Abstract
Here we show that the cell-cycle regulator p21 is involved in immune system function. T lymphocytes from p21−/− mice exhibit significant proliferative advantage over wild-type cells following prolonged stimulation, but not after primary activation. Consistent with this, p21-deficient mice accumulate abnormal amounts of CD4+ memory cells, and develop loss of tolerance towards nuclear antigens. Similar to human lupus, female p21-deficient mice develop antibodies against dsDNA, lymphadenopathy, and glomerulonephritis, leading to decreased viability. These data demonstrate a specialized role for p21 in the control of T-cell proliferation, tolerance to nuclear antigens, and female-prone lupus. These findings could be the basis for new therapeutic approaches to lupus.
Similar content being viewed by others
Main
p21 is considered an important regulator of cell proliferation because of its interactions with complexes of cyclins and cyclin-dependent kinases (CDK). In vitro, p21 efficiently inhibits the activity of all cyclin/CDK complexes involved in the cell cycle. However, it is now recognized that in vivo, in addition to its inhibitory properties, p21 has positive regulatory activities on specific cyclin/CDK complexes1. p21 can also bind the replication factor PCNA and inhibit DNA replication2. Other regulators with similarities to p21, such as p27, have functions that are partially redundant with p21 (refs. 1, 3, 4).
The best-characterized transcriptional activator of the p21 gene is the tumor suppressor protein p53 (ref. 5). Mice genetically deficient in p21 are not predisposed to development of tumors6, but are radiation-sensitive7 and their cells show impaired p53-dependent cell-cycle arrest in response to DNA damage6,8. Beyond this defect, however, p21-deficient mice develop normally and no physiological abnormalities have been reported6.
Lymphocytes, unlike other cell lineages, must cycle in an intense, prolonged, and repeated manner to establish an immune response and to generate immunological memory. It is thus possible that lymphocytes may be especially sensitive to alterations in cell cycle regulation. Upregulation of p21 has been described during T-cell proliferation9 and in memory CD4+ T cells of autoimmune-prone BXSB mice10. Nevertheless, direct involvement of p21 in immune system function has not been shown. Here we examine the role of p21 in immune responses using p21−/− mice8. We find that p21 controls sustained T-cell proliferation, and is indispensable for maintaining tolerance towards nuclear antigens and preventing lupus-like disease in female mice.
p21 controls long-term proliferation of T lymphocytes
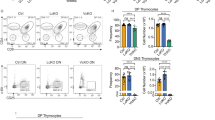
To determine whether p21 plays a role in T lymphocyte stimulation, we performed in vitro proliferation studies of splenocytes obtained from 6–8 week old p21−/− male (p21−/−M), p21−/− female (p21−/−F), and wild-type male (WT-M) and female (WT-F) mice. The absence of p21 did not affect CD4+ T-cell proliferation upon activation with Con A (Fig. 1a) or antibody against CD3 (data not shown). Following this initial activation, CD4+ cells were cultured under conditions of sustained interleukin (IL)-2 stimulation, resulting in an ∼100% increase in proliferation of p21−/− versus wild-type CD4+ cells, with no gender-related differences (Fig. 1b). Concurring with this, cell cycle analysis of IL-2-cultured CD4+ cells showed an increased proportion in S phase in p21−/−F as compared to WT-F mice (65 ± 3% vs. 43 ± 5%, P < 0.0001), with a concomitant decrease in G1 phase (these differences were not gender-dependent) (Fig. 1c and data not shown). Stimulation of p21−/− CD8+ cells using the same protocols and numbers of mice yielded results similar to those of p21−/− CD4+ cells (not shown). These data suggest that, during sustained stimulation of T cells, p21 acts as a negative regulator of proliferation.
a, CD4+ splenocytes were stimulated with Con A and IL-2, and assayed for proliferation after 3 d; b, 3 d Con A-stimulated CD4+ cells were further cultured with IL-2 for 6 d. Proliferation rates were determined by [3H]thymidine incorporation during the last 12 h of the assays. In both a and b, values represent mean ± s.d.,n=3 in one of three independent experiments. Basal values of unstimulated cells ranged from 500 to 900 counts per minute (c.p.m.) and were subtracted from corresponding poststimulation values. c, PI staining of cells following 6 d IL-2 stimulation. FACS profiles represent one WT-F and one p21−/−F. Proportions indicated are the mean ± s.d., n=9.
