Abstract
Malaria control is heavily dependent on chemotherapeutic agents for disease prevention and drug treatment. Defining the mechanism of action for licensed drugs, for which no target is characterized, is critical to the development of their second-generation derivatives to improve drug potency towards inhibition of their molecular targets. Mefloquine is a widely used antimalarial without a known mode of action. Here, we demonstrate that mefloquine is a protein synthesis inhibitor. We solved a 3.2 Å cryo-electron microscopy structure of the Plasmodium falciparum 80S ribosome with the (+)-mefloquine enantiomer bound to the ribosome GTPase-associated centre. Mutagenesis of mefloquine-binding residues generates parasites with increased resistance, confirming the parasite-killing mechanism. Furthermore, structure-guided derivatives with an altered piperidine group, predicted to improve binding, show enhanced parasiticidal effect. These data reveal one possible mode of action for mefloquine and demonstrate the vast potential of cryo-electron microscopy to guide the development of mefloquine derivatives to inhibit parasite protein synthesis.
Similar content being viewed by others
Malaria is a major protozoan parasitic disease that inflicts an enormous burden on global human health. In 2015 the disease resulted in an estimated 429,000 deaths, with several hundreds of millions of people infected1. The causative agents of malaria are a group of protozoan parasites that belong to the genus Plasmodium, a member of the ancient apicomplexan phylum of vertebrate pathogens, with P. falciparum and P. vivax being responsible for the majority of disease mortality and morbidity, respectively2.
Antimalarial chemotherapies have long been the gold-standard utility for the prevention and treatment of malaria. Over many decades, different classes of antimalarials have been clinically approved and deployed as frontline treatments to combat malaria disease3. Despite the long-standing usage of these drugs, their modes of action in mediating parasite killing are not well defined. Mefloquine (MFQ) has been one of the most effective antimalarials since it was first developed and has been used as a chemoprophylactic drug by visitors staying in malaria-endemic areas. Neurological side effects associated with MFQ usage4 have precluded the drug being used widely as a first choice for preventative treatment. MFQ has, however, been used globally in combination with the front-line antimalarial drug artemisinin to treat malaria, constituting one of the many classes of artemisinin-combination therapies (ACT) pivotal to malaria control. Importantly, in regions that have prevalent pools of artemisinin-resistant parasites, recent reports have shown that artemisinin-resistant strains of P. falciparum are sensitive to MFQ due to a decreasing copy number of Pfmdr1, a marker of mefloquine resistance5. In an urgent response to stem the spread of ACT-resistant parasites beyond the province of western Cambodia, the World Health Organization (WHO) has recommended the re-introduction of artesunate and MFQ combination therapy in those regions to combat multi-drug-resistant strains of P. falciparum6. Conversely, in regions where MFQ resistance is prevalent, dihydroartemisinin–piperaquine treatment is preferentially deployed. Despite the major role of MFQ in malaria prevention and its utility in controlling parasites resistant to other ACTs, the molecular basis for its mode of action is not known. Previous studies have suggested that the molecular target(s) for MFQ probably resides in the parasite cytoplasm, because efflux of MFQ from the cytoplasm to the parasite food vacuole by the Pfmdr1-encoded drug transporter Pgh-1 is the predominant mechanism of MFQ resistance7–10. Furthermore, a large-scale screen of antimalarial drugs previously implied that MFQ might be a putative inhibitor of the P. falciparum cytoplasmic ribosome11. To this end, defining the mode of action of MFQ in the malaria parasite, together with high-resolution structural elucidation of the drug bound to its target, would enable structure-guided development of mefloquine derivatives to enhance drug inhibition on the molecular target(s).
Here, we demonstrate that MFQ mediates killing of the malaria parasite by inhibition of parasite protein synthesis through direct binding to the cytoplasmic ribosome (Pf80S) of P. falciparum. We have solved the cryo-electron microscopy (cryo-EM) structure of the Pf80S ribosome in complex with MFQ at 3.2 Å resolution, revealing the interaction between the (+) MFQ enantiomer with residues within the GTPase-associated centre of the Pf80S ribosome. The mechanism of parasite killing by MFQ via Pf80S was confirmed by genetic interrogation of key binding residues, with transgenic parasites possessing amino-acid substitutions predicted to alter MFQ binding showing enhanced resistance to the drug. Furthermore, using the high-resolution cryo-EM structure as a reference, we designed de novo MFQ derivatives with modifications to a critical MFQ piperidine group and demonstrated that these MFQ derivatives have enhanced antimalarial activity correlating with the structure–activity relationship. Collectively, these data establish the Pf80S ribosome as one of the molecular target(s) of MFQ-mediated parasite killing. Our cryo-EM structure of the Pf80S–MFQ complex serves as an important reference for the design of new MFQ-based derivatives, expanding available tools to inhibit parasite protein synthesis.
Inhibition of cytosolic protein synthesis in P. falciparum
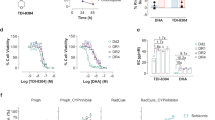
We first determined the half maximum effective concentration (EC50) of MFQ-mediated killing of the 3D7 strain of P. falciparum, and showed potent antimalarial activity with an EC50 of 25.3 nM (Table 1). The effect of MFQ on translation activity was then tested using the incorporation of radiolabelled S35-methionine and S35-cysteine as reporters for protein synthesis. MFQ inhibited protein synthesis by 55%, while parasites cultured in the presence of a non-translation inhibitory compound, chloroquine (CQ), showed no inhibition (Fig. 1a; Student's t-test versus CQ, P < 0.05, both 3D7 and W2mef strains). MFQ-mediated translation inhibition was, however, weaker than other, highly toxic cytosolic translation inhibitors such as cycloheximide (CHX, 90%; t-test versus CQ, P < 0.05, both 3D7 and W2mef strains) (Fig. 1a). Parasites incubated with doxycycline (DOX), a translation inhibitor that is believed to target only the ribosome of the parasite plastid (apicoplast) organelle12,13, showed no effect on cytosolic translation (Fig. 1a). No obvious morphological changes were observed following drug treatment in parasites treated with a range of antimalarials (CQ, CHX, DOX, emetine (EME), MFQ and quinine (QUI)). Indeed, the parasites showed intact mitochondria and nuclei (Supplementary Fig. 1), indicating that the assay conditions did not result in significant non-specific cytotoxicity. Collectively, these results support the hypothesis that MFQ is an inhibitor of cytosolic translation.
a, Translation inhibitory activity of antimalarial compounds cycloheximide (CHX) at 1.3 µM, doxycycline (DOX) at 17 µM, chloroquine (CQ) at 110 nM, emetine (EME) at 105 nM and mefloquine (MFQ) at 90 nM. Statistical significance was calculated by Student's t-test. Data are shown as mean ± s.d. Each assay was undertaken in triplicate for four independent occasions. b, Chemical structure of MFQ.
