Abstract
By combining astigmatism imaging with a dual-objective scheme, we improved the image resolution of stochastic optical reconstruction microscopy (STORM) and obtained <10-nm lateral resolution and <20-nm axial resolution when imaging biological specimens. Using this approach, we resolved individual actin filaments in cells and revealed three-dimensional ultrastructure of the actin cytoskeleton. We observed two vertically separated layers of actin networks with distinct structural organizations in sheet-like cell protrusions.
Similar content being viewed by others
Main
Recent advances in super-resolution fluorescence microscopy have substantially increased the spatial resolution of optical imaging1,2. In optical imaging of biological samples, resolutions down to ∼20 nm have been achieved in lateral directions with axial resolutions of 10–100 nm (refs. 1,2). This enhanced resolution has enabled the visualization of various cellular ultrastructures previously inaccessible to optical methods. However, the current state-of-the-art resolution still cannot resolve many cellular structures, and further improvement of resolution is desirable.
For example, among the three major types of cytoskeletal structures, individual microtubules and intermediate filaments can be observed with optical microscopy; in contrast, individual actin filaments have not been resolved in cells by optical means, including super-resolution methods3,4,5,6, owing to the small diameter and high packing density of actin filaments. However, actin is crucial for many cellular processes7. In particular, the assembly and disassembly of actin filaments in the thin sheet-like cell protrusions drive cell locomotion8. The knowledge of how actin filaments are spatially organized is critical for understanding these processes. Although electron microscopy and cryo-electron tomography can resolve individual actin filaments in cells, three-dimensional (3D) reconstructions are still challenging owing in part to the structural perturbations induced by the dehydration and embedding treatment required for conventional electron microscopy and the difficulty in reconstructing large volumes by cryo-tomography9,10. Therefore, except for the several hundred nanometers near the cell edge, a full 3D reconstruction of actin has not been achieved for the sheet-like cell protrusion10, and how actin is vertically organized in this region is still unclear (Supplementary Discussion).
We set out to increase the image resolution of super-resolution fluorescence microscopy by combining 3D STORM11,12 with a dual-objective detection scheme13,14 (Fig. 1a). In the original 3D STORM approach12, at any given instant, an optically resolvable subset of fluorescent probes are activated and imaged using a single objective. Astigmatism is introduced in the detection path using a cylindrical lens such that the images obtained for individual molecules are elongated in x and y directions for molecules on the proximal and distal sides of the focal plane, respectively. The lateral and axial coordinates of the molecules are determined from the centroid positions and ellipticities of these single-molecule images, respectively. Through iteration of the activation and imaging cycles, the positions of many molecules can be determined and a super-resolution image can be reconstructed from these molecular coordinates. It could be reasoned that total collected fluorescence signal would double by sandwiching the sample between two opposing objectives and detecting the signal from both objectives13,14. Because the localization uncertainty of each molecule scales with the inverse square root of the number of photons detected, doubling the photon count would yield a 1.4-fold improvement in the image resolution.
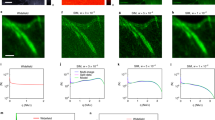
(a) Schematic of setup. Two microscope objectives are placed opposite each other and focused on the same spot of the sample. Astigmatism is introduced into the images collected by both objectives using a cylindrical lens. M, mirror; Obj., objective; LP, long-pass filter; CL, cylindrical lens; BP, band-pass filter. (b) Localization precision of Alexa Fluor 647 molecules in fixed cells measured with dual-objective STORM. Each molecule gives a cluster of localizations owing to repetitive activation of the same molecule. Localizations from 108 clusters (each containing >10 localizations) are aligned by their center of mass to generate the 3D presentation of the localization distribution. Histograms of distribution in x, y and z are fit to Gaussian functions, and the resultant s.d. (σx, σy and σz) is shown. (c) Distribution of number of photons detected for individual Alexa Fluor 647 molecules through both objectives (red; average, 10,600) and from a single objective (black; average, 5,200). (d) Images of activated Alexa Fluor 647 molecules obtained from two objectives in a single frame. A molecule that appears elongated in x through one objective should appear elongated in y through the opposing objective (examples, green and blue arrows). In contrast, if two nearby molecules were mistaken for a single molecule, the images obtained through both objectives would appear elongated in the same direction along the line that connects the two molecules (example, magenta arrows). Scale bar, 2 μm.
