Abstract
Adiponectin (Ad) is a hormone secreted by adipocytes that regulates energy homeostasis and glucose and lipid metabolism. However, the signaling pathways that mediate the metabolic effects of Ad remain poorly identified. Here we show that phosphorylation and activation of the 5′-AMP-activated protein kinase (AMPK) are stimulated with globular and full-length Ad in skeletal muscle and only with full-length Ad in the liver. In parallel with its activation of AMPK, Ad stimulates phosphorylation of acetyl coenzyme A carboxylase (ACC), fatty-acid oxidation, glucose uptake and lactate production in myocytes, phosphorylation of ACC and reduction of molecules involved in gluconeogenesis in the liver, and reduction of glucose levels in vivo. Blocking AMPK activation by dominant-negative mutant inhibits each of these effects, indicating that stimulation of glucose utilization and fatty-acid oxidation by Ad occurs through activation of AMPK. Our data may provide a novel paradigm that an adipocyte-derived antidiabetic hormone, Ad, activates AMPK, thereby directly regulating glucose metabolism and insulin sensitivity in vitro and in vivo.
Similar content being viewed by others
Main
Impaired glucose and lipid metabolism, a hallmark of obesity and type 2 diabetes1, causes increased lipid stores in insulin target tissues such as muscle and liver, thereby leading to insulin resistance2. Obesity and type 2 diabetes are associated with decreased plasma adiponectin (Ad) levels3, which seem to be associated with an elevated triglyceride (TG) content in skeletal muscle and liver4,5. In fact, Ad decreases insulin resistance by increasing fatty-acid oxidation, which reduces the TG content in these tissues in obese and type 2 diabetic mice4,5. Adiponectin also suppresses hepatic glucose production6,7. However, the signaling pathways that mediate the metabolic effects of Ad remain poorly identified. The β-isoform of coenzyme A carboxylase (ACC-β), which is the predominant isoform in skeletal muscle, is an essential regulator of fatty-acid oxidation8. The activation of 5′-AMP-activated protein kinase (AMPK) by muscle contraction and hypoxia has been shown to phosphorylate ACC-β, which leads to the inhibition of ACC activity and a consequent reduction in the malonyl-CoA content, thereby derepressing carnitine palmitoyltransferase 1 activity and increasing fatty-acid oxidation3,9,10. The activation of AMPK has also been shown to stimulate glucose uptake independent of its action on fatty acid in skeletal muscle9,10,11, and to reduce expression levels of molecules involved in gluconeogenesis such as phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) in hepatocytes12.
Here we show that phosphorylation and activation of AMPK are stimulated with globular Ad (gAd) in skeletal muscle and with full-length Ad in the liver. Adiponectin stimulates phosphorylation of ACC, fatty-acid oxidation, glucose uptake and lactate production in myocytes, phosphorylation of ACC and reduction of molecules involved in gluconeogenesis in the liver, and reduction of glucose levels in vivo via activation of AMPK. We conclude that the adipocyte-derived hormone, adiponectin, activates AMPK and thereby directly regulates glucose metabolism and insulin sensitivity in vitro and in vivo.
Ad stimulates β-oxidation and glucose uptake in myocytes
To elucidate the signaling pathways by which Ad regulates fatty acid and glucose metabolism, we incubated C2C12 myocytes in vitro with Ad. Treatment of C2C12 myocytes with Ad for 60 minutes stimulated fatty-acid oxidation in vitro4 (Fig. 1a). Actinomycin D suppressed stimulation of fatty-acid oxidation by peroxisome proliferator-activated receptor (PPAR)-α agonist Wy-14,643, but had no effect on increase in fatty-acid oxidation observed in Ad-treated cells (Fig. 1a). This finding suggests that these effects of Ad may be independent of transcriptional regulation. The treatment of C2C12 myocytes with Ad also increased glucose uptake in vitro (Fig. 1b). Moreover, wortmannin inhibited insulin-stimulated glucose uptake, but had little effect on the increase in glucose uptake induced by Ad (Fig. 1b). These findings indicate that Ad exerts these effects via phosphatidyl inositol 3 (PI3)-kinase independent pathways. As AMPK has been reported to stimulate β-oxidation and insulin-independent glucose uptake9,10, we hypothesized that AMPK might mediate the effects of Ad on lipid and glucose metabolism in muscle.
