Abstract
Synucleinopathies, such as Parkinson's disease and dementia with Lewy bodies, are neurodegenerative disorders that are characterized by the accumulation of α-synuclein (aSyn) in intracellular inclusions known as Lewy bodies. Prefibrillar soluble aSyn oligomers, rather than larger inclusions, are currently considered to be crucial species underlying synaptic dysfunction. We identified the cellular prion protein (PrPC) as a key mediator in aSyn-induced synaptic impairment. The aSyn-associated impairment of long-term potentiation was blocked in Prnp null mice and rescued following PrPC blockade. We found that extracellular aSyn oligomers formed a complex with PrPC that induced the phosphorylation of Fyn kinase via metabotropic glutamate receptors 5 (mGluR5). aSyn engagement of PrPC and Fyn activated NMDA receptor (NMDAR) and altered calcium homeostasis. Blockade of mGluR5-evoked phosphorylation of NMDAR in aSyn transgenic mice rescued synaptic and cognitive deficits, supporting the hypothesis that a receptor-mediated mechanism, independent of pore formation and membrane leakage, is sufficient to trigger early synaptic damage induced by extracellular aSyn.
Similar content being viewed by others
Main
The abnormal accumulation of aggregated aSyn in Lewy bodies (LBs) is a common neuropathological hallmark of synucleinopathies such as Parkinson's disease (PD) and dementia with Lewy bodies (DLB)1,2. LBs can be detected throughout the brain and are frequently observed in the hippocampus and related brain regions. Synucleinopathies are also characterized by progressive neuronal dysfunction and, eventually, the death of affected neuronal populations. In addition to the characteristic motor symptoms of PD, cognitive disturbances also represent an important clinical feature of the disease and occur not only in advanced stages of the disease, but also in early and even pre-motor phases1,2,3,4.
Recent studies have suggested that aSyn oligomers are the most toxic species and that they can be released from neuronal cells, contributing to the major pathological features of synucleinopathies2. In fact, extracellular aSyn oligomers, but not monomers or fibers, impair hippocampal long-term potentiation (LTP)—the molecular mechanism involved in learning and memory5,6—through the activation of NMDARs. This synaptic dysfunction precedes neuronal death or any substantial changes in resting membrane potential or in input resistance values5,6.
Recently, PrPC was reported to act as a receptor for neurotoxic amyloid-beta (Aβ) oligomers, which share structural and functional similarities with aSyn oligomers7. PrPC was also suggested to mediate synaptic dysfunction, memory deficits and neurodegeneration of several β-sheet-rich conformers8,9, acting via NMDAR10. Furthermore, we have previously demonstrated that aSyn oligomers can form complexes with NMDAR at the postsynaptic density6, where PrPC is known to be present10.
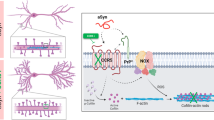
We found that aSyn interacted physically with PrPC to mediate Ca2+ dysregulation and synaptic dysfunction through a mechanism involving Fyn phosphorylation and consequent NMDAR subunit 2B (NMDAR2B) activation, via mGluR5. Moreover, we found that synaptic and cognitive deficits associated with aSyn overexpression in a transgenic mouse model of synucleinopathy were reverted following blockade of mGluR5-evoked phosphorylation of Src kinases and of the toxic activation of NMDAR2B. Overall, our findings shed light into the early pathophysiological mechanisms preceding aSyn-mediated neurodegeneration and implicate PrPC as a molecular target in synucleinopathies.
Results
αSyn oligomers impair LTP through a PrPC-dependent mechanism
We previously demonstrated that extracellular aSyn oligomers impair LTP in rodent hippocampal slices via a mechanism that is dependent on NMDAR5,6. PrPC is known to be involved in NMDAR signaling, and we hypothesized that PrPC could mediate the detrimental effects of aSyn oligomers on synaptic plasticity. For this, we compared synaptic function of hippocampal dorsal slices from wild-type (WT) versus Prnp null mice (Prnp−/−) in the presence of extracellular aSyn oligomers (characterized by atomic force microscopy5,6 and SDS-PAGE; Supplementary Fig. 1a), as previously described5,6. Synaptic function was assessed by electrophysiological recordings of field excitatory postsynaptic potentials (fEPSPs) in the Schaffer collaterals and CA1 pyramid glutamatergic synapses (Fig. 1a). As we previously reported5,6, soluble aSyn oligomers significantly decreased the LTP magnitude in hippocampal slices of WT animals (aSyn olig, 500 nM, 90 min; P < 0.001; Fig. 1a and Supplementary Fig. 1c,d), whereas neither aSyn monomers nor fibrils affected LTP magnitude (P > 0.05; Supplementary Fig. 1b,c). Notably, in slices from Prnp−/− animals, which displayed normal LTP when compared with WT animals (P > 0.05; Fig. 1a,b and Supplementary Fig. 1d), treatment with aSyn oligomers did not affect LTP (P > 0.05; Fig. 1b and Supplementary Fig. 1d).
(a) Left, schematic representation of the simplified circuitry of the hippocampus. DG, dentate gyrus; MF, mossy fibers; SC, Schaffer collaterals; PF, perforant pathway; CA3, cornu ammonis 3; CA1, cornu ammonis 1. A recording electrode and two independent stimulation pathways (S0 and S1), allowing two protocols in the same slice, were placed in the CA1 dendritic area. A representative fEPSP is shown. 1, stimulus artifact; 2, fiber volley; 3, fEPSP slope. Bottom left, schematic representation of hippocampal slices incubation protocol. Right, changes in fEPSP slope induced by theta-burst stimulation recorded from WT mice hippocampal slices pre-incubated with extracellular aSyn oligomers (aSyn olig, 90 min, 500 nM, n = 10) or in control conditions (CTR, n = 7) (means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni's multiple comparison test). Top right, representative traces prior to (1) and after (2) LTP induction. (b) Changes in fEPSP slope were recorded from Prnp−/−slices pre-incubated with extracellular aSyn oligomers (aSyn olig, 90 min, 500 nM, n = 6) or in control conditions (CTR, n = 6) (means ± s.e.m., P > 0.05, one-way ANOVA followed by a Bonferroni's multiple comparison test) obtained as described in a. (c) Left, changes in fEPSP slope were recorded as described in a from hippocampal slices pre-incubated with aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 6), in the presence of the 6D11 antibody against PrPC (6D11, 110 min, 100 nM, n = 4) together with the aSyn oligomers or in control conditions (CTR, n = 4). Right, plot of the LTP magnitude represented in the left panel plus the LTP magnitude of WT hippocampal slices in the presence of immunoglobulin G (IgG, 110 min, 100 nM, n = 4) or the 8B4 (110 min, 10 μM, n = 4) or C-20 (110 min, 10 μM, n = 4) antibodies together with aSyn oligomers (change in fEPSP slope at 50–60 min after theta-burst stimulation, compared to baseline) (means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni's multiple comparison test). Top, schematic representation of hippocampal slices incubation protocol and representative traces prior to (1) and after (2) LTP induction. (d) Schematic diagram of PrP representing the binding sites of 8B4, 6D11 and C-20 antibodies. For complete statistical details, see Supplementary Table 1.
To evaluate whether PrPC was also involved in baseline synaptic efficiency, we recorded input/output (I/O) curves in slices from WT or Prnp−/− animals in the presence or absence of aSyn oligomers. WT slices pre-incubated with aSyn oligomers displayed max slope values higher than control slices (P < 0.001; Supplementary Fig. 1e). This increase in basal excitability was not observed in Prnp−/− slices in the presence of aSyn oligomers, which displayed fEPSP slope values that were similar to those in control slices when stimulated with the same intensity (P > 0.05; Supplementary Fig. 1f). Thus, we concluded that PrPC is essential for the aSyn-oligomer-mediated inhibition of hippocampal LTP.
PrPC 93–109 amino acid region is required for aSyn-oligomer-mediated inhibition of LTP
The absence of sensitivity to aSyn in Prnp−/− slices regarding basal excitability and LTP suggests that PrPC may act as a key mediator for aSyn synaptic toxicity. To investigate the region(s) of PrPC that mediate the aSyn effects, we targeted three regions in the protein using different antibodies against PrPC: 6D11 (epitope targeting the region 93–109 of PrPC; 100 nM), 8B4 (epitope targeting the N terminus of PrPC; 10 μg) and C-20 (epitope targeting the C terminus of PrPC; 10 μg) (Fig. 1d). In slices pre-treated with 6D11 antibody, the effect of aSyn oligomers on LTP was blocked (P < 0.001; Fig. 1c). In contrast, pre-treatment with 8B4 or C-20 had no effect (P > 0.05; Fig. 1c and Supplementary Fig. 1h). This suggests that the 93–109 segment of PrPC is the crucial region for aSyn-induced toxic effects. Accordingly, the 6D11 antibody also prevented the effect of aSyn oligomers on the I/O curve (P > 0.05; Supplementary Fig. 1g). Presynaptic short-term plasticity was not altered by aSyn oligomers or by the 6D11 antibody under these conditions, as evaluated by paired-pulse facilitation (PPF) (P > 0.05; Supplementary Fig. 1i). We also confirmed that 6D11 alone did not affect LTP, nor did IgG modify the aSyn-mediated reduction of LTP (Fig. 1c). Thus, PrPC deletion or blockade at the 93–109 segment prevents aSyn-oligomer-induced impairments in both LTP and basal synaptic transmission, suggesting that PrPC mediates this synaptic dysfunction in a 6D11-sensitive manner.