To characterize the localization precision of the dual-objective 3D STORM setup, we imaged individual Alexa Fluor 647 molecules scattered in fixed cells within ∼150 nm of the focal plane, a range comparable to the thickness of the sheet-like cell protrusions. In this work, we use the term cell protrusion to refer to the thin region of the cell near its edge generally without distinguishing protruding or retracting regions. Although we limited our imaging z range to be comparable to the thickness of cell protrusions here, the imaging depth could be increased by stepping the sample in the z direction. As each Alexa Fluor 647 molecule can be switched on and off multiple times, we experimentally determined the localization precision by the standard deviation (s.d.) of repetitive localizations of the same molecule. Notably, the measured localization precisions, ∼4 nm in the x and y directions and ∼8 nm in the z direction (Fig. 1b), are more than twofold greater than reported values with the same fluorophore using single-objective STORM12. This localization precision corresponds to an image resolution of ∼9 nm in the lateral directions and ∼19 nm in the axial direction, measured in full width at half maximum (FWHM).
By examining the single-molecule images, we found that beyond the expected doubling of photon-collection efficiency (Fig. 1c), the combination of astigmatism imaging and dual-objective detection provided a noise-cancelling mechanism that led to greater image precision. Molecules that were axially closer to one of the objectives were necessarily farther from the opposing one, leading to anticorrelated changes in the ellipticity detected by the two objectives (Fig. 1d). In contrast, noises (such as those caused by sample drift) and abnormalities (such as two nearby molecules with overlapping images that are misidentified as a single molecule) led to correlated changes (Fig. 1d). Using this effect, we cancelled noises by averaging the z measurements from the two channels, and identified and rejected abnormalities by examining the difference in the z positions obtained from the two objectives (Online Methods). In addition, the mechanical stability of the dual-objective setup (with the optical axis of the objectives parallel to the optical table and the sample vertically oriented) was higher than that of the single-objective setup built on a standard inverted microscope. The synergy of these effects led to substantially higher spatial resolution.
Next, we tested these imaging capabilities on the actin cytoskeleton (Fig. 2). To take full advantage of the high image resolution, we labeled the target structure using small organic molecules by staining actin filaments with Alexa Fluor 647 dye–labeled phalloidin, which binds actin filaments with high specificity. We carried out STORM imaging through direct activation of Alexa Fluor 647 (refs. 5,15). Individual actin filaments were clearly resolved in dual-objective STORM images of fixed cells (Fig. 2a,b). The cross-sectional profile of individual filaments showed a 12-nm FWHM (Fig. 2e). After accounting for the 9-nm lateral image resolution, we derived the width of phalloidin-labeled actin filaments as ∼8 nm ((122 – 92)1/2), which agrees with the known diameter of actin (5–9 nm). Nearby filaments with a center-to-center distance of ∼20 nm were also well resolved from each other (Fig. 2f). In comparison, we achieved lower resolution when we relied only on the information collected by one of the two objectives, and actin filaments were completely unresolvable in conventional fluorescent images (Fig. 2c,d).
(a) Dual-objective STORM image of actin (labeled with Alexa Fluor 647-phalloidin) in a COS-7 cell. The z positions are color coded (violet and red, positions closest to and farthest from substratum, respectively). (b) Close-up of boxed region in a. (c) STORM image of same area obtained by using only information collected by Objective 1 of dual-objective setup. (d) Conventional fluorescence image of same area. (e) Cross-sectional profile of eight filaments aligned by the center of each filament. Red line, Gaussian fit with FWHM of 12 nm. (f) Cross-sectional profiles for two nearby filaments in b,c (white arrows). Gray bars, dual-objective images in b; red line, single-objective image in c. Scale bars, 2 μm (a), 500 nm (b–d).
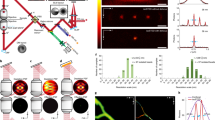
Through volumetric imaging with 9-nm lateral and 19-nm axial resolutions, we obtained a holistic, 3D view of the actin networks with dual-objective STORM. We observed two vertically separated actin layers in the sheet-like cell protrusion despite its small thickness (Fig. 3a–c). The apparent thickness of each layer was 30–40 nm (Fig. 3d–f). The separation between the two layers was generally ∼100 nm, but could be as small as ∼50 nm (Fig. 3d–f). Far from the cell edge and in the interior region, this separation increased to values much greater than 200 nm, suggesting that the two layers evolve into the cortical actin layers in the cell body. We also validated the two-layer organization in living cells (Supplementary Fig. 1). Similar to the x-y case, using only information obtained from one of the objectives led to substantially lower quality of the x-z cross-section images (Supplementary Fig. 2).