a, In vitro fatty-acid oxidation in C2C12 muscle cells treated with the indicated concentrations (μg/ml) of gAd or full-length Ad, or 1 × 10−5 M Wy-14,643 for 6 h, or 0.5 mM AICAR with (□) or without (▪) 5 μg/ml of actinomycin D. Measurements were made at 60 min. b, Glucose uptake in C2C12 cells treated with Ad as indicated, or with 1 × 10−7 M insulin or 0.5 mM AICAR with (□) or without (▪) 100 nM wortmannin. Measurements were made at 30 min. c–g, Immunoblot analyses of AMPK phosphorylation (c–e) and ACC phosphorylation (f and g) in C2C12 muscle cells treated with Ad as indicated. Measurements were made at the indicated time periods (in c, d and f), or at 5 min (e) or 15 min (g). d and f, (○), gAd 0.5; (▵), Ad 25. a–b, e and g, each bar represents the mean ± s.e. (n = 5–10). *, P < 0.05; **, P < 0.01; compared with vehicle, or between 2 groups as indicated.
Ad stimulates phosphorylation of AMPK and ACC in myocytes
The treatment of C2C12 myocytes with AICAR (5-aminoimidazole-4-carboxamide 1-d-ribofuranoside), a cell-permeable activator of AMPK (refs. 9,10), caused sustained phosphorylation of Thr 172 in the α subunit of AMPK (αAMPK); in contrast, treatment with gAd or full-length Ad caused a rapid, robust and transient increase in AMPK phosphorylation (Fig. 1c). Full-length Ad at concentrations of 10 and 25 μg/ml, which are close to the physiological range5,6, increased AMPK phosphorylation in a sequential (Fig. 1d) and dose-dependent fashion (Fig. 1e). Globular Ad increased AMPK phosphorylation more potently than full-length Ad (Fig. 1e).
We next determined whether Ad stimulates phosphorylation of ACC subsequent to the phosphorylation of AMPK (refs. 3,10). The treatment of C2C12 myocytes with gAd or full-length Ad increased ACC phosphorylation in a sequential (Fig. 1f) and dose-dependent fashion (Fig. 1g). The effect peaked with a 2.2-fold rise at 15 minutes with full-length Ad at concentration of 25 μg/ml, which was comparable with the increase induced by AICAR (Fig. 1g), and returned to the baseline value by 60 minutes (Fig. 1f and data not shown).
Ad increases AMPK and ACC phosphorylation in muscle
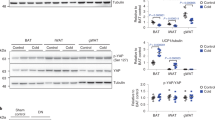
We treated mice with Ad to determine whether Ad could stimulate AMPK phosphorylation in skeletal muscle in vivo. The administration of gAd or full-length Ad in mice increased AMPK phosphorylation in the soleus muscle in a dose-dependent fashion (Fig. 2a and b ). The effect peaked with a 2-fold rise at 5 minutes and returned to the baseline value by 60 minutes (Fig. 2b and data not shown). The effect of Ad on the phosphorylation of AMPK in white (fast twitch, glycolytic) muscles was almost the same as in red (slow twitch, oxidative) skeletal muscles (data not shown).
a and b, Immunoblot analyses of phosphorylation of αAMPK in soleus muscle from mice treated with the indicated concentrations of gAd, full-length Ad, or with AICAR. Measurements were made at 2 min (▪ in b) or 5 min (a, □ in b). c and d, Activity of α1AMPK (c) and α2AMPK (d) in soleus muscle of mice treated with Ad as indicated. Measurements were made at 5 min. e and f, Immunoblot analyses of phosphorylation of ACC in soleus muscle from mice treated with Ad or AICAR as indicated. Measurements were made at 5 min (▪ in f) or 15 min (e, □ in f). In all panels, doses of Ad are μg per 10 g body weight; AICAR given at 1 g per kg body weight. b–d and f, each bar represents the mean ± s.e. (n = 8–10). *, P < 0.05; **, P < 0.01; compared with vehicle.