Cryo-EM structure of the Pf80S–MFQ complex
To demonstrate that MFQ acts directly on the parasite 80S ribosome, we solved the structure of the P. falciparum cytoplasmic ribosome (Pf80S) in the presence of a racemic mixture of MFQ by cryo-EM at an overall resolution of 3.2 Å (Fig. 2a–c and Supplementary Table 1). A difference map calculated between this reconstruction and Pf80S in its apo-form14 showed two independent continuous densities with shape and size congruent with MFQ when visualized at a threshold of five standard deviations (Supplementary Fig. 3a). The well-resolved densities enabled the accurate placement of two MFQ molecules (Fig. 2a,b and Supplementary Fig. 3). The primary MFQ binding site (designated based on location and correlation with MFQ tolerance, as described below) was located within the GTPase-associated centre (GAC) of the large ribosomal subunit (Pf60S) (Fig. 2c,e), composed of the proteins uL13 and uL6, the sarcin-ricin loop of ribosomal RNA helices 94–5 and expansion segment (ES) 13, where this site interacted with a (+) enantiomer of the MFQ molecule ((+) MFQ). This region is critical for translation, coordinating the elongation steps of protein synthesis by binding the translational GTPases and activating the energy-dependent translocation of the tRNA–mRNA complex through the ribosome15,16. A secondary binding site was located at the peripheral surface of the Pf60S subunit (Supplementary Fig. 3b) where this site interacted with a (−) enantiomer of the MFQ molecule. Two residues (Tyr290 and His294) from uL4 form a pocket that accommodates the quinoline ring of MFQ at this secondary site (Supplementary Fig. 3b). In P. vivax, however, His294 is substituted by Ser294 in this distal MFQ binding site (Supplementary Fig. 3c). Because P. vivax is sensitive to MFQ, divergence in this secondary binding site does not, as such, correlate with the inhibitory activity of MFQ on P. falciparum and P. vivax. Furthermore, because this region has no known role in translation, we believe it is unlikely that MFQ binding at such a site could impact protein synthesis. Of note, the observation of primary (functional) and secondary (probably physiologically irrelevant) binding sites for the antibiotic tetracycline have also been reported previously17.
a,b, Cryo-EM density map of the primary MFQ binding pocket in the absence (a) and presence (b) of MFQ. MFQ is represented as yellow sticks and binding residues are in purple. Oxygen, red; nitrogen, blue; fluorine, cyan; magnesium, green. c, Magnified EM density of (+) MFQ depicted in various orientations. d, Ribosomal protein PfuL13 and rRNA ES13 form the MFQ binding pocket. Hydrophobic residues are coloured in grey. Structure is derived from 43,184 particles from 829 micrographs (Supplementary Table 1). e, Atomic model of the Pf80S–MFQ complex, shown from the acceptor site (A-site) entry side. Magnified inset: composition of the GTPase-associated centre (GAC) with bound MFQ.
The identified primary MFQ binding site lies within a crevice formed by a helix of the ribosomal protein uL13 (residues 45–59) and ES13 of 28S rRNA (Figs 2d and 3a). The non-polar residues Leu15 and Ile42 of uL13 interact with the hydrophobic trifluoromethyl group (CF3) located on C8 of the quinoline ring (Fig. 3a). On the opposite end of the quinoline ring, the 2-CF3 group forms a hydrophobic interaction with two aromatic residues (Tyr53 and Phe56) of uL13 (Fig. 3a). The quinoline ring is further stabilized through a cation–π interaction with a magnesium ion coordinated to the backbone phosphate of base C1442 (Fig. 3a). The hydroxyl group of the linker that bridges the quinoline and piperidine ring forms a hydrogen bond with the phosphate backbone of base G1441 (Fig. 3a). Finally, the secondary amine group of the piperidine ring forms a further hydrogen bond with Glu55 of uL13 (Fig. 3a). The inter-atomic distances between (+) MFQ and interacting residues are within the range of 2.6–3.5 Å (Fig. 3b). The nature of this binding site is consistent with structure–activity studies when MFQ was originally conceived18. Thus, all three functional moieties of (+) MFQ (quinoline, piperidine ring and the hydroxyl linker) are required for binding to the GAC, while a combination of hydrophobic and hydrogen bonds form the basis of the interaction. To our knowledge, this site of the eukaryotic 80S ribosome represents a novel binding site for a translation inhibitor. Although the thiopeptide and orthosomycin classes of antibiotics also target the GAC, they interfere directly with the binding of elongation factors to the ribosome (Supplementary Fig. 4)19. However, given that MFQ and thiopeptide/orthosomycin each target the GAC, this suggests that MFQ functions to inhibit parasite protein synthesis by inhibiting the polypeptide elongation step.
a, Amino-acid residues from protein PfuL13 and bases from ES13 of the 28S rRNA involved in binding to (+) MFQ. b, Residues that interact with (+) MFQ with inter-atomic distances indicated. c, MFQ-mediated growth inhibition of control P. falciparum parasites carrying an integrated, wild-type copy of the uL13 gene and four transgenic parasite lines carrying single-amino-acid substitutions at the uL13 MFQ binding pocket. Data are shown as mean ± s.d. of three biological replicates with each biological replicate representing three experimental replicates. d, Divergence in the ES13 part of the MFQ binding pocket between the P. falciparum A-type (blood stage) and S-type (sexual stage) ribosomes. A single nucleotide C1440 in the A-type 28S rRNA is deleted in the S-type 28S rRNA. MFQ binding residues are highlighted in a box. e, Sequence alignment of uL13 from P. falciparum, P. vivax, T. gondii and T. brucei. Residues involved in binding to MFQ are highlighted with asterisks.