(a) Dual-objective STORM image of actin in a BSC-1 cell. The z positions are color coded (color bar). (b,c) Vertical cross sections (each 500-nm wide in x or y) of cell in a along dotted and dashed lines, respectively. When far from cell edge, z position of dorsal layer increases quickly and falls out of imaging range. (d,e) The z profiles for two points along vertical section (red and yellow arrows in b, respectively). Each histogram is fit to two Gaussians (red curves), yielding apparent thickness of ventral and dorsal layers and peak separation between the two layers. (f) Quantification of apparent thickness averaged over two layers and dorsal-ventral separation obtained from x–z cross-section profile in b. (g,h) Ventral and dorsal actin layers of cell in a. (i,j) Ventral and dorsal actin layers of a COS-7 cell treated with blebbistatin. (k,l) Vertical cross sections (each 500-nm wide in x or y) of cell along dotted and dashed lines, respectively. (m) Actin density of ventral and dorsal layers along yellow box in i,j, measured by localization density. Scale bars, 2 μm (a,g–j); 100 nm for z and 2 μm for x and y (b,c,k,l).
Although filaments in the two layers formed well-separated networks, thick filament bundles occasionally connected the two layers (Supplementary Fig. 3). Such bundles typically originated from adhesion plaques, ran through the ventral layer and gradually rose toward and ultimately reached the dorsal layer (Supplementary Fig. 3b–d), likely representing dorsal stress fibers16. We also observed thick bundles connecting adhesion plaques on the ventral surface, likely representing ventral stress fibers16. In addition to these mature focal adhesions at the end of the thick actin bundles17, we also observed smaller and more isotropic adhesion complexes (Supplementary Fig. 3e–h), probably representing nascent adhesion complexes17. Actin filaments attached to these structures diverged in different directions.
Notably, the two layers of actin networks often had highly distinct spatial organizations of actin filaments (Fig. 3g,h). Whereas the dorsal layer appeared as a consistently dense and homogeneous meshwork, the ventral layer typically formed a web-like structure with a lower filament density and highly variable organization. We observed the two-layer arrangement in BSC-1 epithelial cells (Fig. 3a–h and Supplementary Fig. 4) and in COS-7 fibroblast cells (Supplementary Figs. 5 and 6). The actin density in the dorsal layer was often several times higher than that in the ventral layer (Supplementary Fig. 5f,i,j). Additional analysis suggests that the two-layer arrangement spans the lamellum and possibly extends into the lamellipodium (Supplementary Results).
To explore the molecular mechanisms underlying the structural differences observed for the two actin networks, we investigated how these networks responded to different actomyosin-perturbing drugs. Cytochalasin D, a drug that inhibits actin polymerization, led to lower filament density as we expected (Supplementary Fig. 7a)18. Latrunculin A, a drug that sequesters monomeric actin, also led to lower filament density as we expected18, but with the dorsal network substantially more disrupted than the ventral layer (Supplementary Fig. 7b). This suggests that the dorsal layer is potentially more dynamic and thus more readily disrupted by actin monomer depletion. Notably, blebbistatin, an inhibitor for myosin II, removed the structural differences between the ventral and dorsal networks: both networks became uniform actin meshworks of similar density, reminiscent of the dense, uniform dorsal network observed in untreated cells (Fig. 3i–m). This suggests that myosin II has a key role in the structural organization of the ventral actin layer and in maintaining the structural differences between the dorsal and ventral actin networks. This function of myosin II is potentially related to its activities previously found in regulating actin disassembly, actin bundle formation and focal adhesion maturation (Supplementary Discussion).
By combining astigmatism imaging, dual-objective detection and small-molecule labeling in STORM, we have achieved an image resolution of <10 nm in the lateral direction and <20 nm in the axial direction. This lateral resolution is twofold or more higher than previously achieved for biological samples using super-resolution methods. Although higher axial resolutions have been reported using interferometry approaches13,14, the astigmatism-based system reported here is substantially simpler to implement.
The ability to resolve individual actin filaments in cells using fluorescence microscopy opens new possibilities for studying numerous actin-related processes in cells. The two layers of continuous actin networks with distinct structures that we observed in sheet-like cell protrusions support and extend previous understanding of the 3D ultrastructure of the actin cytoskeleton19 (Supplementary Discussion). The high image resolution obtained with dual-objective STORM should also find use in many other biological systems.
