Abstract
The causes of major depression remain unknown. Antidepressants elevate concentrations of monoamines, particularly serotonin, but it remains uncertain which downstream events are critical to their therapeutic effects. We found that endogenous serotonin selectively potentiated excitatory synapses formed by the temporoammonic pathway with CA1 pyramidal cells via activation of serotonin receptors (5-HT1BRs), without affecting nearby Schaffer collateral synapses. This potentiation was expressed postsynaptically by AMPA-type glutamate receptors and required calmodulin-dependent protein kinase–mediated phosphorylation of GluA1 subunits. Because they share common expression mechanisms, long-term potentiation and serotonin-induced potentiation occluded each other. Long-term consolidation of spatial learning, a function of temporoammonic-CA1 synapses, was enhanced by 5-HT1BR antagonists. Serotonin-induced potentiation was quantitatively and qualitatively altered in a rat model of depression, restored by chronic antidepressants, and required for the ability of chronic antidepressants to reverse stress-induced anhedonia. Changes in serotonin-mediated potentiation, and its recovery by antidepressants, implicate excitatory synapses as a locus of plasticity in depression.
Similar content being viewed by others
Main
Depression is a leading cause of mortality and morbidity worldwide1 and probably results from a combination of genetic and environmental risk factors. Although the genes responsible for depression remain difficult to identify, a common risk factor is stress2. Current treatments for depression remain unsatisfactory, partly because the underlying biological changes in brain function in depression remain poorly understood. The discovery that changes in monoamine levels alter the affective state of patients led to the hypothesis that a dysfunction of monoamine signaling, particularly serotonin, causes depression3. Elevation of serotonin levels with conventional antidepressants, such as selective serotonin-reuptake inhibitors (SSRIs) and tricyclic antidepressants, may modulate neuronal excitability and plasticity by altering the transcription, translation and phosphorylation state of target proteins1,3. Neither the principal neural circuits nor the key proteins that are modulated by serotonin are known, however. Serotonin is capable of regulating the glutamatergic system, and evidence of changes in excitatory synaptic transmission in models of depression is accumulating4,5,6, but it remains unclear how these two neurotransmitter systems interact.
Depression is likely caused by dysfunction in a variety of brain regions and cell types, including the hippocampus7,8. Altered hippocampal function may also adversely influence the activity of the cortex, amygdala and other structures associated with reward, motivation and emotionality. For example, the hippocampal formation provides a major source of excitatory input to the nucleus accumbens (NAc)9, where chronic stress weakens AMPA receptor (AMPAR)-mediated excitatory synaptic transmission10.
The highest concentration of serotoninergic fibers in the forebrain is in stratum lacunosum-moleculare (SLM) of hippocampal areas CA1 and CA3 (ref. 11), where the axons of layer III neurons in the entorhinal cortex form excitatory synapses with the distal apical dendrites of pyramidal cells. This temporoammonic pathway is required for some spatial recognition tasks and for long-term consolidation of spatial memory12. To understand the function of serotonin better, we studied its actions at temporoammonic-CA1 (TA-CA1) synapses, and observed that serotonin potentiated these synapses postsynaptically and that this potentiation was altered in a rat model of depression.
Results
Potentiation of TA-CA1 synapses by serotonin
We recorded field excitatory postsynaptic potentials (fEPSPs) in SLM of area CA1 of acutely prepared rat hippocampal brain slices in response to stimulation of the temporoammonic pathway (Supplementary Fig. 1a). Bath application of the tricyclic antidepressant imipramine (2 μM) or the SSRIs fluoxetine (20 μM) or citalopram (10 μM), to acutely elevate extracellular serotonin, all increased the slope of TA-CA1 fEPSPs (Fig. 1a,b and Supplementary Fig. 1b,c). Potentiation of synaptic transmission by serotonin is notable, as most neuromodulators inhibit synaptic transmission in the mammalian CNS, although there is evidence that serotonin can increase glutamate release in some systems13,14.
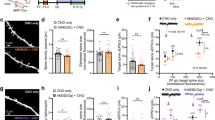
(a) Promoting accumulation of endogenous serotonin by bath application of the tricyclic antidepressant imipramine (2 μM) potentiated TA-CA1 fEPSPs in SLM of area CA1 of acutely prepared hippocampal slices (n = 8 slices) in control saline (black open circles), but not in slices pretreated with the 5-HT1BR antagonist isamoltane (10 μM) (red triangles, n = 9 slices). Sample traces before (black) and 60 min after imipramine application (red) in control saline (top) or in the presence of isamoltane (bottom) are shown at right. TA, temporoammonic. (b) Group data showing the effect of antidepressants on TA-CA1 fEPSPs in control slices (fluoxetine, n = 4 slices; citalopram, n = 3 slices) and the effect of anpirtoline (50 μM) on TA-CA1 fEPSPs in control slices (red, n = 11 slices; ANOVA, F3,20 = 9.751, P < 0.001) or slices pretreated for 60 min with antidepressants (n = 5 slices pretreated with imipramine, n = 5 slices with fluoxetine, n = 3 slices with citalopram, F3,20 = 7.32, P = 0.002). Bonferroni post hoc tests revealed that isamoltane prevented the increase in fEPSP slope observed with antidepressants alone (P < 0.05 versus imipramine, fluoxetine or citalopram) and that anpirtoline treatment differed from each of these pretreatment + anpirtoline conditions (P < 0.05). *P < 0.05, **P < 0.01 compared with before anpirtoline or antidepressant, paired t test. (c) The selective 5HT1BR agonist anpirtoline reversibly increased the slope of TA-CA1 fEPSPs in SLM of area CA1 (open circles, n = 11 slices). Isamoltane (10 μM) blocked the effect of anpirtoline on TA-CA1 fEPSPs (red triangles, n = 5 slices). (d) Anpirtoline selectively enhanced TA-CA1 EPSCs recorded in whole-cell voltage-clamp mode (open circles), but not simultaneously evoked SC-CA1 EPSCs (filled circles) in a two-pathway experimental design (n = 9 cells). Sample traces are shown at right. The selective increase in TA-CA1 fEPSPs by serotonin, but not of SC-CA1 fEPSPs, is consistent with the selective localization of 5-HT1BRs in SLM. SC, Schaffer collateral.
Serotonin receptor type 1B (Hrt1b) mRNA is present in CA1 cells at high levels and 5-HT1BR binding sites are concentrated in SLM15,16. We therefore asked whether 5-HT1BRs mediate the effects of acute elevation of serotonin on TA-CA1 synaptic transmission. Imipramine application did not potentiate TA-CA1 fEPSP slope in slices pretreated with the antagonist isamoltane (10 μM) (Fig. 1a,b), nor did imipramine or fluoxetine affect TA-CA1 fEPSPs in slices of hippocampus taken from mice lacking 5-HT1BRs17 (Supplementary Fig. 2a,b). In contrast, the 5-HT1AR antagonist NAN-190 (10 μM) had no effect on potentiation of TA-CA1 fEPSPs by imipramine (Supplementary Fig. 2c). Consistent with these findings, a selective agonist for 5-HT1BRs, anpirtoline (50 μM)18, enhanced the slope of TA-CA1 fEPSPs (Fig. 1c). Unlike the acute potentiating effects of the antidepressants, the effects of anpirtoline on fEPSP slope were fully reversed after 60 min of washout. Application of isamoltane during washout of imipramine decreased the potentiation (Supplementary Fig. 1d), suggesting that the maintained potentiation induced by antidepressants was a result of a persistent increase in serotonin levels. Anpirtoline also produced a doubling of TA-CA1 EPSP slope in whole-cell voltage-clamp (Fig. 1d) and current-clamp (Supplementary Fig. 2d) recordings. Anpirtoline had no effect on fEPSPs in slices pretreated with isamoltane (Fig. 1c). Another 5-HT1BR selective agonist, CP94253 (5 μM), also potentiated TA-CA1 fEPSP slope (Supplementary Fig. 2e). Anpirtoline antagonizes 5-HT3 receptors at higher concentrations19, but the 5-HT3 receptor antagonist Y-25130 (5 μM) had no effect on TA-CA1 fEPSP slope (Supplementary Fig. 2e), indicating that anpirtoline's effect was mediated by 5-HT1BRs. Anpirtoline-induced potentiation was unaffected by depletion of serotonin with p-chlorophenylalanine20 (Supplementary Fig. 1e), indicating that anpirtoline's effect was mediated by postsynaptic heteroreceptors and not the result of an action on presynaptic inhibitory autoreceptors on serotonergic nerve terminals21. Finally, application of anpirtoline had no effect on TA-CA1 fEPSP slope in slices pretreated for 60 min with antidepressants (Fig. 1b), presumably because the endogenous serotonin had already maximally activated 5-HT1BRs, occluding the potentiation.
