Abstract
Social deficits are observed in diverse psychiatric disorders, including autism spectrum disorders and schizophrenia. We found that mice lacking the excitatory synaptic signaling scaffold IRSp53 (also known as BAIAP2) showed impaired social interaction and communication. Treatment of IRSp53−/− mice, which display enhanced NMDA receptor (NMDAR) function in the hippocampus, with memantine, an NMDAR antagonist, or MPEP, a metabotropic glutamate receptor 5 antagonist that indirectly inhibits NMDAR function, normalized social interaction. This social rescue was accompanied by normalization of NMDAR function and plasticity in the hippocampus and neuronal firing in the medial prefrontal cortex. These results, together with the reduced NMDAR function implicated in social impairments, suggest that deviation of NMDAR function in either direction leads to social deficits and that correcting the deviation has beneficial effects.
Similar content being viewed by others
Main
Social deficits are associated with diverse neuropsychiatric disorders, including autism spectrum disorders (ASDs), schizophrenia, attention deficit hyperactivity disorder (ADHD) and emotional disorders. Because these disorders have a strong genetic component, and a large number of related genetic variations have been reported1,2,3, it has been becoming increasingly feasible to explore the mechanisms underlying social dysfunctions using various approaches, including mouse genetics4,5,6,7,8,9,10,11,12,13,14,15. These studies have also enabled researchers to propose mechanism-based ways to improve social deficits.
IRSp53 (insulin receptor substrate protein, 53 kDa), also known as BAIAP2, is a multi-functional scaffolding protein that has been implicated in the regulation of the actin cytoskeleton and actin-associated membrane protrusions, including lamellipodia and filopodia, through its ability to interact with F-actin, F-actin regulatory proteins and the plasma membrane16. In the brain, IRSp53 is mainly and abundantly present at excitatory synapses17,18,19 and regulates dendritic spines20. IRSp53 directly binds to other abundant excitatory synaptic scaffolds such as PSD-95 and Shank/ProSAP20,21,22, likely forming a platform-like structure that organizes synaptic signaling for the regulation of F-actin, the main cytoskeleton in dendritic spines17,23.
IRSp53−/− mice display enhanced NMDAR function, enhanced NMDAR-dependent long-term potentiation (LTP), and impaired hippocampus-dependent learning and memory24,25, suggesting that IRSp53 is an important regulator of NMDAR function at excitatory synapses. However, IRSp53−/− mice do not show structural abnormalities in dendritic spines or excitatory synapses in the hippocampus26,27, contrary to the expectations of the previous in vitro studies18,19,20,21, although other brain regions need to be observed.
We found that IRSp53−/− mice display deficits in social interaction and social communication. Suppressing the heightened NMDAR function by the NMDAR antagonist memantine or the metabotropic glutamate receptor 5 (mGluR5) antagonist MPEP, which indirectly inhibits NMDARs, rescued the social interaction. Mechanistically, these reagents normalized NMDAR function and plasticity at hippocampal synapses and rescued reduced neuronal firing in the medial prefrontal cortex (mPFC), where excitatory synapse number was reduced. These results implicate NMDAR hyperfunction in the social deficits observed in IRSp53−/− mice.
Results
IRSp53−/− mice display impaired social interaction
To determine whether IRSp53−/− mice display social deficits, we first tested the mice in the three-chambered apparatus, where the social approach of a mouse toward a stranger mouse trapped in a wire cage can be measured28. IRSp53−/− mice showed significantly reduced preference for exploring a stranger mouse relative to an inanimate object compared with wild-type mice, as measured by the amount of time spent in each chamber, or sniffing cages, and the preference index derived from these parameters (Fig. 1a–f). When the inanimate object was replaced with another stranger mouse, IRSp53−/− mice preferred to explore the new stranger to an extent comparable to that of wild-type mice (Fig. 1g–l). These results suggest that IRSp53−/− mice are socially impaired, but display normal social novelty recognition or social anxiety.
(a–f) IRSp53−/− mice displayed impaired social interaction in the three-chamber test. (a,b) Representative heat map images. (c–f) Quantification of the results in a and b, as shown by the amount of time spent in chamber (c) or sniffing the small cage (e) with a novel mouse (S) versus an inanimate object (O), or the preference index derived from the numerical difference between the time spent in chamber (d) or sniffing cages (f) with S1 and O divided by total time spent × 100. (s.e.m., n = 19 for wild type (WT) and 18 for knockout (KO), **P < 0.01, ***P < 0.001; ns, not significant; one-way ANOVA and two-sided Student's t test). (g–l) IRSp53−/− mice displayed normal levels of social novelty recognition or social anxiety in the three-chamber test. (g,h) Representative heat maps. (i–l) Quantification of the results in g and h. S2 and S1, new and old stranger (s.e.m., n = 19 for WT and 18 for KO, **P < 0.01, ***P < 0.001; ns, not significant; one-way ANOVA and two-sided Student's t test). (m–o) IRSp53−/− mice displayed reduced reciprocal dyadic social interaction with a freely moving age-, gender- and genotype-matched stranger, as measured by the duration of social interactions (all activities including sniffing, following, mounting and allogrooming), nose-to-nose sniffing and following (s.e.m., n = 9. *P < 0.05, **P < 0.01, two-sided Student's t test).
In the direct social interaction test, where a mouse is allowed to reciprocally interact with a freely moving stranger of the same gender and genotype, an IRSp53−/− subject mouse spent less time interacting with, nose-to-nose sniffing, and following an IRSp53−/− stranger mouse compared with wild-type mouse pairs (Fig. 1m–o), further suggesting that IRSp53−/− mice have social defects. In the buried food test, IRSp53−/− mice displayed normal olfactory function (Supplementary Fig. 1a), suggesting that the impaired social interaction probably does not involve olfactory dysfunction.
Impaired social communication and hyperactivity
An important domain of social interaction is social communication, and rodents emit ultrasonic vocalizations under social contexts. When exposed to a stranger wild-type female mouse, IRSp53−/− male mice emitted fewer ultrasonic vocalizations and spent a longer time before making the first ultrasonic vocalization than with wild-type mice (Fig. 2a–d), suggesting that IRSp53−/− mice have defects in social communication.
(a,b) Representative images of ultrasonic vocalizations by a WT or KO male mouse encountering a female WT stranger mouse. (c,d) IRSp53−/− mice emitted ultrasonic vocalizations less frequently and spent more time before emitting the first ultrasonic sound than WT mice (s.e.m., n = 13, **P < 0.01, ***P < 0.001, two-sided Student's t test). (e,f) IRSp53−/− mice showed hyperactivity in the open-field test, but spent a comparable amount of time in the center region, relative to WT mice (s.e.m., n = 13, *P < 0.05, **P < 0.01; ns, not significant; repeated measures ANOVA and two-sided Student's t test). (g,h) IRSp53−/− mice showed hyperactivity in home cages during light-off periods (s.e.m., n = 13, *P < 0.05, **P < 0.01, repeated measures ANOVA and two-sided Student's t test).
IRSp53−/− mice showed enhanced locomotor activities in a novel open-field arena, as well as in home cages, compared with wild-type mice (Fig. 2e–h). These mice traveled a distance in the center region of the open field arena comparable to that of wild-type mice, suggesting that they have normal levels of one type of anxiety-like behaviors (Fig. 2f).
We next measured repetitive behaviors in IRSp53−/− mice. However, they did not show enhanced self-grooming, marble burying or digging (Supplementary Fig. 1b–e). In addition, enhanced self-grooming could not be detected in 48-h automated monitoring of mouse movements, although enhanced locomotion could be confirmed (Supplementary Fig. 1f–i).
Lastly, we tested whether heterozygotic IRSp53+/− mice show social impairments similar to those observed in IRSp53−/− mice. The heterozygotes showed normal levels of three-chamber social interaction, repetitive behavior and locomotor activity (Supplementary Fig. 2).
Suppressing NMDAR hyperfunction rescues social interaction
Because IRSp53 is an important component of excitatory synapses17,18,19, the social impairment observed in IRSp53−/− mice may involve defective synaptic structure or function. We previously found that IRSp53−/− Schaffer collateral (SC)-CA1 synapses showed enhanced NMDAR function24. To test whether this contributes to the social deficits in IRSp53−/− mice, we performed pharmacological rescue experiments.
