Abstract
The consolidation of context-dependent emotional memory requires communication between the hippocampus and the basolateral amygdala (BLA), but the mechanisms of this process are unknown. We recorded neuronal ensembles in the hippocampus and BLA while rats learned the location of an aversive air puff on a linear track, as well as during sleep before and after training. We found coordinated reactivations between the hippocampus and the BLA during non-REM sleep following training. These reactivations peaked during hippocampal sharp wave–ripples (SPW-Rs) and involved a subgroup of BLA cells positively modulated during hippocampal SPW-Rs. Notably, reactivation was stronger for the hippocampus–BLA correlation patterns representing the run direction that involved the air puff than for the 'safe' direction. These findings suggest that consolidation of contextual emotional memory occurs during ripple-reactivation of hippocampus–amygdala circuits.
Similar content being viewed by others
Main
Cooperation between amygdala, particularly the BLA, and hippocampus is critical for contextual emotional memory1,2,3,4,5,6,7,8,9,10. It is believed that the emotional and spatial components of an experience are processed by the amygdala and dorsal hippocampal circuits, respectively8,11,12,13. Lesion and other experiments have shown that fine spatial representation in the dorsal hippocampus14,15 is required for spatial and contextual memory, including context–threat associations, with a limited contribution from the ventral hippocampus11,16,17,18,19,20,21. Because only the ventral hippocampus and associated entorhinal outputs project directly to the amygdala, including the BLA and central amygdala10,22,23, it remains to be determined how emotional and contextual stimuli are combined and consolidated to form a stable, integrated representation of the context and associated emotional valence.
Sleep replay of wake sequences of place cells during hippocampal SPW-Rs are instrumental for spatial memory consolidation and the stabilization of newly formed spatial representations24,25,26. By comparison, the consolidation mechanisms of amygdala-dependent memories have only been partially explored1,27,28. We hypothesized that contextual emotional experience is replayed in the interconnected hippocampus–amygdala circuit during sleep. More specifically, because synchronous discharges of neuron populations during SPW-Rs facilitate the combination of neuronal information throughout the entire dorso-ventral axis of the hippocampus29, we hypothesized that spatial information from the dorsal hippocampus may be associated with the threat representation in the BLA during SPW-Rs of non-REM (NREM) sleep.
To study hippocampus–amygdala interactions, we combined a classical spatial task with a location-specific aversive element (air puff). We recorded large neuronal ensembles simultaneously in the amygdala and dorsal hippocampus during training and sleep episodes before and after training. To identify the subpopulations of neurons in the amygdala that are functionally linked to the dorsal hippocampus, we examined their discharge patterns during SPW-Rs. We then investigated whether joint hippocampus–BLA representations of space and threat are reactivated during SPW-Rs of NREM sleep.
Results
Rats learn the daily location of an aversive air puff on a linear track
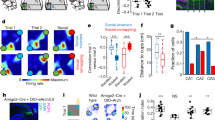
To study hippocampal–amygdala reactivations, we designed a task by combining a classical spatial task with an aversive component to recruit BLA neurons. Rats (n = 4) were pretrained to run back and forth on a linear track for water rewards. After steady performance was achieved, we introduced an aversive air puff at the same location of the track on each lap in one of the running directions. The location and direction of the air puff was changed daily in a pseudo-random manner. Previous work has shown that air-puff-induced contextual fear learning relies on both the amygdala and the hippocampus30. We adapted this task to allow daily behavioral training and recordings of large neuronal ensembles in freely moving animals. Each daily recording session consisted of a pre-run behavioral test session on the track without the air puff, followed by pre-learning sleep in the home cage ('pre-sleep', categorized as pre-REM or pre-NREM), a training session ('run') with the air puff, post-learning sleep ('post-sleep', categorized as post-REM or post-NREM) and a post-run test session without the air puff (Fig. 1a). Because rats slow down before crossing the air puff location if they remember its location, we quantified memory performance from the pre-run and post-run test epochs by comparing the running speed of the rat in the danger zone of the current day (defined as the 20 cm preceding the air puff location) with the speed at the previous day's danger zone (Fig. 1a,b and Supplementary Fig. 1a). The current danger zone, initially neutral during pre-run, acquires an aversive valence during training, while the previous danger zone loses its aversive nature. Therefore, the systematic reversal in the speed ratio (previous/current danger zone) between pre- and post-run indicates learning of the new air puff location (Fig. 1b and Supplementary Fig. 1b). The typical behavioral pattern on the track during run was a reduced speed before the air puff, followed by an acceleration after passing through the danger zone. A similar speed change was maintained during post-run, whereas speed smoothly increased throughout the track during pre-run (Fig. 1c). This was quantitatively reflected by the significantly slower speed in the current danger zone in post-run compared to pre-run (Fig. 1b,c). The aversive valence of the air puff gradually diminished with training days (Supplementary Fig. 1c). The location of the air puff on the track did not correlate with the speed in the current danger zone in pre-run, training or post-run, ruling out a systematic bias of the air puff location on the results.
(a) Rats run back and forth on a linear track for water rewards. Gray line: one-dimensional position of the animal on the track over time in a representative session (one session in one animal out of 55 sessions in 4 animals). An air puff is delivered at the same location on the track in one running direction during the run epoch. The air puff location is changed every day. The run epoch is flanked by two sleep epochs (pre-sleep, post-sleep) and two test run sessions where no air puff is delivered (pre-run, post-run). The danger zones (DZ) are defined as the 20 cm preceding the location of the air puff on the current day (pink) and on the previous day (blue). (b) Left: speed in the current and previous DZ across animals and sessions during the three run epochs (two-way repeated measures ANOVA; n = 55 sessions in 4 animals; significant session effect (pre-run, run or post-run, P = 7.48 × 10−14, d.f. = 2, F = 41.51), air puff location effect (current vs. previous, P = 0.0012, d.f. = 1, F = 11.81) and interaction, (P = 4.46 × 10−11, d.f. = 2, F = 30.53). Post hoc paired t-tests showed significant differences between previous and current DZ speed for pre-run, run and post-run (P = 0.0011, t(52) = 3.45; P = 9.70 × 10−9, t(51) = −6.84; P = 0.00307, t(51) = −3.10), as well as for the current DZ between pre-run and post-run (P = 1.21 × 10−6, t(53) = 5.47). Right: speed ratios across sessions and animals (one-way repeated measure ANOVA; significant session effect, P = 1.31 × 10−13, d.f. = 2, F = 40.48; post hoc t-tests, pre-run vs. run: P = 2.5 × 10−11, t(51) = −8.49; pre-run vs. post-run P = 5.3 × 10−6, t(51) = −5.08; run vs. post-run P = 5.1 × 10−5, t(50) = 4.43; white line, median; black line, mean; black dots, outliers; boxes, first and last quartiles; whiskers, minimum and maximum values excluding outliers). **P < 0.01, ***P < 0.001 with Bonferroni correction. (c) Air-puff-centered mean speed (± s.e.m., pre-run: n = 52 sessions in 4 animals; run and post-run: n = 53 sessions in 4 animals) curves in the air puff direction in the pre-test (no air puff), training and post-test (no air puff) epochs. The current DZ is indicated by the shaded pink bar. Note the slower speed in the DZ post-run compared to pre-run.
BLA recordings and sleep physiology
We recorded ensembles of neurons from both left and right amygdala and the dorsal CA1 hippocampal region during the task (Fig. 2a,b). Eight-shank silicon probes were moved downward by 140-μm steps between each behavioral experiment. This allowed recording from large areas of the amygdala and the piriform cortex in each rat (Supplementary Fig. 2). Over the course of a total of 61 sessions, we recorded 7,390 well-isolated units (rat 1, 2,444; rat 2, 1,294; rat 3, 1,138; rat 4, 2,514). On the basis of histological reconstruction of probe placement and probe movement record, 2,038 of these were in the BLA, 782 in the central nuclei, 1,560 in the piriform cortex and 1,210 in the hippocampus (Supplementary Table 1). Units were further characterized as putative pyramidal cells and interneurons by waveform and physiological criteria (Supplementary Fig. 3 and Online Methods). The firing rates of pyramidal cells followed a skewed distribution during sleep (NREM and REM) and wakefulness31 (Fig. 2c). In addition, we found a specific increase in the firing rate of BLA pyramidal cells, but not interneurons, during REM sleep and, to a lesser extent, NREM sleep relative to wakefulness, as shown by the distributions of REM/wake or NREM/wake firing rate ratios for the two cell types (Fig. 2c and Online Methods).