To examine the susceptibility of p21−/− T cells to apoptosis following prolonged IL-2-dependent culture, CD4+ cells were stimulated with antibody against CD3 and IL-2, resulting in similar apoptosis concentrations in p21−/−F as compared to WT-F mice (40 ± 3% vs. 46 ± 5%, n=3 in three independent assays), detected by propidium iodide (PI) staining. After treatment with antibody against CD3, surviving cells showed an increased S phase, similar to that observed following prolonged IL-2 stimulation. These results indicate that surviving p21−/− cells may continue cycling following repeated stimulation. To further study the possible effect of p21 in T-cell apoptosis, Con A-stimulated p21−/− and wild-type CD4+ and CD8+ splenocytes were treated with antibody against Fas, and no difference was detected in the apoptosis rate in the absence of p21. Similarly, thymocyte culture in the presence of antisera against Fas or CD3 showed p21-independent apoptosis (n=3). Altogether, these data suggest that the absence of p21 has no effect on either primary stimulation or apoptosis in T cells, but considerably influences sustained proliferation of both CD4+ and CD8+ cells.
We also examined the role of p21 in B cell stimulation and apoptosis using assays similar to those for T cells. Specifically, purified B cells from 6–8 week old WT and p21−/− mice proliferated similarly following primary stimulation with IL-4 and antibodies against IgM, as well as after primary or prolonged activation with IL-4 and antibodies against CD40. To examine the possible effect of p21 in B-cell apoptosis, B cells stimulated with IL-4 and antibody against CD40 were treated with antibody against Fas, and purified immature bone marrow IgM+ B cells were IgM antibody-treated. In either case, there were no differences in the apoptosis rate between p21−/− and wild-type B cells (data not shown). p21 thus appears not to be required for in vitro proliferation or apoptosis of B cells.
Accumulation of memory CD4 + cells in p21 −/− mice
To examine whether the in vitro hyperproliferation of p21−/− T cells during sustained stimulation is reflected in vivo, we examined the lymphoid organs of p21−/− mice. Nine-month-old p21−/−F mice exhibited a dramatic increase in lymph node (LN) and spleen size compared to wild-type mice (P=0.005 and P<0.0006 for LN and spleen, respectively), whereas male knockout animals developed moderately enlarged lymph nodes (P=0.0006) (Fig. 2a); increased organ weight correlated with proportionally elevated cellularity (data not shown). LN, but not spleens, showed a moderate size increase in 6–7-month-old p21−/−F as compared to WT-F mice (0.116 ± 0.038 g vs. 0.02 ± 0.03 g, respectively, using cervical LN, n=8, P<0.001). No differences were detected between lymphoid organs of 6–7-month-old p21−/−M and WT-M mice. The data suggest that, in p21−/−F mice, severe lymphadenopathy is age-dependent and is manifested after 7 months of age. In 9-month-old animals, lymphocyte composition of p21−/− spleens was similar to that of wild-type mice, whereas a substantial increase in the proportion of B cells was observed in the LN of p21−/− compared to wild-type mice (p21−/−F, 70.5 ± 2% vs. WT-F 42.3 ± 4%; p21−/−M, 50 ± 7% vs. WT-M 44 ± 3%; n=5).
