two per day, 2 h apart), of three trials each. The mouse was placed into the pool at alternating drop locations for each trial. A trial ended when the mouse located the platform. The maximum trial time was 60 s. Mice that failed to find the platform within 60 s were led to it and placed on it for 15 s. After the initial learning task, a reversal learning protocol (days R1–R4; two per day, 2 h apart) was conducted for the same mice. The hidden platform was moved to the opposite quadrant for reversal learning. For probe trial, 48 h after the final reversal training trial, mice were returned to the pool with a new drop location in the absence of hidden platform. Performance was measured with an EthoVision video-tracking system (Noldus Information Technology). Visible platform training, where the platform was cued with a mounted black-and-white striped mast, was conducted for four trials after completion of probe trials. The pre-established exclusion criterion is that mice that floated or did not swim would be excluded from analysis.
Small Y-maze.
Mice were placed in the center of a small Y-maze (arm length, 15 cm) and spontaneous alternation was recorded in a single continuous 6 min trial by a live observer. Each of the three arms was designated a letter A–C, and entries into the arms were recorded. The percent of spontaneous alternation was calculated over the total number of entries possible. The apparatus was cleaned with 70% ethanol between trials.
Open-field test.
Mice were placed individually into brightly lit automated activity cages equipped with rows of infrared photocells interfaced with a computer (San Diego Instruments). After a 1-min adaptation period, open-field activity was recorded for 20 min. Recorded beam breaks were used to calculate active times, path lengths, rearing times, and rearing events. The apparatus was cleaned with 70% ethanol between trials.
Fixed location dry maze.
Fixed location dry maze (also called DMP dry maze35) experiments were performed at animal facility at Stanford University. Mice were housed at a standard temperature (22 ± 1 °C), in a reverse light-controlled environment (lights off from 8:30 a.m. to 8:30 p.m.) with ad libitum access to food and water. Animals are group-housed with minimal enrichment such as shelter (paper towel tubes and nestlets). The experimenter remained blinded to the experimental groups throughout the study. A custom-built dry maze was used, which is a 122-cm diameter circular platform with 40 escape holes (5 cm in diameter). The target escape tube is attached to one of these holes; all other holes except the target escape hole are left open. High overhead lighting (∼750 lx) and air turbulence created by fans are used as aversive stimuli to encourage the animals to seek out the target escape hole. All aversive stimuli are turned off when the mouse enters the target escape hole. Visual cues are provided around the maze for navigation. The subjects were monitored by a video-tracking system directly above maze, and the parameters were measured using the computer software Ethovision. Subjects are given four trials to find the target escape hole with a 2-min inter-trial-interval. Each trial was 90 s long. Animals that could not find the escape hole were led by the experimenter and allowed to enter the target escape hole. After each trial, the apparatus was cleaned with 1% Virkon to eliminate odor cues. After 2 d of training and 3 d of learning, mice were tested in a probe trial. During the probe, the escape tube was removed from the maze and the mice were allowed to explore the maze. Time in each quadrant was recorded and memory was assessed by the time spent in the target quadrant versus the other three quadrants.
Statistical analysis.
Data were analyzed with GraphPad Prism v.5 (GraphPad) or STATA12 (StataCorp LP). Differences between means were assessed with paired or unpaired Student's t-test, one-way or two-way analysis of variance (ANOVA), followed by post hoc testing of all pairwise comparisons among genotypes (with Tukey-Kramer correction or Dunnett's test for one-way ANOVA and Bonferroni correction for two-way ANOVA), or by mixed effects model, as indicated. Pearson's correlation coefficients were used to quantify the linear relationship between two variables. The Shapiro-Wilk test of normality was applied to all data sets, and in cases where the data did not demonstrate a normal distribution, nonparametric tests were used to analyze statistical differences. The Mann-Whitney test was used for unpaired t-tests, the Wilcoxon matched pairs test was used for paired comparisons, and the Kruskal-Wallis test was used for ANOVAs. Multilevel mixed-effects linear regression model fit using STATA12 were used to compare learning curves in MWM and fixed location dry maze; random intercepts and linear time slopes for each mouse were taken into account for the correlation among repeated observations. Outliers are pre-established as data outside of mean ± 2 s.d. All samples or animals were included for statistical analysis unless otherwise noted in pre-established criteria.
References
Ludolph, A.C. et al. Tauopathies with parkinsonism: clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur. J. Neurol. 16, 297–309 (2009).
Cairns, N.J. et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 114, 5–22 (2007).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Lasagna-Reeves, C.A. et al. Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. FASEB J. 26, 1946–1959 (2012).
Ren, Y. & Sahara, N. Characteristics of tau oligomers. Frontiers Neurol. 4, 102 (2013).