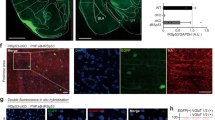
Acute treatment of IRSp53−/− mice with the NMDAR antagonist memantine (10 mg per kg of body weight, intraperitoneal) improved social interaction to levels comparable to those of wild-type mice, as measured by the chamber/sniffing time or the social preference index (Fig. 3a–f). In contrast, memantine had no effect on the social interaction of wild-type mice. A lower dose of memantine (5 mg per kg) had no effect on social interaction in IRSp53−/− mice based on the preference index, although there was a tendency toward improvement (Supplementary Fig. 3). These results suggest that normalization of NMDAR hyperfunction in IRSp53−/− mice rescues the social deficit.
(a,b) Representative heat maps of three-chamber social interactions in WT and KO mice treated with memantine or MPEP. (c–f) Quantification of the results in a and b, as shown by the time spent in chamber (c) or sniffing the cage (e) with a stranger (S) versus an object (O), or the social preference index for the chamber time (d) or sniffing time (f). IRSp53−/− mice were treated with memantine (10 mg per kg, intraperitoneal) or MPEP (30 mg per kg, intraperitoneal) 30 min before the social encounter (s.e.m., WT, n = 8 for saline, 12 for MPEP, and 12 for memantine; KO, n = 7 for saline, 12 for MPEP, and 11 for memantine; *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant; one-way ANOVA).
mGluR5 inhibition rescues social interaction
Because mGluR5 acts synergistically with NMDARs29,30, inhibition of mGluR5 may rescue the social deficits observed in IRSp53−/− mice. Acute administration of MPEP (30 mg per kg), a negative allosteric modulator of mGluR5, improved three-chamber social interaction in IRSp53−/− mice and had no effect on wild-type mice (Fig. 3a–f). A lower dose of MPEP (10 mg per kg) did not improve social interaction in IRSp53−/− mice (Supplementary Fig. 3).
Memantine and MPEP failed to rescue impaired ultrasonic vocalization and hyperactivity in IRSp53−/− mice (Supplementary Fig. 4a–c), suggesting that these defects may not involve NMDAR hyperfunction. However, impaired novel object recognition could be rescued by memantine (Supplementary Fig. 4d), suggesting that memantine can rescue a cognitive function in IRSp53−/− mice.
Suppressed LTD of NMDAR EPSCs in the hippocampus
We next explored potential mechanisms underlying the NMDAR hyperfunction at IRSp53−/− synapses. Given that NMDARs display dynamic trafficking to and from synapses31, we tested whether IRSp53−/− synapses show impaired activity-dependent suppression of NMDAR-mediated synaptic transmission by measuring the LTD of NMDAR-mediated excitatory postsynaptic currents (NMDAR EPSCs), which is distinct from the LTD of AMPA receptors (AMPARs) in underlying mechanisms26.
We found that IRSp53−/− SC-CA1 pyramidal synapses displayed reduced LTD of NMDAR EPSCs compared with wild-type synapses (Fig. 4a–c). In contrast, LTD of AMPAR EPSCs was not affected by IRSp53 deletion (Fig. 4d–f), as we reported previously24. In addition, mGluR-dependent LTD (mGluR-LTD) induced by DHPG (an mGluR1/5 agonist) was not different between genotypes (Fig. 4g–i). These results indicate that IRSp53 deletion leads to a specific suppression in LTD of NMDAR-mediated transmission.
(a–c) Reduced LTD of NMDAR EPSCs. (a,b) Summary graph of LTD of NMDAR EPSCs and representative averaged EPSC traces (baseline 10 min, last 10 min). LTD of NMDAR EPSCs was induced by a 5-Hz, 3-min train of stimulation at the holding potential of −40 mV. (c) Quantification of the results in a and b, as shown by the magnitude of LTD of NMDAR EPSCs (25–35 min; s.e.m., n = 7, **P < 0.01, two-sided Student's t test). (d–f) Normal LTD of AMPAR EPSCs. (d,e) Summary graph of LTD of AMPARs and representative averaged EPSC traces (baseline and last 10 min). LTD of AMPAR EPSCs was induced by a 5-Hz, 3-min train of stimulation at the holding potential of −40 mV. (f) Quantification of the results in d and e, as shown by the magnitude of LTD of AMPAR EPSCs (25–35 min; s.e.m., n = 7; ns, not significant; two-sided Student's t test). (g–i) Normal DHPG-induced mGluR-LTD. (g,h) Summary graph of mGluR-LTD induced by DHPG (50 μM, 5 min) and representative averaged EPSP traces (baseline 20 min, last 10 min). (i) Quantification of the results in g and h, as shown by the magnitude of mGluR-LTD (55–65 min; s.e.m., n = 10 slices from 9 mice for WT, n = 8 slices, 6 mice for KO, ns; not significant; two-sided Student's t test).
Stabilized F-actin in the hippocampus
What might underlie the reduced LTD of NMDARs at IRSp53−/− synapses? Previous studies have shown that synaptic retention of NMDARs requires intact F-actin27 and that LTD of NMDARs requires F-actin depolymerization26. We therefore tested whether IRSp53−/− neurons display abnormal F-actin by treating IRSp53−/− cultured neurons with NMDA (30 μM, 3 min), a condition that is known to induce depolymerization of synaptic F-actin through NMDAR activation32. We found that, prior to NMDA treatment, wild-type and IRSp53−/− neurons displayed similar numbers of dendritic F-actin puncta, or F-actin puncta expressing Shank (an excitatory synapse marker) (Fig. 5a–d). However, following NMDA treatment, F-actin puncta in IRSp53−/− neurons were more resistant to NMDA-induced dispersal (Fig. 5a–d). These results suggest that F-actin at IRSp53−/− hippocampal synapses is more resistant to activity-dependent depolymerization.
(a–d) F-actin puncta in IRSp53−/− hippocampal neurons are resistant to NMDA treatment compared with WT neurons. (a,b) Representative images of phalloidin-labeled F-actin puncta in WT and KO cultured hippocampal neurons at 15 d in vitro in the presence or absence of NMDA (30 μM, 3 min). MAP2 and Shank (pan-Shank) were co-stained for dendrites and excitatory synapses, respectively. Scale bars represent 10 μm. (c,d) Quantification of the results in a and b, as shown by the number of F-actin puncta (c) and Shank-positive synaptic F-actin puncta (d) on secondary or higher (not primary) dendrites of the neurons. (s.e.m., n = 12–13 cells, ***P < 0.001; ns, not significant; one-way ANOVA). Fold decreases in the linear density of F-actin puncta were used to directly compare genotypes. (s.e.m., n = 12–13 cells, ***P < 0.001, one-way ANOVA and two-sided Student's t test). (e,f) Basal levels of cofilin phosphorylation were increased in the IRSp53−/− hippocampus, whereas total levels of cofilin were unchanged (s.e.m., n = 5 for WT and 6 for KO, *P < 0.05; ns, not significant; two-sided Student's t test). (g,h) IRSp53−/− hippocampal slices showed a bigger decrease in NMDA-induced (30 μM, 3 min) cofilin phosphorylation, compared with WT neurons (s.e.m., n = 4, *P < 0.05, **P < 0.01, ns; not significant; one-way ANOVA and two-sided Student's t test).
The enhanced F-actin stability in IRSp53−/− neurons may involve altered actin-regulatory signaling. Activation of cofilin, a negative regulator of F-actin, is required for LTD of NMDARs26. When cofilin activity under basal conditions was measured, the IRSp53−/− hippocampus showed increased cofilin phosphorylation (a measure of inactivation), whereas total cofilin levels were unchanged (Fig. 5e,f and Supplementary Fig. 5). In contrast, IRSp53−/− hippocampal neurons showed normal NMDA-induced (30 μM, 3 min) cofilin dephosphorylation (activation) (Fig. 5g,h and Supplementary Fig. 5). These results suggest that cofilin activity is suppressed under basal conditions, whereas cofilin activation during plasticity occurs normally.
Memantine and MPEP restore NMDAR function and plasticity
We next tested whether the pharmacological rescue of social defects in IRSp53−/− mice involves normalization of the reduced LTD of NMDARs. We found that memantine treatment of IRSp53−/− hippocampal slices normalized the reduced LTD of NMDARs to levels comparable to those of wild-type neurons, as measured by NMDAR-mediated field excitatory postsynaptic potentials (fEPSPs) at SC-CA1 synapses (Fig. 6a–c). However, memantine had no such effect on wild-type synapses.