Mice were 9-months-old unless otherwise indicated. a, Weight of cervical lymph nodes (filled bars) and spleens (shaded bars), n=8; b, CD44 expression concentrations (low, intermediate, high) of gated CD4+ splenocytes. FACS profiles correspond to representative mice, and percentages indicate the mean ± SD, n=5; c, Proportions of CD4+ splenocytes from 4-month-old mice with CD44high expression. Data are derived from FACS analysis and values are the mean ± s.d., n=5; d, Splenocytes were triple-labeled with antibodies against B220, HSA, and IgG. The profile of HSA and IgG expression of gated B220+ cells is shown for representative mice. Proportions represent the mean ± s.d., n=5.
Analysis of lymphocyte phenotype in spleen of 9-month-old animals showed dramatic accumulation of memory/effector CD4+ CD44high T cells (with no appreciable difference between males and females) in p21−/− versus male and female wild-type mice (Fig. 2b and data not shown), whereas CD8+ CD44high cell proportions were similar in both mouse types (data not shown). The increase in CD4+ CD44high splenocytes was evident at 4 months of age, in the order p21−/−F >p21−/−M >wild type (Fig. 2c). These data indicate that the female hormonal environment expedites the generation of memory CD4+ T cells in p21−/− mice. Examination of markers that distinguish effector from memory cells, such as CD25, CD62L, CD69, and CD45RB, showed similar expression in p21−/− and wild-type CD4+ CD44high cells from 4-month-old mice (data not shown). Approximately 70% of the p21−/− and wild-type CD4+ CD44high cells expressed low amounts of the above mentioned markers and can therefore be considered of the memory phenotype11. The effector/memory cell ratio thus appears to be similar in p21−/− and wild-type mice. Based on the in vitro T-cell stimulation data, we expected that p21−/− CD4+ CD44high cells, having previously been activated in vivo, could exhibit increased proliferation compared to their wild-type counterparts following further stimulation. Because memory T cells respond to IL-2 stimulation in vitro12, we compared the response to IL-2 of CD4+ CD44high splenocytes from 4-month-old WT-M and p21−/−M mice, and found that p21 deficiency results in a 28 ± 2% proliferative advantage of p21−/− CD4+ cells (n=6). Similar experiments using CD4+ CD44int cells showed no response to IL-2, irrespective of p21 expression (data not shown). The hyperresponsiveness of CD4+ CD44high cells may be analogous to the in vitro hyperproliferative capacity of p21−/− CD4+ cells following prolonged stimulation.
Analysis of spleen B cells showed an increased proportion of activated HSAlow IgGlow cells in 9-month-old p21−/−F (71 ± 9%) versus WT-F mice (37 ± 3%) (Fig. 2d), with p21−/−M showing an intermediate concentration (57 ± 4%) as compared to WT-M (36 ± 3%); n=5. Nevertheless, the B cell accumulation in LN and the increased p21−/− cell activation were not reflected by defects in in vitro proliferation or apoptosis assays (see previous data), suggesting that B-cell abnormalities may be secondary to the defective regulation of p21−/− T cell proliferation.
Glomerulonephritis in female but not male p21 −/− mice
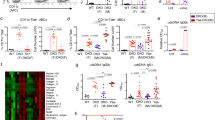
Follow-up of p21−/− mice for up to 12 months of age showed that p21−/−F mice develop severe glomerulonephritis accompanied by proteinuria and 60.7% mortality rate (Table 1). In contrast, most p21−/−M mice were disease-free (see Table 1); all wild-type mice were healthy at 1 year of age, with no signs of glomerulonephritis. Mortality and glomerulonephritis were age-dependent, because 80% of p21−/−F mice that died or had to be killed were >8 months of age, and 4-month-old p21−/−F mice showed no signs of glomerulonephritis (data not shown). Glomerular alterations and inflammatory cell infiltrates were detected in kidneys of p21−/−F but not in p21−/−M or wild-type mice (Fig. 3a, b), as were heavy glomerular IgG deposits, similar in intensity to those found in the MRL-lpr lupus model (Fig. 3c). These results indicate that p21−/−F mice develop a severe lupus-like syndrome. The fact that this phenotype was not reported in the initial description of p21−/−mice6,8 may be because of the fact that previous analysis of p21−/− mice extended only up to 7 months of age6, whereas we observed that lupus manifestations became more evident after this time. In addition, differences in pathogen presence in animal facilities may also influence the severity of autoimmune features.