The phosphorylation of Thr 172 in αAMPK is associated with activation of both the α1 and α2 subunits of AMPK (ref. 13). The administration of gAd in mice significantly increased α1- and α2AMPK activities and full-length Ad also significantly increased α2AMPK activity in the soleus muscle (Fig. 2c and d ). Globular Ad, which has a higher binding affinity to the membrane fractions of skeletal muscles than full-length Ad (see Discussion), had a more pronounced effect on AMPK activation in skeletal muscle (Fig. 2c and d ). The administration of globular or full-length Ad increased ACC phosphorylation in the soleus muscle at 15 minutes (Fig. 2e and f ). The effect peaked with a 2.2-fold rise at 15 minutes with gAd at a concentration of about 10 μg/ml, which was greater than the increase with AICAR (Fig. 2f), and returned to the baseline value by 60 minutes (Fig. 2f and data not shown).
To elucidate the mechanisms by which Ad activates AMPK, we measured the concentrations of cellular AMP (refs. 9,10), a known activator of AMPK. The AMP content of C2C12 myocytes and soleus muscle increased by about two-fold after five minutes of treatment with Ad (Table 1). Neither ATP nor ADP concentrations changed in response to the Ad treatment (Table 1). Thus, the activation of AMPK that occurs five minutes after Ad treatment may result from an increase in the concentration of cellular AMP.
AMPK is necessary for several metabolic effects of Ad
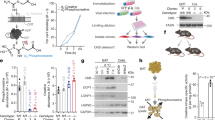
α2AMPK is the predominant isoform in skeletal muscle9,10,11. To determine whether the AMPK pathway is required for the effects of Ad in muscle cells, we blocked AMPK activation using a catalytically inactive α2AMPK, which has a dominant-negative (DN) effect on both α1 and α2AMPK activities in skeletal muscle11. The C2C12 myocytes infected with a retrovirus containing DN-α2AMPK display unaltered levels of αAMPK compared with the cells infected with a retrovirus containing Mock (Fig. 3) as described11. Since the inactive mutated catalytic α subunit has the same affinity toward the βγ subunits, an excess amount of the inactive mutated catalytic α subunit would displace the endogenous form to liberate it as a free α subunit which would readily be degraded as described11. Both globular and full-length Ad induced an increase in AMPK phosphorylation by 1.8-fold in control C2C12 myocytes (Fig. 3a). These effects were indeed blocked by the DN-α2AMPK (Fig. 3a).
a–d, C2C12 myocytes infected with a retrovirus containing mock (▪) or DN-α2AMPK (□) (in b–d) were treated with the indicated concentrations (μg/ml) of gAd or full-length Ad (a–d), 1 × 10−5 M Wy-14,643 (b), or 1 × 10−7 M insulin (c). Immunoblot analysis (a) was performed to measure expression of DN-AMPK (blot 1), phosphorylation (blot 2) and amount (blot 3) of αAMPK and phosphorylation (blot 4) and amount (blot 5) of ACC. Fatty-acid oxidation (b), glucose uptake (c) and lactate production (d) were also measured. e, Isolated hepatocytes, transfected with an adenovirus expressing LacZ or DN-α1AMPK, were treated with the indicated concentrations (μg/ml) of gAd or Ad, and immunoblot analysis was performed to measure expression of DN-AMPK (blot 1) and phosphorylation of αAMPK (blot 2) and ACC (blot 3). f and g, Binding affinity of globular (gAd) (○) or full-length Ad (▵) to the membrane fractions of skeletal muscle (f) or the liver (g). Results are expressed as the percentage of the value in the absence of competitor (f and g). b–d, each bar represents the mean ± s.e. (n = 5–10). *, P < 0.05; **, P < 0.01; between two groups, as indicated, or gAd versus Ad.
