Abstract
Hepatic adeno-associated virus (AAV)-serotype 2 mediatedgene transfer results in transgene product expression that is sustained in experimental animals but not in human subjects. We hypothesize that this is caused by rejection of transduced hepatocytes by AAV capsid–specific memory CD8+ T cells reactivated by AAV vectors. Here we show that healthy subjects carry AAV capsid–specific CD8+ T cells and that AAV-mediated gene transfer results in their expansion. No such expansion occurs in mice after AAV-mediated gene transfer. In addition, we show that AAV-2 induced human T cells proliferate upon exposure to alternate AAV serotypes, indicating that other serotypes are unlikely to evade capsid-specific immune responses.
Similar content being viewed by others
Main
In the first clinical trial of hepatic AAV-2 factor IX (AAV-2-F.IX) gene transfer into subjects with hemophilia B, transgene expression was short lived and after 4–6 weeks started to decline, accompanied by an asymptomatic and reversible transaminitis1. Concomitantly, AAV-2 capsid–specific T cells became detectable in peripheral blood, implying T cell–mediated rejection of AAV-transduced hepatocytes. These findings raise safety questions for the use of AAV in gene transfer to humans who, owing to previous infections, harbor memory T cells to AAVs.
To test if hepatic AAV-2 gene transfer caused expansion of AAV capsid–specific T cells in gene transfer subjects, we used a human leukocyte antigen (HLA)-B*0702 pentamer loaded with the AAV-2 capsid–derived immunodominant peptide VPQYGYLTL to test peripheral blood mononuclear cells (PBMCs) from one of our HLA-B*0702+ subjects for frequencies of circulating AAV-2 capsid–specific CD8+ T cells. Direct staining of PBMCs drawn at week 2 after vector infusion (preinfusion cells were not available) showed that 0.14% of circulating CD8+ T cells were capsid specific (Fig. 1a). Three weeks later, the capsid-specific population had expanded to 0.5% of the circulating CD8+ T cells, indicating proliferation of this T-cell subset (Fig. 1a). By 20 weeks after vector infusion, the capsid-specific CD8+ T-cell population had contracted to the level seen at 2 weeks (Fig. 1b). The expansion and contraction of the capsid-specific CD8+ T-cell population paralleled the rise and fall of serum transaminases in this subject (Fig. 1b).
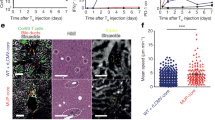
(a) AAV-specific T cells in the peripheral blood of gene transfer subject (sub) G at weeks 2 (top row) and 5 (bottom row) after vector infusion. PBMCs from subject G were stained with three different phycoerythrin-labeled peptide-bound HLA-B*0702 pentamers. Capsid-specific CD8+ T cells were stained with the HLA-B*0702-p74 (VPQYGYLTL) pentamer. The negative control was a stain with an HIV-gag HLA-B*0702-restricted peptide-bound pentamer (subject HIV-negative). Epstein-Barr virus (EBV)-pentamer staining (with an HLA-B*0702-restricted peptide) is also shown for comparison. Plots are gated on forward and side scatter, single-cell events, CD4−CD8+ cells. Numbers in the plots indicate percentage of pentamer+ events within the plot. (b) Time course of serum transaminases, and frequency of AAV-2 peptide-specific CD8+ T cells in PBMCs, in subject G after vector infusion. Time 0, day of vector infusion. Shaded gray area, upper limit of normal for transaminases. (c,d) AAV-specific T cells from two subjects infused with AAV-2 vector undergo robust expansion after in vitro stimulation with a major histocompatibility (MHC) class I–restricted peptide derived from AAV capsid. (c) First panel, HLA-B*0702-p74 (VPQYGYLTL) pentamer staining of unexpanded PBMCs from subject G at 20 weeks after vector infusion. Second panel, pentamer staining after one round of in vitro stimulation (IVS1) with peptide VPQYGYLTL. Third panel, pentamer staining after three rounds of in vitro stimulation (IVS3) with peptide. (d) HLA-A*0101-p99 (SADNNNSEY) pentamer staining of PBMCs from subject E at 2.5 years after vector infusion (left panel) and after two rounds of in vitro stimulation with peptide 99 (right panel). The same populations of cells stained with an HLA-A*0101 pentamer for influenza are negative (data not shown). Plots shown are gated on size, scatter, pulse width, CD4− and CD8+ cells. Numbers shown indicate percentage of AAV-specific CD8+ cells. (e) AAV-specific T cells kill MHC-matched peptide-pulsed target cells. Expanded AAV-2 (peptide VPQYGYLTL)-specific T cells from subject G were tested as effector cells against the HLA-B*0702 lymphoblastoid cell line (JY) pulsed with the same AAV-2 peptide or HIV peptide in a standard chromium-release assay. The x axis shows four effector:target ratios, and the y axis shows the calculated specific lysis of target cells. Data points indicate the mean of triplicates +/− s.e.m. (f) Human CD8+ T-cell cross-reactivity for AAV-2 and AAV-1, 6, 7, 8 antigens in subject previously infused with AAV-2. Cells from subject G were expanded, then stimulated with peptides as shown, and CD8+pentamer+ T cells secreting IFN-γ were identified by intracellular cytokine staining (Supplementary Methods). These studies were reviewed and approved by the Institutional Review Board of the Children's Hospital of Philadelphia. Informed consent was obtained from all human subjects. (g) Frequency of capsid-specific CD8+ T cells in blood of mice transgenic for HLA-B*0702, measured by pentamer described above. Mice were previously injected with an adenoviral vector expressing AAV-2 capsid (triangles), or with an AAV-2-F.IX by intramuscular (IM) injection (squares), or with the same vector by intravenous (IV) injection (circles). These experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia. AAV doses are reported in vector genomes per kilogram body weight (vg/kg) and adenovirus doses in vector particles per kilogram body weight (vp/kg).
We cultured PBMCs drawn at 20 weeks after vector infusion with the VPQYGYLTL peptide. A single round of in vitro stimulation increased the pool of antigen-specific CD8+ T cells by tenfold, and 3 rounds of stimulation increased the percentage to 25% (Fig. 1c). Similar robust expansion of capsid-specific CD8+ T cells responsive to a peptide carrying the AAV capsid sequence SADNNNSEY (identified by intracellular cytokine staining in healthy donors, using capsid-derived peptides, as previously described2) was observed in PBMCs from another subject with a HLA-A*0101 haplotype who had been infused with AAV-2-F.IX 2.5 years earlier (Fig. 1d). Expanded CD8+ T cells were functional, as evidenced by specific lysis of HLA-matched target cells (Fig. 1e) and by interferon (IFN)-γ production in response to AAV epitopes (Fig. 1f). Capsid-specific CD8+ T cells from the HLA-B*0702+ hemophilic subject functionally cross-reacted with the corresponding peptide (IPQYGYLTL) contained in AAV serotypes 1, 6, 7 and 8 (Fig. 1f), which was expected considering the high conservation of this epitope among multiple serotypes of AAV (ref. 3; Supplementary Table 1 online). To determine whether AAV infusion alone results in expansion of capsid-specific CD8+ T cells in naive mice, we infused mice transgenic for human HLA-B*0702 with AAV-2 vector over a range of doses and routes, and analyzed frequency of capsid-specific CD8+ T cells after infusion (Fig. 1g). In contrast to the human response, hepatic AAV-2 vector infusion at a range of doses failed to elicit capsid-specific CD8+ T cells in mice, although such cells were readily elicited in mice immunized with an adenoviral vector expressing AAV capsid (Fig. 1g and ref. 2). Thus, hepatic AAV vector infusion in a naive mouse failed to elicit expansion of capsid-specific CD8+ T cells, supporting our hypothesis that the response in human subjects was mediated by preexisting memory CD8+ T cells.