PrPC modulates aSyn-mediated synaptic impairment through the activation of Src Tyr-kinases and NMDAR2B
The function of PrPC relates to the modulation of phosphorylation cascades, particularly the one governed by Fyn9, a member of the Src tyrosine kinase family (SFK) that is highly expressed in neurons11. Moreover, both Fyn and PrPC localize in lipid rafts, and clustering of PrPC activates Fyn in cell lines12. Fyn has also been reported to colocalize with PrPC at the postsynaptic density (PSD), where it is critical for cognition and LTP, as it mediates NMDAR phosphorylation and, consequently, excitotoxicity13,14. As such, the tyrosine kinase Fyn is a candidate mediator of signal transduction from an aSyn/PrPC interaction. We assessed the involvement of Fyn in aSyn-associated synaptic deficits. For this, we treated rat hippocampal slices with aSyn oligomers alone or in the presence of a selective SFK inhibitor, 1-Naphtlyl PP1 (PP1, 30 μM, 110 min; Fig. 2a), and induced LTP as before. As expected, the LTP magnitude was significantly reduced in slices pre-incubated with aSyn oligomers (aSyn olig, 500 nM, 90 min; P < 0.01; Fig. 2a and Supplementary Fig. 2a). When Fyn was blocked by PP1, the LTP magnitude was restored to control values (P < 0.01; Fig. 2a and Supplementary Fig. 2a). PP1 alone did not affect the LTP magnitude (P > 0.05; Supplementary Fig. 2a). The aSyn-induced shift in I/O curve was also lost when Fyn activation was inhibited by PP1 (Supplementary Fig. 2b).
(a) Top, schematic representation of hippocampal slices incubation protocol. Bottom, representative traces prior to (1) and after (2) LTP induction and changes in fEPSP slope in hippocampal slices from WT rat in control conditions (CTR, n = 4), and pre-incubated with aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 6) or in the presence of the Src-family inhibitor 1-naphthyl PP1 (PP1, 110 min, 30 μM; PP1 + aSyn olig, n = 3) (means ± s.e.m., P < 0.01, one-way ANOVA followed by a Bonferroni's multiple comparison test). (b) Effect of NMDAR antagonist AP5 (50 μM) perfusion on basal fEPSP slope from control WT hippocampal slices in the same conditions as described in a (means ± s.e.m., P < 0.01, one-way ANOVA followed by a Bonferroni's multiple comparison test). (c) Effect of NMDAR antagonist AP5 (50 μM, 30 min) perfusion on basal fEPSP slope in Prnp−/− hippocampal slices in control conditions or in the presence of aSyn oligomers (P > 0.05). (d) Schematic representation of primary neuronal cultures incubation protocol. (e) Representative immunoblots and quantitation of the phospho-Src levels, normalized to Fyn immunoreactivity, in WT and Prnp−/− primary neuronal cultures in control conditions (CTR, n = 6), and treated with aSyn oligomers alone (aSyn olig, n = 5) or in the presence of the 6D11, C-20 and 8B4 antibodies against PrP (n = 3–4) or the selective mGluR5 antagonist (MPEP, n = 4) (means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni's multiple comparison test). (f) Representative immunoblots and quantitation of the phospho-NMDAR2B levels, normalized to NMDAR immunoreactivity, in the same conditions as described in e plus in the presence of the Fyn antagonist 1-naphthyl-PP1 (PP1, n = 3) (means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni's multiple comparison test). (g) Representative western blot of three independent experiments showing immunoprecipitation of PSD-95 in WT hippocampal control slices (CTR) and slices pre-incubated with aSyn oligomers (aSyn olig, 500 nM, 90 min). Membranes were immunoblotted with anti-NMDAR2B, anti-Fyn, anti-PrP and anti-PSD-95 antibodies. (h) Representative western blot of three independent experiments showing immunoprecipitation of aSyn and PrP, in aSyn Tg, Prnp−/− and WT hippocampal slices in control conditions (CTR) and pre-incubated with aSyn oligomers (aSyn olig, 500 nM, 90 min). Membranes were immunoblotted with anti-NMDAR2B, anti-Fyn, anti-PrP, anti-aSyn and anti-α-tubulin antibodies. IgG was used as a negative control (Neg CTR). (i) Immunohistochemistry of WT and Prnp−/− primary neuronal cultures (representative image of n = 3). aSyn is labeled in green, PrPC is labeled in red, and cell nuclei are stained with Hoechst in blue (scale bars represent 15 μm). Bottom, a 63× magnification image (scale bars represent 5 μm). Colocalization is indicated by arrows and was assessed by Mander's overlap coefficient method using ZEN Software (Zeiss). PrPC-bound aSyn was detected in WT neuronal cultures pre-treated with aSyn oligomers for 5 min (WT aSyn oligomers: Mander's overlap coefficient = 0.70), compared with control conditions, where no colocalization was observed (WT CTR: Mander's overlap coefficient = 0). No surface binding of aSyn oligomers was detected in Prnp−/− cultures (Prnp−/− aSyn oligomers: Mander's overlap coefficient = 0). Uncropped gels and blots with molecular weight standards are provided in the Supplementary Figure 7 and full statistical details in Supplementary Table 1.
Next, we hypothesized that PrPC/Fyn activation converged to activate NMDARs15. To test this, we evaluated the effect of the NMDAR antagonist DL-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) on basal synaptic transmission. AP5 did not modify the fEPSP slope in control WT slices (P > 0.05; Fig. 2b and Supplementary Fig. 2c). In contrast, the acute application of AP5 induced a progressive reduction of the fEPSP in aSyn-oligomer-treated WT slices (P < 0.001; Fig. 2b and Supplementary Fig. 2c), indicating that aSyn caused a basal activation of NMDARs5,6. Inhibition of Fyn by PP1 prevented this effect (P < 0.01; Fig. 2b and Supplementary Fig. 2c). This loss of aSyn effect was also observed in slices from Prnp−/− mice compared with their WT littermates (Fig. 2c and Supplementary Fig. 2d,e). Previously, we showed that exposure to aSyn is associated with an increase in NMDAR2B levels6. Here we found that, in Prnp−/− mice, aSyn oligomers lost the ability to alter NMDAR2B levels, whereas the levels of NMDAR subunit 1 (NMDAR1) were not altered by aSyn in WT or Prnp−/− mice (Supplementary Fig. 2f).
We then evaluated Fyn activation by quantifying Src phosphorylation levels. We exposed primary neuronal cultures (12 d in vitro) to aSyn oligomers for different time periods. At this stage, neurons were fully mature, as confirmed by morphological analysis and MAP2 staining (Supplementary Fig. 3a). We detected a maximal level of phospho-Src after a 5-min exposure, with no alterations in Fyn (Supplementary Fig. 3c,d). This was coincident with a significant increase in aSyn levels (P < 0.01; Supplementary Fig. 3b). We asked whether PrPC was required for the observed aSyn-induced Src activation, but we could not detect any effect of aSyn on Src activation in neuronal cultures from Prnp−/− mice (P > 0.05; Fig. 2e). In addition, we found that the 6D11 antibody prevented Src activation by aSyn (P < 0.001; Fig. 2d,e), whereas neither the 8B4 nor C-20 antibodies prevented Src phosphorylation. These effects on Src activation were not a result of alterations in PrPC levels, as similar levels of PrPC were detectable after aSyn exposure for different time periods (Supplementary Fig. 3e).
NMDAR has a key role in synaptic plasticity and in aSyn-induced LTP impairment5. Intracellular segments of the NMDAR2A and NMDAR2B subunits are phosphorylated on tyrosine residues by SFK16. Of these, Y1472 of NMDAR2B is a major phosphorylation site of Fyn kinase15. We examined total and phosphorylated levels of Y1472 NMDAR2B in neuronal cultures exposed to aSyn oligomers for different time periods. After 5 min of exposure, an increase in the levels of phospho-NMDAR2B was already detected, with no changes in the total levels of NMDAR2B (Supplementary Fig. 3f,g). This increase was blocked by the 6D11 antibody and the Fyn inhibitor (PP1, 30 μM, 25 min; P < 0.05; Fig. 2f), but was not affected by the 8B4 or C-20 antibodies. Consistent with this, aSyn oligomers failed to induce further NMDAR2B phosphorylation in neuronal cultures from Prnp−/− mice (Fig. 2f). Thus, aSyn requires PrPC, particularly the 93–109 region, to induce Fyn activation and subsequent NMDAR2B phosphorylation, which may underlie the LTP impairments observed in the presence of aSyn oligomers.
aSyn physically interacts with PrPC to form a complex with NMDAR2B and Fyn kinase at the postsynaptic membrane
Our previous studies demonstrated a postsynaptic action of aSyn oligomers in hippocampal synapses, where aSyn forms a complex with NMDAR2B6. Proteomic analyses have revealed that PrPC is a component of the PSD17. Consistently, PrPC co-fractionates with PSD-95 and Fyn kinase, and is involved in Fyn activation in cell lines and in animal models12.