Maeda, S. et al. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer′s disease. Neurosci. Res. 54, 197–201 (2006).
Lasagna-Reeves, C.A. et al. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 6, 39 (2011).
Berger, Z. et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J. Neurosci. 27, 3650–3662 (2007).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Sydow, A. et al. Reversibility of Tau-related cognitive defects in a regulatable FTD mouse model. J. Mol. Neurosci. 45, 432–437 (2011).
Wang, Y. & Mandelkow, E. Degradation of tau protein by autophagy and proteasomal pathways. Biochem. Soc. Trans. 40, 644–652 (2012).
Lee, B.H. et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467, 179–184 (2010).
Tai, H.C. et al. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am. J. Pathol. 181, 1426–1435 (2012).
Min, S.W. et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966 (2010).
Cohen, T.J. et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2, 252 (2011).
Kim, G.W. & Yang, X.J. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem. Sci. 36, 211–220 (2011).
Mandelkow, E.M. & Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2, a006247 (2012).
Cook, C. et al. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 23, 104–116 (2014).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Jicha, G.A., Bowser, R., Kazam, I.G. & Davies, P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 48, 128–132 (1997).
Weaver, C.L., Espinoza, M., Kress, Y. & Davies, P. Conformational change as one of the earliest alterations of tau in Alzheimer's disease. Neurobiol. Aging 21, 719–727 (2000).
Yeung, F. et al. Regulation of the mitogen-activated protein kinase kinase (MEK)-1 by NAD-dependent deacetylases. Oncogene 34, 798–804 (2015).
Wang, B. et al. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat. Commun. 5, 3479 (2014).
Zhao, Y. et al. p300-dependent acetylation of activating transcription factor 5 enhances C/EBPβ transactivation of C/EBPα during 3T3–L1 differentiation. Mol. Cell. Biol. 34, 315–324 (2014).
Jaworski, T. et al. AAV-tau mediates pyramidal neurodegeneration by cell-cycle re-entry without neurofibrillary tangle formation in wild-type mice. PLoS ONE 4, e7280 (2009).
Lecourtier, L. et al. Intact neurobehavioral development and dramatic impairments of procedural-like memory following neonatal ventral hippocampal lesion in rats. Neuroscience 207, 110–123 (2012).
Takeuchi, H. et al. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS ONE 6, e21050 (2011).
Kasper, L.H. et al. CBP/p300 double null cells reveal effect of coactivator level and diversity on CREB transactivation. EMBO J. 29, 3660–3672 (2010).
Kasper, L.H., Qu, C., Obenauer, J.C., McGoldrick, D.J. & Brindle, P.K. Genome-wide and single-cell analyses reveal a context dependent relationship between CBP recruitment and gene expression. Nucleic Acids Res. 42, 11363–11382 (2014).
Jin, Q. et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 30, 249–262 (2011).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Yoshiyama, Y., Kojima, A., Ishikawa, C. & Arai, K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. J. Alzheimers Dis. 22, 295–306 (2010).
Faizi, M. et al. Thy1-hAPPLond/Swe+ mouse model of Alzheimer's disease displays broad behavioral deficits in sensorimotor, cognitive and social function. Brain Behav. 2, 142–154 (2012).
Grinberg, L.T. et al. Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol. 125, 581–593 (2013).
Irwin, D.J. et al. Acetylated tau neuropathology in sporadic and hereditary tauopathies. Am. J. Pathol. 183, 344–351 (2013).
Irwin, D.J. et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain 135, 807–818 (2012).
Morris, M. et al. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 18, 1183–1189 (2015).
Smith, W.L., DeWitt, D.L. & Garavito, R.M. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69, 145–182 (2000).
in t' Veld, B.A. et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N. Engl. J. Med. 345, 1515–1521 (2001).
Aubry, S. et al. Assembly and interrogation of Alzheimer's disease genetic networks reveal novel regulators of progression. PLoS ONE 10, e0120352 (2015).
Hawley, S.A. et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336, 918–922 (2012).
Zhang, Y., Qiu, J., Wang, X., Zhang, Y. & Xia, M. AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler. Thromb. Vasc. Biol. 31, 2897–2908 (2011).
Lim, J.Y., Oh, M.A., Kim, W.H., Sohn, H.Y. & Park, S.I. AMP-activated protein kinase inhibits TGF-β-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J. Cell. Physiol. 227, 1081–1089 (2012).
Greene, W.C. & Chen, L.F. Regulation of NF-κB action by reversible acetylation. Novartis Found. Symp. 259, 208–217 discussion 218–225 (2004).
Lee, H. et al. Ethanol selectively modulates inflammatory activation signaling of brain microglia. J. Neuroimmunol. 156, 88–95 (2004).