(a) Silicon probe recordings from the dorsal hippocampal CA1 (four-shank probe) and bilateral amygdala (eight-shank probes; total 160 channels) and example local field potentials (LFPs) and units (raster plots) in the hippocampus (Hpc; red) and left and right amygdala (BLA; blue). Hippocampal SPW-R times are indicated by gray lines in NREM sleep. (b) CA1 and BLA spectrograms for an example session (out of 29 sessions in 3 rats with simultaneous BLA and hippocampus recordings). Spectrograms were used to define brain states (wake, NREM or REM sleep; colors represent power in arbitrary units, from green (low) to red (high)). (c) Distributions of firing rates for monosynaptically identified pyramidal cells (orange, n = 675 cells; see Online Methods and Supplementary Fig. 3), interneurons (blue, n = 175 cells) and other cells (gray, n = 1,188 cells) in BLA during NREM vs. wake (top left) and REM vs. wake (top right). The distribution of REM/wake and NREM/wake firing rate ratios (bottom) is skewed toward 1 for pyramidal cells (orange, monosynaptically identified pyramidal cells; n = 675; NREM/wake: P = 4.07 × 10−46, z = 14.25; REM/wake: P = 8 × 10−42, z = 13.54; Wilcoxon signed rank tests), indicating an increase in firing rate during both sleep stages compared to wake. Interneurons do not change firing rates between REM and wake (blue, monosynaptically identified interneurons, n = 175; REM/wake: P = 0.21, z = 1.25) and slightly decrease firing rates during NREM compared to wake (NREM/wake: P = 1.97 × 10−9, z = −6.00; Wilcoxon signed rank tests; dotted lines: medians).
BLA–hippocampus coordinated reactivations during NREM sleep
Reactivations across the hippocampus–amygdala network as well as within-structure networks (Supplementary Fig. 4) were quantified using the explained variance (EV). EV is the percentage of variance in the population of pairwise correlations during post-sleep (REM or NREM) that can be explained by run correlations ('reactivation') while taking into account pre-existing correlations during pre-sleep (REM or NREM32,33,34; Fig. 3a,b and Online Methods). The reverse explained variance (REV), calculated by switching the pre-sleep and post-sleep epochs, is used as a control value. The EV and REV, calculated using hippocampus–BLA pyramidal cell pairs, showed significant reactivations between the hippocampus and BLA during post-NREM (Fig. 3c). The gradual decay in reactivations over the first hour of NREM sleep (Fig. 3c; mean differences between EV and REV, ± s.e.m.: 0–20 min, 2.89 ± 0.47%; 20–40 min, 1.72 ± 0.44%; 40–60 min, 1.31 ± 0.60%; n = 19 sessions, P = 0.014, d.f. = 2, χ2 = 8.53; Kruskal-Wallis test) paralleled the previously described decay for pairs of hippocampal neurons35,36. Reactivations were maintained when both pyramidal cells and interneurons were included in the EV calculation (Supplementary Fig. 5). Neurons in the piriform cortex showed weaker experience-induced reactivation with their CA1 partner neurons (Fig. 3d; Wilcoxon one-tailed rank sum test on EV – REV for hippocampus–BLA (n = 25 sessions, mean EV – REV 3.13 ± 0.73%, s.e.m.) vs. hippocampus–piriform cortex (n = 14, mean EV – REV 0.85 ± 0.34%, s.e.m.), P = 0.0092, z = 2.38), while there were no reactivations at all between the central nuclei and the hippocampus (Fig. 3e). Reactivations during REM sleep27,37,38 were not significant in any structure, despite the robust REM-sleep-specific increase in BLA putative pyramidal cells firing rates (Figs. 2c and 3c–e and Supplementary Fig. 5a).
(a) Correlation matrices were calculated for hippocampus–BLA cell pairs for the pre-NREM, run and post-NREM epochs in 50-ms time bins. These matrices were used to calculate the explained variance (EV) and its control value, reverse explained variance (REV). Red arrows: strong coactivations of a single BLA pyramidal neuron with multiple hippocampal pyramidal (pyr) cells in an example session (1 animal and session out of 3 animals and 25 sessions for NREM sleep). (b) NREM EV and REV for the example session shown in a. (c) Left: EV and REV across all sessions for NREM (n = 25, P = 0.00019, z = 3.72; n = 3 rats) and REM sleep (n = 23, P = 0.377, z = 0.88; n = 3 rats; pyr–pyr pairs). Right: EV and REV for successive 20 min epochs of NREM for sessions where EV > REV in the first 20-min NREM epoch (pyr–pyr pairs; n = 19 sessions in 3 rats, 0–20 min: P = 0.00013, z = 0.38; 20–40 min: P = 0.00046, z = 3.5; 40–60 min: P = 0.0312, z = 2.15). (d) Hippocampus–piriform cortex EV and REV (NREM: n = 17 sessions, P = 0.0052; REM: n = 15 sessions in 3 rats, P = 0.301; pyr–pyr pairs). (e) Same as d but for central nuclei (CeN) (NREM: n = 19 sessions, P = 0.717, z = 0.36 and REM: n = 19 sessions, P = 0.687, z = −0.40; all cell pairs; n = 3 rats). All tests are Wilcoxon signed rank tests, ***P < 0.001, **P < 0.01, *P < 0.05. All box plots show the median (red line), first and last quartiles (box), and minimum and maximum values excluding outliers (whiskers), outliers (black dots).
A subset of BLA cells are modulated during hippocampal SPW-Rs
BLA neurons receive direct input from ventral, but not dorsal, CA1 neurons22. However, spatial location is more precisely coded by dorsal CA1 neurons than ventral ones14,15. Because both dorsal and ventral hippocampal neurons fire together during large-amplitude SPW-Rs29, SPW-Rs may establish functional connections between the dorsal hippocampus and amygdala. To test this hypothesis, we examined the functional relationship between SPW-Rs and BLA neurons. A fraction of BLA neurons were significantly and positively modulated ('upmodulation'; 42 of 163 interneurons (25.8%), 137 of 1,233 pyramidal cells (11.1%)) or negatively modulated ('downmodulation'; 24 of 163 interneurons (14.7%), 102 of 1,233 pyramidal cells (8.3%)) during hippocampal SPW-Rs (Fig. 4). This confirmed the indirect influence of dorsal hippocampal SPW-Rs on BLA cells. Moreover, reactivations calculated using SPW-R-modulated BLA cells were larger than for nonmodulated pairs (Supplementary Fig. 5b).
(a) Percentages of hippocampal (Hpc) and BLA putative pyramidal cells (pyr; red) and interneurons (int; blue) that are significantly upmodulated (up-mod; dark red or dark blue) or downmodulated (down-mod; light red or light blue) during SPW-Rs (Hpc: n = 41 sessions, n = 3 rats; BLA: n = 29 sessions, n = 3 rats). (b) Peri-event gain for two putative BLA pyramidal cells (red: top, upmodulated; bottom, downmodulated) and interneurons (blue: top, upmodulated; bottom, downmodulated). These neurons are indicated by the respective colored asterisks in c. (c) Normalized peri-event gain for all upmodulated (top left) and downmodulated (top right) BLA putative pyramidal cells (top panels) and interneurons (bottom panels). Top black traces: example hippocampal ripples (LFP).