To test whether aSyn interacts with PrPC, we performed co-immunoprecipitation of PSD-95 after teatment with aSyn oligomers. This qualitative approach suggested an augmentation in the levels of the PrPC/Fyn/NMDAR2B complex (Fig. 2g). Next, by pulling down PrPC, we detected Fyn, NMDAR2B and aSyn in the same complex in WT, but not in Prnp−/−, slices in both control and aSyn-exposed slices (Fig. 2h). This was further validated by reverse co-IP, in which we pulled down aSyn and detected NMDAR2B, Fyn and PrPC (Fig. 2h). As expected, a non-interacting protein, α-tubulin, was only detected in the pre-IP lysates, with no detectable signal in either aSyn or PrPC IPs, further confirming the specificity of the aSyn-PrPC association. In all of these conditions, co-IP with IgG (negative control) did not yield any bands (Fig. 2h). In addition, we detected PrPC-bound aSyn in WT neuronal cultures pre-treated with aSyn oligomers (Fig. 2i). In control conditions, no colocalization was observed. Moreover, we did not detect any surface binding of aSyn oligomers in Prnp−/− cultures (Fig. 2i).
To confirm the aSyn-PrPC interaction in vivo, we used transgenic mice expressing human WT aSyn under the control of the Thy1 promoter (Thy1-aSyn mice), which display substantial levels of aSyn in the hippocampus before nigrostriatal modifications18,19. When we immunoprecipitated either PrPC or aSyn from the hippocampus of aSyn transgenic mice, we detected the counterpart of the aSyn-PrPC complex (Fig. 2h). Moreover, we found that the PrPC levels followed the increase in aSyn in the hippocampus (Supplementary Fig. 5a,e), as reported previously for Aβ oligomers20. Taken together, our data confirm an association of aSyn with PrPC and the formation of a PrPC-Fyn-NMDAR2B protein complex at the PSD.
aSyn oligomers impair calcium homeostasis through a PrPC-dependent mechanism
Phosphorylation of NMDAR2B mediates alterations in NMDAR-induced calcium levels. To investigate the involvement of Ca2+ signaling disruption as a consequence of aSyn-oligomer-mediated NMDAR hyperactivation, we measured variations in intracellular calcium concentrations ([Ca2+]i) in primary neuronal cultures. We detected changes in [Ca2+]i by Ca2+ imaging using fura 2-acetoxymethyl ester (Fura 2AM) (Fig. 3a). Application of oligomeric forms of aSyn rapidly elevated intracellular Ca2+ levels (Supplementary Video 1), whereas equivalent amounts of aSyn monomers evoked no detectable changes in fluorescence (P < 0.001; Fig. 3b,d). This aSyn-oligomer-evoked increase in [Ca2+]i was prevented by the 6D11 antibody (P < 0.001; Fig. 3c,d), but not by either the C-20 or 8B4 antibodies (Fig. 3c,d). None of the antibodies alone affected calcium levels (Fig. 3c,d). When the specific NMDAR2B subunit was blocked by ifenprodil (3 μM), the effect of aSyn oligomers on [Ca2+]i was also suppressed (P < 0.001; Fig. 3b,d). Notably, in primary neuronal cultures of Prnp−/− mice, aSyn oligomers failed to induce any increase in intracellular Ca2+ levels (Fig. 3e and Supplementary Video 2), indicating that the aSyn-mediated calcium deregulation depends on PrPC/NMDAR2B-mediated signaling.
(a) Representative images of Ca2+ imaging. Bright regions indicate the location of cytoplasm and organelles, where the concentration of Ca2+ is higher than in the dark regions, indicating the intercellular medium, where diffusion processes take place. Right image corresponds to the ratio between the radiation emitted at 510 nm, when cells are excited at 340 nm, over emission following excitation at 380 nm (F340/F380). (b,c) Graphs showing a 55-min time course of Ca2+-dependent fluorescence recorded and averaged from FURA-2AM WT neurons in response to aSyn monomers (aSyn mon, 20 min, 500 nM), oligomers (aSyn olig, 20 min, 500 nM) and oligomers in the presence of selective NMDAR2B antagonist, Ifenprodil (40 min, 3 μM) or in the presence of the 6D11 (40 min, 100 nM), 8B4 (40 min, 10 μM) or C-20 (40 min, 10 μM) antibodies against PrP. (d) WT representative images of the different conditions shown in b and c. (e) Prnp−/− representative images and graphs showing a 35-min time course of Ca2+-dependent fluorescence recorded and averaged from FURA-2AM Prnp−/− neurons before and after exposure to aSyn oligomers (20 min, 500 nM). Cells were challenged with ionomycin (15 min, 2 μM) at the conclusion of each experiment. Each point represents the means ± s.e.m. of 340/380-nm readings of 20–25 responsive cells per experimental condition from three independent cultures. P < 0.001, one-way ANOVA followed by a Bonferroni's multiple comparison test. For complete statistical details, see Supplementary Table 1.
mGluR5 mediates aSyn/PrPC synaptic dysfunction
Although PrPC and SFK are enriched in the PSD10,17, the connection of aSyn/PrPC to SFK cannot be direct, as PrPC is anchored via glycolipid to the plasma membrane, whereas SFK kinases are cytoplasmic16. Previous studies have identified mGluR5 as a mediator of Fyn activation promoted by PrPC (ref. 11). We hypothesized that mGluR5, a PSD transmembrane protein21,22, could mediate aSyn/PrPC signaling, leading to impaired neuronal function. We treated primary cultures with the selective mGluR5 antagonist MPEP (5 μM) and then exposed them to aSyn oligomers for 5 min. Blockade of mGluR5 prevented both Fyn and NMDAR2B activation, as measured by Src and NMDAR2B phosphorylation levels. To investigate the functional role of this mGluR5-mediated phosphorylation of NR2B subunit, we treated rat hippocampal slices with aSyn oligomers in the presence of MPEP (5 μM, 110 min; Fig. 4a) and induced LTP in Schaffer collaterals/CA1 pyramid glutamatergic synapses by theta-burst stimulation. Notably, mGluR5 blockade by MPEP prevented LTP impairment induced by aSyn oligomers alone (P < 0.001; Fig. 4b and Supplementary Fig. 4a). MPEP alone did not affect the LTP magnitude (P > 0.05; Supplementary Fig. 4a). Furthermore, when mGluR5 was activated by the selective agonist DHPG (10 μM, 110 min; Fig. 4c), the protective effect of the 6D11 antibody against aSyn-induced LTP impairment was no longer detectable (P < 0.001; Fig. 4d and Supplementary Fig. 4c). DHPG alone did not change the effects of aSyn on LTP (P > 0.05; Supplementary Fig. 4c). Taken together, these data suggest that mGluR5 acts downstream of PrPC and probably serves as a bridge between PrPC and SFK/NMDAR2B to impair synaptic function.
(a) Schematic representation of the hippocampal slices incubation protocol used in b. (b) Changes in fEPSP slope induced by theta-burst stimulation recorded from WT hippocampal slices in control conditions (CTR, n = 4), pre-incubated with extracellular aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 6) or in the presence of the mGluR5-selective antagonist MPEP (110 min, 5 μM; MPEP + aSyn olig, n = 4) (means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni's multiple comparison test). Left, representative traces prior to (1) and after (2) LTP induction. (c) Schematic representation of hippocampal slices incubation protocol used in d. (d) Changes in fEPSP slope induced by theta-burst stimulation recorded from WT hippocampal slices in control conditions (CTR, n = 4), pre-incubated with extracellular aSyn oligomers together with the 6D11 antibody (110 min, 100 nM; 6D11 + aSyn olig, n = 4) and in the presence of the mGluR5-selective agonist DHPG (110 min, 10 μM; 6D11 + DHPG + aSyn olig, n = 4) (means ± s.e.m.,P < 0.001, one-way ANOVA followed by a Bonferroni's multiple comparison test). Left, representative traces prior to (1) and after (2) LTP induction. For complete statistical details, see Supplementary Table 1.
Blockade of mGluR5-evoked tyrosine phosphorylation of NMDAR2B reverses memory deficits in a transgenic mouse model of PD
Phosphorylation of the NMDAR2B-Y1472 residue by Fyn kinase is tightly regulated by mGluR5 via adenosine A2A receptors (A2ARs). Blockade of A2ARs effectively inhibits NMDAR2B phosphorylation evoked by mGluR5 (ref. 23) and prevents LTP impairment and NMDAR overactivation mediated by aSyn in vitro6. Furthermore, blocking A2ARs (SCH-58261, 50 nM) in hippocampal slices protected against aSyn-mediated LTP impairments (Supplementary Fig. 4b), and this protective effect was lost if mGluR5 was activated, which is consistent with there being a common signaling pathway for the effects observed (P > 0.05; Supplementary Fig. 4b,c).