Both globular and full-length Ad induced an increase in ACC phosphorylation (Fig. 3a), fatty-acid oxidation (Fig. 3b), glucose uptake (Fig. 3c) and lactate production (Fig. 3d) in C2C12 myocytes. These effects were blocked by the retrovirus- (Fig. 3a–d) or adenovirus-mediated (data not shown) expression of DN-α2AMPK. Thus, the activation of AMPK is necessary for the Ad-induced stimulation of ACC phosphorylation, fatty-acid oxidation, glucose uptake and lactate production in muscle cells.
Only full-length Ad stimulates hepatocyte AMPK
To determine whether Ad also activates AMPK in hepatocytes, we next studied the phosphorylation of AMPK and ACC in isolated hepatocytes. Only full-length Ad was capable of stimulating phosphorylation of AMPK (Fig. 3e) and ACC (Fig. 3e) in isolated hepatocytes, but gAd was not (Fig. 3e), even when higher or equal doses (that is, 25–50 μg/ml) of gAd were used (data not shown).
ACC phosphorylation by Ad requires AMPK in hepatocytes
α1AMPK is the predominant isoform in liver9,10,14. To determine whether the AMPK pathway is required for the effects of Ad on ACC phosphorylation in isolated hepatocytes, we blocked AMPK activation using a catalytically inactive α1-subunit of AMPK, which has a dominant-negative effect on both α1 and α2AMPK activities in hepatocytes14. Full-length Ad induced an increase in AMPK phosphorylation in control, isolated hepatocytes (Fig. 3e). These effects were indeed blocked by the expression of DN-α1AMPK via adenovirus-mediated gene transfer (Fig. 3e).
Full-length Ad induced an increase in ACC phosphorylation in control isolated hepatocytes (Fig. 3e). These effects were markedly reduced by DN-α1AMPK (Fig. 3e). Thus, the activation of AMPK is necessary for the Ad-induced stimulation of ACC phosphorylation in isolated hepatocytes.
Binding of Ad to membrane fractions of muscle/liver
To elucidate the mechanisms by which different forms of Ad are effective in liver versus muscle, we measured binding affinities of gAd and full-length Ad to the membrane fractions of skeletal muscle or the liver. gAd had a higher binding affinity to the membrane fractions of skeletal muscle than full-length Ad (Fig. 3f), whereas full-length Ad had a higher binding affinity to the membrane fractions of liver than gAd (Fig. 3g). These findings were consistent with the data on the potency of gAd or full-length Ad in causing phosphorylation and activation of AMPK in liver and muscle cells.
Ad activates AMPK and phosphorylates ACC in liver in vivo
We next treated mice with Ad to determine whether Ad could stimulate the phosphorylation and activation of AMPK in liver in vivo. Only full-length Ad, which has a higher binding affinity to the membrane fractions of the liver (Fig. 3g) was capable of stimulating phosphorylation (Fig. 4a and b ) and activation of α1 (Fig. 4c) and α2AMPK (Fig. 4d) in the liver, but gAd was not (Fig. 4a–d), even when higher or equal doses of gAd were used (data not shown). The effect peaked with a 2.5-fold increase at 5 minutes and returned to the baseline value by 60 minutes (Fig. 4b and data not shown).
a–f, Immunoblot analyses of phosphorylation of αAMPK (a and b) and ACC (e and f), and activity of α1AMPK (c) and α2AMPK (d), in the liver from mice treated with the indicated concentrations (μg per 10 g body weight) of gAd, full-length Ad, or AICAR (0.5 mM or 1 g/kg body weight). Measurements were made at the following times: in a, c and d, 5 min; in b, 2 min (▪) or 5 min (□); in f, 5 min (▪) or 15 min (□); and in e, 15 min. Each bar represents mean ± s.e. (n = 8–10). *, P < 0.05; **, P < 0.01; compared with vehicle.