Ripple-modulated BLA cells are preferentially involved in reactivations
In a further attempt to characterize the reactivation dynamics during sleep, we used two complementary approaches. In the first approach, we defined the firing properties of individual neurons relative to ripples and then examined how such properties influenced reactivations. The second approach worked from the opposite direction. First, we quantified reactivations for each cell irrespective of their LFP ripple correlates and examined how their reactivation values were related to ripples. During run, a fraction of hippocampal–BLA pyramidal neuron pairs showed significantly positively correlated spike trains (2,521 of 37,660; 6.69%). Another small percentage (1,258 of 37,660; 3.34%) was negatively correlated, while the remaining majority (33,881 of 37,660; 89.96%) was not reliably correlated (Online Methods; total number of pyramidal–pyramidal hippocampus–BLA pairs 37,660: rat 1, 16,056; rat 3, 3,836; rat 4, 17,768). To test whether a selective subgroup of pairs was preferentially involved in sleep reactivations, we separated pairs into nine subgroups based on a combination of run correlation and SPW-R modulation of the BLA partner (Fig. 5a, Supplementary Fig. 6 and Supplementary Table 2). Hippocampus–BLA pairs with significant, positive run correlations and SPW-R upmodulation of the BLA partner showed the largest pre-NREM to post-NREM change (one-way ANOVA, P = 1.1 × 10−45, d.f. = 8, F = 29.09; post hoc comparisons, P < 0.001). The dominant contribution of this specific subgroup of cell pairs to reactivations was confirmed by calculating the EVs across the nine subgroups (pairs grouped across animals and sessions; Supplementary Fig. 6b).
Strongly contributing modulated BLA cells selectively increase their gain during SPW-Rs following training. (a) Mean differences between pre-NREM and post-NREM correlations for each group of cell pairs classified according to (i) the ripple-modulation type of the BLA cell—upmodulation (up-mod), no modulation (no mod) or downmodulation (down-mod)—and (ii) the correlation during the run—significantly positive, nonsignificant or significantly negative. Inset shows the normalized cumulative distributions of the post-NREM – pre-NREM correlation difference for the upmodulated, significantly positive run correlation group (red) and downmodulated, significantly negative run correlation group (blue; error bars: s.e.m.; n = 37,660 pairs, one-way ANOVA P = 1.1 × 10−45, d.f. = 8, F = 29.09, n = 3 rats). The upmodulated, significantly positive correlation group is the only one to be significantly different from all others (post hoc Tukey–Kramer multiple comparison test with **P < 0.01). Details of all distributions are shown in Supplementary Figure 6. (b) Left: peri-ripple gains for upmodulated cells of the highly contributing quartile for pre-NREM SPW-Rs and post-NREM SPW-Rs (color: normalized gain per cell; cells are sorted by timing of the bin with highest gain during the pre-sleep epoch). Right: mean (± s.e.m.) gain during pre-NREM (blue) and post-NREM (red) SPW-Rs for upmodulated cells of the strongly contributing quartile (top) and for the remaining quartiles (bottom). There is a significant increase in gain between pre-NREM and post-NREM SPWRs for strongly contributing BLA upmodulated cells (Wilcoxon one-tail signed rank tests on gain averaged in a 500-ms window around ripple peak; high contribution quartiles: n = 63 cells, ***P = 4.57 × 10−5, z = −3.913; low contribution quartiles: n = 73 cells, P = 0.992, z = 2.408; n = 3 rats).
In the second approach, we evaluated the contribution of each cell pair to the overall EV (calculated with all pairs across animals and sessions) by removing hippocampus–BLA pairs one by one. The change in EV (EVall – EVminus one pair) indicates the individual contribution of the removed pair (the larger the decrease in EV, the larger the contribution of the pair; Supplementary Fig. 7a). To obtain a per-cell contribution measure, contributions were averaged over all the pairs that the cell participated in. We then divided BLA cells into quartiles according to the magnitude of their individual contributions. We found that upmodulated BLA neurons in the most strongly contributing quartile showed a specific increase in SPW-R gain (that is, firing rate during versus outside SPW-R) from pre-NREM to post-NREM compared to the upmodulated cells of the remaining three, low-contribution quartiles (Fig. 5b and Supplementary Fig. 8; Wilcoxon one-tail sign-rank test on gain averaged in a 500-ms window around ripple peak; high-contribution quartile: P = 4.57 × 10−5, z = 3.91; low-contribution quartiles: P = 0.991, z = 2.40). To control for the effects of firing rates, we calculated EVs and REVs for pairs pooled according to their firing rates (individual BLA cell firing rate, hippocampal cell firing rate or combined firing rate). This control showed that EV did not depend on firing rates (Supplementary Fig. 9).
The aversive trajectory is reactivated during SPW-Rs
BLA cells that are upmodulated during hippocampal ripples show a preferential involvement in coordinated reactivations, through an increased gain of their modulation after training. However, these observations offer only indirect support for reactivations during hippocampal SPW-Rs. Furthermore, these findings alone do not directly address the critical role of threat in sleep reactivations. To obtain more direct support, we used a reactivation strength (R) measure (Supplementary Fig. 10 and Online Methods) and analyzed firing patterns separately during the two directions of travel. Since the air puff was presented during only one direction of run on the track (air puff or danger trajectory) on a given day, the opposite run can be considered safe. Therefore, we compared the reactivation strengths of the hippocampus–BLA pairwise correlation patterns of the air puff vs. the safe direction. We found that the reinstatement of the joint hippocampus–BLA neuron representation was significantly enhanced during post-NREM SPW-R compared to pre-NREM SPW-Rs for the air puff direction but not for the safe direction (Fig. 6a,b and Supplementary Fig. 11b,c). Because the firing rates of BLA cells did not significantly differ between safe and air puff trajectories (pyramidal cells: P = 0.270, z = 1.101; all cells: P = 0.763, z = 0.302, Wilcoxon signed rank tests), the reactivation results cannot be explained by air-puff-induced firing rate increase. The occurrence rate of SPW-R was also not significantly different between the pre-NREM and post-NREM epochs (P = 0.192; z = −1.289, n = 41 sessions; Wilcoxon signed rank test; Supplementary Fig. 11d). These observations thus confirm that NREM sleep SPW-Rs are specific time windows within which the place–threat association is reinstated during sleep.
(a) Mean (± s.e.m.) z-scored peri-ripple reactivation strength of the air puff (top) versus safe (bottom) trajectories over animals (n = 3) and sessions (n = 25) for pre-NREM (blue) and post-NREM (red) hippocampal SPW-Rs. Right: reactivation strength in a 500-ms window around ripple peaks (gray bars) was significantly higher in post-NREM compared to pre-NREM for air puff trajectory (***P = 7.42 × 10−5, z = −3.793), but not for safe trajectory (P = 0.217, z = −0.780; Wilcoxon one-tail signed rank tests). Gray lines from pre-NREM to post-NREM indicate single sessions. (b) The pre-NREM vs. post-NREM difference in reactivation strength (RS) at the ripple peak is significantly higher for the air puff trajectory compared to safe trajectory (Wilcoxon one-tail signed rank-test; **P = 0.00257, z = −2.798, n = 25 sessions; box plots show the median (red line), first and last quartiles (box), and minimum and maximum values (whiskers) excluding outliers).
NREM contributes to reinstatement of new place–threat representations
Finally, we examined how sleep reactivations are linked to the place–threat representation during wakefulness. Rats learned a new air puff location every day during the training session (Fig. 1a,b). The joint representation of space and threat is thus expected to be different between the pre-run test, when the new location has not been experienced yet, and the post-run test, when it has been experienced and replayed during sleep. Figure 7a shows examples of highly contributing BLA–hippocampus cell pairs that showed air-puff-related activity (BLA) and air-puff-related place fields (hippocampus). These coordinated patterns developed during training and were maintained in the post-run test in the absence of an air puff. To quantify this relationship, we examined separately the most strongly contributing pairs (represented by the highest 2.5th percentile of the contribution distribution) and the least strongly contributing pairs (the 2.5th percentile of lowest contribution; Supplementary Fig. 7b). We found that for strongly contributing, but not for weakly contributing, pairs the increase between pre-run and post-run coactivity was significantly correlated with the increase in coactivity between pre-NREM and post-NREM (Fig. 7b). This result was maintained when the most strongly and the most weakly contributing quartiles (instead of 2.5th percentiles) of the distribution were compared (Fig. 7c). These coordinated changes indicate that reactivations during sleep play a role in the stabilization of the new space–threat representation.