Methods
Optical setup.
A schematic of the dual-objective STORM setup is in Figure 1a. Two infinity-corrected microscope objectives (Olympus Super Apochromat UPLSAPO 100×, oil immersion, numerical aperture (NA) 1.40) were placed opposite each other and aligned to focus on the same spot of the sample. A piezoelectric actuator (Thorlabs DRV120) was used to control the axial position of the sample with nanometer precision. The 647-nm line from a Kr/Ar mixed-gas laser (Innova 70C Spectrum, Coherent) and the 405-nm beam from a solid-state laser (CUBE 405-50C, Coherent) were introduced into the sample through the back focal plane of the first objective using a customized dichroic mirror that worked at an incident angle of 22.5° (Chroma). Using a translation stage, the laser beams were shifted toward the edge of the objective so that emerging light reached the sample at incidence angles slightly smaller than the critical angle of the glass-water interface, thus illuminating only the fluorophores within a few micrometers of the coverslip surface. The fluorescence emission was collected by both objectives. After passing through long-pass filters (HQ665LP, Chroma), the two parallel light rays from the two objectives were each focused by a 20-cm achromatic lens, cropped by a slit at the focal plane and then separately projected onto two different areas of the same electron-multiplying charge-coupled device (EM-CCD) camera (Andor iXon DU-897) using two pairs of relay lenses. Astigmatism was introduced into the imaging paths of both objectives using a cylindrical lens so that the images obtained by each objective were elongated in the x and y directions for molecules on the proximal and distal sides of the focal plane (relative to the objective), respectively. A band-pass filter (ET700/75m, Chroma) was installed on the camera. See Supplementary Protocol 1 for more details.
Sample preparation.
BSC-1 and COS-7 cells were plated on 18-mm diameter, #1.5 coated glass coverslips at a confluence of ∼20%. After 16–24 h, the cells were fixed and labeled according to protocols for ultrastructural studies of actin cytoskeleton20,21,22 (see Supplementary Protocol 2 for details). Briefly, the cells were initially fixed and extracted for 1–2 min using a solution of 0.3% (v/v) glutaraldehyde and 0.25% (v/v) Triton X-100 in cytoskeleton buffer (CB, 10 mM MES, pH 6.1, 150 mM NaCl, 5 mM EGTA, 5 mM glucose and 5 mM MgCl2), and then post-fixed for 10 min in 2% (v/v) glutaraldehyde in CB. The sample was treated with freshly prepared 0.1% (w/v) sodium borohydride for 7 min to reduce background fluorescence. For vinculin staining (when needed), the sample was first blocked with 3% (w/v) BSA and 0.5% (v/v) Triton X-100, and then was stained with rabbit monoclonal vinculin antibodies (Invitrogen 700062) followed by Cy3-labled goat anti-rabbit secondary antibodies (Invitrogen A10520). Actin filaments were labeled with Alexa Fluor 647-phalloidin (Invitrogen A22287) overnight at 4 °C. A concentration of ∼0.5 μM phalloidin in phosphate buffered saline (PBS pH 7.4) was used. To minimize the dissociation of phalloidin from actin, the sample was briefly washed once with PBS and then immediately mounted for STORM imaging.
For drug-effect studies, cells were incubated with culture medium containing 0.5 μM cytochalasin D (Sigma-Aldrich), 0.25 μM latrunculin A (Invitrogen) or 50 μM (−)blebbistatin (the active enantiomer; Sigma-Aldrich) at 37 °C for 1 h and then were fixed and labeled as described above.
The STORM imaging buffer for fixed cells was PBS with 100 mM cysteamine, 5% (w/v) glucose, 0.8 mg ml−1 glucose oxidase (Sigma-Aldrich) and 40 μg ml−1 catalase (Roche Applied Science). Imaging buffer (∼4 μl) was dropped at the center of a freshly cleaned, #1.5 rectangular coverslip (22 mm × 60 mm), and the sample on the 18-mm diameter coverslip was mounted on the rectangular coverslip and sealed with nail polish.
Image data acquisition.