Only full-length Ad, but not gAd, was capable of increasing ACC phosphorylation in the liver (Fig. 4e and f ). The effect peaked with a 2.2-fold rise at 15 minutes and returned to the baseline value by 60 minutes (Fig. 4f and data not shown).
Glucose-lowering effect of Ad requires liver AMPK activation
To investigate whether the AMPK pathway is required for the effects of Ad on glucose metabolism in vivo, we injected mice with adenoviruses expressing LacZ or DN-α1AMPK (ref. 14) intraperitoneally, and then compared glucose-lowering effect of Ad (refs. 4,6) between them. The administration of full-length Ad triggered a reduction in glucose levels4,6 in mice injected with adenoviruses expressing LacZ by about 40%, compared with the effect of administering phosphate buffered saline (PBS) in vivo (Fig. 5a). Injection with adenoviruses containing DN-α1AMPK partially but significantly reduced the glucose-lowering effect of Ad (Fig. 5a), indicating that this effect was dependent at least in part on AMPK. To clarify the mechanisms by which injection into mice with adenoviruses containing DN-α1AMPK reduced the glucose-lowering effect of Ad, we first determined where DN-α1AMPK was expressed and where it reduced AMPK activation by Ad. Expression of DN-α1AMPK was detected in the liver (Fig. 5b, upper panel), which indeed blocked AMPK activation by Ad in the liver (Fig. 5c), but DN-α1AMPK expression and action were not detected in other tissues we studied, including skeletal muscle (Fig. 5b and d ).
a, Plasma glucose levels during the Ad tolerance test in mice transfected with an adenovirus containing LacZ or DN-α1 AMPK, treated with 84 μg per g body weight of full-length Ad. (○), LacZ+vehicle; (●), LacZ+Ad; (▵), DN-α1AMPK+vehicle; (▴), DN-α1AMPK+Ad. b, Immunoblot analyses of DN-α1AMPK (top blots of each subpanel) and αAMPK protein (bottom blots) in liver or muscle of mice treated with indicated adenoviral expression constructs, plus full-length Ad for 30 min, as in a. c and d, AMPK activity in liver (c) or muscle (d) of mice treated as indicated; measurements were made at 30 min. e, Northern-blot analysis of liver RNA isolated from mice treated with indicated adenoviruses, plus Ad or vehicle, as in a. Representative blots probed for PEPCK (top panel) or G6Pase (middle panel), as well as reference 28S RNA lanes (bottom panel) are shown. Percentages shown are relative to control mice injected with LacZ adenovirus and treated with vehicle. Measurements were made at 4 h. For c–e, each bar represents the mean ± s.e., n = 3–10. In all panels, *, P < 0.05; **, P < 0.01; between two groups, as indicated, or LacZ versus DN-α1AMPK.
We next studied the mechanisms by which the expression of DN-α1AMPK in the liver reduced glucose-lowering effect of Ad. Full-length Ad reduced expression levels of molecules involved in gluconeogenesis such as PEPCK and G6Pase in the liver (Fig. 5e) as reported7. Expression of DN-α1AMPK in the liver blocked these effects of Ad (Fig. 5e), which was consistent with a previous report that activation of AMPK reduced expression levels of PEPCK and G6Pase12. Thus, activation of AMPK in the liver is necessary for Ad to reduce expression levels of molecules involved in gluconeogenesis in the liver, and trigger an in vivo reduction in glucose levels.