(a) Behavioral correlates of an example BLA pyramidal cell (left; red triangle) that forms highly contributing pairs with two hippocampal place cells (center and right; blue and green triangles). Firing maps are shown for pre-run, run and post-run epochs (top to bottom) and firing rate histogram for run. Red line: air puff location. Black arrows: running direction in which the air puff is applied ('air puff trajectory'). Color bars are in hertz. (b) Correlation between changes in pre vs. post-NREM correlations and pre- vs. post-run correlations for strongly contributing pairs (red, 2.5% highest contributions) and weakly contributing pairs (blue, 2.5% lowest contributions). NREM and run changes are correlated for strongly contributing pairs (Pearson r = 0.27, n = 866 pairs, P = 2.11 × 10−16; n = 3 rats), but not for weakly contributing pairs (r = 0.03, n = 845 pairs, P = 0.305; n = 3 rats). Corr diff, correlation difference. (c) Same as b but for strong (n = 9,415 pairs, r = 0.13, P = 1.07 × 10−32; n = 3 rats) and weak (n = 9,415 pairs, r = 0.013, P = 0.22; n = 3 rats) contributing quartiles (25% highest and lowest contributions).
Discussion
We found that correlated neuronal activity between neurons of the dorsal hippocampus and BLA was strengthened during NREM sleep following experience in a spatially anchored threat model39,40. Reactivations involved a subgroup of hippocampus-responsive neurons in BLA and occurred in association with hippocampal SPW-Rs. Notably, the reactivation of hippocampus–BLA coactivity during post-experience sleep was stronger for the patterns of pairwise correlations dominating during the travel through the danger zone, compared to reactivations of the pairwise patterns representing the safe direction.
Previous works have shown that in both spatial memory tasks and contextual threat learning only a small set of neurons is active in the hippocampus and amygdala7,8,9,35. Identifying amygdala neurons that receive hippocampal inputs required recording from an unprecedentedly large number of individual neurons simultaneously in these two structures. We achieved this by using multi-shank silicon probes and an experimental design that allowed us to generate new place–threat associations every day so that we could slowly advance our probes through the full structure of the amygdala and sample new sets of neurons daily. Of the large number of cross-structure neuron pairs, we identified the relevant subset whose coactivation increased significantly from pre-experience to post-experience sleep and thus contributed to the cross-structure reactivations. We further characterized the amygdala members of these pairs as hippocampus-responding because they increased firing rates during hippocampal SPW-Rs. In contrast, BLA neurons that decreased or did not change their activity during SPW-Rs did not show significant change in their correlation with hippocampal neuron partners from pre-experience to post-experience sleep. Furthermore, neuron pairs across the hippocampus and BLA that showed the strongest increase in correlation from pre-experience sleep to post-experience sleep were those that also showed the strongest correlations during learning of the place–threat association. For the reactivated pairs only, the changes in the strength of coactivation during sleep induced by training were correlated with the changes in the coactivations on the test sessions on the track. Moreover, experience-induced reactivations were stronger for hippocampus–BLA pairs correlated during travel that involved the aversive air puff, compared to travel in the safe direction. Overall, our findings suggest that training on the place–threat association creates a novel joint representation between the hippocampus and the amygdala that is subsequently consolidated or reconsolidated during sleep and is reinstated on the track during the test session in absence of the threat.
The dorsal hippocampus is crucially involved in spatial memory, including classical context–threat associations19,41, in line with the observations that neurons in the dorsal hippocampus carry highly specific spatial information13. More specifically, it has been shown that the indirect suppression of sleep hippocampal SPW-Rs, known to consolidate spatial memories24, impairs contextual fear conditioning25. By comparison, the importance of the ventral hippocampus for contextual processing is debated42, again in line with the coarser spatial representation of ventral hippocampal neurons14,15. We show that despite the lack of direct connections from dorsal hippocampus to BLA, a fraction of BLA pyramidal cells and interneurons were effectively entrained by SPW-Rs of the dorsal hippocampus. We hypothesize that this is possible because during large amplitude SPW-Rs populations of the dorsal and ventral hippocampus, likely involving ventral neurons projecting to the amygdala43, robustly synchronize. Indeed, one postulated role of SPW-Rs is to combine neuronal activity across different segments of the hippocampus29,44 that send and receive projections to different parts of the neocortex, amygdala and subcortical structures45.
Alternative explanations should also be considered. In addition to the direct ventral hippocampus–amygdala projections, a hippocampus–entorhinal cortex–amygdala route may also be involved, given that BLA–entorhinal connections have been implicated in the acquisition of contextual threat conditioning46 and given that deep-layer entorhinal neurons also respond robustly to SPW-Rs47. Another potential explanation for the hippocampus–amygdala sleep replay is that both neuronal populations are simply responding to a third party, such as slow oscillations. However, several findings argue against this possibility. First, neuronal responses in BLA to hippocampal SPW-Rs were immediate and short. If firing correlations were driven by UP-DOWN states of sleep or spindles, more prolonged firing rate responses would be expected. Second, DOWN-UP shift induces increases but not decreases in firing rates. In contrast, a large fraction of the amygdala neurons responded with suppression of firing rates during hippocampal SPW-R. Third, only SPW-R-excited BLA neurons showed a significant change from pre-experience to post-experience sleep. Conversely, we found that BLA neurons with high contribution to reactivations were more likely to increase their association with SPW-R during sleep after learning compared to sleep before learning. Overall, our findings suggest that SPW-Rs are instrumental for establishing functional connections between dorsal hippocampus and BLA to consolidate place–threat associations.
Previous findings in humans and other animals have suggested that REM sleep is critical for the consolidation of emotional information27,36,37,48,49. Our results showing an elevated firing rate of BLA pyramidal cells during REM sleep are in line with this hypothesis. However, we did not find significant reactivations during REM sleep. The short duration of REM sleep episodes and the consequently low number of spikes available for the analyses we performed may contribute to the lack of significant reactivations during REM sleep. It is also possible that REM sleep plays a different or complementary role in the consolidation of emotional memories that does not involve an offline reinstatement of the joint hippocampus–BLA representation. Finally, our work did not address the potential role of subcortical neurotransmitters in memory replay and consolidation50 or the role of the entorhinal cortex as a possible mediator of information exchange between hippocampus and amygdala. These questions remain to be answered by future investigations.
In summary, we identified a small subset of hippocampus–BLA neuronal pairs that are reactivated during sleep SPW-Rs following training in a place–threat association task. The BLA partners of these pairs are preferentially upmodulated during SPW-Rs and selectively increase their firing rate during SPW-Rs after training. This finding suggests that SPW-R replay provides a physiological mechanism to integrate place cell activity in the dorsal hippocampus and threat-responsive neurons in the amygdala. We hypothesize that concerted activation of hippocampal and BLA cells during SPW-Rs is responsible for combining spatial/contextual and emotional representations during NREM sleep and thus for the consolidation of contextual fear. Direct support for this hypothesis will require further experiments, such as dynamic perturbation of BLA neurons specifically during SPW-Rs.
Methods
Subjects and electrode implantation.