(a–c) Memantine restored LTD of NMDAR fEPSPs at IRSp53−/− SC-CA1 synapses. (a,b) Summary graph of LTD of NMDAR fEPSPs (a) and representative averaged NMDAR fEPSP traces (b; baseline 10 min and last 10 min of recording). LTD of NMDAR EPSPs was induced by a 5-Hz, 3-min train of stimulation in the presence or absence of memantine (1 μM). (c) Quantification of the results in a and b. Magnitudes of LTD of NMDAR fEPSPs during 50–60 min of the recordings were compared (s.e.m., n = 7 for WT saline, n = 5 for WT memantine, n = 7 for KO saline, n = 6 for KO memantine, *P < 0.05, **P < 0.01; ns, not significant; one-way ANOVA). (d,e) MPEP restored the NMDA/AMPA ratio at IRSp53−/− SC-CA1 synapses. (d) Representative averaged NMDAR and AMPAR EPSC traces. NMDAR and AMPAR EPSCs were measured at the holding potentials of +40 mV and −70 mV, respectively, in the presence or absence of MPEP (20 μM). The shaded regions indicate the time point from which NMDAR currents were sampled. (e) Quantification of the results in d (s.e.m., n = 7 for WT saline, n = 5 for WT MPEP, n = 7 for KO saline, n = 6 for KO MPEP, *P < 0.05; ns, not significant; two-sided Student's t test).
We also tested whether NMDAR function, in addition to NMDAR plasticity, could be normalized. MPEP treatment normalized the NMDA/AMPA ratio at IRSp53−/− SC-CA1 synapses to levels comparable to those of wild-type neurons (Fig. 6d,e). In contrast, MPEP had no effect on the NMDA/AMPA ratio at wild-type synapses. We did not use memantine in this experiment because of its voltage-dependent blockade of NMDARs. Taken together, these results suggest that the drugs that rescue social interaction in IRSp53−/− mice normalize NMDAR function and plasticity at hippocampal synapses.
Reduced dendritic complexity and synapse number in the mPFC
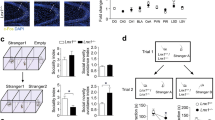
The prelimbic region in the medial prefrontal cortex (mPFC) is an important brain area that has been implicated in social function33,34. We explored structural and functional abnormalities in the IRSp53−/− mPFC. Although the IRSp53−/− hippocampus displays mainly functional changes, such as NMDAR hyperfunction24,25, different brain regions often display heterogeneity in the structural and functional properties of the neurons. We first characterized the dendritic complexity of IRSp53−/− mPFC neurons by Sholl analysis. We found that the apical, but not basal, dendrites of IRSp53−/− layer II/III pyramidal neurons in the prelimbic area had reduced dendritic complexity (Fig. 7a–c).
(a–c) Reduced dendritic complexity in the apical, but not basal, dendrites of IRSp53−/− mPFC prelimbic layer II/III pyramidal neurons. (a) Representative images of biocytin-infused neurons. (b,c) Quantification of the results in a by Sholl analysis, as shown the complexity of apical (b) and basal (c) dendrites (s.e.m., n = 11 cells for WT and n = 14 for KO, *P < 0.05, two-sided Student's t test). (d–f) Reduced frequency and amplitude of mEPSCs in IRSp53−/− mPFC prelimbic layer II/III pyramidal neurons. (d) Examples of mEPSCs from WT and IRSp53−/− neurons. (e,f) Quantification of the results in d, as shown by mEPSC frequency (e) and amplitude (f) (s.e.m., n = 14 cells, 6 mice for WT and 15 cells, 4 mice for KO, *P < 0.05, ***P < 0.001, two-sided Student's t test). (g–k) Reduced numbers and dendritic spines and perforated PSDs in IRSp53−/− mPFC prelimbic layers II/III. PSD, postsynaptic density. (g) Representative electron micrographs from WT and KO mice. Arrowheads indicate normal spines, and arrows indicate perforated spines. Scale bar represents 500 nm. (h–k) Quantification of the results in g. Note that IRSp53−/− neurons showed normal PSD length and thickness (s.e.m., n = 3 mice for WT and KO, *P < 0.05, ***P < 0.001; ns, not significant two-sided Student's t test).
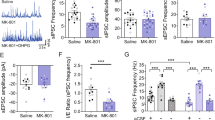
We next characterized synaptic changes in this region. We found that the frequency and amplitude of miniature EPSCs (mEPSCs) were reduced in IRSp53−/− layer II/III pyramidal neurons compared with wild-type neurons, with a greater reduction in frequency (Fig. 7d–f). In addition, electron microscopy revealed decreases in the number of dendritic spines and perforated postsynaptic densities (PSDs) (a measure of maturity) in the IRSp53−/− mPFC, whereas PSD length and thickness were normal (Fig. 7g–k). These results collectively suggest that excitatory synapse number is reduced in the IRSp53−/− mPFC, which contrasts with the normal excitatory synapse number in the hippocampus24,25, but is consistent with the previous in vitro results that IRSp53 regulates excitatory synapses and dendritic spines20,21.
Although displaying a reduced excitatory synapse number, IRSp53−/− layer II/III pyramidal neurons did not show functional alterations in the paired pulse ratio, NMDA/AMPA ratio, cofilin phosphorylation or miniature inhibitory postsynaptic currents (mIPSCs) (Supplementary Figs. 5–8). Together, these results suggest that IRSp53 deletion leads to two distinct changes in the brain: enhanced NMDA function with normal excitatory synapse number in the hippocampus and reduced excitatory synapse number with normal NMDAR function in the mPFC.
Memantine normalizes reduced firing rates in the mPFC
How might the reduction in excitatory synapse number in the IRSp53−/− mPFC be associated with the social deficits observed in IRSp53−/− mice? To address this question, we first tested whether memantine rescues the reduced mEPSC frequency or amplitude in the mPFC and found no effect on these parameters (Supplementary Fig. 9), which suggests that mEPSCs are not associated with the pharmacological rescue.
We next turned to neuronal firing in the IRSp53−/− mPFC, reasoning that the reduced excitatory synapse number may affect neuronal firing. Single-unit recordings using tetrodes in live anesthetized mice revealed reduced firing rate in the IRSp53−/− mPFC relative to wild-type controls (Fig. 8a,b). When the recorded units were grouped into putative excitatory and inhibitory neurons, excitatory neuronal firing was reduced, whereas inhibitory neuronal firing was unchanged (Fig. 8c,d). These results suggest that IRSp53 deletion leads to a selective reduction in excitatory neuronal firing in the mPFC region.
(a–d) Reduced excitatory neuronal firing in the IRSp53−/− mPFC, as determined by in vivo tetrode recordings in urethane-anesthetized animals. Recording sites were verified with DiI marking (a). PL and IL, prelimbic and infralimbic area, respectively. Shown are averaged firing rates of all (b), putative excitatory (pExc, c) and putative inhibitory (pInh, d) neurons (s.e.m., WT, n = 374 total, 262 pExc and 10 pInh from 5 animals; KO, 352, 247 and 15, respectively, from 5 animals; ***P < 0.001; ns, not significant; Mann-Whitney U test). (e–h) Acute memantine treatment (10 mg per kg, intraperitoneal) induced a greater increase in neuronal firing in the IRSp53−/− mPFC, as indicated by average firing rates (f), averaged fold increases in individual units (g), and firing rates of individual units before and after memantine treatment (h). Examples of firing traces from in vivo silicon probe recordings are shown in e (s.e.m., n = 107 from 5 WT and 99 from 4 KO mice; firing rate comparisons, **P < 0.01, ***P < 0.001, Wilcoxon signed-rank test; fold increases, **P < 0.01, Mann-Whitney U test). (i–l) Memantine induced different excitatory neuronal firing between wild-type and IRSp53−/− hippocampus, as determined by in vivo tetrode recordings in dorsal CA1 (s.e.m., WT-saline, n = 255 total, 162 pExc and 8 pInh from 5 mice; WT-memantine, 179, 128 and 5, respectively, from 5 mice; KO-saline, 302, 131 and 22, respectively, from 6 mice; KO-memantine, 59, 28 and 5, respectively, from 4 mice; *P < 0.05, **P < 0.01; ns, not significant; two-way ANOVA with normalization of neuronal firing by log transformation).
We next tested whether memantine treatment affected neuronal firing in the IRSp53−/− mPFC. Memantine increased neuronal firing in both IRSp53−/− and wild-type mPFC, but the increase was larger in IRSp53−/− mice, as indicated by the fold increase (Fig. 8e–h). These results suggest that IRSp53 deletion suppresses excitatory neuronal firing in the mPFC, which can be reversed by memantine treatment.