MFQ acts via the uL13 binding residues
To assess if the primary MFQ binding site in uL13 is the site of action for MFQ-mediated parasite killing, targeted mutagenesis was conducted using CRISPR genome editing technology20. Amino acid substitutions (Leu15Ser and Ile42Ser) were introduced into uL13 in 3D7 parasites (Supplementary Fig. 5 and Supplementary Tables 2–3), and wild-type residues (Leu15 and Ile42) were introduced as a positive control. In the control experiment, parasites were obtained within two weeks under drug selection using the dihydrofolate reductase inhibitor WR99210, but no parasites were recovered in two separate experiments when attempts were made to introduce both Leu15Ser and Ile42Ser. This implies that Ser substitutions of Leu15 and Ile42 in uL13 disrupt the function of GAC of the Pf80S ribosome, leading to parasite death. These data demonstrate the essentiality of the GAC for parasite viability, suggesting that (+) MFQ binding to this site may contribute to parasite death. Consequently, we introduced single substitutions to the MFQ binding pocket (Ile42Ala, Glu55Ala, Phe56Ala, Leu140Phe), creating four transgenic parasite lines. All four transgenic parasite lines carrying single substitutions were recovered in two weeks under WR99210 drug selection. MFQ sensitivity testing of each was compared to control parasites carrying an integrated wild-type uL13 gene to test the significance of the MFQ uL13 binding pocket in parasite killing. Despite numerous attempts to purify pure (+) and (−) enantiomers of MFQ with different methods, purification of the MFQ chiral enantiomers for drug sensitivity testing was not possible for this study. As a result, we performed MFQ sensitivity testing using a racemic mixture. Transgenic parasites carrying each single amino acid substitution were more resistant to MFQ, which gave EC50 values 1.4- to 1.7-fold higher (36.6–43.8 nM) than control parasites transfectant for the wild-type allele (EC50 = 26 nM) (Fig. 3c and Table 1, P < 0.05). These data confirm that the primary MFQ binding pocket in PfuL13 of the 80S ribosome contributes to MFQ-mediated parasite killing.
Previous studies have demonstrated that sexual stages of P. falciparum are insensitive to MFQ (refs 21,22). During this phase, the parasite switches to variant forms of rRNA that, together with the ribosomal proteins, form an S-type ribosome that is distinct from the A-type ribosomes found in asexual stages23. Comparison of the RNA sequence of the MFQ binding pocket between rRNA variants reveals a single base deletion within ES13 of the S-type ribosome (C1440 deletion, A-type numbering) (Fig. 3d). Such a change is expected to disrupt the local conformation of the primary MFQ binding pocket, thus potentially explaining the resistance of gametocytes to MFQ. Finally, structural conservation of the new binding pocket was explored to determine if it was predictive of the tolerance of other major protozoan parasites to MFQ. Each of the binding elements is strictly conserved between P. vivax and P. falciparum (Fig. 3e), which is consistent with the MFQ sensitivity of P. vivax24. Trypanosoma brucei is also sensitive to MFQ (ref. 25), and most of the MFQ-binding elements are identical except for a conservative Glu55Gln substitution (Fig. 3e) that would preserve the hydrogen bond formed with the NH group on the piperidine ring of MFQ. In contrast, Toxoplasma gondii, which is insensitive to MFQ (ref. 26), has a non-conservative Glu55Arg substitution that would be predicted to sterically hinder binding of the piperidine ring moiety (Fig. 3e).
Cryo-EM structure-based design of MFQ derivatives
Our high-resolution cryo-EM structure of the Pf80S–MFQ complex serves as a reference guide to develop MFQ derivatives with improved potency towards the inhibition of the Pf80S ribosome. Comparative structural analysis with the human cytosolic ribosome27 revealed two non-conservative substitutions in uL13 found within the MFQ binding pocket (Supplementary Fig. 6a). The first substituted residue (Glu55Ala; Pf: human) would eliminate the hydrogen bond between the NH group of the piperidine ring and uL13 (Fig. 3a and Supplementary Fig. 6a). As already shown, when a Glu55Ala substitution is performed in P. falciparum, it leads to lower MFQ binding and thus a higher EC50, as expected (Table 1). The second substituted residue (Leu59Lys) would sterically inhibit the binding of MFQ by clashing with C4 of the piperidine ring (Figs 1b and 3a and Supplementary Fig. 6). This suggested that this site could be exploited to enhance drug affinity towards the P. falciparum 80S ribosome. Furthermore, substantial structural differences in the MFQ binding pocket of the human mitochondrial ribosome28 indicate that MFQ should not be able to inhibit human mitochondrial protein synthesis (Supplementary Fig. 6b).
As a proof of concept towards this goal, we designed derivatives of MFQ possessing hydrophobic groups that would extend into the parasite-specific Leu59 region of the binding pocket (Fig. 4a–d and Supplementary Table 4), while maintaining hydrogen-bond interactions with Glu55 and the G1441 nucleotide. Synthesis of these MFQ derivatives (following previously described methods29,30) and evaluation against P. falciparum parasites in culture demonstrated that a subset of these derivatives (MFQ_D3-5) showed 1.9- to 2.4-fold enhancement in potency towards parasite killing (Table 1, P < 0.05). Thus, changes in the parasite inhibitory potency of MFQ derivatives were found to be entirely consistent with the interaction of MFQ and the PfuL13 binding pocket.
a, Chemical structure of MFQ_D1. b, MFQ_D1 docked into the MFQ binding pocket. c, Chemical structure of MFQ_D2. d, MFQ_D2 docked into the MFQ pocket. e, Parasite growth inhibition assay measuring the inhibitory activity of MFQ derivatives on 3D7 parasites. Data are shown as mean ± s.d. of three biological replicates, with each biological replicate representing three experimental replicates.
Discussion
Understanding the modes of action of clinically used antimalarial drugs is important for designing new compound derivatives that can potentially improve inhibition of their molecular targets. To this end, at least two critical pieces of information are required to achieve this goal: (1) identification of the molecular targets inhibited by these drugs and (an equally important second step) (2) the high-resolution structure of the drug bound to the molecular target to enable structure-guided drug design to improve the potency of the drug for target inhibition. Here, we have solved these problems for the antimalarial MFQ by revealing the P. falciparum 80S ribosome as one of the targets of MFQ-mediated parasite killing. With a high-resolution cryo-EM structure of the Pf80S with bound (+) MFQ enantiomer presented in this study, along with a proof-of-principle synthesis of MFQ derivatives with enhanced antimalarial activity, this body of work establishes the foundation for designing new MFQ derivatives to inhibit parasite protein synthesis
The inhibition of Pf80S by MFQ is consistent with the known site of action of MFQ being in the parasite cytoplasm. It has been demonstrated previously that the removal of MFQ from the parasite cytoplasm into the food vacuole by the drug transporter Pgh-1 is the predominant basis for MFQ resistance7–10. Furthermore, the mechanism of MFQ resistance in P. falciparum is inversely correlated with CQ resistance8, suggesting that the primary mode of action of MFQ is not in the parasite food vacuole, the compartment where CQ acts to inhibit haem polymerization. By solving the structure of MFQ bound to the Pf80S, this has led to the identification of binding residues in the 28S ribosomal RNA and protein PfuL13 that interact with the (+) MFQ enantiomer. This site is part of the GAC of the eukaryotic ribosome known for its important role in the polypeptide elongation step during protein synthesis, suggesting that this is the stage that (+) MFQ inhibits. Importantly, CRISPR-cas9-mediated amino-acid substitution of (+) MFQ binding residues in uL13 generated transgenic parasites with increased drug resistance (highest EC50 of 43.8 nM, Table 1). The measured EC50 values of these transgenic parasites (36.6–43.8 nM) in response to MFQ treatment are within the range of published values measured in field isolates (mostly clustered within 35–60 nM) that have a MFQ resistance profile31. It is important to note that the mechanism of MFQ resistance mediated by Pgh-1 in P. falciparum is independent of the molecular target of the drug (mode of killing). Based on genetic evidence from four independent single amino acid substitutions of PfuL13 with significantly higher resistance to MFQ (Fig. 3c and Table 1), the data demonstrate that the Pf80S ribosome is at least one of the targets of MFQ-mediated parasite killing.