The sealed sample was mounted between the two opposing objectives. The 647-nm laser was used to excite fluorescence from Alexa Fluor 647 molecules. Before acquiring STORM images, we used relatively weak 647-nm light (∼0.05 W cm−2) to illuminate the sample and recorded the conventional fluorescence image before a substantial fraction of the dye molecules were switched off. We then increased the 647-nm light intensity (to ∼2 kW cm−2) to rapidly switch the dyes off for STORM imaging. The 405-nm laser was used to reactivate the fluorophores from the dark state back to the emitting state23. The power of the 405-nm laser (0–1 W cm−2) was adjusted during image acquisition so that at any given instant only a small, optically resolvable fraction of the fluorophores in the sample were in the emitting state. The EM-CCD camera acquired images from both objectives simultaneously at a frame rate of 60 Hz. Typically, ∼90,000 frames were recorded to generate the final super-resolution image of the actin ultrastructure. Recording of more frames (for example, 230,000 frames for Fig. 2) further increased the image quality at the expense of longer imaging time.
Image data analysis.
The recorded STORM data were first split into two movies, each of which comprises a sequence of images obtained by one of the two objectives. Each movie was first analyzed separately as described previously12. The centroid positions and ellipticities of the single-molecule images provided lateral and axial positions of each activated fluorescent molecule, respectively. The molecular positions obtained by the second objective were mapped to the coordinates of the first objective through a transformation on the basis of corresponding features (control points) in both images24. The mapped data from the two objectives were then compared frame by frame: molecules that were switched on within one frame of time and that were within ∼50 nm of each other in the mapped x-y plane were identified as the same emitting molecule detected by both objectives. Nonmatching molecules were discarded. For each pair of matched molecules observed by the two objectives, the availability of two z positions obtained through the two objectives provides a new way to identify abnormalities and cancel noise. Because the focal planes of the two opposing objectives coincided, a molecule on the proximal side of the focal plane of one objective would be on the distal side of the other objective. Therefore, its image would appear elongated in x through one objective but elongated in y through the other objective. A real change in the z position would cause anticorrelated changes in ellipticity measured through the two objectives (Fig. 1d, green and blue arrows). Conversely, abnormalities and noise would tend to cause correlated changes in ellipticity. For example, when two nearby molecules with overlapping images were misidentified as a single molecule, the resultant images through both objectives would appear elongated in the same direction along the line connecting the two molecules (Fig. 1d, magenta arrow). Likewise, any x-y drift of the stage or camera would also cause elongation in the same direction. These correlated changes in ellipticity led to apparently different z positions obtained through the two objectives (Δz). The value of Δz can thus be used to identify and reject abnormalities. These abnormalities (identified by Δz greater than ∼100 nm, which is substantially larger than the axial resolution of a single objective) comprised ∼10% of all identified molecules. For molecule pairs that matched well with each other in all four (spatial and temporal) coordinates, the final coordinates were determined as the average of the mapped coordinates from the two objectives, weighted by the width of the image and number of photons obtained by each objective. This averaging procedure further reduced noise caused by errors, such as the correlated changes in ellipticity described above. The final super-resolution images were reconstructed from these molecular coordinates by depicting each location as a 2D Gaussian peak. Analysis software is available as Supplementary Software.
To characterize the localization precision, we used fixed cell samples sparsely labeled with Alexa Fluor 647. Relatively strong activation conditions were used such that each Alexa Fluor 647 molecule was activated multiple times during image acquisition and gave a cluster of localizations owing to repetitive activation. Under this sparsely labeled condition, we could readily identify individual clusters of localizations. Localizations from many such clusters within ∼150 nm of the focal plane were aligned by their center of mass to generate the localization distribution reported in Figure 1b. The localization precision determined from these distributions was ∼4 nm (s.d.) in the x and y directions and ∼8 nm (s.d.) in the z direction. The variation in localization precision across this region was small (within 15% of the average value). The localization precision determined from the sparsely labeled sample also represents the localization precision achieved in densely labeled actin samples as the parameters relevant for the localization precision, such as the number of photons detected from individual molecules and the background fluorescence signal, were measured to be the same for both sparsely and densely labeled samples.
For STORM imaging of the densely labeled actin samples, relatively weak 405-nm activation intensities were used during image acquisition. This led to a typical linear localization density of one localization per 4 nm along individual actin filaments for ∼90,000 frames, which corresponds to a Nyquist criterion–based resolution of 8 nm, smaller than the 9-nm lateral and 19-nm axial resolutions determined above. As we had not exhausted all of the labeled molecules by the end of image acquisition, the number of localizations can be further increased by increasing the imaging time.