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at New York University Medical Center. Four individually housed male Long-Evans rats were used in this experiment, and maintained on a 12h:12h light-dark cycle (lights on at 7 a.m.) throughout the study. Animals (300 g, 3 months old at time of surgery) were deeply anesthetized with isoflurane. Three silicon probes (2 with 8 shanks, 1 with 4 shanks, 160 recording channels total, NeuroNexus H32 and H62, A-style, Buzsaki32 and 64 layout) mounted on individual movable microdrives51 were implanted above the amygdalae bilaterally (AP −2.5 mm ML ± 3.6 to 5.5 mm from bregma) and in the dorsal hippocampus (left or right, CA1, AP −3.5 mm, ML ± 2.5 mm). The drives were secured to the skull using dental cement. Skull screws above the cerebellum were used as ground and reference. The drives and probes were protected by a cement-covered copper-mesh Faraday cage on which the probe connectors were attached. Animals were allowed to recover for at least 5 d with ad libitum food and water. In one animal, the hippocampus probe failed during the course of the experiment. This animal was hence not used for analysis about hippocampus–BLA coordination, but was used for intra-amygdala and intra-piriform cortex physiology and reactivation analysis. Data collection and analysis we not performed blind to the conditions of the experiments.
Recordings and behavior.
All animals were free from prior manipulation before being included in the study. After a week of daily handling, animals were placed on water restriction and trained to run back and forth on a linear track for water rewards (Fig. 1). All experiments were performed during the day (light cycle). Three days before surgery, they regained access to ad libitum food and water. After the recovery period, the probes were slowly lowered in the brain and the recordings started when reaching hippocampal CA1 pyramidal layer and the superior limit of BLA, respectively. During this period, the rats were placed back on water restriction to >85% of their normal weight and re-exposed to the linear track. The position of the animal was tracked using a camera mounted on the ceiling and a red LED attached to the head of the animal. Signals were recorded at 20 kHz using an Amplipex recording system (Amplipex Inc., Szeged, Hungary) and the associated Amplirec software. The amygdala electrodes were lowered by 140 μm at the end of each recording session to ensure a complete spanning of the amygdala region over the course of the experiment. The hippocampal probe was adjusted daily to optimize ripple and unit recording.
Preprocessing.
20-kHz signals were resampled at 1,250 Hz to extract LFP data. Spikes were extracted by high-pass filtering (800 Hz) and thresholding the signal, then clustered using Klustakwik (http://sourceforge.net/projects/klustakwik/) followed by manual clustering using Klusters (http://neurosuite.sourceforge.net/) Data were visualized and preprocessed using Neuroscope (http://neurosuite.sourceforge.net/) and NDManager (http://neurosuite.sourceforge.net/)52. Units were classified into putative pyramidal cells and putative interneurons using monosynaptic connections (Supplementary Fig. 3). The remaining, unidentified cells were sorted using k-means clustering (two clusters) on the inverse frequency (that is, duration; fast Fourier transform) and peak-to-trough values (in milliseconds) of the mean waveform of the spikes. Sleep stages were manually scored through visual inspection of the hippocampus and amygdala spectrograms and accelerometer signal using the visual scoring custom program TheStateEditor. Periods of NREM were associated with immobility and high theta/delta ratio (in hippocampus) or gamma (45–65 Hz)/broad low frequency (1–12 Hz) band ratio (in amygdala). REM sleep was characterized by sleep posture and regular theta waves.
Statistical analysis.
Non-parametric Wilcoxon rank sum or signed rank sum (two-tailed, unless otherwise specified) tests were used throughout the paper. All tests used are specified in the figure legends or in the text. Sample sizes were not predetermined, but our sample sizes are similar to (n animals) or higher than (n cells) those generally employed in the field. When parametric tests were used, the data satisfied the criteria for normality (Kolmogorov–Smirnov test) and equality of variance (Bartlett's test for equal variance). For multiple comparisons in the post hoc tests, the original P-values are shown but the significance thresholds *P < 0.05, **P < 0.01, ***P < 0.001 are indicated with either a Bonferroni corrections or Tukey–Kramer test for multiple comparisons. All data are represented with box plots showing the median with central and dispersion statistics. Some extreme data points are not shown in the figures for clarity but all data points were included in the analyses. Bar plots (Fig. 5a) are shown only in combination with the full distributions (Supplementary Fig. 6). P-values for Pearson's correlations are computed using a Student's t distribution for a transformation of the correlation (Matlab “corr” function). A Life Sciences Reporting Summary is available for an overview of ethics and statistics.
Analysis.
All analyses were performed using Chronux (http://chronux.org/), the FMAToolbox (http://fmatoolbox.sourceforge.net/) and Matlab (The MathWorks, Inc., Natick, MA, USA) built-in functions and custom-written scripts.
For behavioral measures, speed ratios were calculated as (pDZ speed – cDZ speed)/(pDZ speed + cDZ speed), with pDZ the previous danger zone (20 cm preceding the air puff location of the previous training day) and cDZ the current danger zone (20 cm preceding the air puff location of the current training day). Because rats run more and faster laps when habituated to the air puff, a habituation index was calculated for each training session and animal as the total number of back-and-forth laps divided by the total time spent on the maze × 100. To obtain the air-puff-centered speed curves, the track positions were normalized and aligned to the air puff location for each session. In this plot (Fig. 1c), two sessions are missing due to corruption of the animal position data.
Firing rate (FR) changes between wakefulness and REM sleep were evaluated using the REM/wake ratios, calculated as (REM FR – wake FR)/(REM FR + wake FR). Positive ratios indicate REM FR > wake FR.
Ripple detection was performed by band-pass filtering (∼100–200 Hz), squaring and normalizing, followed by thresholding of the field potential recorded in CA1 pyramidal layer. SPW-Rs were defined as events starting at 1 s.d., peaking at >4 s.d., and remaining at >1 s.d. for <130 ms and >20 ms around the peak. A control detection was performed on a nonhippocampal channel and all events simultaneously recorded from the hippocampal and control channels (for example, muscular noise) were removed. Ripple modulation was assessed using a Poisson test with P < 0.001. This approach tests whether the parameters for the Poisson cumulative distribution function of spikes outside SPW-Rs (baseline) are the same as for the Poisson cumulative function during SPW-Rs (custom program calling the “poiscdf” Matlab function). The baseline (inter-ripple) firing rate was computed during NREM sleep epochs excluding SPW-Rs. To avoid contamination of rate changes around SPW-Rs, the 100-ms periods before and after each ripple were also excluded.
Explained variance (EV) and reverse explained variance (REV) were calculated per session using subsets of cell pairs selected from the structures of interest. Only sessions with a minimum of 1 shank and 6 cells in each structure were included in the analysis. This criterion accounts for the variation in the number of sessions depending on the subset of cells the EV and REV are calculated for (pyramidal cell only vs. all cells). For REM sleep, only sessions with a minimum of 3 min of total REM sleep (all REM sleep epochs were pooled together) in both pre- and post-sleep were included. Pairwise correlations for EV and REV were calculated using the Pearson correlation coefficient on 50-ms-binned spike trains. The coefficients were separately calculated for pre-sleep (NREM or REM), training, and post-sleep (NREM or REM) periods and assembled into correlation matrices. The correlations between all combinations of these three matrices were then calculated and were used to assess the percentage of variance in the post-sleep period that could be explained by the patterns established during training while controlling for pre-existing correlations in the pre-sleep session (EV):

where R variables are the correlation coefficients between training (T), pre-sleep (S1) and post-sleep (S2) pairwise correlation matrices. The control value (REV) is obtained by switching the temporal order of the pre- and post-sleep session17,18. Only sessions with EV > REV for the first 20 min NREM epoch were used to calculate the decay of reactivations (EV/REV in first and subsequent 20-min NREM epochs; 6 sessions with EV > REV were excluded). Reactivations were considered significant when EV was significantly different from REV (Wilcoxon sign rank test). Comparisons of reactivation across time or structures were performed on the difference EV – REV (Wilcoxon rank sum tests).