Memantine induces a firing difference in the hippocampus
We next measured neuronal firing in the hippocampal CA1 region, where NMDAR function is enhanced, and examined how it is affected by memantine treatment. Two-way ANOVA revealed a significant main effect of genotype (P < 0.01) and a genotype × treatment interaction (P < 0.05). Post hoc Tukey tests indicated that CA1 neuronal firing was not different between memantine-treated and non-treated wild-type mice, or between memantine-treated and non-treated IRSp53−/− mice. However, neuronal firing was higher in memantine-treated IRSp53−/− mice, compared to memantine-treated wild-type mice (Fig. 8i,j).
Similar results were obtained for putative excitatory neurons (Fig. 8k). No group difference was detected for putative inhibitory interneurons (Fig. 8l). These results suggest that memantine induces a difference in excitatory neuronal firing between wild-type and IRSp53−/− hippocampi.
Discussion
Our data suggest that enhanced NMDAR function contributes to the development of social deficits in IRSp53−/− mice. In support of this hypothesis, memantine normalized the social interaction in IRSp53−/− mice (Fig. 3) and NMDAR plasticity (LTD of NMDARs) in the hippocampus (Fig. 6a–c). In addition, MPEP rescued the social deficits (Fig. 3) and NMDAR function (NMDA/AMPA ratio) (Fig. 6d,e). The fact that MPEP reduced the NMDA/AMPA ratio suggests that reduced mGluR5 signaling suppresses NMDAR activity rather than the signaling pathways downstream of NMDAR activation. In addition, the normal mGluR-LTD at IRSp53−/− hippocampal synapses (Fig. 4g–i) suggests that MPEP does not rescue social interaction in IRSp53−/− mice by modulating mGluR-LTD.
Memantine and MPEP normalized social interaction, but not ultrasonic vocalization or hyperactivity in IRSp53−/− mice (Supplementary Fig. 4a–c), suggesting that these behaviors do involve enhanced NMDAR function. This is reminiscent of phenotype-specific rescues observed in other mouse lines with social deficits35,36. For instance, in Cntnap2−/− mice, the antipsychotic risperidone rescues repetitive behavior and hyperactivity, but not social interaction36. These selectively rescued phenotypes may provide a platform with which we can further study the mechanisms.
The IRSp53−/− hippocampus showed reduced LTD of NMDARs responsive to memantine (Fig. 6a–c). Given that IRSp53−/− hippocampal neurons displayed F-actin puncta resistant to NMDA-induced dispersal (Fig. 5a–d), the abnormally stabilized synaptic F-actin at IRSp53−/− synapses may act against the synaptic loss of NMDARs during LTD of NMDARs, promoting synaptic enrichment of NMDARs. Alternatively, the stabilized F-actin may functionally enhance the activity of synaptic NMDARs through their interaction mediated by α-actinin23.
Notably, cofilin activation (dephosphorylation) in response to NMDA treatment occurred normally in the IRSp53−/− hippocampus, whereas F-actin puncta were resistant to NMDA treatment (Fig. 5). This suggests that F-actin puncta at IRSp53−/− synapses are somehow resistant to activated cofilin. How this cofilin resistance arises is unclear. Perhaps, it represents compensatory changes that occur in response to the loss of IRSp53, a positive regulator of F-actin and actin-rich dendritic spines16,20, restoring normal F-actin levels at the cost of F-actin plasticity.
The IRSp53−/− mPFC displayed substantial decreases in dendritic complexity and excitatory synapse number and maturity (Fig. 7). Thus, our study provides, to the best of our knowledge, the first in vivo evidence that IRSp53 regulates excitatory synapses and dendritic spines, which is consistent with the previous in vitro results that IRSp53 positively regulates dendritic spines20. This contrasts with the fact that the IRSp53−/− hippocampus displays normal neuronal morphology or excitatory synapse number24,25, which might be attributable to the fundamental differences in the ultrastructure and composition of the PSD in different brain regions and cell types37.
IRSp53 deletion does not induce changes in NMDAR function at mPFC synapses, contrary to the NMDAR hyperfunction observed at hippocampal synapses. This regional difference is reminiscent of a previous report that neuroligin-3 mutant mice (R451C) with social deficits display distinct synaptic changes in the somatosensory cortex and hippocampus38. How is the mPFC related with the memantine-dependent rescue of the social deficit in IRSp53−/− mice? We found that memantine normalized the suppressed neuronal firing in the mPFC (Fig. 8a–h), suggesting that it may contribute to social rescue in IRSp53−/− mice. In the hippocampal CA1 region, memantine induced neuronal firings in IRSp53−/− neurons that were more frequent than those in wild-type neurons (Fig. 8i–l), consistent with the results from the mPFC. These results suggest that mPFC and hippocampus might act synergistically to rescue the social deficits of IRSp53−/− mice in response to memantine treatment.
Our results suggest that enhanced NMDAR function may lead to social deficits in IRSp53−/− mice. Suppressed NMDAR function has been suggested to cause social deficits in other mouse lines. For instance, Shank2−/− mice (exons 6 + 7 deletion) display NMDAR hypofunction and social deficits that are rescued by NMDAR-enhancing reagents D-cycloserine (NMDAR agonist) and CDPPB (positive allosteric modulator of mGluR5)35. In addition, ASD-like social deficits in Tbr1+/− and Glud1−/− mice are rescued by D-cycloserine, although NMDAR hypofunction in these animals have not been directly measured39,40. These results suggest that NMDAR function in a normal range is important for social function, and that deviation of NMDAR function in either direction may lead to social impairments.
IRSp53 has been associated with ASDs41,42,43. Whether NMDAR hyperfunction is associated with human ASDs is an open question. However, the fact that memantine has been somewhat beneficial for ASD44, and that it is now under clinical trials for ASD-associated social deficits13, points to the possibility. In addition, although there is no robust statistical support for IRSp53's involvement in schizophrenia or ADHD as of yet2,45,46, exploring a potential link between IRSp53 and these disorders using IRSp53−/− mice might be an interesting research direction to pursue. Notably, memantine has been shown to be beneficial for schizophrenia. This relatively new approach, however, needs further investigations47, as reduced (not enhanced) NMDAR function has long been implicated in schizophrenia48. Similarly, ADHD has recently been suggested to involve NMDAR dysregulation, in addition to its long-known dopamine dysfunction49, although therapeutic usefulness of NMDAR modulators such as memantine in ADHD treatment remains controversial44.
In conclusion, our data suggest that NMDAR hyperfunction in IRSp53−/− mice leads to social deficits that are responsive to pharmacological NMDAR inhibition, suggesting a mechanism for social deficits and its therapeutic potential.
Methods
Animals.
IRSp53−/− mice (C57BL/6J) have been previously reported50. Mice were bred and maintained according to the Requirements of Animal Research at KAIST, and all procedures were approved by the Committee of Animal Research at KAIST (KA2012-19). Mice were fed ad libitum, and 2–4 animals were housed in a cage under 12-h light cycle. Male mice were used for all behavioral experiments, and, for non-behavioral experiments, which mainly used mice younger than 4 weeks, both male and female mice were used.
Behavioral assays.
All behavioral assays were performed with age-matched male mice (8–16 weeks) generated by hetero × hetero mating. All behavior studies were performed during day times (light-on periods) except for the open field test. There were at least 1-d-long rest periods between tests.
Three-chambered social interaction.
Three-chambered apparatus was slightly modified from the one previously described51; 40 cm width × 20 cm height × 26 cm depth with a 12-cm-wide center chamber and 14-cm-wide side chambers. Both side chambers contained a plastic cage in the corner, with a plastic cup with a weight on it, to prevent the subject mouse from climbing. The assay consisted of four sessions. The first session began with 10-min habituation in the center chamber followed by the second 10 min session where the subject mouse could freely explore all three chambers. The mouse was then gently confined in the center chamber while a novel object and a wild-type stranger mouse, stranger 1, was placed in the two plastic cages. The subject mouse was then allowed to freely explore all three chambers for 10 min. Before the last session, the subject mouse was again gently guided to the center chamber while the object was replaced with a wild-type mouse, stranger 2. The subject mouse again freely explored all three chambers for 10 min. All stranger mice were males at the same age and previously habituated to the plastic cage during the previous day (30 min). The positions of object and stranger 1 were alternated between tests to prevent side preference.
Social interaction in pairs.
This test was performed as previously described52. Each individual mouse spent 10 min in a gray box (30 × 30 × 30 cm) for two consecutive days for habituation. On the third day, pairs of mice of the same genotype (unfamiliar to each other) were placed in the test box for 10 min. Social interactions were defined as activities involving sniffing, following, mounting and allogrooming.
Ultrasonic vocalization.