For the characterization of the widths of individual actin filaments, we chose short (∼200 nm), straight segments of filaments in cells along which no crossing or branching of filaments was observed. Analysis of eight such segments yielded FWHM widths of 12 ± 2 nm.
References
Hell, S.W. Science 316, 1153–1158 (2007).
Huang, B., Babcock, H. & Zhuang, X.W. Cell 143, 1047–1058 (2010).
Betzig, E. et al. Science 313, 1642–1645 (2006).
Gould, T.J. et al. Nat. Methods 5, 1027–1030 (2008).
Heilemann, M. et al. Angew. Chem. Int. Ed. 47, 6172–6176 (2008).
Vogelsang, J., Cordes, T., Forthmann, C., Steinhauer, C. & Tinnefeld, P. Proc. Natl. Acad. Sci. USA 106, 8107–8112 (2009).
Chhabra, E.S. & Higgs, H.N. Nat. Cell Biol. 9, 1110–1121 (2007).
Pollard, T.D. & Borisy, G.G. Cell 112, 453–465 (2003).
Svitkina, T. Methods Cell Biol. 79, 295–319 (2007).
Urban, E., Jacob, S., Nemethova, M., Resch, G.P. & Small, J.V. Nat. Cell Biol. 12, 429–435 (2010).
Rust, M.J., Bates, M. & Zhuang, X.W. Nat. Methods 3, 793–795 (2006).
Huang, B., Wang, W.Q., Bates, M. & Zhuang, X.W. Science 319, 810–813 (2008).
Shtengel, G. et al. Proc. Natl. Acad. Sci. USA 106, 3125–3130 (2009).
Aquino, D. et al. Nat. Methods 8, 353–359 (2011).
Zhuang, X.W. Nat. Photonics 3, 365–367 (2009).
Pellegrin, S. & Mellor, H. J. Cell Sci. 120, 3491–3499 (2007).
Geiger, B., Spatz, J.P. & Bershadsky, A.D. Nat. Rev. Mol. Cell Biol. 10, 21–33 (2009).
Svitkina, T.M. & Borisy, G.G. J. Cell Biol. 145, 1009–1026 (1999).
Giannone, G. et al. Cell 128, 561–575 (2007).
Small, J.V., Rottner, K., Hahne, P. & Anderson, K.I. Microsc. Res. Tech. 47, 3–17 (1999).
Koestler, S.A., Auinger, S., Vinzenz, M., Rottner, K. & Small, J.V. Nat. Cell Biol. 10, 306–313 (2008).
Auinger, S. & Small, J.V. Methods Cell Biol. 88, 257–272 (2008).
Dempsey, G.T. et al. J. Am. Chem. Soc. 131, 18192–18193 (2009).
Goshtasby, A. 2-D and 3-D Image Registration for Medical, Remote Sensing, and Industrial Applications (Wiley, 2005).
Acknowledgements
We thank G. Danuser for helpful discussion. This work was supported in part by the US National Institutes of Health and a Collaborative Innovation Award (43667) from Howard Hughes Medical Institute and Gatsby Charitable Foundation (to X.Z.). X.Z. is funded by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
K.X., H.P.B. and X.Z. designed research. K.X. did experiments and data analysis. H.P.B. assisted with the optical setup. K.X. and X.Z. prepared the manuscript. X.Z. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Results, Supplementary Discussion and Supplementary Protocols 1–2 (PDF 23681 kb)
Supplementary Software
Analysis software (ZIP 4 kb)
Rights and permissions
About this article
Cite this article
Xu, K., Babcock, H. & Zhuang, X. Dual-objective STORM reveals three-dimensional filament organization in the actin cytoskeleton. Nat Methods 9, 185–188 (2012). https://doi.org/10.1038/nmeth.1841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1841
This article is cited by
-
HOPE-SIM, a cryo-structured illumination fluorescence microscopy system for accurately targeted cryo-electron tomography
Communications Biology (2023)
-
Maximum-likelihood model fitting for quantitative analysis of SMLM data
Nature Methods (2023)
-
Quantitatively mapping local quality of super-resolution microscopy by rolling Fourier ring correlation
Light: Science & Applications (2023)
-
4polar-STORM polarized super-resolution imaging of actin filament organization in cells
Nature Communications (2022)
-
Simple methods for quantifying super-resolved cortical actin
Scientific Reports (2022)