Serotonin-mediated potentiation was specific for the TA-CA1 excitatory synapses in SLM, where 5-HT1BRs are concentrated. Neither anpirtoline (Fig. 1d and Supplementary Fig. 2d) nor fluoxetine (Supplementary Fig. 2f) had any effect on Schaffer collateral–CA1 (SC-CA1) cell responses to stimuli delivered in stratum radiatum. Thus, activation of 5-HT1BRs by serotonin produces a highly localized potentiation of excitatory synapses.
Temporoammonic inputs are relatively ineffective at inducing action potential firing in CA1 pyramidal cells, although modest Schaffer collateral cell activation facilitates this process22. Anpirtoline enhanced the supralinear summation of temporoammonic–Schaffer collateral EPSPs and promoted temporoammonic–Schaffer collateral–induced action potential firing (Supplementary Fig. 3a,b). Activation of 5-HT1BRs by endogenously released serotonin and subsequent potentiation of the TA-CA1 pathway therefore promotes action potential output from area CA1.
Molecular basis of 5-HT–mediated potentiation
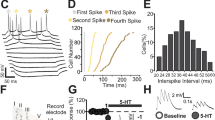
Is 5-HT1BR–mediated potentiation of TA-CA1 fEPSPs mediated pre- or postsynaptically? Neither the amplitude of the temporoammonic fiber volley (106 ± 3% of control, n = 7 slices, P > 0.05) nor the paired-pulse ratio of TA-CA1 fEPSPs (1.39 ± 1.3 before, 1.34 ± 1.0 after anpirtoline, n = 11 slices, P > 0.05; elicited with pairs of temporoammonic stimuli separated by 50 ms) were changed significantly by anpirtoline (Fig. 2a). Furthermore, anpirtoline significantly potentiated the AMPAR-mediated component of the excitatory postsynaptic current (EPSC), recorded under voltage clamp at −70 mV (161 ± 5% of control, n = 7 slices, t6 = 4.808, P = 0.003), whereas the NMDA receptor–mediated component of the EPSC at +40 mV was unaffected (83 ±13% of control, n = 5 cells, t4 = 1.838, P = 0.14; Fig. 2b). A similar selective potentiation of pharmacologically isolated AMPAR-mediated fEPSPs was also observed in extracellular recordings (Supplementary Fig. 3c). 5-HT1BR–induced potentiation is therefore inconsistent with classical presynaptic expression mechanisms.
(a) Time course of changes of TA-CA1 fEPSP slope (black circles), fiber volley (open triangles) and paired-pulse ratio (PPR, red circles) before and after anpirtoline application in a typical experiment. Representative traces are shown at right, with the fiber volley at higher resolution above. Neither fiber volley (t6 = 0.039, P = 0.97) nor PPR (t10 = 1.17, P = 0.27) changed with anpirtoline treatment. (b) Whole-cell voltage-clamp recordings of EPSCs at −70 and +40 mV in the same cell before (black) and 40 min after application of anpirtoline (red). (c) Left, image of a CA1 cell filled with Alexa 594 from a somatic whole-cell pipette indicating sites of microphotolysis of caged glutamate on distal apical dendrites in SLM or oblique dendrites in stratum radiatum. Scale bar represents 20 μm. Right, bath application of anpirtoline increased photolysis-evoked EPSPs elicited from dendrites in SLM in acutely prepared hippocampal brain slices (n = 5 cells), but not from dendrites in stratum radiatum (n = 6 cells in separate experiments), indicating that 5-HT1BR activation selectively enhanced postsynaptic glutamate responses at sites of temporoammonic synapse formation. Sample EPSP-like photolysis responses elicited from dendrites in SLM and stratum radiatum before (black) and after anpirtoline application (red) are shown at right.
Selective 5-HT1BR–induced enhancement of AMPAR-mediated responses at temporoammonic synapses may result from a postsynaptic increase in AMPAR function. We tested this hypothesis using microphotolysis of caged glutamate23. With photostimuli delivered in SLM, anpirtoline application produced a near doubling of the slope of AMPAR-mediated photolysis-evoked EPSPs (phEPSPs; Fig. 2c). Consistent with its lack of effect on SC-CA1 EPSPs, anpirtoline did not increase the slope of AMPAR-mediated phEPSPs elicited by microphotolysis of caged glutamate onto proximal oblique dendrites in stratum radiatum (Fig. 2c). Potentiation of phEPSPs was not accompanied by changes in either holding current or input resistance (data not shown). Thus, activation of 5-HT1BRs specifically and selectively increases the number or efficacy of postsynaptic AMPARs in the distal apical dendrites of CA1 pyramidal neurons in a highly localized manner.
Stimulation of 5-HT1BRs can activate several protein kinases24,25, including calcium/calmodulin-dependent protein kinase (CaMK). We first analyzed threonine 286 on CaMKII, which is autophosphorylated when CaMK is activated26, in SLM tissue wedges subdissected from hippocampal slices. Anpirtoline increased CaMK T286 phosphorylation with a time course comparable to the induction of the potentiation (Fig. 3a,b). Incubation of hippocampal slices with KN62 (5 μM), a specific CaMK inhibitor, blocked the effect of anpirtoline on TA-CA1 fEPSP slope (Fig. 3c). Similarly, intracellular dialysis with a selective CaMK inhibitor peptide, autocamtide-2-related inhibitory peptide (AIP, 2 μM; Fig. 3c) blocked potentiation of TA-CA1 EPSP slope by anpirtoline. In contrast, anpirtoline-induced potentiation of fEPSPs was not affected by the protein kinase A (PKA) inhibitor PKI 14-22 (1 μM; Fig. 3c). We conclude that CaMK is activated rapidly in response to 5-HT1BR activation and that this activation is necessary for the potentiation of TA-CA1 transmission.
(a,b) Representative western blots (a) and quantification (b) showing that anpirtoline (50 μM, 2 h) time dependently increased phosphorylation of alpha and beta CaMK at T286 (molecular weights 52 and 60 kDa; F5,18 = 4.651, P = 0.007) and GluA1 at S831 (F5,24 = 3.706, P = 0.013), but did not affect total GluA1 expression (F5,18 = 0.863, P = 0.524) in isolated CA1 SLM tissue sections, subdissected from whole hippocampal slices. *P < 0.05, **P < 0.01 compared with before anpirtoline. (c) The effect of anpirtoline on TA-CA1 fEPSPs was blocked by bath application of the specific CaMK inhibitor KN62 (5 μM) (n = 6 slices), but not by bath application of the cell-permeable PKA inhibitor PKI 14-22 (PKI, 1 μM, n = 3 slices). Intracellular dialysis of the CaMK inhibitor autocamtide-2–related inhibitory peptide (AIP, 2 μM) via patch-pipettes (n = 5 cells) blocked potentiation of TA-CA1 EPSPs recorded in whole-cell mode. (d) Anpirtoline increased phosphorylation of GluA1 at S831 in isolated CA1 SLM tissue sections, but not at S845. (e) Anpirtoline (Anp, 50 μM for 60 min) increased phosphorylation of CaMK T286 (left, Mann-Whitney U test, z = 2.09, P = 0.04) and GluA1 S831 (right, Mann-Whitney U test, z = 2.09, P = 0.04) in tissue punches taken from the core of the NAc (n = 6 slices each). Con, control. (f) Anpirtoline did not potentiate TA-CA1 fEPSPs in slices from GluA1 S831A mice (n = 7 slices from 4 animals), but produced strong potentiation in slices from wild-type littermate mice (n = 4 slices from 4 animals). Full-length blots are presented in Supplementary Figure 7.