Interestingly, the potency of MFQ in inhibiting parasite protein synthesis (55%) is relatively lower than the highly toxic translation inhibitor CHX (90%) (Fig. 1a). Mechanistically, CHX and MFQ work distinctly based on the mode of their interactions with the ribosome. CHX competitively blocks the binding of deacylated tRNA to the E-site of the 60S subunit32, whereas binding of (+) MFQ to its primary binding site in the PfuL13 pocket of the GAC does not directly compete for binding with any factors. This difference in the binding mode probably explains the variation in inhibition potency of the two translation inhibitors with radically distinct modes of interaction with the 60S subunit. Similar to other antibiotics such as thiopeptide and orthosomycin, (+) MFQ also binds to the GAC of the Pf60S subunit. Although thiopeptide/orthosomycin use a different binding site within the GAC compared to (+) MFQ (ref. 19), nevertheless, (+) MFQ would similarly be expected to function by inhibiting the polypeptide elongation step during parasite protein synthesis. Furthermore, we have demonstrated the functional importance of the PfuL13 MFQ pocket by introducing amino-acid substitutions to residues that form this pocket. By replacing Leu15 and Ile42 with Ser, this resulted in a lethal phenotype after transfection, indicating the essential nature of this pocket of the GAC for generating viable parasites, implying an essential function of the GAC for protein synthesis. Single amino-acid substitutions to the PfuL13 pocket, with the resultant change in EC50 values, in response to MFQ treatment, also demonstrate the functional importance of this site of the GAC.
The nature of (+) MFQ binding to the primary binding site is dominated by a number of hydrophobic residues of PfuL13 that form this pocket (Leu15, Ile42, Tyr53, Phe56, Leu59, Leu140), whereas a charge residue (Glu55) of PfuL13 and the sugar phosphate backbone of the 28S ribosomal RNA (G1441, C1442) also contribute to (+) MFQ binding. Importantly, comparison of uL13 between the human and P. falciparum 80S ribosomes reveals significant differences exist in the MFQ pocket. This observation provides a foundation for improving the potency of MFQ towards better inhibition of parasite protein synthesis. Two divergent residues in human and parasite uL13 (Glu55Ala and Leu59Lys Pf: human) are readily identifiable (Supplementary Fig. 6a), and form the basis for increasing the potency of (+) MFQ on the parasite translation machinery. We have shown, by introducing Glu55Ala, which mimics the human ribosome, an increase in the EC50 value of this transgenic parasite from 26 to 38.3 nM compared to isogenic wild-type control. We hypothesize that, by means of an iterative optimization process, (+) MFQ derivatives that effectively engage with Glu55 and Leu59 of the P. falciparum uL13 pocket may generate more potent compounds that inhibit parasite protein synthesis (Fig. 4 and Table 1). Together with recent structural analyses of the Pf80S (refs 14,33) showing the many parasite-specific features along with structural dynamics unique to the parasite ribosome, these data reinforce the idea that the P. falciparum 80S ribosome is an increasingly attractive target for antimalarial drug development. Although improving the potency of (+) MFQ towards inhibition of parasite protein synthesis is important for the improvement of on-target inhibition, other factors, such as safety concerns regarding MFQ-associated neurological toxicity due to off-target effects, will also need to be overcome to develop second-generation MFQ derivatives with clear clinical benefit over the parental form. Furthermore, noting that a 90 nM MFQ (EC90) concentration only inhibited translation by 55% (Fig. 1a), this suggests other unidentified targets are likely to be inhibited by a racemic mixture of MFQ. Because the cryo-EM structure of the Pf80S–MFQ complex presented in this study shows the (+) form of the MFQ enantiomer bound to the GTPase-associated centre–PfuL13 pocket (Figs 2 and 3), this suggests that the (−) form of the MFQ enantiomer may be a key factor inhibiting other molecular targets in the parasite.
The identification of MFQ as a protein synthesis inhibitor raises the question of whether other related antimalarials such as quinine (QN) and lumefantrine (LF) may also inhibit parasite protein synthesis through the PfuL13 pocket. Although these compounds have a related chemical scaffold, the substantial alterations in their structure would argue against their ability to interact with the PfuL13 MFQ pocket. Further biochemical characterization of QN and LF would be required to determine their effect on parasite protein synthesis.
Finally, in this study, we have demonstrated how cryo-EM can function as an attractive tool for the development of MFQ-based improved protein-synthesis inhibitors. The low yield of Pf80S using cultured parasites has so far precluded the ability to crystallize the Pf80S for structural studies of drug interaction, although sufficient ribosome material may now be feasible for use in biological assays34. Together with recent elucidation of the structure of the Pf80S–EME complex14, cryo-EM is now the method of choice for the design of new inhibitors for the Pf80S ribosome.
Methods
Parasite culture and ribosome purification
The wild-type 3D7 strain of P. falciparum parasites, a clone itself derived from NF54, provided by the late D. Walliker at Edinburgh University, was maintained in human erythrocytes (blood group O) at a haematocrit of 4% with 10% Albumax. Saponin-lysed parasite pellets were incubated with lysis buffer (20 mM HEPES, pH 7.4, 250 mM KCl, 25 mM Mg(Ac)2, 0.15% Triton, 5 mM 2-mercaptoethanol) at 4 °C for 1 h. Ribosomes were purified by ultracentrifugation, initially with a sucrose cushion (20 mM HEPES pH 7.4, 1.1 M sucrose, 40 mM KAc, 10 mM NH4Ac, 10 mM Mg(Ac)2 and 5 mM 2-mercaptoethanol) followed by a 10–40% sucrose gradient separation step using the same buffer.
Drug sensitivity assay
Trophozoite-stage parasites at 0.5% parasitaemia were grown in a 50 µl culture at 2% haematocrit in 96-well round bottom microtitre plates (Falcon) with doubling dilutions of each drug. After incubation for 48 h, each well was fixed at room temperature for 30 min with 50 µl of 0.25% glutaraldehyde (ProSciTech) diluted in PBS. Following centrifugation at 1,200 r.p.m. for 2 min, supernatants were discarded and trophozoite-stage parasites were stained with 50 µl of 5X SYBR Green (Invitrogen) diluted in PBS. The parasitaemia of each well was determined by counting 50,000 cells by flow cytometry using a Cell Lab Quanta SC–MPL Flow Cytometer (Beckman Coulter). Growth was expressed as a percentage of the parasitaemia obtained using a drug-free control. All samples were tested in triplicate.