An alternative approach was used to assess the contribution of individual cell pairs to replay. For this analysis, cell pairs were pooled (across sessions and animals) into 9 groups based on (i) the nature of hippocampal ripple-modulation of the BLA cell of the pair (up, down or none) and (ii) the significance of the Pearson correlation during training (positively correlated, negatively correlated (P < 0.01) or uncorrelated (P > 0.01) pairs). The nine groups and the number of pairs in each of these groups are summarized in Supplementary Table 2. Next, we computed (i) the difference between pre-NREM and post-NREM correlations for all pairs in each group, where the groups were compared by ANOVA followed by Bonferroni corrected multiple comparisons, and (ii) EV and REV for each subgroup, where the contribution of each pair was evaluated by calculating a global EV (all pairs) and then taking the difference between the global EV and recalculated EV without that pair (Supplementary Fig. 10a). A decrease in EV without that pair indicates a positive contribution of that pair. Since a single cell can participate in several pairs, a contribution per cell was also calculated by averaging the contributions of all pairs in which the cell participated. Cells and cell pairs were then pooled according to the magnitude of their contributions, using percentiles of the distribution of contributions (quartiles or 2.5th percentile of the left and right tails of the distribution). The gain in ripple upmodulation between pre- and post-NREM was calculated for each 10-ms bin by dividing the firing rate in each bin by the baseline firing rate outside SPW-Rs (inter-ripple NREM intervals). The mean peri-ripple gain was then calculated for each cell and averaged across cells. The data are shown for ±2-s windows for clarity. The statistics were performed on pre- and post-NREM ripple mean gains using a one-sided Wilcoxon signed rank test on the mean smoothed (20-ms Gaussian window) gain in a ±250-ms window centered at the ripple peak.
To calculate the reactivation strength R in pre-experience sleep and post-experience sleep epochs, BLA and hippocampal pyramidal cell spike trains were binned (50-ms bins) and z-scored. This gives Zbla and Zhpc, the nPyr × nBinsz-scored spike count matrices for hippocampus and BLA. The hippocampus–BLA correlation matrix Cbla-hpc for the training epoch (whole epoch or safe runs or air puff runs) was calculated as Chpc–bla = ZblaZhpcT/nBins. The similarity between the training correlation matrix (whole run, safe or air puff trajectories) and the correlations at each time point of the pre-sleep and post-sleep epochs (reactivation strength R) was then calculated as R(t) = zbla(t)Cbla–hpczhpc(t)T, where zbla(t) and zhpc(t) are the population firing rate vectors of BLA and hippocampus neurons for the time bin t of either pre-experience sleep and post-experience sleep epoch. The reactivation strength R over time was then z-scored over the whole pre-NREM or post-NREM epochs. The peri-ripple reactivation strength was calculated for SPW-Rs occurring during pre-NREM and SPW-Rs occurring during post-NREM as the average R in a ±2-s window around ripple peaks (Supplementary Fig. 10) Finally, the mean peri-ripple reactivation strength R was computed over all rats and sessions. The significance of the difference in R between pre-experience NREM and post-experience NREM SPW-Rs was calculated on the mean R in a 500-ms window centered on the ripple peak using a Wilcoxon signed rank test. These methods used for evaluation of the reactivation strength were previously described for the reactivation of individual components following an ICA or PCA on the correlation matrix26,53,54. Because we were specifically interested in cross-structure reactivations and these previously used methods could not be directly applied, we used the raw correlation matrix instead of individual or principal components of the training correlation matrix to calculate reactivations strength.
Histology.
At the end of experiments, small electrolytic lesions were made to mark the final position of the probes. Rats were euthanized with pentobarbital and perfused using saline and then 10% paraformaldehyde. The brains were extracted, sliced (70 μm), DAPI-stained and coverslipped. The sequential positions of the electrodes were reconstructed for all shanks from adjacent slices using the final position of the probe and the expected depth of the probe location for each recording day. This allowed the construction of histology maps showing the putative recorded location for each shank and each recording day (Fig. 1f and Supplementary Fig. 2). These maps were then used to restrict the analyses to specific amygdala nuclei.
Data availability.
The data that support the main findings of this study will be publicly available on the CRCNS server (http://crcns.org/).
Code availability.
All custom code is freely available on the Buzsáki Laboratory Github (https://github.com/buzsakilab/papers/tree/master/GGirardeau-BLAHpcInteractions-Package).
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Vazdarjanova, A. & McGaugh, J.L. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J. Neurosci. 19, 6615–6622 (1999).
Paré, D., Collins, D.R. & Pelletier, J.G. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn. Sci. 6, 306–314 (2002).
Maren, S. & Fanselow, M.S. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci. 15, 7548–7564 (1995).
Ikegaya, Y., Saito, H. & Abe, K. Attenuated hippocampal long-term potentiation in basolateral amygdala-lesioned rats. Brain Res. 656, 157–164 (1994).
Ikegaya, Y., Saito, H. & Abe, K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci. Res. 22, 203–207 (1995).
Goshen, I. et al. Dynamics of retrieval strategies for remote memories. Cell 147, 678–689 (2011).
Reijmers, L.G., Perkins, B.L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Redondo, R.L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014).
Hsiang, H.-L.L. et al. Manipulating a “cocaine engram” in mice. J. Neurosci. 34, 14115–14127 (2014).
Xu, C. et al. Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell 167, 961–972 (2016).
Phillips, R.G. & LeDoux, J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285 (1992).
Zelikowsky, M., Hersman, S., Chawla, M.K., Barnes, C.A. & Fanselow, M.S. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J. Neurosci. 34, 8462–8466 (2014).
O'Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Oxford Univ. Press, 1978).
Kjelstrup, K.G. et al. Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. USA 99, 10825–10830 (2002).
Royer, S., Sirota, A., Patel, J. & Buzsáki, G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J. Neurosci. 30, 1777–1787 (2010).
Moser, M.B., Moser, E.I., Forrest, E., Andersen, P. & Morris, R.G. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci. USA 92, 9697–9701 (1995).
Moser, E.I.E., Krobert, K.A., Moser, M.B. & Morris, R.G. Impaired spatial learning after saturation of long-term potentiation. Science 281, 2038–2042 (1998).
Small, S.A. The longitudinal axis of the hippocampal formation: its anatomy, circuitry, and role in cognitive function. Rev. Neurosci. 13, 183–194 (2002).
Kim, J.J. & Fanselow, M.S. Modality-specific retrograde amnesia of fear. Science 256, 675–677 (1992).
Corcoran, K.A., Desmond, T.J., Frey, K.A. & Maren, S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J. Neurosci. 25, 8978–8987 (2005).
Maren, S. & Quirk, G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 5, 844–852 (2004).
Pitkänen, A., Pikkarainen, M., Nurminen, N. & Ylinen, A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. NY Acad. Sci. 911, 369–391 (2000).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008).
Girardeau, G. & Zugaro, M. Hippocampal ripples and memory consolidation. Curr. Opin. Neurobiol. 21, 452–459 (2011).
Wang, D.V. et al. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat. Neurosci. 18, 728–735 (2015).
van de Ven, G.M., Trouche, S., McNamara, C.G., Allen, K. & Dupret, D. Hippocampal offline reactivation consolidates recently formed cell assembly patterns during sharp wave-ripples. Neuron 92, 968–974 (2016).
Popa, D., Duvarci, S., Popescu, A.T., Léna, C. & Paré, D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc. Natl. Acad. Sci. USA 107, 6516–6519 (2010).
Huff, M.L., Miller, R.L., Deisseroth, K., Moorman, D.E. & LaLumiere, R.T. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl. Acad. Sci. USA 110, 3597–3602 (2013).
Patel, J., Schomburg, E.W., Berényi, A., Fujisawa, S. & Buzsáki, G. Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J. Neurosci. 33, 17029–17041 (2013).
Lovett-Barron, M. et al. Dendritic inhibition in the hippocampus supports fear learning. Science 343, 857–863 (2014).
Mizuseki, K., Royer, S., Diba, K. & Buzsáki, G. Activity dynamics and behavioral correlates of CA3 and CA1 hippocampal pyramidal neurons. Hippocampus 22, 1659–1680 (2012).
Kudrimoti, H.S., Barnes, C.A. & McNaughton, B.L. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 19, 4090–4101 (1999).