Multiple phosphorylation sites in AMPARs, including two serines in the C terminus of the GluA1 subunit, are important for synaptic long-term potentiation (LTP)27,28. Anpirtoline produced a time-dependent increase in the phosphorylation of GluA1 at serine 831 (142 ± 11% of untreated, n = 11 slices, t10 = 3.17, P = 0.01), the target of protein kinase C and CaMK, but not at the PKA site serine 845 (116 ± 8% of untreated, n = 4 slices, P > 0.05), as revealed in western blots of SLM tissue wedges (Fig. 3a,b,d). Consistent with the lack of potentiation of SC-CA1 synapses, anpirtoline had no effect on CaMK autophosphorylation or GluA1 S831 phosphorylation in isolated tissue from stratum radiatum (Supplementary Fig. 4a) or in SLM in tissue from Htr1b−/− mice (Supplementary Fig. 4b). Consistent with the persistent potentiation induced by acute application of antidepressants (Fig. 1), acute fluoxetine treatment resulted in activated CaMK and phosphorylation of GluA1 S831 that persisted after fluoxetine washout, unlike anpirtoline (Supplementary Fig. 4c). Comparable time-dependent increases in CaMK activation and GluA1 S831 phosphorylation in response to anpirtoline were obtained with tissue from the NAc core (Fig. 3e) and medial prefrontal cortex (Supplementary Fig. 4d), suggesting that local regulation of the strength of synaptic excitation by serotonin may occur in multiple brain regions that are important for cognitive and affective function.
We next tested the hypothesis that CaMK-mediated phosphorylation of S831 is required for anpirtoline-induced potentiation using transgenic mice in which GluA1 S831 was mutated to alanine (GluA1 S831A mice), rendering it incapable of being phosphorylated by CaMK29. Neither anpirtoline (Fig. 3f) nor fluoxetine (Supplementary Fig. 4e) had any effect on TA-CA1 fEPSP slope in slices prepared from these mice, although they potentiated TA-CA1 fEPSP slope in slices from wild-type littermate mice. We conclude that serotonin enhances AMPAR function by activating CaMK and increasing GluA1 phosphorylation at serine 831.
Serotonin action influences in memory consolidation
Activation of CaMK and phosphorylation of GluA1 are required for both serotonin-induced potentiation of TA-CA1 synapses and conventional LTP at SC-CA1 synapses28,30. Indeed, induction of LTP at TA-CA1 synapses prevented subsequent potentiation by anpirtoline (Fig. 4a,b) and TA-CA1 LTP could not be induced after potentiating fEPSPs with anpirtoline (Fig. 4c). In contrast, anpirtoline did not potentiate SC-CA1 fEPSPs or occlude SC-CA1 LTP (Fig. 4d). We conclude that serotonin-induced potentiation and conventional synaptic LTP occlude each other at TA-CA1 excitatory synapses, probably because they share common signaling and expression mechanisms.
(a) After induction of LTP with high-frequency stimulation (HFS, four trains, 1 s per train at 100 Hz, 5-min interval), bath application of anpirtoline (50 μM) failed to induce further potentiation of TA-CA1 fEPSP slope (n = 8 slices). (b) The effect of anpirtoline on TA-CA1 fEPSP slope remained occluded after induction of LTP, even when the stimulation intensity was decreased to return the fEPSP to the baseline level (n = 5 slices). Representative traces are shown below. Scale bars represent 0.1 mV and 20 ms. (c) Anpirtoline-induced potentiation of TA-CA1 fEPSP slope occluded tetanus-induced LTP at TA-CA1 synapses (n = 4 slices). (d) Anpirtoline neither enhanced SC-CA1 fEPSPs nor occluded LTP of SC-CA1 synapses (red, n = 7 slices). LTP of SC-CA1 fEPSPs in control slices shown in black (n = 5 slices). Only every other data point is plotted to allow the two data sets to be distinguished.
TA-CA1 synapses are strongly activated during cognitive tasks, including both spatial and nonspatial tasks that involve working and associative memory in primates31. In rodents, TA-CA1 synapses support learning in the Morris water maze32 and are required for long-term memory consolidation12. To determine whether serotonin activates 5-HT1BRs at TA-CA1 synapses in intact rats, we asked whether a 5-HT1BR antagonist would have any influence on consolidation of spatially cued memory using the Morris water maze (Fig. 5). The time required for rats to locate a hidden platform became shorter after 6 d of training. Successful memory of the location was verified in a probe trial 24 h after the final training session. Rats were then divided into two groups: one group received the 5-HT1BR antagonist SB216641 (4 mg kg of body weight, intraperitoneal, daily for 2 weeks starting immediately after the probe trial)33 and the others did not (controls, saline, intraperitoneal, daily). The rats underwent a second probe trial to test consolidation 28 d after the end of training. Both groups performed better in this trial than they had before training, indicating that they had consolidated some memory of the platform location. Rats treated with SB216641, however, displayed a shorter latency to find the platform location than the untreated controls. Furthermore, the latency of the SB216641-treated rats to the platform location was not substantially different 28 d after training than it was in the 24 h probe trial. Treated rats also crossed through the platform area significantly more times than the control rats (P < 0.05), and spent more time in the target quadrant, indicating a more accurate search pattern. Consistent with previous reports that Htr1b−/− mice have improved short-term learning in the Morris water maze34, we conclude that activation of 5-HT1BRs by endogenous serotonin interacts with the memory consolidation process, perhaps through interaction with LTP at TA-CA1 synapses.
(a) Latency to find the target platform in the Morris water maze decreased significantly between the first (naive) and last training trial (trained), indicating that the rats learned the location of the platform. Latency remained short during a probe trial administered 24 h after the last training trial, indicating that the rats remembered the platform location. Finally, latency remained significantly shorter than naive when tested 28 d after the final training trial, demonstrating memory consolidation. Rats administered a 5-HT1BR antagonist (SB216641, 4 mg per kg, intraperitoneal) daily for 14 d starting after the 24-h probe trial displayed a significantly shorter escape latency in the consolidation trial than the control group (0.9% NaCl, intraperitoneal). Representative examples of the swim path in the maze from the start (white) to stop (black) positions are shown at left for control and SB216641-treated individuals. (b,c) SB216641-treated rats also spent more time in the target quadrant during the 28-d consolidation probe trial than controls (b) and swam a significantly shorter distance than controls (c). Both groups had identical swim speeds (c), however, indicating that the difference in performance was not a result of altered motor function. Full swim paths for two different individuals in the probe trials are shown at left. Dashed line indicates random performance. **P < 0.05, *P = 0.06, n = 8 rats per group, post hoc t test. Further statistical information can be found in the Online Methods.
Potentiation is altered in a depression model
The serotonin hypothesis of depression postulates that changes in the actions of serotonin contribute to its pathology. Depression is a purely human condition, but a depressive-like behavioral state can be induced in rodents. We therefore investigated serotonin-induced potentiation in rats subjected to the chronic unpredictable stress (CUS) model of depression, which has face, construct and predictive validity35. After 3 weeks of CUS, rats displayed both a decrease in sucrose preference and increased latency in novelty suppressed feeding (Supplementary Fig. 5a,b), as reported previously for rodent models of depression35,36. We observed that the anpirtoline-induced potentiation of TA-CA1 EPSPs was much greater in slices from CUS rats than in unstressed littermates, and that EPSP slope remained enhanced even after 100 min of washout in rats subjected to CUS, but not control rats, in both whole-cell (Fig. 6a,b) and extracellular (Supplementary Fig. 5c,d) recordings. The persistence of the potentiation was independent of its magnitude (Fig. 6b) and was resistant to isamoltane treatment (Supplementary Fig. 5e). Despite the increased magnitude of the response in CUS rats, the potentiation remained specific to the TA-CA1 synapse (Supplementary Fig. 5d). Elevation of extracellular serotonin with acute application of imipramine also produced a large enhancement of TA-CA1 fEPSP slope in slices from rats subjected to CUS (naive, 230 ± 46%, n = 4 slices; CUS, 370 ± 88%, n = 3 slices; 2 × 2 ANOVA main effect of imipramine F1,5 = 18.22, P = 0.008; main effect of group naive versus CUS, F1,5 = 2.264, P = 0.2). We conclude that endogenous serotonin can still be released from dorsal raphe afferents in the hippocampus in rats subjected to CUS and that the resting level of extracellular serotonin is still regulated by the serotonin transporter, but that serotonin's downstream signaling at excitatory synapses is increased in rats subjected to CUS.
(a) Changes in TA-CA1 EPSP slope in slices from CUS rats (red, n = 11 cells) and control littermates (black, n = 10 cells) showing the larger amplitude and persistence of potentiation in CUS rats compared with controls. Representative traces before and after anpirtoline application and after 60 min of washout are shown at right. Scale bars represent 2 mV and 50 ms. (b) Changes in TA-CA1 EPSP slope induced by anpirtoline in slices from CUS rats (red) were larger and more persistent than the potentiation seen in slices from controls (black) (controls, peak change in fEPSP slope = 162 ± 3%, fEPSP slope after 60-min wash = 106 ± 3%, n = 10 slices; CUS, peak change = 226 ± 23%, 218 ± 21% after 60-min wash, n = 11 slices). (c) Anpirtoline produced no significant effect on fEPSP slope in slices from rats treated for 21–28 d with the antidepressants imipramine (100 mg l−1, red) or fluoxetine (80 mg l−1, blue), whereas it doubled fEPSP slope in slices from controls in interleaved experiments (black) (imipramine, n = 9 slices; fluoxetine, n = 5 slices; controls, n = 7 slices). (d) Administration of fluoxetine for the final 3 weeks in rats subjected to 6 weeks of CUS (blue, n = 12 slices) resulted in a restoration of transient anpirtoline-induced potentiation (black, n = 11 slices), which was clearly different from the irreversible potentiation in CUS rats (red, n = 5 slices).