Parasite translation assay
Synchronous trophozoite-stage 3D7 parasites were dispensed into a 24-well plate to a final parasitaemia of 4–6%, 2% haematocrit with EC90 concentrations of CQ (110 nM), CHX (1.3 µM), DOX (17 µM), EME (105 nM) or MFQ (90 nM) in a final volume of 1 ml. Parasites were cultured for 2 h at 37 °C in a humidified atmosphere of 5% CO2, 1% O2 and 94% N2. Following 2 h incubation, 800 µl aliquots were transferred to rubber-sealed 1.5 ml tubes, to which 16.5 µCi EasyTag(TM) EXPRE35S35S Protein Labeling Mix [3S] (PerkinElmer) was added, then incubated for 2 h at 37 °C in growth medium made from RPMI HEPES lacking l-cysteine and l-methionine. Infected erythrocytes were washed twice with 1× PBS, resuspended in 6× protein sample loading buffer, and proteins were separated by SDS–PAGE. Gels were fixed (40% (vol/vol) methanol and 10% (vol/vol) acetic acid) for 15 min and stained with Invitrogen SimplyBlue Safe Stain according to the manufacturer's instructions. Gels were dried between cellophane, exposed to a phosphor plate for 3 days and imaged with a GE Typhoon phosphorimager. Densitometric analysis was performed using ImageJ software. Experiments were conducted four times, independently.
Electron microscopy
Aliquots of 3 µl purified Pf80S at a concentration of ∼160 nM were incubated with a 2 mM solution of MFQ (BioBlocks) in 20 mM HEPES pH 7.4, 40 mM KAc, 10 mM NH4Ac, 10 mM Mg(Ac)2 and 5 mM 2-mercaptoethanol for 15 min at 25 °C. Samples were incubated for 30 s on glow-discharged holey carbon grids (Quantifoil R1.2/1.3), on which a home-made continuous carbon film (estimated to be ∼30 Å thick) had previously been deposited. Grids were blotted for 2.5 s and flash-frozen in liquid ethane using an FEI Vitrobot. Pf80S–MFQ grids were transferred to an FEI Tecnai Polara electron microscope operated at 300 kV. Images were recorded manually during two non-consecutive days on a back-thinned FEI Falcon II detector at a calibrated magnification of 104,478 (yielding a pixel size of 1.03 Å). Defocus values in the final data set ranged from 1.0 to 3.3 µm. During the data collection sessions, all images that showed signs of significant astigmatism or drift were discarded. An in-house system was used to intercept the video frames from the detector at a rate of 16 s−1.
Image processing
We used RELION (version 1.3-beta) for automated selection of 112,347 particles from 829 micrographs for the Pf80S–MFQ sample. Contrast transfer function parameters were estimated using CTFFIND3 (ref. 35). All two- and three-dimensional classifications and refinements were performed using RELION (ref. 36). We used reference-free two-dimensional class averaging and three-dimensional classification to discard suboptimal particles. A 60 Å low-pass-filtered cryo-EM reconstruction of the P. falciparum 80S ribosome (EMDB-2661; ref. 14) was used as an initial model for the three-dimensional refinement. The final refinement for the Pf80S–MFQ sample contained 43,184 particles.
For the correction of beam-induced movements, we used statistical movie processing, as described previously37, with running averages of five movie frames and a standard deviation of one pixel for the translational alignment. To further increase the accuracy of the movement correction, we used RELION particle polishing to fit linear tracks through the optimal translations for all running averages38, and included neighbouring particles on the micrograph in these fits. In addition, we employed a resolution- and dose-dependent model for the radiation damage, where each frame was weighted with a different B-factor, as estimated from single-frame reconstructions. These procedures yielded a map with an overall resolution of 3.2 Å for the Pf80S–MFQ complex.
Reported resolutions are based on the gold-standard Fourier shell correlation (FSC) = 0.143 criterion39 and were corrected for the effects of a soft mask on the FSC curve using high-resolution noise substitution39. Before visualization, all density maps were corrected for the modulation transfer function (MTF) of the detector and then sharpened by applying a negative B factor (Supplementary Table 1), which was estimated using automated procedures40.
To locate MFQ in the Pf80S–MFQ reconstruction, we calculated a difference map between the reconstructions of empty Pf80S (ref. 14) and Pf80S–MFQ. For this purpose, the two MTF-corrected and B-factor sharpened maps were aligned with respect to each other using the ‘Fit in Map’ functionality in UCSF Chimera 7. Before subtraction, the empty Pf80S map was re-interpolated on the Cartesian grid of the Pf80S–MFQ map, and the power spectrum of the empty Pf80S map was re-scaled to match the power spectrum of the Pf80S–MFQ map. For visualization purposes, the resulting difference map was low-pass-filtered at 4 Å and the threshold was set at five standard deviations as calculated within the area of the Pf80S ribosome (Supplementary Fig. 3). At this threshold, only two continuous density features were visible. The highest difference density inside these features extended to 6.5 and 9.3 standard deviations in the difference map for the primary and secondary sites, respectively.
Local resolution variations in the reconstruction were estimated using ResMap (ref. 41). To improve the resolution of the Pf80S–MFQ reconstruction, a ‘focused’ refinement was performed, where we masked out the large subunit at every iteration. This generated a map (Supplementary Fig. 2) with improved density for the large ribosomal subunit (at an overall resolution of 3.2 Å) and this map was used for the refinement of the atomic model.
Model building and refinement
The available Pf60S atomic model (PDB accession code 3J79; ref. 14) was used as a starting model for refinement of the Pf80S–MFQ reconstruction. MFQ was first real-space-refined in Coot (ref. 42) and the model was subsequently stereo-chemically refined using REFMAC v.5.8, which was modified for structures determined by cryo-EM (refs 43,44). The Pf60S–MFQ atomic model was refined in the map that was obtained in the focused refinement of the cryo-EM reconstruction. Structure factors for the (reciprocal-space) refinement in REFMAC were obtained by cutting out sections of the corresponding maps with a 3 Å radius from the centre of each atom in the model. Structure factor phases were not altered during refinement.
Throughout refinement, reference and secondary structure restraints were applied to the ribosomal proteins using the Sc80S structure as a reference model45. Base pair and parallelization restraints obtained using LIBG were also applied throughout refinement44. The stereochemistry of the rRNA model was further improved using the ERRASER–PHENIX pipeline46. Ramachandran restraints were not applied during refinement to preserve backbone dihedral angles for validation.