To provide in vivo evidence for this mechanism, we assessed the effect of the A2AR blocker KW-6002 (refs. 24, 25, 26), which is particularly suited for chronic administration because of its bioavailability and brain penetration27, to rescue memory and synaptic impairments in Thy1-aSyn mice. These animals express human aSyn in the whole hippocampus (Supplementary Fig. 5a), with an enrichment in cell bodies and axons of pyramidal neurons, whereas mouse aSyn mainly colocalizes with the pre-synaptic marker SNAP25 in WT mice (Supplementary Fig. 5b). We treated 5-month-old Thy1-aSyn mice and their WT littermates for 1 one month with KW-6002, delivered in the drinking water, at a dose known to be effective in vivo (3 mg per kg per d; Fig. 5a)24,25.
(a) Schematic representation of the groups of animals used and the corresponding oral pharmacological treatment (vehicle or KW-6002, istradefylline). (b) Spatial memory performance was assessed by the Y-maze test. Schematic representation of the Y-maze test (top). Representative traces (left) and quantification of the time spent in novel arm (N) versus the other arm (O) in the different groups of animals represented in a (n = 8–12; means ± s.e.m., P < 0.05, two-way ANOVA followed by a Tukey post hoc comparison test). (c) Quantification of the number of transitions between arms observed in each group (n = 8–12; means ± s.e.m., P > 0.05, two-way ANOVA followed by a Tukey post hoc comparison test). (d) Quantification of the average swimming speed during MWM probe test (n = 6–11; means ± s.e.m., P > 0.05, two-way ANOVA followed by a Tukey post hoc comparison test). (e,f) Hippocampal-dependent memory performance was assessed by the MWM test, in which acquisition (e) and retention (f) were evaluated (n = 6–11; means ± s.e.m., P < 0.05, two-way ANOVA followed by a Tukey post hoc comparison test). (g) Representative immunoblots and quantification of aSyn levels in the hippocampus of WT and aSyn Tg mice treated with vehicle (n = 5 and 4) or KW-6002 (n = 5 and 7) (means ± s.e.m., P < 0.001, two-way ANOVA followed by a Tukey post hoc comparison test). α-tubulin was used as a loading control. Uncropped gels and blots with molecular weight standards are provided in the Supplementary Figure 7 and full statistical details in Supplementary Table 1.
Hippocampal-dependent memory was assessed by the Morris water maze (MWM) test, in which memory acquisition and retention were evaluated. Chronic blockade of A2ARs completely reverted the learning and memory deficits induced by aSyn overexpression. During the acquisition phase, treatment of Thy1-aSyn animals with KW-6002 restored learning (P < 0.001; Fig. 5e), when compared with animals treated with vehicle, which exhibited a slower learning performance at finding the hidden platform, showing deficits at days 3, 4 and 5 (P < 0.001; Fig. 5e). Furthermore, KW-6002 treatment reestablished the retention ability of Thy1-aSyn mice, as observed in the probe test, measured by the time spent in the target quadrant compared with the other quadrants (Fig. 5f). No changes were observed in the overall swimming speed among groups (Fig. 5d).
Short-term reference memory was assessed in a spontaneous novelty-based spatial preference Y-maze test. Thy1-aSyn mice performed worse than WT mice, revealing no preference for the novel arm (P < 0.05; Fig. 5b). Notably, KW-6002 restored memory impairments in Thy1-aSyn animals, as observed by the increased time spent in the novel arm (P < 0.05; Fig. 5b). No changes were observed in the number of transitions between arms (P > 0.05; Fig. 5c), discarding significant motor impairments as described, given that Thy1-aSyn mice exhibit mostly cognitive and memory deficits at this early stage, before nigrostriatal pathology18. This correlated with the absence of substantial dopaminergic neuronal loss, as evaluated by tyrosine hydroxylase levels (Supplementary Fig. 5c,d). Furthermore, we did not detect any influence of KW-6002 treatment on the levels of aSyn in the hippocampus (P > 0.05; Fig. 5g).
Treatment with KW-6002 also rescued LTP deficits induced by aSyn overexpression, without affecting LTP magnitude in WT animals (P < 0.05; Fig. 6a,b). Moreover, the treatment also rescued the I/O alterations observed in Thy1-aSyn mice, without changing the I/O curve in WT mice (P < 0.001; Fig. 6c). No changes were observed in the magnitude of long-term depression (LTD) or in PPF across genotypes or treatment (P > 0.05; Fig. 6d–f).
(a) Changes in the fEPSP slope following LTP induced by theta-burst stimulation from hippocampal slices. (b) LTP magnitude after theta-burst stimulation (change in fEPSP slope at 50–60 min) (n = 4–5; means ± s.e.m., P < 0.05, two-way ANOVA followed by a Tukey post hoc comparison test). (c) I/O curves corresponding to fEPSP slope evoked by different stimulation intensities (60–300 μA) (n = 3–5; means ± s.e.m., P < 0.001, F test). (d) Changes in fEPSP slope following LTD induction obtained for WT and Thy1-aSyn (aSyn Tg) mice treated with vehicle or KW-6002. (e) LTD magnitude (change in fEPSP slope at 70–80 min) (n = 3–4; means ± s.e.m., P > 0.05, two-way ANOVA followed by a Tukey post hoc comparison test). (f) PPF plotted against different interpulse intervals in WT and aSyn Tg mice treated with vehicle or KW-6002 (n = 3; means ± s.e.m., P > 0.05, two-way ANOVA followed by a Tukey post hoc comparison test). (g) Representative immunoblot and quantification of NMDAR2B, NMDA receptor subunit 1 (NMDAR1) and PrPC levels in WT and aSyn Tg mice hippocampus in the same conditions as described in a (n = 4–8; means ± s.e.m., P < 0.01, two-way ANOVA followed by a Tukey post hoc comparison test). GAPDH or α-tubulin was used as a loading control. (h) Representative immunoblots and quantitation of the phospho-Src levels, normalized to Fyn immunoreactivity, in WT and Prnp−/− primary neuronal cultures in control conditions (CTR, n = 3), and treated with aSyn oligomers alone (aSyn olig, n = 3) or in the presence of the SCH-58261 (SCH, 50 nM, n = 3) (means ± s.e.m., P < 0.05, one-way ANOVA followed by a Bonferroni's multiple comparison test). Uncropped gels and blots with molecular weight standards are provided in the Supplementary Figure 7 and full statistical details in Supplementary Table 1.
Finally, we found that the Thy1-aSyn mice had increased levels of NMDAR2B in the hippocampus when compared with WT littermates. Selectively blocking mGluR5-Fyn signaling with KW-6002 restored the levels of NMDAR2B and of PrPC in the Thy1-aSyn animals (Fig. 6g), suggesting that this pathway may regulate the aSyn-mediated effects in vivo. Accordingly, we observed that A2AR blockade prevented aSyn-mediated Src kinase phosphorylation in primary cultures (Fig. 6h).
Discussion
The main finding of this study is that aSyn oligomeric species interact with PrPC through mGluR5, activating SFK kinases and, subsequently, NMDAR2B. We discovered a physical interaction between aSyn, PrPC, NMDAR2B and Fyn kinase in the PSD of the hippocampus, supporting a role of this interaction in the pathophysiological effects induced by aSyn. Furthermore, we found, to the best of our knowledge for the first time, that genetic or antibody-mediated inactivation of PrPC prevented the toxic effects of aSyn on synaptic function. This protective effect afforded by PrPC inhibition is a result of prevention of aberrant SFK/NMDAR2B signaling triggered by mGluR5 and the reestablishment of intracellular Ca2+ homeostasis. Finally, we confirmed the importance of this mechanism in vivo, in a mouse model of synucleinopathies based on the overexpression of human aSyn, by rescuing synaptic and cognitive deficits following blockade of mGluR5-evoked phosphorylation of NMDARs. Together, these data support the hypothesis that a receptor-mediated mechanism, independent of pore formation and membrane leakage, is sufficient to trigger early synaptic damage induced by extracellular aSyn, which could occur as part of the normal biology of the protein or during the spreading of pathology in PD and other synucleinopathies.
aSyn aggregation, synaptic dysfunction and consequent neuronal cell loss are key neuropathological hallmarks of synucleinopathies, but the precise molecular mechanisms and nature of the toxic species produced during aggregation remain unclear28. Nevertheless, extracellular soluble aSyn oligomers are attracting much attention because of their potential role in disease pathogenesis and progression2,29,30. In fact, it is now widely accepted that aSyn is secreted and propagates between neurons in a prion-like manner31,32. Thus, different aSyn species (monomer, oligomers and fibrils) are predicted to gain access to the extracellular space and act postsynaptically to impair neuronal communication and plasticity. Consistent with this hypothesis, we have previously shown that extracellular aSyn oligomers impair LTP, via NMDAR activation, before the occurrence of any neuronal death or changes in membrane conductance5,6. Recent findings demonstrate that PrPC can act as a cell surface binding partner for β-sheet-rich protein aggregates, namely soluble oligomeric protein species7,8,20,33. Moreover, PrPC is involved in age-dependent behavioral abnormalities34, memory impairment in animal models of neurodegeneration35 and mediates Ca2+ influx via NMDARs36. These observations suggest that PrPC might act as a mediator of the synaptotoxic effects triggered by aSyn oligomers. We now establish a previously undocumented link between aSyn and PrPC, whereby extracellular aSyn oligomers disturb Ca2+ homeostasis, affecting synaptic plasticity. These toxic effects depend on PrPC, as LTP and calcium impairments are lost in Prnp−/− or by blocking PrPC with an antibody. In addition, our data suggest that the amino acid region 93–109 of PrPC is involved in mediating the toxic effects of aSyn. Whether the reported interaction also affects the spreading of aSyn in the brain37 still needs to be investigated.