Many antidepressants elevate extracellular serotonin levels, and this chronic elevation of serotonin increases AMPAR phosphorylation in the hippocampus37. We asked what effect chronic antidepressant treatment of naive rats might have on 5-HT1BR–mediated potentiation. In hippocampal slices from rats given imipramine or fluoxetine via their drinking water for 21–28 d, anpirtoline-induced potentiation of TA-CA1 fEPSP slope was absent, but was present in slices from untreated littermate controls (Fig. 6c). In contrast, anpirtoline produced normal potentiation in slices from rats treated with imipramine for only 36 h (data not shown), insufficient time for most therapeutically relevant behavioral effects in rodents38 and depressed humans. Chronic antidepressant treatment therefore prevents 5-HT1BR activation from potentiating TA-CA1 synapses, an effect that is directly opposite to the enhancement of 5-HT1BR–induced potentiation seen after CUS.
Finally, we observed that restoration of normal sucrose preference and novelty suppressed feeding behaviors in rats subjected to CUS for 3 weeks, followed by an additional 3 weeks of CUS and fluoxetine administration (Supplementary Fig. 5a,b), was accompanied by restoration of normal magnitude, reversible potentiation of TA-CA1 synapses in response to anpirtoline (Fig. 6d). A brief period of fluoxetine administration (36 h), in contrast, failed to restore either sucrose preference or the reversible anpirtoline response (Supplementary Fig. 6). Thus, the response of TA-CA1 synapses to 5-HT1BR activation correlates with the affective state of the rats under several conditions.
Potentiation required for antidepressant action
Is the 5-HT1BR–dependent potentiation of excitatory synaptic transmission observed in brain slices in response to acute elevation of serotonin involved in the action of chronically administered SSRIs in assays of antidepressant response? The sucrose preference test was chosen as a behavioral endpoint because of its predictive validity. To answer this question, we first used a pharmacological approach in C57BL6j mice subjected to the social defeat stress (SDS) model of depression39 (Fig. 7a). Sucrose preference was high in control mice, and remained high in the absence of SDS, but became decreased after 1 week of SDS and remained low during succeeding weeks of SDS. Administration of fluoxetine via the drinking water restored a high level of sucrose preference to SDS mice after 1 week. Administration of the 5-HT1BR antagonist SB224289 (4 mg l−1)40 had no effect on the high sucrose preference of naive mice, but prevented the increase in sucrose preference produced by chronic fluoxetine administration in mice that had lost their sucrose preference after SDS. Similarly, naive Htr1b−/− mice had a high sucrose preference41 that was lost after 3–5 weeks of CUS (Fig. 7b). Sucrose preference was restored by 3 weeks of fluoxetine administration in wild-type Sv129Imj mice subjected to CUS, but not in Htr1b−/− mice. We conclude that 5-HT1BR activation is necessary for the efficacy of SSRIs in this behavioral test, consistent with previous evidence that acute activation of 5-HT1BRs is sufficient for an antidepressant-like effect of SSRIs in the forced swim test42.
(a) Sucrose preference was high in vehicle-treated control C57BL6j mice (black) and remained high. SDS eliminated sucrose preference after 1 week (light blue). Fluoxetine (80 mg l−1) restored sucrose preference in SDS mice in which it had been lost (red). The 5-HT1BR antagonist SB224289 (4 mg l−1 in drinking water) had no effect on sucrose preference in non-SDS mice (gray), but prevented restoration of sucrose preference in SDS mice treated with fluoxetine (dark blue). Asterisk indicates significantly less than corresponding baseline. (b) Sucrose preference was high in naive Htr1b−/− mice and wild-type (Sv129Imj) mice, and was decreased in both strains after CUS. Fluoxetine restored sucrose preference in wild-type, but not in Htr1b−/−, mice. *P < 0.05, different than baseline in same genotype (Bonferroni corrected post hoc). **P < 0.05, different than after CUS (Bonferroni corrected post hoc). Thus, 5-HT1BR activation is required for fluoxetine to exert its therapeutic action. (c) CUS decreased sucrose preference in control C57BL6j mice (Bonferroni corrected post hoc, P < 0.02), which was restored by fluoxetine (baseline versus fluoxetine, P > 0.5). Sucrose preference was lower in GluA1 S831A mice than in C57BL6j mice (Student's t test, t19 = 3.37, P < 0.005). Sucrose preference in GluA1 S831A mice was not affected by fluoxetine (t14 = 0.335, P > 0.7 with paired t test), and antidepressant-treated GluA1 S831A mice had lower sucrose preference than controls (Student's t test, t25 = 2.42, P < 0.05 with Bonferroni adjustment for multiple comparisons). * indicates significantly less than C57BL6j baseline, ** indicates significantly different than C57BL6j baseline, but not GluA1 S831A baseline. (d) In the tail-suspension test, C57BL6J littermates treated with imipramine spent less time immobile than those treated with saline (t10 = 4.10, P < 0.005). GluA1 S831A mice treated with imipramine spent less time immobile than GluA1 S831A mice treated with saline (t13 = 2.35, P < 0.05). * indicates significantly different than control in same genotype. A behavioral response to acute administration of antidepressants was preserved in this assay, unlike the response to chronic antidepressants in the sucrose preference test. (e) Latency to feeding in the novelty-suppressed feeding test was longer in GluA1 S831A mice (n = 14) than in wild-type C57BL6j mice (n = 13) (t27 = −2.10, P < 0.05). GluA1 S831A mice did not differ from wild-type littermates with regard to time spent in the center in the open field test, but completed fewer line crossings than littermates, consistent with a hypo-locomotor phenotype, but not increased anxiety (t13 = −2.29, P < 0.05). * indicates significantly different than wild type. Taken together with lower sucrose preference, GluA1 S831A mice displayed a depressive-like behavioral phenotype. Further statistical information can be found in the Online Methods.
Is 5-HT1BR–mediated potentiation of AMPARs also required? GluA1 S831A mice displayed a low preference for 1% sucrose during the baseline period (Fig. 7c). Administration of fluoxetine for 3 weeks failed to induce an increase in preference for 1% sucrose in GluA1 S831A mice, but did restore sucrose preference in wild-type C57BL6j mice that had lost their sucrose preference after CUS. In contrast, GluA1 S831A mice did respond to acute imipramine administration in the tail suspension test (Fig. 7d), indicating that serotonin uptake remains sensitive to inhibition by tricyclics in these mice and highlighting the importance of serotonin-induced plasticity of glutamatergic transmission in the ability of antidepressants to reverse anhedonia. The lack of preference for 1% sucrose in naive GluA1 S831A mice suggested that they might have a depressive-like phenotype. We therefore compared novelty suppressed feeding in these mice with their wild-type controls (Fig. 7e). The GluA1 S831A mice required almost twice as long as wild-type C57BL6j mice to begin eating food in the brightly lit, novel arena, consistent with behavioral changes induced in several animal models of depression36. They also displayed a hypo-locomotor phenotype in the open field test, without evidence of anxiety (Fig. 7e).
Taken together, our data suggest that 5-HT1BR activation and subsequent phosphorylation of GluA1 receptors by CaMK is required for the ability of chronically administered SSRIs and tricyclic antidepressants to reverse anhedonia in these assays. Because the sucrose preference test does not depend on the hippocampus, these results support the conclusion that serotonin-induced potentiation of excitatory synaptic transmission occurs in multiple brain regions involved in hedonic behaviors.