We analyzed the prevalence of AAV-specific memory T cells in the general human population by testing 46 adults for AAV-2 neutralizing antibodies and for frequencies of circulating capsid-specific T cells by a screening ELISpot assay (see Supplementary Methods online). Approximately half of the subjects (25/46) had readily detectable serum titers of AAV-2 neutralizing antibodies (Supplementary Table 2 online) while only 2/46 subjects had detectable T-cell responses in blood (Supplementary Table 2). We identified peptide GSGAPMADNNEGADG as containing the immunodominant T-cell epitope in one of these subjects (Supplementary Fig. 1 online). AAV-specific CD8+ T cells that produced IFN-γ in response to the peptide were CD45RA+CCR7−CD27+, a phenotype consistent with a subset of resting central memory CD8+ T cells4.
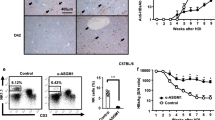
To increase the sensitivity of the T-cell detection method, we expanded PBMCs from seven HLA-B*0702+ healthy donors with peptide VPQYGYLTL and tested for AAV capsid–specific CD8+ T cells after 1–3 rounds of expansion (Fig. 2a). In two subjects that scored negative for AAV-specific CD8+ T cells after direct ex vivo testing, such T cells could be detected after in vitro expansion (Fig. 2a and data not shown), whereas five remained negative. Two of these five subjects had high-titer neutralizing antibodies to AAV-2, documenting prior exposure to AAV-2 (data not shown). Healthy-donor, capsid-specific CD8+ T cells that were expanded produced IFN-γ upon stimulation with the epitopic peptide (Fig. 2b): healthy-donor cells that were expanded with AAV-2 vector capsids produced IFN-γ upon stimulation with AAV-2 or AAV-8 capsids (Fig. 2c,d); similarly, healthy-donor cells that were expanded with AAV-8 capsid produced IFN-γ upon stimulation with AAV-1, AAV-2 or AAV-8 capsids, demonstrating that all three vectors could be processed appropriately in vitro to present the epitopic peptide to capsid-specific T cells (see Supplementary Note online).
(a,b) Healthy donors negative by ELISpot carry a pool of expandable AAV-specific T cells. (a) PBMCs from a healthy donor (ND1) with the HLA-B*0702 haplotype were stained with the HLA-B*0702-p74 (VPQYGYLTL) pentamer either directly (unexpanded) or after 1, 2 or 3 in vitro stimulations (IVS) with the p74 (VPQYGYLTL) peptide. Population of AAV-2–specific CD8+ T cells progressively rises. Plots shown are gated on forward and side scatter, pulse width and CD4−CD8+ cells. Numbers shown indicate percentage of pentamer+ cells within the plot. (b) Intracellular cytokine stain of PBMCs from a healthy HLA-A*0101 donor (ND2) previously expanded three times in vitro with peptide 99 (SADNNNSEY). Cells were stimulated with medium or peptide 99, surface stained for CD8 and CD4 antigens and stained intracellularly for IFN-γ. Plots shown are gated on forward and side scatter, pulse width and CD4−CD8+ cells. Numbers indicate percentage of IFN-γ–producing CD8+ cells. (c,d) CD8+ T cells from healthy donors cross-react with alternative serotypes. (c) Cells from a healthy donor (ND3) were expanded with AAV-2 whole capsid and restimulated with either AAV-2 or AAV-8 whole capsid, and IFN-γ production was measured by intracellular cytokine staining. Results reported as fold increase over medium control of percentage of CD8+ T cells producing IFN-γ. (d) Cells from a healthy donor (ND4) were expanded with AAV-8 whole capsid, then restimulated with whole capsid of AAV-8, AAV-2 or AAV-1, and IFN-γ synthesis was measured by intracellular cytokine staining. Results reported as fold increase over medium control of percentage of CD8+ T cells producing IFN-γ. Note that IFN-γ+ cells for subject G (Fig. 1f) represent a subset of CD8+pentamer+ T cells, whereas for ND3 and ND4 (Fig. 2c,d), IFN-γ+ cells are a subset of all CD8+ T cells. These studies were reviewed and approved by the Institutional Review Board of the Children's Hospital of Philadelphia. Informed consent was obtained from all human subjects.