Discussion
Here we show that Ad increases phosphorylation and activity of AMPK, and that it simultaneously increases glucose uptake and lactate production in C2C12 myocytes as well as in skeletal muscle in vivo and also reduces the expression of molecules involved in gluconeogenesis in the liver7, thereby reducing glucose levels in vivo4,6. Ad also increases phosphorylation of ACC and fatty-acid oxidation in C2C12 myocytes as well as in isolated hepatocytes. These actions appear to be mediated by AMPK, as the dominant-negative AMPK blocks each of these effects. Thus, we demonstrate that the adipocyte-derived hormone Ad activates AMPK, and thereby directly regulates glucose metabolism and insulin sensitivity. From the data presented, the primary effect of Ad may not be to increase AMP kinase activity, but to increase cellular AMP levels by an unidentified mechanism. Mitochondrial uncoupling or activation of adenine nucleotide phosphatases are potential mechanisms by which Ad increased AMP levels; however, this remains to be clarified.
The results of this study also suggest that while gAd activates AMPK and stimulates glucose uptake and fatty-acid oxidation more potently than full-length Ad in skeletal muscle, for the most part, only full-length Ad activates AMPK in the liver. These results can be explained by the finding that gAd has a higher binding affinity to the membrane fractions of skeletal muscle than does full-length Ad (Fig. 3f), whereas full-length Ad has a higher binding affinity to the membrane fractions of the liver than gAd (Fig. 3g). However, the possibility could not be excluded that differences in binding affinity may not be the sole explanation for the differential effects of globular and full-length Ad on muscle versus liver. Moreover, it remains to be determined whether these differential forms correspond to physiologically relevant isoforms. Whether the putative skeletal muscle and liver membrane receptors for Ad are structurally and functionally distinct is now under investigation.
The observations that exercise9,10, antidiabetic adipokines such as Ad and leptin15 and the antidiabetic drug metformin16,17 and rosiglitazone17 can all activate AMPK suggest that AMPK plays crucial and central roles in the regulation of energy expenditure and glucose and lipid metabolism. The AMPK pathway might therefore provide useful targets for therapeutic agents intended to reduce lipotoxicity in patients with obesity and type 2 diabetes.
Methods
Animals.
Male C57BL/6 mice (aged 8–10 wk) were purchased from Japan CREA. To measure AMPK activity in vivo, the indicated amounts of recombinant murine Ad or 1 g per kg (body weight) of AICAR were injected intravenously in mice through an inferior vena cava catheter15. Administration of a given amount, 'x' μg per 10 g body weight of Ad resulted in increase of approximately 'x' μg/ml of plasma Ad levels, thus the doses used in this study were comparable with those of endogenous Ad levels. The Animal Care Committee of the University of Tokyo approved the animal care and experimental procedures.
Generation of recombinant Ad.
Bacterially expressed murine Ad and Ad produced in a mammalian expression system were generated as described5,6. As reported18, immunoblot analysis after cross-linking using Bis (Sulfosuccinimidyl) suberate (BS3) indicated that full-length Ad expressed in and purified from E. coli and NIH3T3 cells forms trimers and hexamers; NIH3T3 cells also produce a higher molecular weight (HMW) species (Supplementary Fig. A). In contrast, gAd expressed in and purified from E. coli and NIH3T3 cells exists as both monomer and trimer (Supplementary Fig. A). Importantly, no significant differences were observed between the bacterially expressed gAd/Ad and the gAd/Ad produced by the mammalian expression system in the binding and biological activity (data not shown).
Western-blot analysis and measurement of AMPK activities.
Phosphorylation and protein levels of αAMPK and ACC, the expression levels of DN-AMPK, isoform-specific AMPK activity, and AMPK activity in the study with DN-α1AMPK were determined as described15,18,19,20. Immunoblot analyses were performed using anti-myc, anti-p-AMPK, anti-αAMPK and p-ACC and streptavidin.
RNA preparation and northern-blot analysis.
The total RNA from 5–10 mice in each group was pooled, and aliquots were subjected to northern-blot analysis5 using probes for mouse PEPCK and G6Pase (from K. Motojima). The radioactivity in each band was quantified7, and the fold change in each mRNA was calculated after correcting for loading differences by measuring the amount of 28S rRNA. Representative data from 1 of 3 independent experiments are shown.
Lipid and glucose metabolism.