Discussion
We observed that SSRIs, tricyclic antidepressants, agonists of 5-HT1BRs and conventional LTP all potentiate excitatory synaptic transmission at TA-CA1 synapses and occlude each other, suggesting that the molecular events triggered by serotonin and conventional antidepressants overlap with those underlying activity-dependent synaptic plasticity to a notable degree5. Activation of 5-HT1BRs enhanced AMPAR-mediated synaptic excitation by activating CaMK and causing phosphorylation of GluA1 at serine 831. We also observed that long-term memory consolidation, which depends on intact TA-CA1 synaptic circuits12,32, was enhanced by inhibiting 5-HT1BRs. We suggest that release of endogenous serotonin and activation of 5-HT1BRs occurs during memory consolidation, after learning has taken place, and influences the ability of TA-CA1 synapses to participate in the consolidation process. Unlike LTP, 5-HT1BR–mediated potentiation was reversed rapidly after withdrawal of agonist. Given the similarity of the biochemical pathways, this difference is surprising and cannot be readily explained. Induction of LTP requires CaMK activity, whereas maintenance of LTP does not43. It therefore appears that LTP induction is accompanied by recruitment of some additional signaling process(es) that are not recruited by 5-HT1BR activation. A better understanding of the second-messenger pathways downstream of this form of potentiation may reveal key biochemical steps distinguishing the induction of potentiation, which seems to occur via a common mechanism in both forms of potentiation, from the maintenance of potentiation, which only occurs after induction of LTP.
TA-CA1 synapses were potentiated by serotonin, but nearby SC-CA1 synapses were not. The signaling processes underlying this phenomenon must therefore be highly localized in postsynaptic CA1 cell dendrites. Serotonin release can be predicted to change the flow of information through the hippocampal circuitry, favoring the so-called direct pathway at the expense of the indirect pathway. Regulating information flow through a neural circuit is a unique mechanism of action for neuromodulatory transmitters and may be generally applicable in other brain regions. Conversely, a dysregulation of information flow as a result of altered serotonin receptor signaling or altered regulation of excitatory synapses may contribute to the cognitive dysfunction of depression.
Animals subjected to chronic stress exhibit elevated glucocorticoid levels, and elevated glucocorticoid levels damage neuronal structure and function in the hippocampus44, including impairing Schaffer collateral LTP45. We observed that serotonin-induced potentiation of TA-CA1 synapses was altered in two distinct ways in chronically stressed rats: the potentiation was increased in magnitude and became irreversible. We do not yet know the explanation for these changes. A decrease in initial synaptic strength6 could potentially account for the increase in the magnitude of the potentiation, but the persistence of the potentiation in CUS mice was independent of its magnitude (Fig. 6). Chronic antidepressant administration in naive mice decreased the potentiation and restoration of sucrose preference in CUS mice treated chronically with antidepressants was accompanied by a restoration of the normal transient potentiation. Short-term administration of antidepressants had no effect on serotonin-induced potentiation in either naive or CUS mice. The response of TA-CA1 synapses to 5-HT1BR activation correlates well with the affective state of the animals under five experimental conditions, and therefore represents a unique endophenotype of this model of depression.
There is increasing evidence that excitatory synapses are altered in models of depression4,5. Depression of AMPAR-mediated excitation of neurons in the reward circuitry of the NAc is required for the genesis of some depressive-like behaviors after chronic restraint stress, including anhedonia in the sucrose preference test10. The source of the inputs that become depressed was not identified, but the NAc receives prominent projections from the hippocampus and frontal cortex. The serotonin-mediated plasticity that we observed may act to counteract this depression of NAc excitatory synapses in two ways. First, serotonin-induced potentiation of TA-CA1 synapses increased hippocampal action potential discharge (Supplementary Fig. 3b), and should increase the excitatory drive onto NAc cells and promote de-depression of NAc inputs, thereby acting to reverse anhedonia and restore the rewarding values of peripheral stimuli, such as sucrose. Consistent with this suggestion, activation of hippocampal 5-HT1BRs has been shown to increase dopamine levels in the NAc46, and increased dopamine alleviates depressive-like behaviors47. The enhanced and persistent action of serotonin in the CUS mice (Fig. 6) may be interpreted not only as a unique stress-induced form of plasticity of excitatory synapses, but also as a potential compensatory response, acting to promote the de-depression of hippocampal-NAc inputs. Second, medium spiny cells in the NAc express high levels of Hrt1b mRNA. If the plasticity that we observed also occurs in NAc D1-expressing neurons, then direct potentiation of excitatory synapses should counteract stress-induced depression of excitatory synapses10. Consistent with this possibility, we observed a 5-HT1BR–induced activation of CaMK and phosphorylation of S831 of GluA1 receptors in the NAc.
Our results link antidepressant-induced elevation of serotonin to functional potentiation of excitatory synapses in vivo for the first time, to the best of our knowledge. We found that pharmacological inhibition or genetic deletion of 5-HT1BRs prevented the ability of chronically administered fluoxetine to restore sucrose preference in two chronic stress models. Furthermore, sucrose preference was low in GluA1 S831A mice and was not increased by 3 weeks of fluoxetine administration. We therefore suggest that conventional antidepressants exert at least some of their therapeutic actions by promoting on-going serotonin-induced, 5-HT1BR–mediated potentiation of excitatory synaptic transmission. It is noteworthy that acute application of SSRIs produced a rapid potentiation of excitatory synaptic transmission in brain slices, whereas the therapeutic action of SSRIs in depressed humans and in animal models of depression requires several weeks. We do not yet have an explanation, but suggest that induction of potentiation is a critical first step that improves affective state by promoting action potential firing, which subsequently triggers other slower, synergistic, activity-dependent processes1, such as growth factor signaling, neurogenesis and changes in gene expression. Repeated bouts of potentiation may be required to produce lasting changes, as has been observed with deep brain stimulation and electroconvulsive therapy.
Given the plethora of behavioral changes that define depression, it is unlikely that there is one specific brain region that would be critical to the ability of antidepressants to restore these functions. The plasticity that we observed for TA-CA1 synapses is attractive as a potential explanation for some of serotonin's therapeutic actions because it is generalizable: similar to LTP for learning and memory, this form of plasticity may, in principle, take place at any excitatory synapse at which 5-HT1BRs are expressed and thereby re-normalize activity in almost any region in the depressed brain. This is in contrast with the well-documented ability of serotonin to promote dentate gyrus neurogenesis, which does not occur in any other brain region. The TA-CA1 synapse serves as a useful archetype to study the actions of serotonin on excitatory synaptic transmission and the correlates of a depression-like state.
Elevating monoamine levels improves the affective state of depressed patients, which led to the serotonin hypothesis of depression3, but the evidence of a deficiency in serotonin synthesis or release in depression is modest. Instead, our results suggest an excitatory synapse hypothesis of depression. We postulate that changes in excitatory synapses are fundamental to the genesis of depression and that their restoration is critical to the relief of depression. We found that induction of serotonin-mediated potentiation was abnormally enhanced and prolonged after CUS and that GluA1 S831A mice, similar to Gria1−/− mice48, displayed a depressive-like phenotype. Similarly, glutamate receptor expression is decreased6 and excitatory synaptic transmission is depressed10 in other systems after repeated stress. Serotonin-mediated potentiation would be predicted to restore the normal strength of synapses weakened by chronic stress. Indeed, we observed that 5-HT1BR activation and GluA1 phosphorylation at S831 were necessary for fluoxetine to restore sucrose preference in chronically stressed animals, suggesting that this action may be critical to the therapeutic efficacy of SSRIs. It is noteworthy that these effects of conventional, slow-acting antidepressants, such as SSRIs and tricyclics, are shared by newly identified, fast-acting antidepressants. For example, ketamine potentiates excitatory synapses in hippocampal slices and its immediately effective therapeutic actions have been attributed to enhanced activation of glutamatergic circuits49. Potentiation of excitatory synaptic transmission by serotonin in multiple brain regions involved in cognitive function, and its bidirectional alteration by stress and chronic antidepressants, provides a link between the pathology of depression and its treatment, and represents a new perspective on depression and antidepressant action.
Methods
All protocols were approved by the University of Maryland School of Medicine and St. Mary's College of Maryland Institutional Animal Care and Use Committees.
Acute slice electrophysiology.