The average overall Fourier shell correlation (FSCaverage) was monitored during refinement (Supplementary Table 1) and the final model was validated using MolProbity (ref. 47). For cross-validation against overfitting, we randomly displaced the atoms of our final model (with an r.m.s.d. of 0.5 Å) and performed a fully restrained refinement against a map that was reconstructed from only one of the two independent halves of the data that were used in our gold-standard FSC procedure. We then calculated FSC curves between the resulting model and the half-map against which it had been refined (FSCwork), as well as the FSC curve between that model and the other half-map (FSCtest). The observation that the FSCwork and FSCtest curves overlap demonstrates the absence of overfitting of the model (Supplementary Fig. 2b).
CRISPR mutagenesis of PfuL13
Cas9-expressing plasmid. The DHOD selectable marker was removed from the pUF1-cas9 plasmid20, by self-ligating the XbaI/SpeI-cut plasmid. A NcoI/AatII fragment containing the cas9-gRNA cassette was then removed from the pL6-eGFP plasmid20 and cloned into the pUF1-cas9 plasmid lacking DHOD above, resulting in the pUF1-cas9-gRNA plasmid. The 20 nucleotide guide sequence (GAATATGTTATCGATTGCAA) was cloned into the BtgZI sites of this plasmid using the In-Fusion method (Clontech).
HDR plasmids
The plasmids for homology directed repair were assembled in the p1.2 plasmid used to tag genes at the 3′ end with Strep II and 3HA tags48. The 5′ homology flanks were synthesized (Geneart) as the 5′ portion of the recodoned uL13 sequence (Supplementary Table 3). The 3′ homology flank was amplified using primers p9 and p10 (Supplementary Table 2) and cloned into the EcoRI/KasI sites of the p1.2 plasmid. Subsequently, the 5′ flanks bounded by BglII/XhoI sites (Supplementary Table 3) were cloned into the 3′ flank-containing p1.2 plasmid. This resulted in six plasmids encoding either wild-type (WT), L15S/I42S, I42A, E55A, F56A or L140F uL13 genes.
Transfection
E64-treated magnet-purified schizonts49 were transfected with 100 µg circular guide-containing plasmid and 50 µg of each of the six linearized plasmids containing the homology flanks. A Nucleofector 1 device was used according to the manufacturer's protocol (Amaxa). Cultures were selected with WR99210.
General chemistry methods
Analytical thin-layer chromatography was performed on Merck silica gel 60F254 aluminium-backed plates, which were visualized by fluorescence quenching under ultraviolet light. Flash chromatography was performed with silica gel 60 (particle size 0.040–0.063 µm). NMR spectra were recorded on a Bruker Avance DRX 300 (1H NMR at 300 MHz) with the solvents indicated. Chemical shifts are reported in ppm on the δ scale and referenced to the appropriate solvent peak. High-resolution electrospray mass spectrometry (HRESMS) were acquired by J. Dang at the Monash Institute of Pharmaceutical Sciences Spectrometry Facility using an Agilent 1290 infinity 6224 TOF LCMS. The column used was an RRHT 2.1 × 50 mm × 1.8 µm C18. The gradient was applied over 5 min with a flow rate of 0.5 ml min–1. For MS, the conditions were as follows: gas temperature of 325 °C; drying gas rate of 11 l min–1; nebulizer 45 p.s.i.g.; fragmentor 125 V. LCMS were recorded on a Waters ZQ 3100 using a 2996 Diode Array Detector. LCMS conditions used to assess purity of compounds were as follows: column, XBridge TM C18 5 µm × 4.6 × 100 mm; injection volume 10 µl; gradient 10–100% B over 10 min (solvent A: water 0.1% formic acid; solvent B: AcCN 0.1% formic acid); flow rate 1.5 ml min–1; detection 100–600 nm. All final compounds were analysed using ultrahigh-performance liquid chromatography/ultraviolet/evaporative light scattering detection coupled to mass spectrometry. Unless otherwise noted, all compounds were found to be >95% pure by this method. 2-(2,8-Bis(trifluoromethyl)-4-quinolyl)oxirane was purchased commercially and used without further purification.
Synthesis of MFQ analogues
MFQ analogues MFQ_D1 and MFQ_D2 were generated according to the method by Milner and co-authors29,30:

General procedure A
4-(Oxiran-2-yl)-2,8-bis(trifluoromethyl)quinoline (30 mg, 0.10 mmol) and the appropriate amine (0.49 mmol) in iPrOH (2 ml) were irradiated in a microwave reactor (CEM) for 30 min at 130 °C. The reaction mixture was concentrated in vacuo and purified by silica chromatography gradient eluting with 100% dichloromethane (DCM) to 7.5% MeOH/DCM/0.1% NH4OH to obtain the quinoline.
1-(2,8-Bis(trifluoromethyl)quinolin-4-yl)-2-(cyclohexylamino)ethanol (MFQ_D1)
General procedure A was followed using cyclohexylamine (56 µl, 0.49 mmol) to obtain MFQ_D1 as a solid (30 mg, 76%). 1H NMR (CDCl3): δ 8.27 (d, J = 8.3 Hz, 1H), 8.19–8.14 (m, 2H), 7.73 (t, J = 8.1 Hz, 1H), 5.50 (dd, J = 8.6 and 3.3 Hz, 1H), 3.29 (dd, J = 12.5 and 3.8 Hz, 1H), 2.76–2.50 (m, 3H), 1.97–1.93 (m, 2H), 1.79–1.64 (m, 3H), 1.31–1.10 (m, 5H). 13C NMR (75 MHz, CDCl3) δ 151.6, 148.9, 148.4, 143.7, 129.8, 128.7, 127.0, 125.3, 123.0, 121.7, 119.4, 114.5, 67.8, 56.7, 52.6, 34.1, 33.7, 25.9, 24.9. MS, m/z = 407 [M+H]+. HRMS found: (M+H) 407.1561; C19H21F6N2O requires (M+H), 407.1558.
1-(2,8-Bis(trifluoromethyl)quinolin-4-yl)-2-((2,3-dihydro-1H-inden-2-yl)amino)ethanol (MFQ_D2)
General procedure A was followed using 2-aminoindane (64 µl, 0.49 mmol) to obtain MFQ_D2 as a solid (35 mg, 81%). 1H NMR (CDCl3): δ 8.23–8.17 (m, 2H), 8.14 (s, 1H), 7.75 (t, J = 7.8 Hz, 1H), 7.22–7.17 (m, 4H), 5.50 (dd, J = 8.9 and 3.3 Hz, 1H), 3.76–3.68 (m, 1H), 3.33–3.19 (m, 3H), 2.88–2.74 (m, 3H). 13C NMR (75 MHz, CDCl3) δ 151.2, 148.8, 148.4, 143.7, 141.0, 129.9, 129.5, 128.7, 127.1, 126.7, 125.3, 123.1, 121.7, 119.4, 114.5, 67.9, 59.3, 53.9, 40.2. MS, m/z = 441 [M+H]+. HRMS found: (M+H) 441.1403; C22H19F6N2O requires (M+H), 441.1402.