The measurement of [14C]CO2 production from [1-14C]palmitic acid was performed using cell lysates, as described5. The lactate concentration was measured using a calorimetric method (Lactate C; Wako Pure Chemical Industries, Osaka, Japan). Glucose uptake was determined as described21. The effect of Ad on stimulation of glucose uptake peaked at 30 min, whereas AICAR had a much more sustained effect on stimulation of glucose uptake (data not shown).
Studies with C2C12 myocytes and isolated hepatocytes.
cDNA encoding α1 or α2AMPK, containing a mutation that alters aspartic acid residue 157 to alanine or lysine residue 45 to arginine, was used as a DN-α1AMPK (ref. 14) or α2AMPK (ref. 11), respectively.
The DN-α2AMPK expression vector for retrovirus-mediated gene transfer was constructed by ligating into the EcoRI/NotI site of pMX-puro22. C2C12 cells were infected with equal titers of retrovirus containing a mock vector or DN-α2AMPK, as previously described23, with some modifications. Induction of differentiation was carried out as described1. 5 d later, cells were treated with the indicated concentrations of Ad, and then subjected to analysis. The retrovirus-mediated expression of DN-α2AMPK had little effect on expression levels of MyoD and myogenin protein in C2C12 myocytes. Isolated hepatocytes14 were transfected with adenovirus containing LacZ or DN-α1AMPK. The cells were treated with the indicated concentrations of Ad, and phosphorylation of AMPK and ACC was examined.
Adenovirus-mediated gene transfer in vivo and Ad tolerance test.
Mice were injected intraperitoneally with adenoviruses containing LacZ or DN-α1AMPK at a concentration of 3 × 108 plaque-forming units (p.f.u.) per gram of body weight, as described24. 3–5 d after injection, mice were subjected to a 3-hour fast before testing. Adiponectin (84 μg per g body weight) was injected intraperitoneally6 followed by glucose measurement at 0, 4, 8, and 12 h or at 4 h; the mouse tissues were then collected and subjected to northern-blot analysis. The liver from mice injected with adenoviruses showed no significant changes, including in their morphology. Livers of mice infected with an adenovirus containing DN-α1AMPK display unaltered levels of αAMPK compared with livers of mice infected with an adenovirus containing LacZ (Fig. 4b) as described14. Since the inactive mutated catalytic α subunit has the same affinity toward the βγ subunits, an excess amount of the inactive mutated catalytic α subunit would displace the endogenous form to liberate it as a free α subunit, which would readily be degraded as described14.
Binding affinity of Ad to membrane fractions of skeletal muscle and the liver.
Recombinant gAd/Ad was biotinylated with NHS-LC-Biotin (Pierce, Rockford, USA). Biotinylation of Ad had little effect on the higher order structures, binding affinity to the membrane fractions of skeletal muscle or the liver, or AMPK phosphorylation in C2C12 myocytes (data not shown). Adiponectin binding assay was carried out as described21. The binding affinity of Ad to membrane fractions of skeletal muscle and the liver seems comparable, with a concentration expected of typical receptor–ligand interactions (Fig. 3f and g ).
Measurement of AMP, ADP and ATP content.
Soleus muscles or C2C12 myocytes treated with Ad were homogenized in a Krebs-Ringer bicarbonate (KRB) buffer containing ice-cold HClO4. The lysates were then neutralized by the addition of NaOH. The AMP, ADP and ATP contents in the supernatant were measured using a bioluminescent assay kit, as described25.
Note: Supplementary information is available on the Nature Medicine website.
References
Kahn, B.B. & Flier, J.S. Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 (2000).
Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 (2000).
Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 (1999).
Fruebis, J. et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 98, 2005–2010 (2001).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Med. 7, 941–946 (2001).
Berg, A.H., Combs, T.P., Du, X., Brownlee, M. & Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature Med. 7, 947–953 (2001).
Combs, T.P., Bergm, A.H., Obici, S., Scherer, P.E. & Rossetti, L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108, 1875–1881 (2001).