Hippocampal slices were prepared from 3–6-week-old male Sprague-Dawley rats or mice (C57BL6j or Sv129Imj, as described). Dissection was done in ice-cold artificial cerebrospinal fluid (ACSF; 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.5 mM MgCl2, 2.5 mM CaCl2, 26 mM NaHCO3 and 10 mM glucose) bubbled with 95% O2/5% CO2. Brain slices (400 μM) were cut on a vibratome and kept in a holding chamber at 20–22 °C at the interface of ACSF and humidified 95% O2/5% CO2 for >1 h. The slices were then transferred to a submersion-type recording chamber and perfused at 20–22 °C with ACSF (flow rate = 1–2 ml min−1). Picrotoxin (0.1 mM) and CGP52432 (2 μM) were included to block GABAA and GABAB receptors. Area CA3 and the dentate gyrus were cut from the slice to prevent spontaneous epileptiform discharge (Supplementary Fig. 1a). Concentric bipolar tungsten electrodes were placed either in SLM to stimulate temporoammonic afferents or in stratum radiatum to stimulate Schaffer collateral afferents (Supplementary Fig. 1a). Extracellular recording pipettes were filled with ACSF (3–5 MΩ) and placed 100–150 μm from the stimulating electrodes. Stimuli (100-μs duration) were delivered at 0.05 Hz. The stimulus intensity was set at 150% of threshold intensity, resulting in a fEPSP of 0.1–0.2 mV. All compounds were applied by perfusion. fEPSPs were recorded using Axoclamp 2B (Molecular Devices) or n.p.i. (NPI) amplifiers, filtered at 10 kHz, and amplified 100× before digitization. Whole-cell intracellular recordings were obtained with patch pipettes (tip resistances = 3–6 MΩ) filled with 135 mM cesium or potassium methanesulphate (for voltage- or current-clamp recording, respectively), 10 mM HEPES, 10 mM NaCl, 1 mM MgCl2, 0.1 mM K4BAPTA, 2 mM Mg2+-ATP and 10 mM phosphocreatine (adjusted to pH 7.3 with KOH). All experiments were performed at 20–22 °C. Whole-cell recordings were made from cell bodies and were made blind.
Photolysis.
As described previously23, an argon ion laser fitted with ultraviolet optics was used to produce a continuous 300-mW beam of ultraviolet light at the 355- and 361-nm lines. The light was focused into a 25-μm multimode quartz fiber and delivered to the preparation via a dichroic mirror, so as to permit simultaneous wide-field excitation with an HBO lamp. Laser flash duration was controlled by a high-speed electromechanical shutter. The proximal end of the fiber was focused via a relay lens assembly in a conjugate image plane with respect to the cell and positioned with micromanipulators near the center of the field of view through the 60× (1.0 N.A.) water-immersion objective of an upright microscope (Nikon). The location and focus of the ultraviolet spot in the tissue was determined from excitation of a dye-filled dendritic shaft by an attenuated beam. Intracellular Alexa 568 fluorescence was imaged with a CCD camera (Hamamatsu Orca ER II, effective pixel size = 0.108 μm2) controlled by Simple PCI software (Compix). Caged glutamate (0.5 mM N-Ncm-Glu) and the antioxidant Trolox (0.1 mM) were added via perfusion.
Western blotting.
Protein expression was quantified using western blotting. Area SLM or stratum radiatum tissue wedges were dissected from hippocampal slices (3–4) under ice-cold saline, pooled, and homogenized in ice-cold lysis solution containing phosphatase and protease inhibitors (PPI, Sigma) and sample buffer (Laemmli, Sigma), boiled, and loaded into 4–12% Bis-Tris gel (Invitrogen). After running in 1× NuPage MOPS SDS buffer, the gel was transferred onto polyvinylidene difluororide membranes in 1× Nupage transfer buffer (in 10% methanol, wt/vol). The membrane was blocked with 5% nonfat dry milk (wt/vol) in buffer containing 1 M Tris-buffered saline and 0.05% Tween (wt/vol), and probed with antibodies to Ser845-phosphorylated GluA1 (1:1,000, Millipore AB5849), Ser831-phosphorylated GluA1 (1:1,000, Sigma A4352) and Thr286-phosphorylated CaMKII (1:5,000, Cell Signaling Technology #3361) at 4 °C overnight. After rinses in TBS-Tween, the membrane was incubated for 1 h at 20–22 °C in horseradish peroxidase–conjugated goat antibody to rabbit IgG (1:1,000, Cell Signaling Technology #7074). The immunoblot was developed with enhanced chemiluminescence (Amersham). Membranes were then stripped, blocked and reprobed with antibodies to GluA1 (0.5 μg ml−1, Thermo Scientific PA1-37776), CaMKII (1:2,000, Cell Signaling Technology #3362) or β-actin (1:2,000, Cell Signaling Technology #4967). Levels of phosphorylation, expressed as the ratio of phospho-specific intensity divided by total protein intensity and computed with ImageJ, were used for statistical analysis. For display purposes, blots were cropped (see Supplementary Fig. 7) and brightness and contrast were adjusted globally using Photoshop.
Serotonin depletion.
Rats were given two injections of the tryptophan hydroxylase inhibitor p-chlorophenylalanine (300 mg per kg, intraperitoneal, 24 h apart)20 before preparing slices for electrophysiological recording. Tissue from the same animals was fixed, mounted in gelatin, sectioned at 50 μm, and stained with a polyclonal antibody to serotonin (1:1,000, Millipore AB125), followed by labeling with a secondary antibody conjugated with the fluorophore Cy2 and epifluorescence microscopy.
CUS procedure.
Adult male Sprague-Dawley rats (3–4 weeks old) were randomly divided into control and CUS groups. CUS rats were treated as follows: day 1, cage rotation (3 h), forced swim (5 min), food deprivation (16 h); day 2, strobe light (30 min), restraint (30 min), food and water deprivation (16 h); day 3, strobe light (30 min), social isolation (16 h); day 4, strobe light (30 min), restraint (30 min); day 5, cage rotation (3 h), water deprivation (16 h); day 6, restraint (3 h), social isolation (16 h); day 7, cage rotation (3 h), restraint (30 min). The cycle was repeated over 3–5 weeks. Electrophysiological experiments were then performed and analyzed with the experimenter blinded to the experimental condition of the rats.
SDS procedure.
Individual male test mice (>4 weeks old) were introduced into the home cage of an unfamiliar resident mouse for 5 min and observed to ensure that they were physically attacked and defeated, as indicated by freezing behavior and submissive posture. Resident mice were CD1 breeders selected for short attack latencies. After 5 min of physical interaction, the test mouse was kept for 1 h in the resident's cage while being separated by a perforated screen that protected the test mouse from physical contact, but permitted olfactory, visual and auditory contact. The SDS protocol was maintained for 5 weeks. To avoid individual differences in intensity of defeat, the test animals were confronted with a different resident each day.
Sucrose preference.
After 3 h of water deprivation, rats were given a choice between two bottles for 3–4 h, one with 1% sucrose solution (wt/vol) and another with normal drinking water. To prevent the possible effects of side preference in drinking, the position of the bottles was reversed after 2 h. Mice were tested over 12 h (6 h light/6 h dark) with bottle positions switched after 6 h. The consumption of water and sucrose were measured by weighing the bottles. Preference for sucrose was calculated as a percentage of consumed sucose-containing solution relative to the total amount of liquid intake. 50% means that they drank equally from both bottles, that is, they had no preference for sucrose. Naive rats were tested repeatedly in group housing until the cage had a consistent preference for 1% sucrose, but were always tested individually thereafter. For experiments with only wild-type rats, any individual that did not demonstrate a sucrose preference >65% was discarded. In some experiments, rats were first trained to the task using 2% sucrose. Tests were repeated once per week during the course of CUS or antidepressant administration.
Novelty supressed feeding.
Novelty suppressed feeding tests were performed as previously50. The test apparatus was a brightly lit arena (60 × 60 × 35 cm for rats, 50 × 40 × 25 cm for mice) with a solid floor placed in a dimly lit room. The floor of the box was covered with a layer of bedding. Two laboratory chow pellets were placed on a white paper circle platform positioned in the center of the box. Rats that had been food deprived for 24 h, and mice that were food deprived for 3 h, were gently placed in a corner of the arena. The latency to begin eating, defined as active chewing of the pellet, was recorded. A maximum time allowance was set at 600 s. Immediately after the test, animals were returned to their home cage and allowed to feed for 5 min. Food pellets were weighed before and after the 5 min, and the amount of food consumed was calculated. Rats that ate less than 0.3 g of food in this 5-min period were removed from all analyses to ensure that only sufficiently hungry animals were included. Similarly, mice that failed to actively chew on a pellet were not included.
Open field test.
Mice were placed in a 60 × 60 × 35 cm plexiglass box for 5 min. The box was divided into 12 squares using tape on the bottom of the box. Mice were video recorded and a blinded experimenter calculated the time spent in center two squares and the number of line crossings for each animal.
Tail suspension test.
Mice were intraperitoneally injected with saline or 30 mg per kg of imipramine 30 min before testing. Each mouse was taped to a wooden horizontal bar 2 inches from the base of its tail. A blinded experimenter recorded the amount of time spent immobile for a 6-min period.