This test was performed as described previously35. In brief, each male WT or KO mouse was isolated in home cages for three days before the recording. On the recording day, an isolated male mouse was placed in the recording chamber for 30 min. After 5-min background recording, one randomly chosen estrus B6 female was placed in the recording chamber. Signals were filtered from 1 Hz to 100 kHz and digitized with a sampling frequency of 250 kHz, 16 bits per sample, using a 1,000-Hz high-pass digital filter (model 1322A, Axon Instruments). Low frequencies were filtered out to reduce background white noise and audible squeaking from females.
Self-grooming.
This test was performed in a standard home cage or a clear and small chamber (14 × 7 × 20 cm), as described previously53,54. Mice were individually placed in a home cage and given a 10-min habituation period. Mouse behavior during the following 10 min was recorded and analyzed by an observer blind to the identity of subject mice. Self-grooming behavior was defined as stroking or scratching of face, head or body with the two forelimbs, or licking body parts. To minimize the possibility that other activities such as hyperactivity affect self-grooming, we also measured self-grooming behavior in a small chamber for 30 min without habituation.
Marble burying and digging.
Digging and marble burying were measured as previously described55. For marble burying, each mouse was placed in a standard home cage with 20 marbles placed on wood-chip bedding with the depth of 5 cm. The number of marbles buried to the 2/3 depth of their height were counted. Digging was measured in cages without marbles. Digging behavior was defined as the behavior of a mouse where it coordinately uses two fore legs or hind legs to dig out or displace bedding materials.
Buried food finding.
This experiment was performed as previously described52. Briefly, mice were given chocolate cookies and water ad libitum for 4 d before testing. Mice were given cookies every day of an amount that can be consumed in 24 h. Mice were deprived of cookies and water for 12 h before testing. For testing, a small piece of cookie was buried at either end of home cages under 1.5 cm of wood-chip bedding. Subject mouse was placed in the opposite end of the site of cookie burial. Food finding time was defined as the time taken to locate the cookie and begin to burrow. The mouse was removed as soon as the mouse discovered the cookie. Fresh cage and bedding were used for every test.
Open-field and home-cage locomotor activity.
Mice were placed in the center region of an open field box (40 × 40 × 40 cm). The center zone in the open field was defined as the area 10 cm or more apart from the wall. Open-field and home-cage locomotor activities were measured for 60 min and 24 h, respectively, and analyzed using EthoVision 3.1 software (Noldus).
Novel object recognition.
Mice at 2–3 months of age were habituated in the open field box without objects for 30 min a day before training session. Before 30 min of training session, each mouse was injected saline or memantine (10 mg per kg, intraperitoneal). At the training session, mice were placed in the box with two identical objects for 10 min. 24 h after the training, one of the objects was replaced with a different object. Before 30 min of this test session, saline or memantine was injected (10 mg per kg, intraperitoneal). Object recognition was scored by the amount of time with the nose of the mouse pointed and located within 2 cm from the object.
Automated 48-h movement analysis.
For a long-term movement analysis, we used the LABORAS system (Metris), designed to detect and analyze vibrations from the movements such as grooming, locomotion, rearing, and climbing of a mouse placed in a cage on a carbon-fiber plate. Before the data collection, mice were isolated and habituated for a week in the same booth that LABORAS system was set up. After the habituation, each mouse was placed in the cage, and data were collected uninterrupted for 48 h. Collected data were analyzed by the LABORAS software.
Mouse neuron culture and immunostaining.
Cultured hippocampal mouse neurons were prepared from postnatal day 0–3 littermates. Dissociated neurons were cultured on poly-d-lysine (0.1 mg ml−1, Sigma) and laminin (10 μg ml−1, BD Biosciences)-coated coverslips with complete neurobasal media-A (Gibco) supplemented with B27 (Invitrogen), 0.5 mM L-glutamine, and penicillin-streptomycin. Chemical LTD was induced by incubating neurons in a preexisting medium with NMDA (30 μM) for 3 min. After transferring the neurons to an NMDA-free medium for another 3 min, neurons were fixed with 4% paraformaldehyde/sucrose (vol/vol) and permeabilized with 0.2% Triton X-100 (vol/vol). For immunostaining, neurons were incubated with primary antibodies and rhodamine phalloidin (Molecular Probes) followed by incubation with dye-conjugated secondary antibodies. For quantification of the linear density of F-actin puncta, we analyzed dendritic regions containing secondary or greater than secondary branches, excluding the primary branches connected to the cell body.
Electrophysiology with acute hippocampal and cortical slices.
LTD experiments were performed using slices from 21–28-d-old mice, and mEPSCs, mIPSCs, AMPA/NMDA ratio were measured using 14–21-d-old mice. Sagittal slices (hippocampus, 300 μm for whole cell recordings, 400 μm for field recordings) and coronal slices (mPFC, 300 μm for whole cell recordings) were prepared using a vibratome (VT1200S, Leica) in ice-cold dissection buffer (in mM: 212 sucrose, 25 NaHCO3, 5 KCl, 1.25 NaH2PO4, 10 D-glucose, 2 sodium pyruvate, 1.2 sodium ascorbate, 3.5 MgCl2, 0.5 CaCl2 bubbled with 95% O2/5% CO2). The slices were recovered at 32 °C in normal artificial cerebrospinal fluid (aCSF; in mM: 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 D-glucose, 1.3 MgCl2, 2.5 CaCl2) and thereafter maintained at room temperature. Whole-cell patch-clamp recordings and field potential recordings were performed as previously described50. In brief, recordings were made by using MultiClamp 700B amplifier and Axopatch 200B (Molecular Devices) under visual control with differential interference contrast illumination in an upright microscope (BX50WI; Olympus). Field recordings were performed with only a MultiClamp 700B amplifier. Data were acquired by Clampex 9.2 (Molecular Devices) and analyzed by Clampfit 9 (Molecular Devices) or using custom macros written in Igor (Wavemetrics).
Whole-cell recordings with hippocampal slices.
For LTD experiments, recording pipettes (3–5 MΩ) were filled with a solution containing (in mM) 117 CsMeSO4, 10 TEA-Cl (tetraethylammonium chloride), 8 NaCl, 10 HEPES, 5 QX-314-Cl, 4 Mg-ATP, 0.3 Na-GTP, 0.5 EGTA. For NMDA/AMPA experiments, pipettes were filled with 117 CsMeSO4, 10 TEA-Cl, 8 NaCl, 10 HEPES, 5 QX-314-Cl, 4 Mg-ATP, 0.3 Na-GTP, 10 EGTA. mIPSC was measured with pipette solution containing (in mM) 115 CsCl, 10 TEA-Cl, 8 NaCl, 10 HEPES, 5 QX-314-Cl, 4 Mg-ATP, 0.3 Na-GTP, 10 EGTA. All pipette solutions were adjusted to acquire a pH and osmolarity of 7.3 and 290–300 mOsm, respectively. Signals were filtered at 2.0 kHz and digitized at 10 kHz. Series resistance was monitored in each sweep by measuring the peak amplitude of capacitance currents in response to short hyperpolarizing step pulse (5 mV, 40 ms). Picrotoxin (50 μM) was added during NMDAR/AMPAR ratio experiments, and picrotoxin and NBQX (10 μM) were added during LTD of NMDAR/AMPAR EPSC recordings. Slices received LTD stimulation as previously described56. Briefly, EPSCs were evoked with a glass pipette containing external aCSF to activate SC-CA1 pyramidal synapses. AMPA and NMDA EPSCs were recorded at −70-mV and −40-mV holding potentials. After obtaining a stable baseline response (1/15 Hz), LTD of AMPAR and NMDAR EPSCs were both induced by a 5-Hz, 3-min train at a −40-mV holding potential. For NMDA/AMPA ratio experiments, AMPA EPSCs were recorded at a −70 mV of holding potential. After obtaining a stable baseline for 10 min, holding potential was shifted to 40 mV to record NMDA EPSCs. 20 consecutive traces were averaged to obtain mean current trace of AMPA and NMDA EPSCs. NMDA EPSC currents were measured 50 ms after stimulus. mIPSCs were recorded with 10 μM NBQX, 25 μM AP5, 0.5 μM TTX at −70 mV. For experiments regarding pharmacological treatment of electrophysiological phenotypes (NMDA/AMPA ratio), either 1 μM memantine or 20 μM MPEP was added to the aCSF, and the cells/slices were exposed to the drug-infused aCSF at least 30 min before the recording of the experiment, as well as throughout the entirety of the recording itself.