Abu-Elheiga, L., Matzuk, M.M., Abo-Hashema, K.A.H. & Wakil, S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291, 2613–2616 (2001).
Hardie, D.G., Carling, D. & Carlson, M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Ann. Rev. Biochem. 67, 821–855 (1998).
Winder, W.W. & Hardie, D.G. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 277, E1–E10 (1999).
Mu, J., Brozinick, J.T. Jr., Valladares, O., Bucan, M. & Birnbaum, M.J. A role for AMP-activated protein kinase in contraction—and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell. 7, 1085–1094 (2000).
Lochhead, P.A., Salt, I.P., Walker, K.S., Hardie, D.G. & Sutherland, C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes 49, 896–903 (2000).
Stein, S.C., Woods, A., Jones, N.A., Davison, M.D. & Carling, D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 345, 437–443 (2000).
Woods, A. et al. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell. Biol. 20, 6704–6711 (2000).
Minokoshi, Y. et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 (2002).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Fryer, L.G., Parbu-Patel, A. & Carling, D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277, 25226–25232 (2002).
Tsao, T.S, Murrey, H.E., Hug, C., Lee, D.H. & Lodish, H.F. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J. Biol. Chem. 277, 29359–29362 (2002).
Woods, A., Salt, I., Scott, J., Hardie, D.G. & Carling, D. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 397, 347–351 (1996).
Hayashi, T. et al. Metabolic stress and altered glucose transport. Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49, 527–531 (2000).
Kaburagi, Y. et al. Site-directed mutagenesis of the juxtamembrane domain of the human insulin receptor. J. Biol. Chem. 268, 16610–166222 (1993).
Onishi, M. et al. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol. Cell. Biol. 18, 3871–3879 (1996).
Tontonoz, P., Hu, E. & Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR γ2, a lipid-activated protein transcription factor. Cell 79, 1147–1156 (1994).
Ueki, K. et al. Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J. Clin. Invest. 105, 1437–1445 (2000).
Tsubamoto, Y. et al. Hexamminecobalt(III) chloride inhibits glucose-induced insulin secretion at the exocytotic process. J. Biol. Chem. 276, 2979–2985 (2001).
Acknowledgements
We thank M.J. Birnbaum, O. Ezaki and N. Kubota for helpful suggestions; K. Motojima for the PEPCK and G6Pase cDNA probes; and K. Kirii, M. Shibata, A. Okano and T. Nagano for technical assistance. This work was supported by a grant from the Human Science Foundation (to T.K.), a Grant-in-Aid for the Development of Innovative Technology from the Ministry of Education, Culture, Sports, Science and Technology (to T.K.), a Grant-in-Aid for Creative Scientific Research 10NP0201 from the Japan Society for the Promotion of Science (to T.K.), and by Health Science Research Grants (Research on Human Genome and Gene Therapy) from the Ministry of Health and Welfare (to T.K.) and NIH grants PO1 DK 56116 and RO1 DK 43051.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yamauchi, T., Kamon, J., Minokoshi, Y. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8, 1288–1295 (2002). https://doi.org/10.1038/nm788
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm788
This article is cited by
-
Adiponectin attenuates H2O2-induced apoptosis in chicken skeletal myoblasts through the lysosomal-mitochondrial axis
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Epididymal RNase T2 contributes to astheno-teratozoospermia and intergenerational metabolic disorder through epididymosome-sperm interaction
BMC Medicine (2023)
-
Sex differences in obesity-induced renal lipid accumulation revealed by lipidomics: a role of adiponectin/AMPK axis
Biology of Sex Differences (2023)
-
The effects of sodium butyrate supplementation on the expression levels of PGC-1α, PPARα, and UCP-1 genes, serum level of GLP-1, metabolic parameters, and anthropometric indices in obese individuals on weight loss diet: a study protocol for a triple-blind, randomized, placebo-controlled clinical trial
Trials (2023)
-
Two-year changes in body composition and future cardiovascular events: a longitudinal community-based study
Nutrition & Metabolism (2023)