Antidepressant treatment.
Animals were given antidepressants via their drinking water to minimize stress (imipramine, 100 mg l−1; fluoxetine, 80 mg l−1). Animals were housed singly and drinking water was changed every 3 d. Animals were given antidepressants continually for 3–4 weeks. Control animals received water only. Experiments were then performed and analyzed with the experimenter blinded to the experimental condition of the animals.
Data analysis.
Experiments were performed with the investigators blinded to the genotype of the animals or the identity of the substance being applied whenever possible. The blind was not broken until data analysis was complete. For quantification of anpirtoline and antidepressant actions in electrophysiological experiments, fEPSP or EPSC slope values were averaged and quantified over a 3-min period preceding application of the substance (control) and a 3-min period at the end of the substance application (effect).
Memory consolidation.
16 male 70–80-d-old Sprague Dawley rats were housed two per cage in a temperature-controlled room. Rats were kept on a 12 h:12 h light-dark schedule and all testing was done during the light phase. All rats were handled for approximately 5 min d−1 for the 4 d before testing. The rats were given unlimited access to food and water throughout the testing periods. The water maze (139.7 cm in diameter) was filled with black-colored water created with non-toxic black paint. During training, a clear Plexiglas platform (10.16 cm in diameter) was submerged 0.5 cm below the water surface. Three black and white geometric spatial cues were placed around the maze and remained in a fixed location throughout training and testing.
Each rat was randomly assigned a quadrant (NW, NE, SE, SW) in which the platform would always be located. As described previously12, rats were then given ten training blocks in the water maze across 6 d. Days 1 and 6 contained one training block. Days 2–5 contained two training blocks separated by a minimum of 2 h. Each training block consisted of four training trials in which the animal was placed in a random starting location in the pool (NW, NE, SE, SW) and given 120 s to swim to the platform. The animal was allowed to remain on the platform for 30 s. If the rat did not reach the platform in 120 s, it was guided to the platform and left to sit on the platform for 30 s. Animals were given a 2-min intertrial interval. Two probes tests were conducted in which the platform was removed, animals were placed in a random starting location and were then allowed to swim in the maze for 60 s. One probe test was given 24 h after the completion of the final training block. The second probe was given 28 d after the original probe trial. Latency to the target, distance to target, time spent in the target quadrant and swim speed were recorded by HVS Image software.
Following completion of the first probe test, animals were treated with either SB216641 (Tocris) or 0.9% physiological saline (wt/vol) for 14 consecutive days. Intraperitoneal injections of SB216641 (4 mg per kg, 4 mg ml−1) or saline were given at the same time each day immediately after light onset in the housing room.
The animals did not differ in performance during the original training blocks. A 2 × 2 mixed ANOVA (Block × Group) was used to examine performance on the original and final training block. The escape latency decreased significantly from block 1 (mean = 44.34, s.e.m. = 3.1) to block 10 (mean = 8.1, s.e.m. = 0.9) (F1,13 = 107.4, P < 0.001). There was no difference between untreated (mean = 26.4, s.e.m. = 2.1) and future SB216641-treated (mean = 26.0, s.e.m. = 2.1) animals (F1,13 = 0.02, P > 0.05). There was no interaction (F1,13 = 0.36, P > 0.05).
Examination of performance on the probe trial given 24 h after the final training block also showed no group differences. There was no difference in the percentage of time spent in the target quadrant (t14 = 0.91, P > 0.05). There was no difference in path taken to the target area (t14 = 0.46, P > 0.05). There was no difference in latency to the target area (t14 = 0.3, P > 0.05) and no difference in swim speed between the animals (t14 = 0.51, P > 0.05).
The animals were tested for consolidation of original training 28 d after the end of training in the water maze. Animals treated with SB216641 demonstrated significantly improved retention of spatial information. During the probe trial, animals treated with SB216641 (mean = 42.66, s.e.m. = 3.4) spent an increased percentage of time in the target quadrant compared with the untreated animals (mean = 32.41, s.e.m. = 3.69) (t14 = 2.04, P = 0.06). Animals treated with SB216641 (mean = 1.77, s.e.m. = 0.42) used a shorter path to the target area than the untreated animals (mean = 4.05, s.e.m. = 0.82) (t14 = 2.48, P = 0.27). The SB216641-treated animals (mean = 11.11, s.e.m. = 2.06) also demonstrated a significantly shorter latency to the target area than the untreated animals (mean = 26.26, s.e.m. = 5.89) (t14 = 2.41, P = 0.03). There were no differences in swim speed between the two groups (t14 = 0.5, P > 0.05).
Therapeutic actions of 5-HT1BRs.
SB224289 was used as the 5HT1BR antagonist and was given to mice via drinking water. SB224289 was administered at 4 mg l−1. Water levels were checked daily to ensure each mouse was receiving the intended SB224289 dose throughout the allotted period.
Preference for sucrose was analyzed in the experiments shown in Figure 7a by a 5 × 5 mixed ANOVA (time × group). There was a significant effect of time (F4,100 = 9.33, P < 0.001) as the overall sucrose preference decreased across weeks. There was a significant effect of group (F4,25 = 47.3, P < 0.001) and there was a significant interaction between time and group (F16,100 = 2.99, P < 0.001). Each time point was then analyzed by a between-subjects one-way ANOVA. There were no differences between the groups in sucrose preference at week 1 (baseline) (F4,32 = 2.26, P > 0.05).
The groups were significantly different in sucrose preference levels at week 2 (F4,32 = 9.2, P < 0.001). At week 2, all socially defeated animals (SDS, SDS + fluoxetine, fluoxetine + SB224289) had a significantly lower sucrose preference than both control groups as measured by independent t tests (all P's < 0.003). This pattern remained at week 3, as the overall groups were significantly different (F4,26 = 36.2, P < 0.001) and all socially defeated animals had significantly lower sucrose preferences than the controls (all P's < 0.001).
At week 4, the groups were significantly different (F4,26 = 6.78, P = 0.001). Independent t tests indicated that the control animals had a significantly higher sucrose preference than the SDS group (t8 = 9.95, P < 0.001) and a significantly higher sucrose preference than the SB224289 + fluoxetine group (t12 = 2.5, P = 0.03). The SB224289 group had a significantly higher sucrose preference than the SDS group (t8 = 4.57, P = 0.002). However, the SDS + fluoxetine group had a significantly higher sucrose preference than the SDS group (t9 = 5.6, P < 0.001), but was not different than the SB224289 + fluoxetine group (t13 = 1.889, P = 0.081).
Groups differed in overall sucrose preference at week 5 (F4,25 = 6.9, P = 0.001). The control animals had a significantly higher sucrose preference than the SDS group (t8 = 5.02, P = 0.001) and the SB224289 + fluoxetine group (t11 = 4.19, P = 0.002). The SB224289 group had a significantly higher sucrose preference than SB224289 + fluoxetine group (t11 = 2.77, P = 0.018), but was not different from the SDS group (t8 = 2.09, P = 0.07). The SDS + fluoxetine group had a significantly higher sucrose preference than the SDS group (t9 = 3.34, P = 0.009) and the SB224289 + fluoxetine group (t12 = 3.28, P = 0.007).
Finally, dependent t tests indicated that sucrose preference significantly increased in the SDS + fluoxetine group from week 3 to week 4 (t6 = 4.28, P = 0.005) and remained higher at week 5 compared to week 3 (t6 = 3.11, P = 0.02). However, no increase in sucrose preference was seen in the SB + fluoxetine animals between week 3 and 4 (t7 = 1.77, P = 0.12) or between week 3 and week 5 (t6 = 1.7, P = 0.15).
In the experiment with Htr1b−/− mice (Fig. 7b), two-factor ANOVA revealed a significant effect of genotype (F1,12 = 2.965, P = 0.111) and condition (F2,24 = 12.594, P < 0.001), and an interaction between genotype and condition (F2,24 = 8.090, P = 0.002). In the experiment with GluA1 S831A mice (Fig. 7c), there was a significant effect of condition in the control mice by repeated-measures ANOVA (F2,10 = 8.67, P < 0.01). Finally, a one-way ANOVA revealed a significant group effect in the tail suspension test (F3,23 = 7.70, P = 0.001; Fig. 7d).
References
Krishnan, V. & Nestler, E.J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Billings, A.G., Cronkite, R.C. & Moos, R.H. Social-environmental factors in unipolar depression: comparisons of depressed patients and nondepressed controls. J. Abnorm. Psychol. 92, 119–133 (1983).