Field potential recordings with hippocampal slices.
mGluR-LTD was induced with DHPG (50 μM, 5 min) in the presence of picrotoxin (50 μM)57. fEPSPs were evoked with a glass pipette containing external aCSF to activate SC-CA1 pyramidal synapses. Average responses (±s.e.m.) were expressed as percentage of baseline response (after confirming 20 min of stable responses, 1/20 Hz). AMPAR LTD and NMDAR-dependent LTD were measured using protocols previously described also after achieving a stable response for 20 min56. Pharmacological rescue of NMDAR-dependent LTD with memantine was achieved as described in the previous section.
Whole-cell recordings with cortical slices.
Recordings were obtained from layer II/III pyramidal neurons of the prelimbic mPFC and performed as described above. All whole-cell patch-clamp recordings were performed with biocytin (0.5%, vol/vol) added to the internal solution (in mM: 117 CsMeSO4, 10 TEA-Cl (tetraethylammonium chloride), 8 NaCl, 10 HEPES, 5 QX-314-Cl, 4 Mg-ATP, 0.3 Na-GTP, 10 EGTA or EPSCs and 115 CsCl, 10 TEA-Cl, 8 NaCl, 10 HEPES, 5 QX-314-Cl, 4 Mg-ATP, 0.3 Na-GTP, 10 EGTA for mIPSC). Pyramidal neurons were first identified by shape and the presence of single apical dendrites and later confirmed by biocytin staining. Somatic whole-cell recordings of mEPSCs were performed at a holding potential of −70 mV after adding TTX 0.5 μM, picrotoxin 50 μM in the aCSF. For paired-pulse ratio and NMDA/AMPA ratio experiments, EPSCs were evoked with a glass pipette containing aCSF positioned 50–100 μm away from pyramidal cells. AMPA EPSCs were recorded at −80 mV of holding potential. After obtaining a stable baseline for 10 min, holding potential was shifted to 40 mV to record NMDA EPSCs. 20 consecutive traces were averaged to obtain mean current trace of AMPA and NMDA EPSCs. Picrotoxin (50 μM) and glycine (20 μM) were added to the aCSF. NMDA EPSC currents were measured 50 ms after stimulus. mIPSCs were recorded with 10 μM NBQX, 25 μM AP5, 0.5 μM TTX at −70 mV. Pharmacological treatment of mEPSCs with memantine was achieved as described in the previous section.
Sholl analysis.
Layer II/III pyramidal neurons in the prelimbic mPFC were randomly chosen and infused with biocytin during electrophysiological recordings. After recording, the slices were collected and fixed in 4% paraformaldehyde at 4 °C for at least 24 h. Fixed slices were washed three times with phosphate-buffered saline (PBS) for 5 min and permeablized with 0.5% Triton X-100/PBS for one hour at 24 °C. After permeablization, slices were washed three times with PBS for 5 min and immersed in streptavidin antibody to biocytin with 5% bovine serum albumin/PBS (vol/vol) for 2 h at 4 °C. Images were captured by LSM 510 and 20× objectives. Dendritic branch intersections were evaluated by performing the Sholl analyses as described previously58.
Electron microscopy.
2–3-week-old mice were deeply anesthetized with sodium pentobarbital (80 mg per kg, intraperitoneal) and were intracardially perfused with 100 ml of heparinized normal saline, followed by 500 ml of a freshly prepared fixative with 2.5% glutaraldehyde (vol/vol) and 1% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Removed brains were sectioned with a vibratome at 70 μm thickness setting. The sections were postfixed in the same fixative for 2 h, osmicated with 1% osmium tetroxide (vol/vol) in 0.1 M phosphate buffer for 1 h, dehydrated in graded alcohols, flat embedded in Durcupan ACM (Fluka), and cured for 48 h at 60 °C. Small pieces containing layers II/III in the prelimbic region of mPFC were cut out of the wafers and glued onto the plastic block by cyanoacrylate. Ultrathin sections were cut and mounted on Formvar-coated single-slot grids, stained with uranyl acetate and lead citrate, and examined with an electron microscope (Hitachi H-7500, Hitachi) at 80 kV accelerating voltage. 24 micrographs representing 368.9 μm2 neuropil regions in each mouse were photomicrographed at a 40,000× and 72 electromicrographs for each three WT and IRSp53−/− mice were used for quantification. Number of spines (PSD density), proportion of perforated spines, PSD length and PSD thickness were measured. The measurements were all performed by an experimenter blind to the genotype. Digital images were captured with GATAN DigitalMicrograph software driving a CCD camera (SC1000 Orius, Gatan) and saved as TIFF files. Brightness and contrast of the images were adjusted in Adobe Photoshop 7.0 (Adobe Systems).
Pharmacological rescue.
Intraperitoneal injections of 30 mg per kg MPEP (Tocris) or 10 mg per kg memantine (Sigma), or of 10 mg per kg MPEP or 5 mg per kg memantine for low doses, were performed 30 min before open field and ultrasonic vocalization tests and 10 min before the three-chamber test, considering 20 min habituation period in this test. Supporting that these drug concentrations can lead to significant receptor blockade in the brain, acute systemic administration of MPEP (10 mg per kg; intraperitoneal, mice) has been shown to maintain >75% mGluR5 receptor occupancy for 30 min59. In addition, memantine (10 mg per kg, intraperitoneal, rats) is known to lead to peak CNS concentration of 1.2 μM, which is higher than the Ki value of memantine for NMDARs60. Mice first received MPEP (or memantine) in the first round. After a 7–14-d period, in the second round, the mice received saline injection. After another 7–14-d rest period, the mice received memantine (or MPEP) injection. We used saline in the second round to make sure that the effects of drug injection in the first round were completely finished, and to use the results as control data.
Antibodies.
Commercial antibodies were purchased from the following sources: MAP2 (Sigma, mouse, M4403), α-tubulin (1:10,000, Sigma, mouse, T9026), cofilin (1:1,000, Cell Signaling, rabbit, #3312) and phospho-cofilin (1:1,000, Cell Signaling, rabbit, #3311). Shank antibody has been previously described (#3856)61.
Electrophysiological recording in vivo.
Male mice (8 weeks) were used for in vivo recording by fixing the heads on a stereotaxic device under urethane anesthesia (1.5 g per kg). The body temperature of the mice was monitored and maintained by a temperature controller (TC-1000, CWE). Neuronal activity was recorded in the right mPFC (in mm, 1.7 anterior and 0.3 lateral from bregma and 1.8–2.5 ventral from brain surface) and dorsal CA1 (1.4 posterior and 1.0 lateral from bregma and 0.8–1.3 ventral from brain surface) with two sets of four tetrodes with EIB-16 (Neuralynx) made of 4 nichrom wires. To localize the site of the recordings, the tips of the tetrodes were briefly dipped in fluorescence dye solution (DiI, 50 mg ml−1, Sigma) before tissue penetration, and electrode tracks in the brain slices were visualized under a confocal microscope using a rhodamine filter, as previously described14. Unit signals were amplified 10,000×, filtered between 600 and 6,000 Hz, digitized at 32 kHz, and stored on a personal computer using a Cheetah data acquisition system (Neuralynx). Spike clustering was performed using Mclust (L ratio < 0.05, isolation distance > 30), and units were classified according to spike width and discharge frequency (putative excitatory neurons: spike width > 280 μs, frequency < 5 Hz; putative inhibitory neurons: spike width < 280 μs, frequency > 5 Hz) as previously described14.
To measure in vivo firing during the pharmacological rescue (memantine) in the mPFC, we used multi-array silicon probes (Neuronexus, Buzaski 32) and 8-week-old male mice under urethane anesthesia. Neuronal activity was recorded with multi-array silicon probes because this method yielded higher sampling of neuronal activity compared to tetrode recording and thus is ideal for monitoring acute drug effects, although it did not allow clear isolation of single units preventing direct comparison of neuronal activity across animals and differentiation between excitatory and inhibitory neurons. After inserting a silicon probe in the prelimbic cortex and recording neural activity for 20 min, memantine was administered (10 mg per kg, intraperitoneal) and neural activity was recorded for 20 min starting from 30 min after the drug injection. The recording and targeting were performed as described above. Unit signals were isolated by manual clustering of various spike waveform parameters (3D-spike sort, Neuralynx), but unit separation quality was generally poor so that some individual clusters were likely to contain multiple units. Nevertheless, only stable clusters were included in the analysis to ensure measurement of memantine treatment effect against the same set of neurons.