a, upper, glomerulus of a 9-month-old WT-F mouse, lower, glomerulus of a 9-month-old p21−/−F mouse exhibiting typical diffuse proliferative glomerulonephritis with hypercellular glomerulus, increased mesangial cells, leukocyte infiltration, and thickening of capillary walls. Arrows indicate typical nuclear fragments. Hematoxylin and eosin staining (original magnification ×240). b, massive infiltration of inflammatory cells in the kidney of a 9-month-old p21−/−F mouse. c, Typical IgG deposits in glomeruli of 4-month-old MRL-lpr (upper) and 9-month-old p21−/−F mice (lower).
Autoantibody development in p21 −/− mice
To further establish the lupus-like syndrome developed by p21−/−F mice, concentrations of IgG and antibodies against dsDNA autoantibody were determined in 9–12-month-old mice. The data (Fig. 4a) show a significant increase in the IgG1 (Th2-dependent) isotype in p21−/− vs. wild-type mice (P<0.00001), whereas gender differences were not significant (statistical significance was tested in a two-way ANOVA test). Using the same test, differences in IgG2a, the major Th1-dependent isotype, were not found to be significant. These results indicate a shift toward a Th2 response in both male and female p21−/− mice. Concentrations of IgE, an immunoglobulin associated with a Th2 response, were similar in p21−/− and wild-type mice (p21−/−F, 0.164 ± 0.02 vs. WT-F, 0.123 ± 0.02 mg/ml and p21−/−M, 0.12 ± 0.01 vs. WT-M, 0.15 ± 0.02 mg/ml, n=15). This indicates that IgG1 and IgE concentrations are regulated differently in p21−/− mice. Older p21−/−F mice developed elevated concentrations of antibodies against dsDNA, in some cases similar to those of diseased MRL-lpr mice, whereas most same-age male mice produced very low concentrations of these autoantibodies (P<0.002, Fig. 4b). dsDNA specificity was verified by the Crithidia luciliae test, because all representative sera from p21−/−F and p21−/− M mice found to be anti-DNA positive or negative in enzyme-linked immunosorbent assay (ELISA) showed identical specificity by fluorescent staining in the Crithidia test (n=3). In female mice, antibodies against dsDNA of the IgG1 isotype appear increased; nevertheless, some mice developed elevated amounts of the Th1-dependent IgG2a and, notably, IgG3 antibodies against dsDNA (Fig. 4c). Analysis of these data showed that 6 of the 15 mice tested had higher concentrations of antibodies against dsDNA IgG2a + IgG3 isotypes than of IgG1; in these cases, autoantibody production may thus be considered Th1-dependent. This is significant, because IgG2a, and especially IgG3 antibodies against dsDNA have been considered of increased pathogenicity in glomerulonephritis development13.
Mice were 9–12 months old unless otherwise specified. a, Serum concentrations of IgG isotypes. Data represent the mean ± s.d., n=15. b, Serum concentrations of IgG antibody against dsDNA (n=15). For MRL-lpr mice, a serum pool from five 4-month-old mice was tested. c, Concentrations of anti-dsDNA IgG isotypes of 15 p21−/−F mice. d, Indirect immunofluorescence intensity of serum reactivity against anti-nuclear autoantibodies using fixed Hep-2 cells (n=10). e, Anti-histone antibody concentrations measured by ELISA (n=10). f, Immunofluorescence intensity of serum reactivity from 4-month-old mice against Hep-2 cells (n=10).