Heninger, G.R., Delgado, P.L. & Charney, D.S. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29, 2–11 (1996).
Duman, R.S. & Aghajanian, G.K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72 (2012).
Sanacora, G., Zarate, C.A., Krystal, J.H. & Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 7, 426–437 (2008).
Yuen, E.Y. et al. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73, 962–977 (2012).
Campbell, S. & Macqueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 29, 417–426 (2004).
Hickie, I. et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br. J. Psychiatry 186, 197–202 (2005).
Phillipson, O.T. & Griffiths, A.C. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16, 275–296 (1985).
Lim, B.K., Huang, K.W., Grueter, B.A., Rothwell, P.E. & Malenka, R.C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012).
Ihara, N., Ueda, S., Kawata, M. & Sano, Y. Immunohistochemical demonstration of serotonin-containing nerve fibers in the mammalian hippocampal formation. Acta Anat. 132, 335–346 (1988).
Remondes, M. & Schuman, E.M. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature 431, 699–703 (2004).
Lambe, E.K., Goldman-Rakic, P.S. & Aghajanian, G.K. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb. Cortex 10, 974–980 (2000).
Kobayashi, K., Ikeda, Y., Haneda, E. & Suzuki, H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J. Neurosci. 28, 6272–6280 (2008).
Aït Amara, D., Segu, L., Naili, S. & Buhot, M.C. Serotonin 1B receptor regulation after dorsal subiculum deafferentation. Brain Res. Bull. 38, 17–23 (1995).
Sari, Y. et al. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88, 899–915 (1999).
Saudou, F. et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265, 1875–1878 (1994).
Svenningsson, P. et al. Alterations in 5-HT1B receptor function by p11 in depressive-like states. Science 311, 77–80 (2006).
Göthert, M. et al. 5-HT3 receptor antagonism by anpirtoline, a mixed 5-HT1 receptor agonist/5-HT3 receptor antagonist. Br. J. Pharmacol. 114, 269–274 (1995).
Dewar, K.M., Grondin, L., Carli, M., Lima, L. & Reader, T.A. [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J. Neurochem. 58, 250–257 (1992).
Sharp, T., Bramwell, S.R. & Grahame-Smith, D.G. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br. J. Pharmacol. 96, 283–290 (1989).
Jarsky, T., Roxin, A., Kath, W.L. & Spruston, N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci. 8, 1667–1676 (2005).
Cai, X. et al. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44, 351–364 (2004).
Hsu, E.H., Lochan, A.C. & Cowen, D.S. Activation of Akt1 by human 5-hydroxytryptamine (serotonin)1B receptors is sensitive to inhibitors of MEK. J. Pharmacol. Exp. Ther. 298, 825–832 (2001).
Leone, A.M., Errico, M., Lin, S.L. & Cowen, D.S. Activation of extracellular signal-regulated kinase (ERK) and Akt by human serotonin 5-HT(1B) receptors in transfected BE(2)-C neuroblastoma cells is inhibited by RGS4. J. Neurochem. 75, 934–938 (2000).
Giese, K.P., Fedorov, N.B., Filipkowski, R.K. & Silva, A.J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279, 870–873 (1998).
Roche, K.W., O'Brien, R.J., Mammen, A.L., Bernhardt, J. & Huganir, R.L. Characterization of multiple phosphorylation sites on the AMPA receptor GluA1 subunit. Neuron 16, 1179–1188 (1996).
Lee, H.K., Barbarosie, M., Kameyama, K., Bear, M.F. & Huganir, R.L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959 (2000).
Lee, H.-K., Takamiya, K., Hen, K., Song, L. & Huganir, R.L. Specific roles of AMPA receptor subunit GluR1(GluA1) phosphorylation sites in regulation synaptic plasticity in the CA1 region of the hippocampus. J. Neurophysiol. 103, 479–489 (2010).
Malinow, R. & Malenka, R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002).
Sybirska, E., Davachi, L. & Goldman-Rakic, P.S. Prominence of direct entorhinal-CA1 pathway activation in sensorimotor and cognitive tasks revealed by 2-DG functional mapping in nonhuman primate. J. Neurosci. 20, 5827–5834 (2000).
Nakashiba, T., Young, J.Z., McHugh, T.J., Buhl, D.L. & Tonegawa, S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–1264 (2008).
Tatarczyńska, E., Kłodzińska, A., Stachowicz, K. & Chojnacka-Wójcik, E. Effects of a selective 5–HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav. Pharmacol. 15, 523–534 (2004).
Buhot, M.C. et al. Spatial learning in the 5–HT1B receptor knockout mouse: selective facilitation/impairment depending on the cognitive demand. Learn. Mem. 10, 466–477 (2003).
Willner, P., Towell, A., Sampson, D., Sophokleous, S. & Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl.) 93, 358–364 (1987).
David, D.J. et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493 (2009).
Svenningsson, P. et al. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac). Proc. Natl. Acad. Sci. USA 99, 3182–3187 (2002).
Bondi, C.O., Rodriguez, G., Gould, G.G., Frazer, A. & Morilak, D.A. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33, 320–331 (2008).
Malatynska, E. & Knapp, R. Dominant-submissive behavior as models of mania and depression. Neurosci. Biobehav. Rev. 29, 715–737 (2005).
Gaster, L.M. et al. The selective 5–HT1B receptor inverse agonist 1′-methyl-5-[[2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydro-spiro[furo[2,3-f]indole-3,4′-piperidine](SB-224289) potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo. J. Med. Chem. 41, 1218–1235 (1998).
Bechtholt, A.J., Smith, K., Gaughan, S. & Lucki, I. Sucrose intake and fasting glucose levels in 5-HT(1A) and 5-HT(1B) receptor mutant mice. Physiol. Behav. 93, 659–665 (2008).
O'Neill, M.F. & Conway, M.W. Role of 5-HT1A and 5-HT1B receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology 24, 391–398 (2001).
Buard, I. et al. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J. Neurosci. 30, 8214–8220 (2010).
Krugers, H.J., Lucassen, P.J., Karst, H. & Joëls, M. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front. Synaptic Neurosci. 2, 1–10 (2010).
Pavlides, C., Nivon, L.G. & McEwen, B.S. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus 12, 245–257 (2002).
Boulenguez, P. et al. Modulation of dopamine release in the nucleus accumbens by 5HTlB agonists: Involvement of the hippocampo-accumbens pathway. Neuropharmacology 35, 1521–1529 (1996).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Chourbaji, S. et al. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 22, 3129–3134 (2008).
Autry, A.E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 (2011).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Acknowledgements
We thank S. Ahmari and R. Hen (Columbia University) for providing Hrt1b−/− mice, B. Alger, T. Blanpied, T. Gould, J. Kim and M. McCarthy for their comments, S. Aungst for advice on immunohistochemistry, and H. Zimmerman, L. Mok and M. Taylor for technical assistance. This work was funded by a Mr. and Mrs. Robert and Lee Peterson Southwest Florida National Alliance for Research on Schizophrenia and Depression Young Investigator Award (X.C.), the Howard Hughes Medical Institute (R.L.H.) and the US National Institutes of Health (R.L.H., H.-K.L., X.C. and S.M.T.).
Author information
Authors and Affiliations
Contributions
X.C., A.J.K., M.D.K., A.M.B. and S.M.T. designed the study. X.C., A.J.K., M.D.K., S.G., K.G. and A.M.B. performed the experiments and analyzed the data. H.-K.L. and R.L.H. provided the GluA1 S831A mice. X.C., A.J.K. and S.M.T. prepared the manuscript. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 8719 kb)
Rights and permissions
About this article
Cite this article
Cai, X., Kallarackal, A., Kvarta, M. et al. Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci 16, 464–472 (2013). https://doi.org/10.1038/nn.3355
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3355
This article is cited by
-
Single-nucleus transcriptomic analysis reveals the relationship between gene expression in oligodendrocyte lineage and major depressive disorder
Journal of Translational Medicine (2024)
-
16S rRNA gene sequencing reveals the effect of fluoxetine on gut microbiota in chronic unpredictable stress-induced depressive-like rats
Annals of General Psychiatry (2023)
-
SSRIs differentially modulate the effects of pro-inflammatory stimulation on hippocampal plasticity and memory via sigma 1 receptors and neurosteroids
Translational Psychiatry (2023)
-
Plasticity of synapses and reward circuit function in the genesis and treatment of depression
Neuropsychopharmacology (2023)
-
Receptor-informed network control theory links LSD and psilocybin to a flattening of the brain’s control energy landscape
Nature Communications (2022)