Lansink, C.S., Goltstein, P.M., Lankelma, J.V., McNaughton, B.L. & Pennartz, C.M.A. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 7, e1000173 (2009).
Hoffman, K.L. & McNaughton, B.L. Coordinated reactivation of distributed memory traces in primate neocortex. Science 297, 2070–2073 (2002).
Wilson, M. & McNaughton, B. Reactivation of hippocampal ensemble memories during sleep. Science (80- ) 5, 14–17 (1994).
Shen, J., Kudrimoti, H.S., McNaughton, B.L. & Barnes, C.A. Reactivation of neuronal ensembles in hippocampal dentate gyrus during sleep after spatial experience. J. Sleep Res. 7 (Suppl. 1), 6–16 (1998).
Genzel, L., Spoormaker, V.I., Konrad, B.N. & Dresler, M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiol. Learn. Mem. 122, 110–121 (2015).
Hutchison, I.C. & Rathore, S. The role of REM sleep theta activity in emotional memory. Front. Psychol. 6, 1439 (2015).
LeDoux, J.E. Coming to terms with fear. Proc. Natl. Acad. Sci. USA 111, 2871–2878 (2014).
Moita, M.A., Rosis, S., Zhou, Y., LeDoux, J.E. & Blair, H.T. Putting fear in its place: remapping of hippocampal place cells during fear conditioning. J. Neurosci. 24, 7015–7023 (2004).
Wang, M.E. et al. Differential roles of the dorsal and ventral hippocampus in predator odor contextual fear conditioning. Hippocampus 23, 451–466 (2013).
Bannerman, D.M. et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci. Biobehav. Rev. 28, 273–283 (2004).
Ciocchi, S., Passecker, J., Malagon-Vina, H., Mikus, N. & Klausberger, T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348, 560–563 (2015).
Buzsáki, G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015).
Logothetis, N.K. et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012).
Sparta, D.R. et al. Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Front. Behav. Neurosci. 8, 129 (2014).
Chrobak, J.J. & Buzsáki, G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J. Neurosci. 16, 3056–3066 (1996).
Boyce, R., Glasgow, S.D., Williams, S. & Adamantidis, A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science (80- ) 23, 812 (2016).
Maquet, P. et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat. Neurosci. 3, 831–836 (2000).
Atherton, L.A., Dupret, D. & Mellor, J.R. Memory trace replay: the shaping of memory consolidation by neuromodulation. Trends Neurosci. 38, 560–570 (2015).
Vandecasteele, M. et al. Large-scale recording of neurons by movable silicon probes in behaving rodents. J. Vis. Exp. 3568, e3568 (2012).
Hazan, L., Zugaro, M. & Buzsáki, G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J. Neurosci. Methods 155, 207–216 (2006).
Peyrache, A., Khamassi, M., Benchenane, K., Wiener, S.I. & Battaglia, F.P. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat. Neurosci. 12, 919–926 (2009).
Lopes-dos-Santos, V., Ribeiro, S. & Tort, A.B.L. Detecting cell assemblies in large neuronal populations. J. Neurosci. Methods 220, 149–166 (2013).
Acknowledgements
We thank J. LeDoux, C. Léna, E. Stark, A. Peyrache and L. Roux for comments and discussions on the analyses and manuscript and all the members of the Buzsáki laboratory for their support. This work was supported by the Fondation pour la Recherche Médicale (FRM), the Fyssen Foundation, a Charles H. Revson Senior Fellowship in Biomedical Science (G.G.), NIH MH54671 and MH107396, NS 090583 and the Simons Foundations (G.B.).
Author information
Authors and Affiliations
Contributions
G.G. and G.B. designed the study, G.G. and I.I. performed the experiments, G.G. analyzed the data and G.B. and G.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Behavioral performance and habituation for each rat.
a. The speed of the animal was measured in the 20-cm segment preceding the airpuff location (current or previous danger zone: DZ) during pre-RUN, RUN and post-RUN. b. Ratios were calculated as indicated. The reversal of ratio between pre and post RUN indicates that all animals learned the new location during RUN and remembered it during post-RUN (one-way repeated measure ANOVAS; Rat1: n=11 sessions, p=0.000214, df=2, F=13.26; Rat2: n=10 sessions, p=0.00556 df=2, F=7.02; Rat3: n=14 sessions, p=3.9469x10-07, df=2, F=27.41; Rat4: n=16 sessions, p=0.021754, df=2, F=4.36; followed by Bonferroni-corrected post-hoc paired t-test *p<0.05, **p<0.01, ***p<0.001; white lines: median, black dotted lines: mean; box: upper and lower quartiles; whiskers: minimum and maximum values). c. Habituation index across days for each animals. The index is calculated as the number of airpuff laps divided by the total time spent on the track x100. Habituated animals run more laps in a given time, yielding to increased habituation index values. Empty dots: training days where the physiological data was not considered for analysis for technical reasons.
Supplementary Figure 2 Histological reconstruction from DAPI-stained brain slices and reconstructed anatomical maps of recording sites for all four animals.
The probe was moved after each session and the depth of the recording sites of each shank (8 shanks with a 140 μm depth span of recording sites) was estimated from the histological reconstructions of the shanks from the lesion-marked final position of the probe (see Online Methods). The maps were reconstructed from consecutive brain slices. Only one slice per side and animal is shown here, without (upper pictures) and with (lower picture) the superimposed reconstruction grid. The group memberships of neurons and LFPs for analyses were defined according to the boundaries of the nuclei (black lines).
Supplementary Figure 3 Cell-type classification.
a. Example excitatory (left) and inhibitory (right) connections detected by cross-correlograms (black line: predicted values; red dotted lines: upper and lower significance thresholds with p=0.001) Significant bins in the monosynaptic time window (0.001-0.004s) are plotted in red and light blue. Autocorrelograms for the reference (black) and target (grey) cells are also shown. b. The putative type of the remaining cells was identified using a kmeans clustering with 2 clusters on the waveform properties of the cells (spike width and peak to trough time), separately for amygdala (BLA, BLV, LaDL, BMA and BMP; n=4 rats), olfactory areas (Pir, VEn and Den; n=4 rats) and hippocampal neurons (n=3 rats). Monosynaptically and kmeans-identified pyramidal cells and interneurons are shown in dark and light pink and blue, respectively.
Supplementary Figure 4 Intra-structure reactivations for hippocampus and BLA.
a. EV and REV calculated using all hippocampus-hippocampus cell pairs (left; NREM: n= 38 sessions, p=7.75x10-05, z=3.95; REM: n= 34 sessions, p=0.965, z=0.04; n=3 rats) or putative pyramidal cells only (right; NREM: n=38 sessions, p=6.45x10-05, z=3.99; REM: n=34 sessions, p=0.499, z=-0.67; 3 rats). b. EV and REV calculated using all BLA-BLA cell pairs (left; NREM: n=34 sessions, p=0.00606, z=2.74; REM: n=28 sessions, p=0.39948, z=0.84; n=4 rats) or putative pyramidal cells only (right; NREM: n=32 sessions, p=0.00157, z=3.16; REM: n=26 sessions, p=0.158, z=1.40; n=4 rats). Some outliers (included in the analysis) are represented above the upper y-axis limit for the clarity of the scale. All boxplots show the median (red line), upper and lower quartiles (box) and maximal and minimal values excluding outliers (whiskers).