We then evaluated autoantibodies to nuclear antigens. In 9- to 12-month-old p21−/− mice, anti-nuclear (Fig. 4d), anti-histone (Fig. 4e) and anti-ssDNA antibodies (data not shown) were detected, and statistical analyses showed no significant differences in antibody expression among sexes. This indicates that histone and ssDNA are among the nuclear antigens requiring p21 for tolerance. Analysis of sera from 4-month-old mice showed that only female, but not male p21−/− mice produced anti-nuclear autoantibodies (P=0.0003, Fig. 4f), in agreement with the elevated proportion of effector/memory CD4+ CD44high cells in p21−/−F mice at this age (Fig. 2c). Overall, the data suggest that the breakdown of tolerance toward nuclear antigens occurs in both male and female p21−/− mice. The female hormonal environment clearly influences the early onset of autoantibody production, and more importantly, the subsequent production of antibodies against dsDNA and development of renal disease.
Discussion
Three major conclusions arise as a result of this work: a) p21 negatively regulates T cell proliferation following long-term stimulation, b) p21 is essential for maintaining tolerance toward nuclear antigens, and c) similar to the human lupus syndrome, the female hormonal environment exacerbates the tolerance defect in p21-deficient mice, leading to the production of antibodies against dsDNA and severe lupus-like disease.
It is of interest that p21 affects sustained T-cell stimulation, but not primary T-cell stimulation. p21 is expressed similarly during both types of stimulation (our unpublished observations and ref. 9), although p21 deficiency appears to influence only long-term stimulation. Differential expression of other cell cycle-associated regulators during sustained proliferation may enhance the requirement for p21 function.
Tolerance to an antigen can be induced following T-cell activation under incomplete costimulatory conditions (in the absence of a proper inflammatory environment), leading to nonsustained T-cell proliferation, followed by deletion of activated cells14. Peripheral tolerance to autoantigens may occur in an analogous manner15. Consequently, it could be suggested that break of tolerance is provoked by defects in either T-cell proliferation or apoptosis. Based on our observations of the enhanced, sustained T-cell proliferation in the absence of p21, we propose that, following repeated encounters with autoantigen, autoreactive p21-deficient T cells hyperproliferate, provoking break of tolerance. We propose that this defect is limited mainly to anti-nuclear antigens, and may be related to the persistent presentation of nuclear antigens, which are exposed on the surface of the abundant apoptotic bodies that are generated in the organism16. In vitro experiments show defects in both CD4+ and CD8+ p21−/− T cells; however, only CD4+, memory T cells accumulate in vivo. This may be the result of differences in the manner in which antigens are presented to these two cell types.
We have observed that female p21−/− mice, in contrast to their male counterparts, develop a lupus-like syndrome before 1 year of age, characterized by lymphadenopathy, antibodies against dsDNA, and glomerulonephritis, leading to fatal renal failure. A factor that may contribute to lupus development is the mixed 129/Sv × C57BL/6 genetic background, although no autoimmune predisposition was detected in our control animals. We are currently crossing p21−/− mice onto other backgrounds to address this point. In addition, lack of p21 may increase kidney cell proliferation, thus contributing to glomerulonephritis development. p27 or p21 deficiency has recently been shown to exacerbate experimentally induced inflammatory kidney disease, as a result of increased proliferation of kidney cells17,18,19. Nonetheless, the severe immunological defects in p21−/− mice, comparable to those in other lupus models, appear enough for the development of glomerulonephritis.
Lupus predisposition has recently been described in mice deficient for the C1q complement component20 or for serum amyloid P component (SAP)21. Lupus susceptibility in these knockout mice is considered to be due to defects in the clearance of apoptotic debris22. In contrast, p21−/− mice show immune system abnormalities characteristic of lupus.