As resting memory T cells may reside preferentially in lymphatic tissues, we tested splenocytes from 18 children and 10 adults (Supplementary Table 3 online). AAV-specific T cells were detected in only two subjects when cells were analyzed directly ex vivo. Upon expansion of cells with single AAV capsid peptides, however, responses were elicited in splenic samples of 5/8 children and 4/7 adults. Epitope mapping of the 5 pediatric samples and 1 adult sample showed that most subjects responded to one epitope; responses to two or more epitopes were detected in only one subject each (for example, Supplementary Fig. 1). Epitopes differed depending on the subjects' HLA type, and whereas some epitopes were highly conserved among different AAV serotypes, others were variable (Supplementary Table 1). Ongoing analysis of human splenocytes will allow definition of capsid epitopes for the most common HLA alleles.
Our data show that human subjects carry AAV-2–specific memory CD8+ T cells at frequencies that are commonly too low for detection by conventional direct ex vivo methods. The in vitro expansion method we employed to detect circulating AAV capsid–specific T cells may still not be sensitive enough to fully appreciate the prevalence of such T cells in humans, as indicated by the discrepancy between the presence of AAV neutralizing antibodies and circulating CD8+ T cells. In vitro stimulation with a single peptide requires a frequency of memory T cells at or above 1 in 106; lower levels will escape detection even upon in vitro expansion, since expansion typically begins with 106 cells.
Based on studies in experimental animals that have resulted in sustained transgene expression5,6,7, it has been assumed that AAVs fail to induce cellular immune responses because they are unable to trigger inflammatory reactions needed for differentiation of dendritic cells into professional antigen-presenting cells. Indeed, using immature human dendritic cells, we showed that AAV vectors, even if used at high doses, fail to induce dendritic cell maturation (data not shown). In humans, initial exposure to AAV-2 occurs in the context of a helper virus infection8, in which the robust inflammatory response to the helper virus most likely insures that CD8+ T cells to reactive AAV and the helper virus are primed concomitantly. It could be this preexisting memory CD8+ T-cell response that accounts for the difference in vector-infusion outcome between humans, the only natural hosts for AAV-2 infection, and other species.
It has recently been proposed that infusion of AAV-8 will lead to a different outcome, based on studies in nonhuman primates demonstrating a difference in the interactions of AAV-2 and AAV-8 with dendritic cells9. However, our data on PBMCs expanded from healthy human subjects by exposure to whole capsid (Fig. 2c,d) imply that alternative serotypes currently being considered for clinical applications (for example, AAV-1, AAV-8) are processed in a manner appropriate for presentation of the same epitope(s), at least by human cells, resulting in expansion of functional CD8+ T cells that are indistinguishable from those elicited by AAV-2 capsid. Moreover, and perhaps more to the point, since presentation by dendritic cells is not essential for activation of memory CD8+ T cells (see Supplementary Note), differential uptake of vectors by dendritic cells would be irrelevant to humans with immunological memory to AAV undergoing a second exposure to AAV capsid as a consequence of vector infusion. Our data would thus predict that alternate AAV serotypes will fare no better in hepatic gene transfer trials than AAV-2 vectors. Other target tissues may escape destruction by capsid-specific CD8+ T cells (see Supplementary Note).
In summary, we have documented a CD8+ T-cell response to AAV capsid in humans, and have presented evidence suggesting that memory CD8+ T cells become reactivated upon AAV gene transfer (seeSupplementary Note). We have shown that this population of cells can recognize other AAV serotypes, whether processed from whole capsid, or presented on peptide-loaded cells. Management of these CD8+ T-cell responses to capsid will ultimately be required for optimal use of AAV vectors in liver, and transient immunosuppressive regimens may be needed to achieve sustained AAV-mediated gene transfer in humans.
Note: Supplementary information is available on the Nature Medicine website.
References
Manno, C.S. et al. Nat. Med. 12, 342–347 (2006).
Sabatino, D.E. et al. Mol. Ther. 12, 1023–1033 (2005).
Gao, G.P. et al. Proc. Natl. Acad. Sci. USA 99, 11854–11859 (2002).
Appay, V. et al. Nat. Med. 8, 379–385 (2002).