Apostolova, L.G. et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch. Neurol. 63, 693–699 (2006).
Kerchner, G.A. et al. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology 75, 1381–1387 (2010).
Leung, K.K. et al. Cerebral atrophy in mild cognitive impairment and Alzheimer disease: rates and acceleration. Neurology 80, 648–654 (2013).
Reagan-Shaw, S., Nihal, M. & Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 22, 659–661 (2008).
Schilling, B. et al. Platform-independent and label-free quantitation of proteomic data using MS1 extracted ion chromatograms in skyline: application to protein acetylation and phosphorylation. Mol. Cell. Proteomics 11, 202–214 (2012).
Shilov, I.V. et al. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell. Proteomics 6, 1638–1655 (2007).
Perkins, D.N., Pappin, D.J., Creasy, D.M. & Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Pagans, S. et al. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol. 3, e41 (2005).
Chen, J. et al. SIRT1 Protects against microglia-dependent amyloid-β toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 280, 40364–40374 (2005).
Barghorn, S. et al. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry 39, 11714–11721 (2000).
Moore, C.L. et al. Secondary nucleating sequences affect kinetics and thermodynamics of tau aggregation. Biochemistry 50, 10876–10886 (2011).
Winsor, C.P. The Gompertz curve as a growth curve. Proc. Natl. Acad. Sci. USA 18, 1–8 (1932).
Sun, A., Nguyen, X.V. & Bing, G. Comparative analysis of an improved thioflavin-s stain, Gallyas silver stain, and immunohistochemistry for neurofibrillary tangle demonstration on the same sections. J. Histochem. Cytochem. 50, 463–472 (2002).
Kim, S.W. et al. Robust protective effects of a novel multimodal neuroprotectant oxopropanoyloxy benzoic acid (a salicylic acid/pyruvate ester) in the postischemic brain. Mol. Pharmacol. 79, 220–228 (2011).
Acknowledgements
We thank V. Haroutunian (The Icahn School of Medicine at Mount Sinai) and W.W. Seeley (UCSF) for human brain samples; B. Hann and J. Freimuth (UCSF) for oral gavage; L. Petrucelli (Mayo Clinic) for ac-KIGS antibody; P. Davies (Feinstein Institute for Medical Research) for MC1 and PHF-1 antibodies, Prothena Biosciences for 12E8 antibody; R. Ponnusamy and N. Devidze for advice on behavioral analyses; D. Song for PK analysis (PPL Inc); M. Finucane and S. Liu for advice on statistical analysis; L. Mucke and S. Meada for insightful discussions; L. Grinberg for technical advice on pathological analysis; C. Brennecka, G. Howard and S. Ordway for editorial review; J. Carroll and G. Maki for graphics assistance; E. Nguyen for administrative assistance and AB SCIEX for evaluation of the TripleTOF 5600 at the Buck Institute. This work was supported by a grant from the Tau Consortium (to L.G.) and US National Institutes of Health (NIH) grants 1R01AG036884 and R01AG030207 (to L.G.). We acknowledge the support of the NIH to L.E. (NIH NS40251 and NIH NS062413), and instrumentation from the NCRR shared instrumentation grant S10 RR024615 (to B.W.G.). Behavioral data were obtained with the help of the Gladstone Institutes' Neurobehavioral Core (supported by NIH grant P30NS065780).
Author information
Authors and Affiliations
Contributions
L.G., S.-W.M. and X.Chen. conceived the project. L.G., S.-W.M. and X.Chen. designed experiments. S.-W.M., X.Chen., T.E.T., P.D.S., S.A.M., C.W., K.S., S.S.M., E.D., B.S., X.Cong., J.J., Y.Z., Y.L. and E.M. performed experiments. L.E., B.W.G., M.S., J.G., N.K. and E.V. developed experimental tools or reagents. L.G., S.-W.M. and X.Chen. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 17988 kb)
Rights and permissions
About this article
Cite this article
Min, SW., Chen, X., Tracy, T. et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med 21, 1154–1162 (2015). https://doi.org/10.1038/nm.3951
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3951
This article is cited by
-
Palmitic Acid Induces Posttranslational Modifications of Tau Protein in Alzheimer’s Disease–Related Epitopes and Increases Intraneuronal Tau Levels
Molecular Neurobiology (2024)
-
Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation
Journal of Neuroinflammation (2023)
-
Nucleic acid drug vectors for diagnosis and treatment of brain diseases
Signal Transduction and Targeted Therapy (2023)
-
Acetylation discriminates disease-specific tau deposition
Nature Communications (2023)
-
Tau-targeting therapies for Alzheimer disease: current status and future directions
Nature Reviews Neurology (2023)