Because the number of recorded CA1 units under the baseline condition (that is, without memantine treatment) was relatively small (75 in wild-type and 171 in IRSp53−/− mice), and because the silicon probe did not yield satisfactory CA1 unit signals for unknown reasons when examining memantine-treatment effect, we performed additional unit recordings by implanting eight tetrodes in the dorsal CA1 of urethane-anesthetized wild-type and IRSp53−/− mice under baseline and memantine-treatment conditions. We first recorded multiple single-unit signals while gradually advancing the tetrodes in CA1 cell body layer without memantine treatment to maximize sample sizes (for comparison between wild-type and IRSp53+/− mice in the baseline condition). When a seemingly sufficient number of unit signals were obtained, memantine was treated (10 mg per kg, intraperitoneal) and, 30 min after the treatment, unit signals were recorded for 20 min (mematine-treatment condition). Putative excitatory and inhibitory neurons were grouped as follows; pExc, spike width > 250 μs, firing rate < 5 Hz; pInh, spike width < 250 μs, firing rate > 5 Hz (ref. 62).
Representative figures and replication.
For representative images in figures, experiments associated with behavior, electrophysiology, Sholl analysis and electron microscopy were performed once with the n number of animals/slices/cells indicated. Western analysis and immunohistochemistry experiments were repeated three times, and the results could be replicated.
Data acquisition, analysis, statistics and general methods.
We performed all the behavior experiments by researchers blind to the identity of the animals, and analyzed the data in a blind manner. Data collection and analysis for slice and in vivo physiology, dendritic complexity, spine counting, and electron microscopic analysis were not performed blind to the conditions of the experiments. Statistical details including statistical methods used are described in Supplementary Table 1. Statistical tests were justified for every figures, and whether the data meet the assumptions of the statistical methods were tested.
A Supplementary Methods Checklist is available.
References
Huguet, G., Ey, E. & Bourgeron, T. The genetic landscapes of autism spectrum disorders. Annu. Rev. Genomics Hum. Genet. 14, 191–213 (2013).
Fromer, M. et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184 (2014).
Lionel, A.C. et al. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci. Transl. Med. 3, 95ra75 (2011).
Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Zoghbi, H.Y. & Bear, M.F. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 4, a009886 (2012).
Jiang, Y.H. & Ehlers, M.D. Modeling autism by SHANK gene mutations in mice. Neuron 78, 8–27 (2013).
Ehninger, D. & Silva, A.J. Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends Mol. Med. 17, 78–87 (2011).
Delorme, R. et al. Progress toward treatments for synaptic defects in autism. Nat. Med. 19, 685–694 (2013).
Kleijer, K.T. et al. Neurobiology of autism gene products: towards pathogenesis and drug targets. Psychopharmacology (Berl.) 231, 1037–1062 (2014).
Darnell, J.C. & Klann, E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat. Neurosci. 16, 1530–1536 (2013).
Bagni, C. & Oostra, B.A. Fragile X syndrome: from protein function to therapy. Am. J. Med. Genet. A. 161A, 2809–2821 (2013).
Ting, J.T., Peca, J. & Feng, G. Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annu. Rev. Neurosci. 35, 49–71 (2012).
Spooren, W., Lindemann, L., Ghosh, A. & Santarelli, L. Synapse dysfunction in autism: a molecular medicine approach to drug discovery in neurodevelopmental disorders. Trends Pharmacol. Sci. 33, 669–684 (2012).
Silverman, J.L., Yang, M., Lord, C. & Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11, 490–502 (2010).
Ebert, D.H. & Greenberg, M.E. Activity-dependent neuronal signaling and autism spectrum disorder. Nature 493, 327–337 (2013).
Scita, G., Confalonieri, S., Lappalainen, P. & Suetsugu, S. IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 18, 52–60 (2008).
Sheng, M. & Hoogenraad, C.C. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 76, 823–847 (2007).
Abbott, M.A., Wells, D.G. & Fallon, J.R. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J. Neurosci. 19, 7300–7308 (1999).
Burette, A.C., Park, H. & Weinberg, R.J. Postsynaptic distribution of IRSp53 in spiny excitatory and inhibitory neurons. J. Comp. Neurol. 522, 2164–2178 (2013).
Choi, J. et al. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J. Neurosci. 25, 869–879 (2005).
Soltau, M. et al. Insulin receptor substrate of 53 kDa links postsynaptic shank to PSD-95. J. Neurochem. 90, 659–665 (2004).
Bockmann, J., Kreutz, M.R., Gundelfinger, E.D. & Bockers, T.M. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J. Neurochem. 83, 1013–1017 (2002).
Cingolani, L.A. & Goda, Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9, 344–356 (2008).
Kim, M.H. et al. Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J. Neurosci. 29, 1586–1595 (2009).
Sawallisch, C. et al. The insulin receptor substrate of 53 kDa (IRSp53) limits hippocampal synaptic plasticity. J. Biol. Chem. 284, 9225–9236 (2009).
Morishita, W., Marie, H. & Malenka, R.C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat. Neurosci. 8, 1043–1050 (2005).
Allison, D.W., Gelfand, V.I., Spector, I. & Craig, A.M. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J. Neurosci. 18, 2423–2436 (1998).
Nadler, J.J. et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 3, 303–314 (2004).
Jia, Z. et al. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn. Mem. 5, 331–343 (1998).
Alagarsamy, S. et al. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat. Neurosci. 2, 234–240 (1999).
Lau, C.G. & Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 8, 413–426 (2007).
Halpain, S., Hipolito, A. & Saffer, L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18, 9835–9844 (1998).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Shalom, D.B. The medial prefrontal cortex and integration in autism. Neuroscientist 15, 589–598 (2009).
Won, H. et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 486, 261–265 (2012).
Peñagarikano, O. et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147, 235–246 (2011).
Harris, K.M. & Weinberg, R.J. Ultrastructure of synapses in the mammalian brain. Cold Spring Harb. Perspect. Biol. 4, a005587 (2012).
Etherton, M. et al. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. USA 108, 13764–13769 (2011).
Huang, T.N. et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat. Neurosci. 17, 240–247 (2014).
Yadav, R. et al. Deletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviors. PLoS ONE 7, e32969 (2012).
Toma, C. et al. Association study of six candidate genes asymmetrically expressed in the two cerebral hemispheres suggests the involvement of BAIAP2 in autism. J. Psychiatr. Res. 45, 280–282 (2011).
Levy, D. et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70, 886–897 (2011).
Gilman, S.R. et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70, 898–907 (2011).
Hosenbocus, S. & Chahal, R. Memantine: a review of possible uses in child and adolescent psychiatry. J. Can. Acad. Child Adolesc. Psychiatry 22, 166–171 (2013).
Ribasés, M. et al. Case-control study of six genes asymmetrically expressed in the two cerebral hemispheres: association of BAIAP2 with attention-deficit/hyperactivity disorder. Biol. Psychiatry 66, 926–934 (2009).
Liu, L. et al. BAIAP2 exhibits association to childhood ADHD especially predominantly inattentive subtype in Chinese Han subjects. Behav. Brain Funct. 9, 48 (2013).
Shim, S.S. & Nadeem, R. Are NMDA receptor antagonists beneficial in the treatment of schizophrenia? J. Psychiatr. Res. 51, 19–20 (2014).
Coyle, J.T. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 26, 365–384 (2006).
Chang, J.P., Lane, H.Y. & Tsai, G.E. Attention deficit hyperactivity disorder and N-methyl-d-aspartate (NMDA) dysregulation. Curr. Pharm. Des. 20, 5180–5185 (2014).
Kim, M.H. et al. Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J. Neurosci. 29, 1586–1595 (2009).
Moy, S.S. et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 191, 118–129 (2008).
Jamain, S. et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA 105, 1710–1715 (2008).
McFarlane, H.G. et al. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 7, 152–163 (2008).
Pearson, B.L. et al. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 10, 228–235 (2011).
Deacon, R.M. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc. 1, 122–124 (2006).
Morishita, W., Marie, H. & Malenka, R.C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat. Neurosci. 8, 1043–1050 (2005).
Huber, K.M., Roder, J.C. & Bear, M.F. Chemical induction of mGluR5- and protein synthesis–dependent long-term depression in hippocampal area CA1. J. Neurophysiol. 86, 321–325 (2001).
Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 87, 387–406 (1953).
Anderson, J.J. et al. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine). Eur. J. Pharmacol. 473, 35–40 (2003).
Parsons, C.G., Danysz, W. & Quack, G. Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist–a review of preclinical data. Neuropharmacology 38, 735–767 (1999).
Lim, S. et al. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J. Biol. Chem. 274, 29510–29518 (1999).
Lee, J.W., Kim, W.R., Sun, W. & Jung, M.W. Disruption of dentate gyrus blocks effect of visual input on spatial firing of CA1 neurons. J. Neurosci. 32, 12999–13003 (2012).