Mount, J.D. et al. Blood 99, 2670–2676 (2002).
Wang, L., Nichols, T.C., Read, M.S., Bellinger, D.A. & Verma, I.M. Mol. Ther. 1, 154–158 (2000).
Snyder, R.O. et al. Nat. Med. 5, 64–70 (1999).
Muzyczka, N. & Berns, K.I. Parvoviridae: The Viruses and Their Replication, 2327–2359 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Vandenberghe, L.H. et al. Nat. Med. 12, 967–971 (2006).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grants P01 HL078810, M01-RR00240 (NIH GCRC award to Children's Hospital of Philadelphia), M01-RR000056 (NIH GCRC award to University of Pittsburgh), a grant to the Penn Center for AIDS Research P30 AI045008 and the Howard Hughes Medical Institute. D.J.H. was supported by training grant NIH T32 HL07439, S.L.M. by training grant NIH T32 DK07748 and D.E.S. by NIH F32 HL69647. We thank F. Lemonnier (Institute Pasteur) for kindly providing HLA-B*0702 transgenic mice. We thank M. Lasaro and M. Tigges for scientific input, and J. Sun for assistance in manuscript preparation.
Author information
Authors and Affiliations
Contributions
F.M. and M.V.M. performed the in vitro expansion experiments on normal donors and expansions on subjects with hemophilia B. They performed intracellular cytokine assays, the cytotoxic T lymphocyte (CTL) assay, the pentamer staining, the immunophenotyping of memory CD8+ T cells specific to the AAV capsid and the cross-reactivity experiments. D.J.H. performed experiments on human splenocytes and the AAV epitope characterization. D.E.S. performed in vitro expansion experiments on subjects with hemophilia B enrolled in the gene transfer study and part of the ELISpot studies on healthy donors. S.L.M. performed the experiments on HLA-B*0702 transgenic mice. H.J. and J.S. performed part of the ELISpot studies and AAV antibody titer determination on normal donors. C.S.M., M.V.R. and J.E.J.R. directed and/or participated in the clinical gene transfer study, including the care of hemophilic subjects, and collection of PBMCs used in the study. They provided assistance in drafting the manuscript. H.C.J.E. and G.F.P. collaborated on experimental design and interpretation, and helped to draft the manuscript. K.A.H. directed experimental design, conduct, data analysis and interpretation, and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.S., H.J. and G.F.P. were employees of Avigen, Inc. at the time the work was done. F.M., D.J.H. and K.A.H. hold pending patent applications that may be affected by publication of this manuscript.
Supplementary information
Supplementary Fig. 1
Immunophenotyping of capsid-specific CD8+ T cells in a healthy donor. (PDF 192 kb)
Supplementary Table 1
AAV capsid epitopes defined by IFN-γ ELISpot assays of normal donors and of AAV-infused subjects (PDF 56 kb)
Supplementary Table 2
Neutralizing antibody and cellular immune responses, sera and PBMCs from adult human subjects (PDF 48 kb)
Supplementary Table 3
IFN-γ ELISpot responses to AAV capsid in unexpanded and peptide-expanded human splenocytes (PDF 58 kb)
Rights and permissions
About this article
Cite this article
Mingozzi, F., Maus, M., Hui, D. et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med 13, 419–422 (2007). https://doi.org/10.1038/nm1549
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1549
This article is cited by
-
Multicenter assessment and longitudinal study of the prevalence of antibodies and related adaptive immune responses to AAV in adult males with hemophilia
Gene Therapy (2024)
-
Prednisolone and rapamycin reduce the plasma cell gene signature and may improve AAV gene therapy in cynomolgus macaques
Gene Therapy (2024)
-
Molecular functions of microRNAs in colorectal cancer: recent roles in proliferation, angiogenesis, apoptosis, and chemoresistance
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Immune profiling of adeno-associated virus response identifies B cell-specific targets that enable vector re-administration in mice
Gene Therapy (2023)
-
Clonal selection of hematopoietic stem cells after gene therapy for sickle cell disease
Nature Medicine (2023)