Our findings show that the disease in p21−/− mice is remarkably similar to the lupus syndrome, including female propensity to disease, as is also the case for human lupus erythematosus. Recent genetic analyses of NZB × NZW mice and human lupus patients have identified several lupus susceptibility loci23,24,25. The p21 gene maps within a recently identified human MHC susceptibility locus (6p11-p21) with the strongest evidence for disease linkage5,25, which may be related to genes other than HLA. It is thus possible that p21 contributes to lupus susceptibility; we are currently analyzing possible abnormalities in this gene in lupus patients. Furthermore, therapeutic strategies may be envisaged for intervention in lupus progression to control CDK activity. As a final point, because of their single gene defect, p21−/− mice constitute a simplified lupus model that may provide insight into the biological mechanism of tolerance, as well as into the etiology and gender bias of this disease.
Methods
Animals.
Three breeding pairs of p21−/− mice on a mixed 129/Sv × C57BL/6 background were obtained from the laboratory of Gregory Hannon (Cold Spring Harbor Laboratory, New York). The p21−/− mice used in the study were generated following two to three crosses of the original breeding pairs. All wild-type mice were of the same 129/Sv × C57BL/6 mixed genetic background. MRL-lpr mice were obtained from the Jackson Laboratory. All mice were maintained under pathogen-free conditions in a barrier zone.
Proliferation and cell death assays.
Sorted CD4+ or CD8+ cells (1 × 106/ml) were stimulated with Con A (5 μg/ml; Sigma, St. Louis, Missouri) in the presence of irradiated splenocytes (0.5 × 106 ml) in conditioned medium containing 10% supernatant of IL-2-producing transfected X63 cells. Analogous stimulation was done with antisera to CD3, and PMA (1 ng/ml; Sigma) in culture wells coated with antibodies against CD3 (145.2C11, 2.5 μg/well; PharMingen, San Diego, California). Proliferation was quantified after 3 d in culture by uptake of [3H]thymidine (1 μCi/100 μl) added during the final 12 h of culture. Con A-stimulated cells were washed and further cultured in the presence of 10% IL-2 supernatant for 6 d; during this period cells were split every 2 d. Cells were then recultured with IL-2 or stimulated with plate-coated antibodies against CD3 (10 μg/well), and IL-2. After 24 h, cells were collected for determination of [3H]thymidine incorporation or stained with PI for determination of apoptosis and cell cycle analysis. Anti-Fas-triggered apoptosis of Con A- and IL-2-activated cells was quantified after 2 d of culture with antibodies against Fas (1 μg/ml, Pharmingen). Amount of cell death was determined in all cases by PI staining. Thymocyte apoptosis was assayed by culturing cells for 24 h either in plates coated with antibodies against CD3 or in the presence of antibodies against Fas.
Sorted B cells (1 × 106/ml) were cultured with antibodies against IgM (50 μg/ml, Jackson Immunoresearch, West Grove, Pennsylvania), and IL-4 (60 U/ml) for 3 d, or with antibodies against CD40 (10 μg/ml, PharMingen), and IL-4 (60 U/ml) for 3 or 8 d, respectively (splitting the cultures every 2 d). [3H]thymidine incorporation was determined at the end of each culture period. Anti-Fas-triggered apoptosis of cells cultured with antibodies against CD40 and with IL-4 was determined after 24 h incubation. Apoptosis of immature IgM+ bone marrow cells from 4-week-old mice was determined following 24 h culture with antibodies against IgM. These cells were purified by sorting of bone marrow cells expressing intermediate and high amounts of B220; the immature phenotype of the purified cells was confirmed because >95% of purified cells expressed IgM as detected by fluorescent-antibody cell sorter (FACS) using a labeled IgM antibody (Pharmingen).
CD4+ CD44high cells purified from 4-month-old mice were sorted and cultured for 4 d in the presence of 30% IL-2-containing supernatant. [3H]thymidine was added 12 h before cell collection.
Flow cytometric analysis.