Supplementary Figure 5 Details of hippocampus–BLA reactivations.
a. EV and REV calculated using all cell pairs (i.e., including pyramidal cells and putative interneurons) for NREM (n=27 sessions, p=0.00014, z=3.79; n=3 rats) and REM-sleep (n=25 sessions, p=0.47583, z=-0.71; n=3 rats), and reactivation decay over the first hour of NREM (right; n=17 sessions; p=8.85x10-05, z=3.91; p=0.00282, z=2.98; p=0.14458, z=-0.71; Wilcoxon signed rank tests; n=3 rats). b. EV and REV for pairs between hippocampus and ripple-modulated/non-ripple modulated BLA cells. Pyramidal cell pairs only (right; n=18 sessions, p=0.00053, z=3.46;p=0.00377, z=2.89; n=3 rats) and all pairs (including pyramidal and interneuron; left; n=19 sessions, p=0.00034, z=3.58; p=0.00194, z=3.09; Wilcoxon signed rank tests; n=3 rats) are shown separately. Some outliers are displayed above the y-axis limit for clarity. Reactivations were significantly higher for pairs with ripple-modulated BLA cell in both pyr-pyr and all cell conditions (Wilcoxon ranksum test on EV-REV differences; All cells: n=19 sessions, p=0.00662, z=2.71; Pyr-pyr only: n=18 sessions, p=0.00992, z=2.57; Mean EV-REV: all cells, ripple-mod 6.07+/-1.3%; non-ripple mod 1.76+/-0.56%; pyr-pyr ripple-mod 7.16+/-2.19%, non-ripple mod 1.90+/-0.71%; s.e.m; n=3 rats); All boxplots show the median (red line), upper and lower quartiles (box), maximal and minimal values excluding outliers (whiskers).
Supplementary Figure 6 A subset of cell pairs with high run correlations and ripple up-modulated BLA cell partner contribute to the reactivations.
a. Distributions of pre/post nonREM correlation differences shown separately for 9 subgroups of cell pairs based on 1) the type of modulation of the BLA cell during hippocampal ripples (up-, down- or no modulation) and 2) type of correlation during training (RUN; significantly positive, non-significant or significantly negative; see Table S2 for detailed numbers of pairs; n=3 rats, 29 sessions). b. EV and REV calculated on pairs sorted into the same 9 subgroups as shown in a.
Supplementary Figure 7 Highly contributing pairs show correlated changes between pre/post NREM correlation and pre/post run correlation.
a. Replay contributions of each pair were calculated by removing hippocampus-BLA cell pairs (pyramidal-pyramidal pairs) one by one when calculating global EV and REV (left & middle, see Methods). Right, Distribution of EV contribution of all pyramidal cell pairs, divided into four quartiles (red lines indicate division) or separating the highest and lowest 2.5% groups of the distribution (green lines; n=37,660 pairs in 3 rats). b. Changes in pre/post RUN correlation vs changes in pre/post nonREM correlations, shown separately for the four quartiles (1st: r=0.13, p=1.07x10-32; 2nd: r=0.02, p=0.042; 3rd: r=0.005, p=0.61; 4th: r=0.013, p=0.22; n=9415 pairs for each quartile, n=3 rats). c. Changes in pre/post RUN correlation vs changes in pre/post-nonREM correlations for the 2.5% highest and lowest contributing cell pairs (red/blue – same as in Fig. 7; high contribution: n=866 pairs, low contributions: n=845 pairs; n=3 rats), and the remaining 95% (grey; r=0.021, p=0.00027).
Supplementary Figure 8 Highly contributing pairs show correlated changes between pre/post NREM correlation and pre/post run correlation.
a. Average gain of up-modulated cells during pre-NREM hippocampal SPW-Rs (blue) and post-NREM hippocampal SPW-Rs (red), shown separately for the four contribution quartiles (Q). The significance of the pre/post gain difference was calculated on the avergage gain in a 500ms window centered on the ripple peak (Wilcoxon signed rank one-tailed tests; Q1: n=63 cells, p=4.5663x10-5;z=-3.9125 Q2, n=18 cells, p=0.7834 z=0.7839; Q3, n=16 cells, p=0.9704 z=1.8873; Q4,n=39 cells, p=0.9384, z=1.5420; n=3 rats) b. Normalized gain for all up-modulated BLA cells of the 3 lower contribution quartiles during pre-NREM and post-NREM SPW-Rs. Compare these plots with the those of the highest contribution quartile shown in Figure 4c.
Supplementary Figure 9 Reactivation measures are independent of firing rate changes.
a. EV/REV for eight firing rate groups (octiles) averaged across the individual rates of the hippocampus-BLA neuron pairs. The distribution of pairwise averaged firing rates is shown on the right (red lines: octile boundaries) b. EV/REV per octiles of firing rates of BLA cells. c. EV/REV per firing rate octiles of hippocampal cells. The distribution of hippocampal putative pyramidal cells firing rates is shown on the right (n=37,660 pairs in 3 rats for a, b and c).
Supplementary Figure 10 Reactivation strength: methods summary.
A template correlation matrix is calculated for all hippocampus-BLA pairs using the binned and z-scored spike trains during training (RUN). The reactivation during each time bin of the "match" epoch, which can be pre-NREM or post-NREM, is calculated as the match between the global correlations during RUN (Crun) and the BLA-HPC correlation a time t. This gives a measure of reactivation strength over time, i.e. the match epoch variance explained by the RUN correlation. The peri-ripple reactivation strength in a +/- 2s window around ripple peaks is computed for pre-NREM (blue) and post-NREM (red) of each session.
Supplementary Figure 11 Reactivations of the air puff trajectory peak during hippocampal ripples.
a. Mean z-scored peri-ripple reactivation strength of the whole training session (including safe runs, airpuff runs and reward platforms) over animals and sessions for pre-NREM (blue) and post-NREM hippocampal ripples (red; mean±sem). The analysis was first restricted to pyramidal cell pairs (left, n=25 sessions in 3 rats) and then confirmed including all cell types (right, n=27 sessions in 3 rats). Barplots: reactivation strength in a 500ms window around ripple peaks (grey bar in a) was significantly higher in post-NREM compared to pre-NREM for pyramidal cell pairs (left; n=25 sessions in 3 rats, p=7.49x10-4, z=-3.175) and all cell pairs (n=27 sessions in 3 rats, p=0.00189, z=-2.895, Wilcoxon one-tailed signed rank tests). Lines from pre-NREM to post-NREM indicate single sessions. b. Mean z-scored peri-ripple reactivation strength over animals and sessions (n=27 sessions, 3 rats) for pre-NREM (blue) and post-NREM hippocampal ripples (red, ±sem) for all cell type pairs (i.e. including interneurons) for safe vs airpuff trajectories. Barplots: Reactivation strength in a 500ms window around ripple peaks (grey bar in a) was significantly higher in post-NREM compared to pre-NREM for airpuff (n=27 sessions in 3 rats, p=4.38x10-4, z=-3.327) and safe trajectories (n=27 sessions in 3 rats, p=0.036, z=-1.789; Wilcoxon one-tail signed rank tests). Lines from pre-NREM to post-NREM indicate single sessions. c. Pre/Post peak differences (postNREM - preNREM; see methods) in reactivation strength are not significantly different for safe and airpuff trajectories when all cell types are included (n=27 sessions in 3 rats, p=0.0912, z=-1.333; Wilcoxon one-tailed signed rank test). d. SPW-R occurrence rate was not different between pre-NREM and post-NREM. (Wilcoxon signed rank test, n=41 sessions in 3 rats, p=0.1972, z=-1.289). All boxplots show the median (red line), upper and lower quartiles (box), maximal and minimal values excluding outliers (whiskers).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1 and 2. (PDF 6987 kb)
Rights and permissions
About this article
Cite this article
Girardeau, G., Inema, I. & Buzsáki, G. Reactivations of emotional memory in the hippocampus–amygdala system during sleep. Nat Neurosci 20, 1634–1642 (2017). https://doi.org/10.1038/nn.4637
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4637
This article is cited by
-
Endogenous cannabinoids in the piriform cortex tune olfactory perception
Nature Communications (2024)
-
Awake ripples enhance emotional memory encoding in the human brain
Nature Communications (2024)
-
Hippocampal sharp wave ripples underlie stress susceptibility in male mice
Nature Communications (2023)
-
Closed-loop brain stimulation augments fear extinction in male rats
Nature Communications (2023)
-
CA3 hippocampal synaptic plasticity supports ripple physiology during memory consolidation
Nature Communications (2023)