Acknowledgements
This study was supported by the National Research Foundation of Korea (to D.K., 2012-0008795; to Y.C.B., 2012-0009328) and the Institute for Basic Science (IBS-R002-D1 to E.K. and IBS-R002-G1 to M.W.J.).
Author information
Authors and Affiliations
Contributions
W.C., Haram Park, Hanwool Park, Y.C., D.L. and M.-H.K. conducted electrophysiological experiments. S.Y.C. conducted neuron culture, immunoblot and behavior experiments. E.L., J.W.L., S.L. and I.R. conducted in vivo recording experiments. J.K., R.K. and J.C. conducted mouse behavior experiments. S.-G.P. conducted ultrasonic vocalization experiments. Y.S.C. conducted electron microscopy experiments. M.W.J., D.K., Y.C.B. and E.K. supervised the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 IRSp53−/− mice display normal olfactory function in the buried food test and normal repetitive behavior.
(a) Time taken for mice to find buried food and begin to dig was measured. (SEM, n = 10, ns, not significant, two-sided Student's t-test). (b) WT and IRSp53−/− mice spend similar amounts of time in grooming in home cages without bedding. (SEM, n = 10, ns, not significant, two-sided Student's t-test). (c) IRSp53−/− mice show normal total time spent in grooming in a novel and small chamber without bedding, which was used to suppress other activities such as space exploration. (SEM, n = 13, ns, not significant, two-sided Student's t-test). (d) IRSp53−/− mice show normal marble burying behavior comparable to WT mice. (SEM, n = 6, ns, not significant, two-sided Student's t-test). (e) IRSp53−/− mice show digging behavior comparable to WT mice. (SEM, n = 7, ns, not significant, two-sided Student's t-test). (f-i) In automated 48-hr monitoring of movements, which uses weight displacement, IRSp53−/− mice show normal levels of grooming, rearing, and climbing, but did show enhanced locomotion. (SEM, n = 3, **P < 0.01, ns, not significant, two-sided Student's t-test).
Supplementary Figure 2 Heterozygous IRSp53+/− mice show normal social interaction, locomotor activity, and repetitive behavior.
(a and b) IRSp53+/− mice show normal social interaction and social novelty recognition in the three chamber test, as shown by the social preference index based on sniffing time (SEM, n = 6 for WT and 7 for HT, ns, not significant, two-sided Student's t-test). (c) IRSp53+/− mice show normal locomotor activity in the open field test. (SEM, n = 8 for WT and HT, Two-way ANOVA, repeated measures). (d) IRSp53+/− mice show normal levels of grooming in home cages. (SEM, n = 8 for WT and HT, ns, not significant, two-sided Student's t-test).
Supplementary Figure 3 Lower doses of memantine and MPEP do not rescue social interaction in IRSp53−/− mice.
(a and b) Representative heat maps of three-chambered social interactions in WT and KO mice treated with low-dose memantine and MPEP. (c-f) Quantification of the results in (a) and (b), as shown by the time spent in chamber (c), or sniffing the cage (e), with a stranger (S) vs. an object (O), or the social preference index for the chamber time (d), or sniffing time (f). KO mice treated with low-dose memantine (5 mg/kg; i.p.) or MPEP (10 mg/kg; i.p.) show no rescues in social interactions. Note that there is a tendency towards rescue for low dose memantine. (SEM, n = 10 for WT saline, 8 for WT memantine, 10 for WT MPEP, 9 for KO saline, 8 for KO memantine, and 11 for KO MPEP, *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant, one way ANOVA).
Supplementary Figure 4 Memantine and MPEP do not normalize impaired ultrasonic vocalization and open-field hyperactivity in IRSp53−/− mice, but memantine rescues novel object recognition.
(a-d) Animals were treated with memantine (10 mg/kg; i.p.) or MPEP (30 mg/kg; i.p.) 30 min before the indicated behavioral measurements. (a and b) Ultrasonic vocalization (SEM, n = 8 for WT saline, 8 for WT memantine, 6 for WT MPEP, 8 for KO saline, 8 for KO MPEP, and 8 for KO memantine, **P < 0.01, ns, not significant, two-sided Student's t-test). (c) Hyperactivity (SEM, n = 8 for WT saline, 10 for WT memantine, 14 for WT MPEP, 9 for KO saline, 9 for KO memantine, and 14 for KO MPEP, *P < 0.05, **P < 0.01, two-sided Student's t-test). (d) Novel object recognition (SEM, n = 7 for WT saline and memantine, 6 for KO saline and memantine, **P < 0.01, ***P < 0.001, ns, not significant, two-sided Student's t-test).
Supplementary Figure 5 Full images of cropped blots presented in other figures.
(a) Full blots for Figure 5e, f. (b) Full blots for Figure 5g, h. (c) Full blots for Supplementary Figure 6a. (d) Full blots for Supplementary Figure 6d. (W; WT, K; KO, U; untreated control, N; NMDA treatment)
Supplementary Figure 6 Normal levels of paired-pulse ratio and NMDA/AMPA ratio of evoked EPSCs at IRSp53−/− mPFC synapses.
(a) Normal paired-pulse ratio in the IRSp53−/− mice. Representative current traces are shown. (SEM, n = 7 cells, 6 mice for WT and 7 cells from 5 mice for KO, Two-way ANOVA, repeated measures). (b) Normal NMDA/AMPA amplitude ratio of evoked EPSCs in the IRSp53−/− mice. Representative current traces are shown. The grey block inside the representative trace indicates 50 msec after stimulus. (SEM, n = 10 cells, 9 mice for WT and 11 cells from 9 mice for KO, ns, not significant, two-sided Student's t-test).
Supplementary Figure 7 Normal levels of total and phosphorylated cofilin in the IRSp53−/− mPFC and whole brain.
(a-c) mPFC, (d-f) whole brain (SEM, n = 6 for WT, KO cofilin/p-cofilin in mPFC, and n = 4 for WT, KO cofilin/p-cofilin in whole brain, ns, not significant, two-sided Student's t-test).
Supplementary Figure 8 Normal miniature inhibitory postsynaptic currents (mIPSCs) in the IRSp53−/− mPFC and hippocampus.
(a and d) Examples of mIPSCs from WT and KO layer II/III pyramidal neurons in the prelimbic region of the mPFC (a) and in the CA1 pyramidal neurons of the hippocampus (d). (b, c, e, and f) Quantification of mIPSC frequencies and amplitudes. (SEM, n = 15 cells, 4 mice for WT and KO mPFC; 14 cells, 4 mice for WT hippocampus; 13 cells, 4 mice for KO hippocampus, ns, not significant, two-sided Student's t-test).
Supplementary Figure 9 Memantine does not rescue mEPSCs in the IRSp53−/− mPFC.
(a) Examples of mEPSCs. WT and IRSp53−/− mPFC slices in the presence or absence of memantine (1 μM) were measured of mEPSCs in layer II/III pyramidal neurons. (b and c) Quantification of the results in (a), as shown by the frequency (b) and amplitude (c) of mEPSCs. (SEM, n = 15 for WT saline, n = 11 for WT memantine, n = 14 for KO saline, n = 13 for KO memantine, * P < 0.05, ** P < 0.01, ns, not significant, two-sided Student's t-test).
Supplementary information
Supplementary Figures and Table
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 5438 kb)
Supplementary Methods Checklist
Statistics Checklist (PDF 732 kb)
Rights and permissions
About this article
Cite this article
Chung, W., Choi, S., Lee, E. et al. Social deficits in IRSp53 mutant mice improved by NMDAR and mGluR5 suppression. Nat Neurosci 18, 435–443 (2015). https://doi.org/10.1038/nn.3927
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3927
This article is cited by
-
Lateral hypothalamic proenkephalin neurons drive threat-induced overeating associated with a negative emotional state
Nature Communications (2023)
-
Latent toxoplasmosis impairs learning and memory yet strengthens short-term and long-term hippocampal synaptic plasticity at perforant pathway-dentate gyrus, and Schaffer collatterals-CA1 synapses
Scientific Reports (2023)
-
Adult re-expression of IRSp53 rescues NMDA receptor function and social behavior in IRSp53-mutant mice
Communications Biology (2022)
-
Early postnatal serotonin modulation prevents adult-stage deficits in Arid1b-deficient mice through synaptic transcriptional reprogramming
Nature Communications (2022)
-
Expression of actin- and oxidative phosphorylation-related transcripts across the cortical visuospatial working memory network in unaffected comparison and schizophrenia subjects
Neuropsychopharmacology (2022)