The lymphocyte composition of lymphoid organs was determined following triple staining with antibodies against CD4, CD8, and B220. Effector/memory CD4+ T cells were identified by staining with antibodies against CD4, CD44, and CD25, CD69, CD62L, or CD45RB. To identify activated B cells, splenocytes were stained with antibodies against B220, IgG, and HSA. All antibodies were purchased from Pharmingen, and stained cells were analyzed on a Coulter EPICS XL flow cytometer (Coulter, Miami, Florida). PI staining was used to detect apoptotic cells and for cell cycle analysis using Coulter Multicycle software.
Histological and serological analysis.
Histological examination of kidneys was done in a blind manner; the severity of glomerulonephritis was evaluated on a scale from 0 to 4+ (ref 26). Proteinuria was determined using Urispec GP+A strips (Henry Schein, Port Washington, New York). For immunohistochemistry, 6 μm frozen kidney sections were processed as described27 and stained with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Southern Biotechnology, Birmingham, Alabama). Total serum IgG, as well as IgG isotypes, were captured with a goat anti-mouse κ chain antibody (Caltag, Burlingame, California), and concentrations were determined by ELISA using HRP-conjugated antibodies against IgG (Dako), IgG1, IgG2a, IgG2b, and IgG3 (Caltag). Reactivity of these antibodies was normalized for equivalent optical density (OD) against total IgG and the corresponding myeloma isotype controls (Caltag). IgE serum concentrations were measured by sandwich ELISA using an anti-mouse IgE monoclonal antibody as capture antibody (clone R35-72) and, for detection, a biotinylated antibody against mouse IgE (clone R35-118) followed by streptavidin-HRP; purified mouse IgE was used for standard controls. All reagents were from Pharmingen and the manufacturer's protocol was used. Total and isotype-specific antibodies against dsDNA autoantibodies were measured as described27 and expressed as relative units against a standard positive serum pool derived from five 4-month-old MRL-lpr mice. Similarly, antibodies against ssDNA were measured using plates coated with denatured DNA (15 min boiling, 5 min on ice). For anti-histone antibody detection, plates were coated with histone type II-S from calf thymus (Sigma). Antibodies against dsDNA were also detected by the Crithidia luciliae test, by binding to the Crithidia kinetoplast, and visualized by indirect immunofluorescence. Crithidia luciliae slides were from DiaSorin Inc. (Stillwater, Minnesota); serum serial dilutions were assayed according to manufacturer's protocols. Serum IgG anti-nuclear antibody concentrations were measured by indirect immunofluorescence using Hep-2 cells (The Binding Site, Birmingham, UK) and a Cy3-conjugated antibody against mouse IgG F(ab′)2 (Jackson ImmunoResearch). Serum samples were diluted to 1:5000 or 1:1000 for 9–12 and 4-month-old mice, respectively. Antinuclear antibody concentrations were quantitated according to fluorescence intensity using Leica TCS NT software on a Leica confocal microscope.
Statistics.
The Mann-Whitney U test was used unless otherwise indicated. When the two-way ANOVA test was used, the normality of distribution and the homogeneity of variances of the data were verified by the K-S Lilliefors and Cochran C tests, respectively.
References
Sherr, C.J. & Roberts, J.M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–12 (1999).
Li, R., Waga, S., Hannon, G.J., Beach, D. & Stillman, B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature 371, 534–537 (1994).
Cheng, M. et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18, 1571–1583 (1999).
Coats, S. et al. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr. Biol. 9, 163–173 (1999).
El-Deiry, W.S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Deng, C., Zhang, P., Harper, J.W., Elledge, S.J. & Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675–684 (1995).
Wang, Y.A., Elson, A. & Leder, P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc. Natl. Acad. Sci. USA 94, 14590–14595 (1997).
Brugarolas, J. et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377, 552–557 (1995).
Nourse, J. et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 372, 570–573 (1994).
Sabzevari, H., Propp, S., Kono, D.H. & Theofilopoulos, A.N. G1 arrest and high expression of cyclin kinase and apoptosis inhibitors in accumulated activated/memory phenotype CD4+ cells of older lupus mice. Eur. J. Immunol. 27, 1901–1910 (1997).