It has been suggested that the putative receptor function of PrPC relates to the modulation of phosphorylation cascades, particularly that governed by Fyn38,39, a member of the SFK family that highly expressed in neurons11. Through this pathway, PrPC is thought to have a key role in the regulation of several cellular processes, ranging from embryogenesis to neuroprotective signaling9. In fact, under physiological conditions, PrPC depresses Fyn activity and, consequently, attenuates Ca2+ influx via NMDARs36. Our findings are consistent with these results, as we found that the interaction of PrPC with extracellular aSyn oligomers led to SFK phosphorylation and, consequently, to NMDAR hyper-activation followed by a rise in postsynaptic Ca2+ levels. Moreover, either Fyn or PrPC inhibition completely prevented aSyn-mediated synaptic deficits. This toxic Fyn signaling cascade can be attributed to the relief of the PrPC constitutive block of Fyn or, alternatively, to aberrant Fyn activation, as previously proposed for Aβ oligomers10,40. Our results support the latter, as Prnp−/− mice did not display LTP impairments even in the presence of aSyn oligomers, suggesting that no substantial constitutive SFK inhibition occurred. Moreover, Fyn-mediated intracellular Ca2+ flux can occur via store-operated Ca2+ entry (SOCE)41 or via NMDARs36. We found that the PrPC-dependent effect on Ca2+ influx arose via NMDAR2B rather than SOCE, as NMDAR2B blockade rescued Ca2+ increase and both PrPC and SFK blockade prevented NMDAR2B phosphorylation induced by aSyn.
aSyn/PrPC and SFK cannot interact directly, given that PrPC is extracellularly anchored to the plasma membrane, whereas SFK is cytosolic. Our data identify mGluR5 as the protein linking PrPC and SFK on opposite sides of the plasma membrane. Previous studies have shown that mGluR5 mediates Fyn activation promoted by PrPC (ref. 11). Consistently, when we blocked mGluR5 activation, we prevented aSyn-induced NMDAR2B phosphorylation and synaptic impairment. Conversely, we bypassed the PrPC blockade of the aSyn effects by activating mGluR5 directly, suggesting that mGluR5 acts downstream of aSyn/PrPC and upstream of Fyn/NMDAR2B activation.
Notably, the identification of the mGluR5-Fyn-NMDAR2B pathway as a mediator of the aSyn-PrPC signaling uncovers new targets for therapeutic intervention. However, interfering directly with Fyn kinase, PrPC or NMDARs impairs basal neuronal function and memory, even in WT mice, as these proteins are crucial components of the PSD36. Accordingly, Prnp−/− mice exhibit deficits in hippocampal-dependent spatial learning, alterations in hippocampal physiology42, synaptic alterations43,44, social recognition memory deficits, and impaired motor coordination and activity34,45. Thus, this deleterious phenotype of the Prnp−/− mice may occlude any attempt to rescue in vivo aSyn-induced toxicity in a Prnp null background. Likewise, a failure to rescue this particular aSyn phenotype by using a Fyn kinase inhibitor, PrPC antibody or NMDAR antagonists in vivo would prove inconclusive, as their constitutive activity is essential for synaptic function. Thus, we chose to interfere with mGluR5 for rescuing memory and synaptic impairments in vivo. mGluR5 are important players in cognitive and synaptic plasticity processes, and their direct antagonism impairs LTP and memory in vivo21. However, their functional interaction with adenosine A2ARs, which regulate mGluR5-mediated effects via NMDAR2B phosphorylation23,46, provides a suitable alternative for regulating aberrant mGluR5 signaling without disrupting its constitutive activity. A similar approach was used to determine the involvement of PrPC in synaptic impairment driven by Aβ oligomers21.
We found that an A2AR antagonist (KW-6002) was able to rescue synaptic and cognitive deficits in aSyn-transgenic mice, providing the crucial evidence that the toxic effects of aSyn are indeed modulated by downstream effectors of PrPC (mGluR5/Fyn), as we described in vitro. KW-6002, also known as istradefyline, is approved in Japan for the adjunctive treatment of motor deficits in PD47 and has been shown to be particularly suited to target the CNS, based on its bioavailability, half-life and brain penetration in animal studies27.
Previous evidence that blockade of A2ARs effectively inhibits NMDAR2B phosphorylation evoked by mGluR5 (ref. 46) and prevents LTP impairment and NMDAR overactivation mediated by aSyn in vitro6 further supports this crosstalk. Notably, deletion of A2AR is protective against neuronal degeneration induced by a mutant human α-synuclein transgene, although the underlying mechanisms were unknown at the time48.
Our evidence that KW-6002 treatment normalizes NMDAR2B and PrPC levels in Thy1-aSyn mice, preventing Fyn phosphorylation while rescuing memory and LTP, supports our hypothesis and the involvement of this pathway in vivo (Supplementary Fig. 6). In addition, the fact that aSyn was found to be increased in cerebrospinal fluid of prion disease patients reinforces the pathophysiological relevance of our findings49.
Notably, at the time of treatment, the aSyn transgenic mice that we used already displayed detectable cognitive deficits19, suggesting that memory impairments elicited by aSyn overexpression are reversible and do not result from cell death. Indeed, aSyn mice only present neuronal loss later in life18. In accordance with this, we found that aSyn oligomers activated SFK/NMDARs as early as 5 min after exposure, whereas neuronal death occurs only after 24 h of exposure6. These data support the hypothesis that a receptor-mediated mechanism, independent of pore formation and membrane leakage50, is sufficient to trigger early synaptic damage induced by extracellular aSyn. Furthermore, we identified previously unknown components of the signaling cascade triggered by aSyn, suggesting that PrPC signaling might be involved in early stages of PD and DLB.
In total, our findings provide new options for therapeutic intervention aimed at neutralizing the downstream effects occurring at synapses, rather than relying solely on the disease-causing agent. Thus, this strategy may prove to be more effective at preventing or delaying the onset of synucleinopathy-associated memory deficits.
Methods
Animals.
Animal procedures were performed in accordance with the European Community guidelines (Directive 2010/63/EU), Portuguese law on animal care (DL 113/2013), and approved by the Instituto de Medicina Molecular Internal Committee and the Portuguese Animal Ethics Committee (Direcção Geral de Veterinária). Environmental conditions were kept constant: food and water ad libitum, 21 ± 0.5 °C, 60 ± 10% relative humidity, 12-h light/dark cycles, 2–3 rats per cage or 3–4 mice per cage. Only male animals were used in all experiments. Mice were sacrificed by cervical dislocation and rats sacrificed by decapitation after anesthesia under halothane atmosphere. Male Sprague Dawley rat (SD; Harlan, Barcelona, Spain) with 8–12 weeks old were used for electrophysiological experiments. Transgenic mice overexpressing human aSyn under the Thy-1 promoter were generated on a mixed C57BL/6-DBA/2 background as described previously51. Animals were maintained on this background by breeding mutant females with type (WT) with C57BL/6-DBA/2 males. Offspring were genotyped with polymerase chain reaction (PCR) amplification analysis of tail DNA (40 cycles, 60 °C annealing temperature). The sequences of primers used were: Thy-1-F: 5′-CTG GAA GAT ATG CCT GTG GA-3′, Thy-1-R: 5′-GAG GAA GGA CCT CGA GGA AT-3′ (Invitrogen). Male transgenic Thy1-aSyn mice and their WT littermates with matched age (6-8 month old) were used for behavioral experiments. Prion protein knockout mice (designated Prnp−/−) homozygous for the disrupted Prnp gene (Zurich I) were produced on a 129/Sv and C57BL/6 (Zurich I) background as previously described52. Male Prnp−/− mice and their WT littermates with matched age (6–8 months old) were used for electrophysiological experiments.
Oral administration of the drug.
KW-6002 (istradefylline; Tocris Bioscience), a selective adenosine A2AR antagonist27, was orally administered, diluted in the drinking water, being continuously available, as before24,25. The weight of the animals and the volume intake were assessed twice a week and the concentration of the solution was adjusted so that the drug intake was maintained at 3 mg/kg/d. Animals were divided in four groups: WT mice drinking vehicle (0.025% methylcellulose) or drinking KW-6002 (3 mg/kg/d, 0.025% methylcellulose), and aSyn Tg mice drinking vehicle or KW-6002 at the same concentration (Fig. 5a). The treatment started at 5 months of age, 1 month before behavior assessment started. The KW-6002 administration was kept until sacrifice.