Mefloquine derivatives in Supplementary Table 4
The MFQ analogues, MFQ_D3 to MFQ_D7, were generated according to the method by Milner and co-authors29,30.
1-(2,8-Bis(trifluoromethyl)quinolin-4-yl)-2-(isobutylamino)ethanol (MFQ_D3)
General procedure A was followed using isobutylamine (49 µl, 0.49 mmol) to obtain MFQ_D3 as a solid (21 mg, 55%). This compound has data identical to that previously described50.
1-(2,8-Bis(trifluoromethyl)quinolin-4-yl)-2-(butylamino)ethanol (MFQ_D4)
General procedure A was followed using n-butylamine (48 µl, 0.49 mmol) to obtain MFQ_D4 as a solid (29 mg, 76%). 1H NMR (CDCl3): δ 8.27 (d, J = 8.6 Hz, 1H), 8.19–8.14 (m, 2H), 7.73 (t, J = 7.9 Hz, 1H), 5.5–5.52 (m, 1H), 3.19 (dd, J = 12.5 and 3.5 Hz, 1H), 2.79–2.69 (m, 2H), 1.57–1.38 (m, 4H), 0.97–0.93 (m, 3H). MS, m/z = 381 [M+H]+.
1-(2,8-Bis(trifluoromethyl)quinolin-4-yl)-2-((2-methylbutyl)amino)ethanol (MFQ_D5)
General procedure A was followed using 2-methylbutylamine (58 µl, 0.49 mmol) to obtain MFQ_D5 as a solid (31 mg, 79%). This compound has data identical to that previously described29.
1-(2,8-Bis(trifluoromethyl)quinolin-4-yl)-2-(isopentylamino)ethanol (MFQ_D6)
General procedure A was followed using isopentylamine (57 µl, 0.49 mmol) to obtain MFQ_D6 as a solid (32 mg, 82%). 1H NMR (CDCl3): δ 8.25–8.14 (m, 3H), 7.73 (t, J = 7.9 Hz, 1H), 5.54–5.49 (m, 1H), 3.24–3.17 (m, 1H), 2.83–2.64 (m, 2H), 1.70–1.65 (m, 2H), 1.47–1.40 (0.94–0.93(m, 6H). MS, m/z = 395[M+H]+.
1-(2,8-Bis(trifluoromethyl)quinolin-4-yl)-2-(cyclopentylamino)ethanol (MFQ_D7)
General procedure A was followed using cyclopentylamine (48 µl, 0.49 mmol) to obtain MFQ_D7 as a solid (31 mg, 79%). 1H NMR (CDCl3): δ 8.27 (d, J = 8.4 Hz, 1H), 8.19–8.14 (m, 2H), 7.73 (t, J = 8.0 Hz, 1H), 5.53 (dd, J = 8.9 and 3.5 Hz, 1H), 3.26–3.17 (m, 2H), 2.76–2.69 (m, 1H), 1.92–1.38 (m, 8H). MS, m/z = 393 [M+H]+.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. A cryo-EM density map has been deposited in the Electron Microscopy Data Bank under accession no. EMD-8576; and atomic coordinates have been deposited in the Protein Data Bank, under entry code 5UMD.
Additional information
How to cite this article: Wong, W. et al. Mefloquine targets the Plasmodium falciparum 80S ribosome to inhibit protein synthesis. Nat. Microbiol. 2, 17031 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
World Malaria Report (WHO, 2016); http://www.who.int/malaria/publications/world-malaria-report-2016/en
White, N. J. et al. Malaria. Lancet 383, 723–735 (2014).
Wells, T. N., Hooft van Huijsduijnen, R. & Van Voorhis, W. C. Malaria medicines: a glass half full? Nat. Rev. Drug Discov. 14, 424–442 (2015).
Nevin, R. L. & Byrd, A. M. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol. Ther. 5, 69–83 (2016).
Lim, P. et al. Decreasing pfmdr1 copy number suggests that Plasmodium falciparum in western Cambodia is regaining in vitro susceptibility to mefloquine. Antimicrob. Agents Chemother. 59, 2934–2937 (2015).
Roberts, L. Malaria wars. Sci. Transl. Med. 352, 398–405 (2016).
Sanchez, C. P., Rotmann, A., Stein, W. D. & Lanzer, M. Polymorphisms within PfMDR1 alter the substrate specificity for anti-malarial drugs in Plasmodium falciparum. Mol. Microbiol. 70, 786–798 (2008).
Cowman, A. F., Galatis, D. & Thompson, J. K. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl Acad. Sci. USA 91, 1143–1147 (1994).
Reed, M. B., Saliba, K. J., Caruana, S. R., Kirk, K . & Cowman, A. F. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403, 906–909 (2000).
Sanchez, C. P., Dave, A., Stein, W. D. & Lanzer, M. Transporters as mediators of drug resistance in Plasmodium falciparum. Int. J. Parasitol. 40, 1109–1118 (2010).
Gamo, F.-J. et al. Thousands of chemical starting points for antimalarial lead identification. Nature 465, 305–310 (2010).
Dahl, E. L. et al. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob. Agents Chemother. 50, 3124–3131 (2006).
Goodman, C. D., Su, V. & McFadden, G. I. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 152, 181–191 (2007).
Wong, W. et al. Cryo-EM structure of the Plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. eLife 3, e03080 (2014).
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 334, 1524–1529 (2011).
Spahn, C. M. et al. Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 23, 1008–1019 (2004).
Brodersen, D. E. et al. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 103, 1143–1154 (2000).
Lutz, R. E., Ohnmacht, C. J. & Patel, A. R. Antimalarials. 7. Bis(trifluoromethyl)-α-(2-piperidyl)-4-quinolinemethanols. J. Med. Chem. 14, 926–928 (1971).
Harms, J. M. et al. Translational regulation via L11: molecular switches on the ribosome turned on and off by thiostrepton and micrococcin. Mol. Cell 30, 26–38 (2008).
Ghorbal, M. et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR–Cas9 system. Nat. Biotechnol. 32, 819–821 (2014).
Lelievre, J. et al. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence ‘transmission blocking’ assay. PLoS ONE 7, e35019 (2012).
Delves, M. J. et al. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob. Agents Chemother. 57, 3268–3274 (2013).