Dutton, R.W., Bradley, L.M. & Swain, S.L. T cell memory. Annu. Rev. Immunol. 16, 201–223 (1998).
London, C. A., Pérez, V.L. & Abbas, A.K. Functional characteristics and survival requirements of memory CD4+ T lymphocytes in vivo. J. Immunol. 162, 766–773 (1999).
Takahashi, S. et al. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J. Clin. Invest. 97, 1597–1604 (1996).
Pape, K.A. et al. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cells for the study of T-cell activation in vivo. Immunol. Rev. 156, 67–78 (1997).
Ohashi, P.S. & Sarvetnick, N. Autoimmunity: a bias from tolerance to immunity. Curr. Opin. Immunol. 8, 815–817 (1997).
Casciola-Rosen, L.A., Anhalt, G. & Rosen, A. Autoantigens targeted in systemic lupus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 179, 1317–1330 (1994).
Ophascharoensuk, V., Fero, M.J., Hughes, J., Roberts, J.M. & Shankland, S.J. The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nature Med. 4, 575–580 (1998).
Megyesi, J., Safirstein, R.L. & Price, P.M. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J. Clin. Invest. 101, 777–782 (1998).
Kim, Y.G. et al. The cyclin kinase inhibitor p21CIP1/WAF1 limits glomerular epithelial cell proliferation in experimental glomerulonephritis. Kidney Int. 55, 2349–2361 (1999).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19, 56–59 (1998).
Bickerstaff, M.C. et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 5, 694–697 (1999).
Paul, E. & Carroll, M.C. SAP-less chromatin triggers systemic lupus erythematosus. Nat. Med. 5, 607–608 (1999).
Theofilopoulos, A.N. & Kono, D.H. Mechanisms and genetics of autoimmunity. Ann. NY Acad. Sci. 13, 225–235 (1998).
Moser, K.L. et al. Genome scan of human systemic lupus erythematosus: Evidence for linkage on chromosome 1q in African-American pedigrees. Proc. Natl. Acad. Sci. USA. 95, 14869–14874 (1998).
Gaffney, P.M. et al. A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc. Natl. Acad. Sci. USA. 95, 14875–14879 (1998).
Berden, J.H., Hang, L., McConahey, P.J. & Dixon, F.J. Analysis of vascular lesions in murine SLE. I. Association with serologic abnormalities. Immunology 130, 1699–1705 (1983).
Balomenos, D., Rumold, R. & Theofilopoulos, A.N. Interferon-γ is required for lupus-like disease and lymphoaccumulation in MRL/lpr mice. J. Clin. Invest. 101, 364–371 (1998).
Acknowledgements
We thank M. Cohn and A.N. Theofilopoulos for discussion and comments. We are also grateful to G. Hannon for the gift of p21−/− mice, A. Rebollo for IL-4, M.C. Moreno-Ortiz for assistance with FACS analysis C. Alonso for help with statistical analyses following FACS analysis and C. Mark for editorial assistance. The Department of Immunology and Oncology was founded and is supported by the Spanish Research Council (CSIC) and Pharmacia & Upjohn.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balomenos, D., Martín-Caballero, J., García, M. et al. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat Med 6, 171–176 (2000). https://doi.org/10.1038/72272
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72272
This article is cited by
-
c-Myc uses Cul4b to preserve genome integrity and promote antiviral CD8+ T cell immunity
Nature Communications (2023)
-
Regulation of immunological tolerance by the p53-inhibitor iASPP
Cell Death & Disease (2023)
-
Increased expression of CDKN1A/p21 in HIV-1 controllers is correlated with upregulation of ZC3H12A/MCPIP1
Retrovirology (2020)
-
Systemic Inflammation as a Driver of Brain Injury: the Astrocyte as an Emerging Player
Molecular Neurobiology (2018)
-
Emerging roles of p53 and other tumour-suppressor genes in immune regulation
Nature Reviews Immunology (2016)