Behavioral assessments.
Mice were first handled for 5 d before behavioral tests. Mazes were cleaned with a 70% ethanol solution between each animal. Animals were randomized and the experimenter blinded to genotype for the duration of behavioral testing. All behavioral tests were performed during the light phase between 8 a.m. and 6 p.m. in a sound attenuated room.
Y-maze behavioral assessment.
Short-term reference memory was assessed in a spontaneous novelty-based spatial preference Y-maze test. The Y-maze was performed in a two-trial recognition test in a Y-shaped maze with 3 arms (each with 15 cm length × 5 cm wide × 12 cm height), angled at 120° and with opaque walls. Different cues were placed on the surrounding walls. Allocation of arms was counterbalanced within each group. During the first trial (learning trial), mice were placed at the end of the 'start' arm and were allowed to explore the maze for 10 min with only two arms opened (start and 'other' arm). Access to the third arm of the maze (novel arm) was blocked by an opaque door. The mouse was then removed from the maze and returned to its home cage. After 1 h, the animal was placed again in the start arm of the maze, the door of the novel arm was removed and the mouse was allowed to explore the maze for 5 min (test trial). Mice tracings were continuously monitored by an automated tracking system (Smart 2.5, PanLab). Preference for the novel arm is considered a measure of short-term reference memory. To exclude the possible confounding effect of alterations of locomotor activity, we used the frequency of entrance into the arms (number of transitions) as an indirect indicator of the general locomotor activity. The animal's behavior was performed by an observer blind to the treatment conditions and genotype.
MWM.
Spatial memory ability was evaluated in the MWM test53. The test was performed over the course of six consecutive days and consisted of a 5-d acquisition phase and a one-day probe test. The test was performed in a circular pool, with 100 cm in diameter, filled with water opacified with non-toxic white paint (Luxens) and kept at 24 °C. A round 8 cm in diameter platform was hidden 1 cm beneath the surface of the water at a fixed position. Four positions around the edge of the tank were used, dividing the tank into four quadrants: target quadrant (T, quadrant here the platform was hidden), left quadrant (L, quadrant on the left of the target quadrant), right quadrant (R, quadrant of the right of the target quadrant) and opposite quadrant (O, quadrant on the opposite side of the target quadrant). During the acquisition phase, each mouse was given four swimming trials per day (30-min intertrial interval). A trial consisted of placing the mouse into the water facing the outer edge of the pool and allowing the mouse to explore and reach for the hidden platform. If the animal reached the platform before 60 s, it was allowed to remain there for 10 s, if the animal failed to find the target before 60 s, it was manually guided to the platform, where it was allowed to remain for 20 s. After the end of each trial, mice were removed from the pool and placed back to their home cages beneath heat lamps in order to prevent temperature loss. On the probe test the platform was removed and animals were allowed to swim freely for 60 s while recording the percentage of time spent on each quadrant. The latency to find the platform during the acquisition phase and the percentage of time in the platform quadrant in the probe test were recorded and analyzed using the Smart 2.5 tracking system (PanLab) and used to evaluate hippocampal-dependent memory. Swimming speed, measure of possible motor defects that could interfere with the ability to perform the task, was also registered.
Histological procedures.
Mice were transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS (0.1 mol/l PBS, pH 7.4) under deep pentobarbital anesthesia. Brains were removed, post-fixed for 24 h in 4% paraformaldehyde, embedded in paraffin and cut into coronal sections of 1.5 μm. For immunohistochemistry, slides were deparaffinized, rehydrated and antigen retrieval was performed by heating at 70 °C for 1 h in 0.01M Citrate Buffer pH 6. Slices were incubated with primary antibodies specific for aSyn (1:200, mouse monoclonal IgG1, BD Biosciences, 610787) and SNAP25 (1:5,000, rabbit polyclonal IgG, Sigma-Aldrich, S9684) or PrPC (C-20, 1:50; goat polyclonal IgG, Santa Cruz Technology, #sc-7693) overnight at 23–26 °C and washed with PBS before being incubated overnight at 23–26 °C with secondary antibodies (Alexa Fluor 568 donkey anti-mouse and Alexa Fluor 488 donkey anti-rabbit or anti-goat, 1:400, Invitrogen). After washing, slices were incubated with Hoechst (Hoechst 33342, Thermo Scientific; 12 μg/ml final concentration), washed once and mounted in Dako Fluorescent Mounting Medium (Dako). Z-stack images at 63× magnification were acquired with a Zeiss LSM 880 Confocal Microscope and compositional images of hippocampal formation were produced by tile stitching of images at 10× magnification acquired using Zeiss Cell Observer Widefield Fluorescence Microscope. For tyrosine hydroxylase (TH) immunohistochemistry, coronal sections of 4 μm were used. Slices were then deparaffinized, rehydrated and antigen retrieval was performed by heating at 70 °C for 1 h in 0.01M Citrate Buffer pH 6. Slices were incubated with primary antibody specific for TH (1:750, rabbit polyclonal IgG, abcam, ab112). Quantification of the TH staining intensity was performed using the NDP view Software (Hamamatsu Photonics).
Electrophysiological fEPSPs recordings.
The experiments were performed in acute transverse hippocampal slices from male SD rats (8–12 weeks old), in Prnp−/− mice, and in Thy1-aSyn (aSyn Tg) mice and their respective WT littermates. The experimenter was blind to genotype and/or treatment. After decapitation, the brain was rapidly removed and the hippocampi were dissected free in ice-cold artificial CSF, known also as Krebs solution, which is composed of (mM): NaCl 124; KCl 3; NaH2PO4 1.25; NaHCO3 26; MgSO4 1; CaCl2 2; and D-glucose 10, previously gassed with 95% O2 and 5% CO2, pH 7.4. Slices (400 μm thick) were obtained with a McEwan tissue chopper and were pre-incubated with or without extracellular aSyn oligomers (500 nM) for 90 min at 23–26 °C in gassed artificial CSF. Treatment with the different drugs started 20 min before aSyn oligomers pre-incubation and was kept throughout the 90 min of aSyn pre-incubation (Figs. 1a,2a and 4a,c). The range of concentration and time-course chosen for aSyn incubation was based on previous data in which we tested and optimized for obtaining the max effect without compromising the slice viability crucial for the electrophysiology recordings5. In a set of preliminary experiments, we tested 10–50 nM aSyn in fEPSPs but did not detect significant changes. Following this incubation period, slices were then washed, placed in the recording chamber and superfused with artificial CSF (3 ml/min) at 32 °C. fEPSPs were recorded in the stratum radiatum of the CA1 area (Fig. 1a), as previously described5. An I/O protocol was performed to determine the synaptic response parameters for each slice. The Schaffer collaterals were stimulated (stimulus rate of 1 pulse per 30 s) at a range of stimulus intensities. The stimulus strength was increased until the maximum population spike amplitude was reached. The I/O curve was plotted as the relationship of fEPSP slope versus stimulus intensity, which provides a measure of synaptic efficiency. The max slope values were obtained by extrapolation upon nonlinear fitting of the I/O curve and an F-test was used to determine differences between the parameters. Short-term synaptic plasticity at the dendritic synapses was assessed by measuring PPF using a standard paired-pulse stimulation protocol applied to the Schaffer collaterals. Paired-pulse interval of 200 ms was used at a test stimulus intensity that elicited a fEPSP equal to 50% of the maximal fEPSP amplitude, as determined from I/O protocols. Three paired-pulse responses were averaged in each slice. Long-term plasticity was evaluated by a LTP and a LTD protocol. LTP was induced by a theta-burst stimulation protocol (TBS, ten trains with four pulses each at 100 Hz, separated by 200 ms) applied to the Schaffer collaterals at the test stimulus intensity (50% half-maximal fEPSP). LTD was induced using a low frequency stimulation protocol (LFS, three trains with 10-min interval of 2 Hz, 1,200 pulses) as previously described54. The specificity of these effects was previously validated by testing the same concentration of insulin oligomers, which caused no significant changes in LTP magnitude5. Stimulation, data acquisition and analysis was performed using the electrophysiology software program WinLTP program or pClamp (Molecular Devices).
Primary neuronal cultures.
Hippocampal neurons were cultured from 18 d Sprague Dawley rat (Harlan, Barcelona, Spain) and Prnp−/− mice embryos as previously described55,56. Briefly, embryos were collected in Hank's Balanced Salt Solution (HBSS, Corning) and rapidly decapitated. Meninges were removed, and whole cortices (hippocampi and attached cortex) were dissociated and incubated for 15 min in HBSS with 0.025% trypsin. Cells were washed once with HBSS with 30% Fetal Bovine Serum (FBS), centrifuged three times, re-suspended in Neurobasal Medium (Gibco – Life Technologies) supplemented with 2% B-27 supplement, 25 μM glutamate, 0.5 mM glutamine, and 2 U/ml Penicillin/Streptomycin, gently dissociated and filtered through a 70-μm strainer (VWR). Cells were plated on poly-D-lysine-coated plates and grown for 10 d at 37 °C in a 5% CO2-humidified atmosphere in the previously described supplemented Neurobasal medium, in the absence of any positive selection for neurons. Medium was not replaced and cultures were treated with aSyn species (500 nM) and drugs at day 12.