Waters, A. P., Syin, C. & McCutchan, T. F. Developmental regulation of stage-specific ribosome populations in Plasmodium. Nature 342, 438–440 (1989).
Aguiar, A. C., Pereira, D. B., Amaral, N. S., De Marco, L. & Krettli, A. U. Plasmodium vivax and Plasmodium falciparum ex vivo susceptibility to anti-malarials and gene characterization in Rondonia, west Amazon, Brazil. Malar. J. 13, 73 (2014).
Otigbuo, I. N. & Onabanjo, A. O. The in vitro and in vivo effects of mefloquine on Trypanosoma brucei brucei. J. Hyg. Epidemiol. Microbiol. Immunol. 36, 191–199 (1992).
Holfels, E., McAuley, J., Mack, D., Milhous, W. K. & McLeod, R. In vitro effects of artemisinin ether, cycloguanil hydrochloride (alone and in combination with sulfadiazine), quinine sulfate, mefloquine, primaquine phosphate, trifluoperazine hydrochloride, and verapamil on toxoplasma gondii. Antimicrob. Agents Chemother. 38, 1392–1396 (1994).
Khatter, H., Myasnikov, A. G., Natchiar, S. K. & Klaholz, B. P. Structure of the human 80S ribosome. Nature 520, 640–645 (2015).
Brown, A. et al. Structure of the large ribosomal subunit from human mitochondria. Science 346, 718–722 (2014).
Milner, E. et al. Structure–activity relationships amongst 4-position quinoline methanol antimalarials that inhibit the growth of drug sensitive and resistant strains of Plasmodium falciparum. Bioorg. Med. Chem. Lett. 20, 1347–1351 (2010).
Milner, E. et al. Anti-malarial activity of a non-piperidine library of next-generation quinoline methanols. Malar. J. 9, 51 (2010).
Na-Bangchang, K., Muhamad, P., Ruaengweerayut, R., Chaijaroenkul, W. & Karbwang, J. Identification of resistance of Plasmodium falciparum to artesunate–mefloquine combination in an area along the Thai–Myanmar border: integration of clinico-parasitological response, systemic drug exposure, and in vitro parasite sensitivity. Malar. J. 12, 263 (2013).
Garreau de Loubresse, N. et al. Structural basis for the inhibition of the eukaryotic ribosome. Nature 513, 517–522 (2014).
Sun, M . et al. Dynamical features of the Plasmodium falciparum ribosome during translation. Nucleic Acids Res. 43, 10515–10524 (2015).
Ahyong, V. et al. Identification of Plasmodium falciparum specific translation inhibitors from the MMV malaria Box using a high throughput in vitro translation screen. Malar. J. 15, 173 (2016).
Mindell, J. A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Bai, X. C., Fernandez, I. S., McMullan, G. & Scheres, S. H. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. eLife 2, e00461 (2013).
Scheres, S. H. Beam-induced motion correction for sub-megadalton cryo-EM particles. eLife 3, e03665 (2014).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D 67, 355–367 (2011).
Amunts, A. et al. Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014).
Nicholls, R. A., Long, F. & Murshudov, G. N. Low-resolution refinement tools in REFMAC5. Acta Crystallogr. D 68, 404–417 (2012).
Chou, F. C., Sripakdeevong, P., Dibrov, S. M., Hermann, T. & Das, R. Correcting pervasive errors in RNA crystallography through enumerative structure prediction. Nat. Methods 10, 74–76 (2013).
Chen, V. B. et al. Molprobity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Baum, J. et al. Reticulocyte-binding protein homologue 5—an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 39, 371–380 (2009).
Boyle, M. J. et al. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc. Natl Acad. Sci. USA 107, 14378–14383 (2010).
Gillespie, R. J. et al. 4-quinolinemethanol derivatives as purine receptor antagonists (II) US patent 6,608,085 (2003).
Acknowledgements
The authors thank I. Lucet, J. Boddey, S. Herrmann, G. McFadden, J. Rayner, A. Ruecker, M. Delves, H. Baumann, G. Murshudov and P. Emsley for discussions and experimental assistance, S. Chen and C. Savva for help with microscopy, and J. Grimmett and T. Darling for help with computing. The experimental data were made possible by Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. The research was directly supported by a National Health and Medical Research Council of Australia (NHMRC) Project Grant (APP1024678 to J.B. and W.W.), the Australian Cancer Research Foundation, a Human Frontier Science Program (HFSP) Young Investigator Program Grant (RGY0071/2011, to J.B.) and grants from the UK Medical Research Council (MC_UPA0251013, to S.H.W.S.). W.W. is an Early Career Development Awardee (APP1053801) from the NHMRC and was in receipt of a travel award from OzEMalaR to visit MRC–LMB UK to conduct experiments. X.-C.B. is supported by an EU FP7 Marie Curie Postdoctoral Fellowship. A.B. and I.S.F. are supported by grants to V. Ramakrishnan from the Wellcome Trust (WT096570) and the UK Medical Research council (MC_U105184332). J.B. was supported by a Future Fellowship (FT100100112) from the Australian Research Council (ARC) and is currently supported by an Investigator Award from the Wellcome Trust (100993/Z/13/Z). Additional support for this work came from a Pathfinder Award from the Wellcome Trust (105686).
Author information
Authors and Affiliations
Contributions
W.W., X.-C.B., B.E.S., K.E.J., T.T., D.S.M., S.A.R., S.H.W.S. and J.B. designed all experiments. W.W., X.-C.B., B.E.S., K.E.J., A.B., T.T., D.S.M., J.K.T., E.H. and I.S.F. performed experiments. W.W., X.-C.B., B.E.S., K.E.J., T.T., A.B., J.K.T., S.A.R., A.F.C., S.H.W.S. and J.B. contributed to manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-6, Supplementary Data Tables 1-4. (PDF 10094 kb)
Rights and permissions
About this article
Cite this article
Wong, W., Bai, XC., Sleebs, B. et al. Mefloquine targets the Plasmodium falciparum 80S ribosome to inhibit protein synthesis. Nat Microbiol 2, 17031 (2017). https://doi.org/10.1038/nmicrobiol.2017.31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2017.31
This article is cited by
-
Deaggregation of mutant Plasmodium yoelii de-ubiquitinase UBP1 alters MDR1 localization to confer multidrug resistance
Nature Communications (2024)
-
Malaria therapeutics: are we close enough?
Parasites & Vectors (2023)
-
Nonclassical mechanisms to irreversibly suppress β-hematin crystal growth
Communications Biology (2023)
-
A Legionella toxin exhibits tRNA mimicry and glycosyl transferase activity to target the translation machinery and trigger a ribotoxic stress response
Nature Cell Biology (2023)
-
CRISPR-based oligo recombineering prioritizes apicomplexan cysteines for drug discovery
Nature Microbiology (2022)