Ca2+ imaging.
Primary neuronal cultures from WT and Prnp−/− mice were plated at a density of 20 × 103 cells per well in glass bottom microwell chambers previously coated with poly-D-lysine. At the 12–16 d in vitro neurons were loaded with Fura-2AM (5 μM, in external physiological solution with the following composition in mM: NaCl 125, KCl 3, NaH2PO4 1.25, CaCl2 2, MgSO4 2, D-(+)-glucose 10 and HEPES 10; pH 7.4 adjusted with NaOH) and incubated at 37 °C for 1 h. Cells were then placed on a heated chamber installed in an inverted microscope with epifluorescent optics and equipped with a high speed multiple excitation fluorimetric system (Lambda DG4, with a 175W Xenon arc lamp). Data was recorded by a CDD camera. Fura-2AM leaded neurons were sequentially excited both at 340 nm and 380 nm, for 250 ms at each wavelength, and the emission fluorescence was recorded at 510 nm. Experiments were performed on cells with a baseline fluorescence ratio around 0.5, which corresponds approximately to a [Ca2+]i of about 100nM, considered the normal [Ca2+]i57,58. Cells with a baseline fluorescence ratio above 1 were discarded from the experiment. Experiments were performed at 37 °C in a 5% CO2-humidified atmosphere. Drugs, anti-PrPC antibodies and aSyn species were applied directly to the cells medium. All cells were challenged with ionomycin (an effective Ca2+ ionophore) at the end of the experiment and only those that responded were included, confirming neuronal viability. Image data were recorded and analyzed using the MetaFluor software (Universal Imaging).
Immunocytochemistry.
Primary neuronal cultures with 12 d in vitro were fixed for 10 min with 4% paraformaldehyde diluted in PBS. After washing with PBS, cells were permeabilized for 10 min with 0.05% Triton-X in PBS, blocked for 30 min with 10% FBS in PBS and incubated overnight at 4 °C with the mature neuronal marker, rabbit anti-MAP-2 (microtubule-associated protein 2; abcam ab32454; 1:200 dilution), and the astrocytic and the immature neuronal marker, mouse anti-GFAP antibody (glial fibrillary acidic protein; Millipore MAB360; 1:250 dilution) or, with the primary antibodies specific for aSyn (1:50, rabbit polyclonal IgG, Cell Signaling Technology, #2628S) and PrPC (1:50; Santa Cruz Technology, #sc-7693), diluted in PBS with 0.05% Tween-20 (PBS-T) and 4% FBS. After washing with PBS-T, cells were incubated for 1 h with Alexa Fluor 488 donkey anti-rabbit and Alexa Fluor 568 donkey anti-mouse or anti-goat antibodies (Invitrogen) diluted 1:400 in PBS-T with 4% FBS. To label cell nucleus, after washing with PBS-T, coverslips were incubated for 5 min with Hoechst (Hoechst 33342, Thermo Scientific; 12 μg/ml final concentration) and washed for 30 min with PBS -T. After a final washing step with PBS, coverslips were mounted with Dako Fluorescent Mounting Medium (Dako) and let to dry for 24 h at 23–26 °C, protected from light exposure. Cells were observed with a Zeiss Cell Observer Widefield Fluorescence Microscope and a Zeiss LSM 880 Confocal Microscope. For culture characterization purposes, fifteen arbitrary photographs were acquired at 20× magnification and different cell subsets were counted and analyzed using Image-J software (NIH). Approximately, 50% of cells were mature neurons (MAP-2-positive cells) and 30% were GFAP positive cells. The remaining cells were microglia, as confirmed by nuclei identification based on their morphological characteristics59.
Purification and oligomerization of recombinant aSyn.
aSyn was prepared as previously5,60. Monomeric aSyn was readily used or stored at −80 °C until further use. Oligomerization was induced by continuous shaking of monomeric aSyn (140 μM) for 6 d at 37 °C in a thermomixer (Eppendorf) at 900 rpm. Samples were ultracentrifuged to obtain fibrillary aSyn. The supernatant containing monomeric and oligomeric aSyn was centrifuged in Amicon filter unit with Ultracel membrane NMWL of 30 kDa (Millipore). The fibrillary aSyn (>180 kDa) and the retained fraction containing aSyn oligomers (>30 kDa) was readily used or stored at −80 °C in small aliquots to avoid freeze/thaw cycles until further use. The concentration of aSyn was determined using its molar extinction coefficient at 280 nm (that is, ɛ280 = 5960 L/mol/cm). The composition of different aSyn species, monomers, oligomers and fibrils was evaluated by SDS–PAGE (Supplementary Fig. 1a). 5 μg of each aSyn sample was separated by SDS-PAGE using a Tetra Cell (Bio-Rad) in a precast 4–15% polyacrylamide gel (Bio-Rad), using standard procedures. To ensure consistency and stability of the effects of the oligomer preparation, each batch was pre-screened for toxicity using LTP as readout before any further testing.
Co-IP.
Briefly, WT rat hippocampal slices were homogenized in IP buffer (NP40 1%, SDS 0.1%, Tris–HCl 50 mM, NaCl 150 mM, sodium deoxycholate 0,5%, EDTA 1 mM, protease inhibitors - Complete, EDTA-free Protease Inhibitor cocktail tablets; Roche) (pre-IP lysates, see in the Supplementary Fig. 7). Protein extracts were incubated with protein G PLUS-Agarose (Santa Cruz, Biotechnology) for 1 h at 4 °C to eliminate nonspecific binding. After incubation, the precleared supernatants containing 1 mg of protein were incubated with anti-PSD-95 antibody (1:50; Cell Signaling Technology, #D27E11), anti-PrP C-20 (1:50; Santa Cruz Technology, #sc-7693), anti-aSyn (1:50; Cell Signaling Technology, #4179), anti-α-tubulin (1:50, Abcam, #ab52866), or IgG (for negative control; Santa Cruz Biotechnology) overnight at 4 °C under rotation. The day after, lysates were incubated with protein G PLUS-Agarose for 3 h with rotation at 4 °C. Beads were washed three times with IP buffer and resuspended in 1.5× sample buffer pH (Tris 70 mM pH 6.8, glycerol 6%, sodium dodecyl sulfate 2%, dithiothreitol 120 mM, and bromophenol blue 0.0024%). Pre-IP lysates and bound proteins eluted from the immune complexes were denatured by heating to 95 °C for 5 min and used for western blot analysis.
Western blotting.
Neuronal cells were washed with cold PBS and then mechanically scrapped in radioimmunoprecipitation assay buffer pH 8.0 (RIPA buffer: NaCl 150 mM, Tris-base 50 mM, EDTA 1 mM, Nonidet P40 1%, sodium dodecyl sulfate 0.1%, proteases inhibitors - Complete, EDTA-free Protease Inhibitor cocktail tablets; Roche). Hippocampus from Thy1-aSyn (aSyn Tg) and WT mice were homogenized in the same buffer by sonication. After protein quantification using BioRad DC Protein Asay kit, lysates were denatured with 5x sample buffer pH 6.8, as above and heated at 95 °C for 5 min and further processed as before56. Samples and the prestained molecular weight marker (BIO-RAD) were separated by SDS–PAGE (15% gel) under reducing conditions and electro-transferred to polyvinylidene difluoride membranes (0.45 μm, GE Healthcare Life Sciences) using standard procedures. Thereafter, nonspecific binding was blocked with 3% bovine serum albumin (fatty acid free) in Tris-buffered saline (pH 7.6) containing 0.1% Tween 20 (TBS-T) for 1 h at 23–26 °C. Membranes were then incubated overnight at 4 °C with the corresponding primary antibody, namely mouse anti-aSyn (1:1000; BD Biosciences, #610787), rabbit anti-aSyn (1:1,000; Cell Signaling Technology, #4179), rabbit anti-α-tubulin (1:5,000; Abcam, #ab52866), mouse anti-GAPDH (6C5; 1:1,000; ThermoFisher Scientific, #AM4300), rabbit anti-PSD-95 (1:1,000; Cell Signaling Technology, #2507), rabbit anti-NMDA receptor subunit 2B (D15B3; 1:1,000; Cell Signaling Technology, #4212), rabbit anti-phospho-NMDA receptor subunit 2B (Tyr1472; 1:1,000; Cell Signaling Technology, #4208), mouse anti-NMDA receptor subunit 1 (1:500; BD Biosciences, #556308), rabbit anti-Fyn kinase (H-80; 1:1,000; Santa Cruz Biotechnology, #sc-28791), rabbit anti-phospho-Src (Tyr416; 1:100; Cell Signaling, #2101), mouse anti-PrP 6D11 (6D11; 1:1,000; BioLegend, #SIG-39810), goat anti-PrP C-20 (C-20; 1:500; Santa Cruz Biotechnology, #sc-7693) and/or mouse anti-tyrosine hydroxylase (TH; 1:200; abcam, #ab112) diluted in blocking solution. After three washing periods of 10 min with TBS-T, membranes were incubated with horseradish peroxidase (HRP) - conjugated anti-mouse, anti-rabbit or anti-goat secondary antibodies (1:10,000; Santa Cruz Biotechnology) (in 5% nonfat dry milk) for 1 h at 23–26 °C. After 40 min of washing with TBS-T, chemiluminescent detection was performed with ECL western blotting detection reagent (GE Healthcare Life Sciences) using X-Ray films (Fujifilm). Densitometric quantification was determined using Image-J software and normalized to the corresponding control band density. Full-length gels and blots with molecular weight standards are provided in the Supplementary Figure 7.
Drugs and PrPC antibodies.
The mGluR5-selective agonist, (S)-3,5-Dihydroxyphenylglycine (DHPG), and the ionophore Ionomycin were purchased from Sigma-Aldrich. The A2AR-selective antagonists, (E)-8-(2-(3,4-dimethoxyphenyl)-vinyl)-1,3-diethyl-7-methyl-3,7-dihydropurine-2,6-dione (KW-6002, istradefylline), and 5-Amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5c]pyrimidine (SCH-58261) plus the mGluR5-selective antagonist, 2-Methyl-6-(phenylethynyl)pyridine (MPEP), were purchased from Tocris Bioscience. The Src-family inhibitor 1-(1,1-Dimethylethyl)-3-(1-naphthalenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (1-Naphthyl PP1), the selective NMDAR antagonist, AP5, and the selective NMDAR subunit NR2B, Ifenprodil, were purchased from Abcam.
The following anti-PrP antibodies were used: 6D11 (mouse monoclonal; epitope targeting between amino acids 93 and 109; BioLegend, #SIG-39810), C-20 (goat polyclonal; epitope targeting the C terminus; Santa Cruz Biotechnology, #sc-7693), and 8B4 (mouse monoclonal; Santa Cruz Biotechnology, epitope targeting the N terminus, #sc-47729).
Statistical analysis.
All statistical analyses were performed with GraphPad Prism software. Values are presented as dot blots with individual values plus bar, with mean ± s.e.m. in figure legends. Statistical analyses were designed using the assumption of normal distribution and similar variance among groups, as previously tested. Statistical comparisons included two-sided unpaired t test, one or two-way ANOVA followed by a Bonferroni's, Dunnett's or Tukey's multiple comparison post hoc tests as specified in the figure legends. P values of < 0.05 were considered to be statistically significant. The complete statistical values are provided in Supplementary Table 1. The sample size was determined based on preliminary results or similar experiments carried-out in the past. Power analysis was performed using G-power in order to estimate the number of animals required, for a signal-to-noise ratio of 1.4 and 80% to 90% power assuming a 5% significance level.
Data availability.
For detailed information on experimental design please see the provided Life Sciences Reporting Summary. Full-length gels and blots with molecular weight standards are provided in the Supplementary Figure 7. All the software used to data analysis is commercially available and the respective information is provided in each respective section. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Yang, W. & Yu, S. Synucleinopathies: common features and hippocampal manifestations. Cell. Mol. Life Sci. 74, 1485–1501 (2017).
Lee, H.-J., Bae, E.-J. & Lee, S.-J. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 10, 92–98 (2014).
Braak, H., Rüb, U., Jansen Steur, E.N.H., Del Tredici, K. & de Vos, R.A. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64, 1404–1410 (2005).
Goldman, J.G., Williams-Gray, C., Barker, R.A., Duda, J.E. & Galvin, J.E. The spectrum of cognitive impairment in Lewy body diseases. Mov. Disord. 29, 608–621 (2014).
Diógenes, M.J. et al. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J. Neurosci. 32, 11750–11762 (2012).
Ferreira, D.G. et al. Adenosine A2A receptors modulate α-synuclein aggregation and toxicity. Cereb. Cortex 27, 718–730 (2017).
Resenberger, U.K. et al. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J. 30, 2057–2070 (2011).
Laurén, J., Gimbel, D.A., Nygaard, H.B., Gilbert, J.W. & Strittmatter, S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009).
Wulf, M.-A., Senatore, A. & Aguzzi, A. The biological function of the cellular prion protein: an update. BMC Biol. 15, 34 (2017).
Um, J.W. et al. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 15, 1227–1235 (2012).
Um, J.W. & Strittmatter, S.M. Amyloid-β induced signaling by cellular prion protein and Fyn kinase in Alzheimer disease. Prion 7, 37–41 (2013).
Williamson, R., Usardi, A., Hanger, D.P. & Anderton, B.H. Membrane-bound beta-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. FASEB J. 22, 1552–1559 (2008).
Grant, S.G. et al. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258, 1903–1910 (1992).
Suzuki, T. & Okumura-Noji, K. NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem. Biophys. Res. Commun. 216, 582–588 (1995).
Nakazawa, T. et al. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 276, 693–699 (2001).
Salter, M.W. & Kalia, L.V. Src kinases: a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5, 317–328 (2004).
Collins, M.O. et al. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem. 97 (Suppl. 1), 16–23 (2006).
Chesselet, M.-F. et al. A progressive mouse model of Parkinson's disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics 9, 297–314 (2012).
Magen, I. et al. Cognitive deficits in a mouse model of pre-manifest Parkinson's disease. Eur. J. Neurosci. 35, 870–882 (2012).
Caetano, F.A. et al. Amyloid-beta oligomers increase the localization of prion protein at the cell surface. J. Neurochem. 117, 538–553 (2011).
Um, J.W. et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron 79, 887–902 (2013).
Emes, R.D. et al. Evolutionary expansion and anatomical specialization of synapse proteome complexity. Nat. Neurosci. 11, 799–806 (2008).
Sarantis, K., Tsiamaki, E., Kouvaros, S., Papatheodoropoulos, C. & Angelatou, F. Adenosine A2A receptors permit mGluR5-evoked tyrosine phosphorylation of NR2B (Tyr1472) in rat hippocampus: a possible key mechanism in NMDA receptor modulation. J. Neurochem. 135, 714–726 (2015).
Batalha, V.L. et al. Adenosine A(2A) receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol. Psychiatry 18, 320–331 (2013).
Coelho, J.E. et al. Overexpression of adenosine A2A Receptors in rats: effects on depression, locomotion, and anxiety. Front. Psychiatry 5, 67 (2014).
Batalha, V.L. et al. The caffeine-binding adenosine A2A receptor induces age-like HPA-axis dysfunction by targeting glucocorticoid receptor function. Sci. Rep. 6, 31493 (2016).
Yang, M. et al. Characterization of the potency, selectivity, and pharmacokinetic profile for six adenosine A2A receptor antagonists. Naunyn Schmiedebergs Arch. Pharmacol. 375, 133–144 (2007).
Schulz-Schaeffer, W.J. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol. 120, 131–143 (2010).
Kayed, R. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 (2003).
Outeiro, T.F. et al. Formation of toxic oligomeric α-synuclein species in living cells. PLoS One 3, e1867 (2008).
Marques, O. & Outeiro, T.F. Alpha-synuclein: from secretion to dysfunction and death. Cell Death Dis. 3, e350 (2012).
Martin, Z.S. et al. α-Synuclein oligomers oppose long-term potentiation and impair memory through a calcineurin-dependent mechanism: relevance to human synucleopathic diseases. J. Neurochem. 120, 440–452 (2012).
Beraldo, F. H. et al. Regulation of Amyloid β oligomer binding to neurons and neurotoxicity by the complex prion protein/mGluR5. J. Biol. Chem. 291, 21945–21955 (2016).
Schmitz, M. et al. Loss of prion protein leads to age-dependent behavioral abnormalities and changes in cytoskeletal protein expression. Mol. Neurobiol. 50, 923–936 (2014).
Gimbel, D.A. et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 30, 6367–6374 (2010).
Khosravani, H. et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J. Cell Biol. 181, 551–565 (2008).
Urrea, L. et al. Involvement of cellular prion protein in α-synuclein transport in neurons. Mol. Neurobiol. http://dx.doi.org/10.1007/s12035-017-0451-4 (2017).
Linden, R. et al. Physiology of the prion protein. Physiol. Rev. 88, 673–728 (2008).
Sorgato, M.C. & Bertoli, A. From cell protection to death: may Ca2+ signals explain the chameleonic attributes of the mammalian prion protein? Biochem. Biophys. Res. Commun. 379, 171–174 (2009).
Larson, M. et al. The complex PrP(c)-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer's disease. J. Neurosci. 32, 16857–71a (2012).
De Mario, A. et al. The prion protein constitutively controls neuronal store-operated Ca2+ entry through Fyn kinase. Front. Cell. Neurosci. 9, 416 (2015).
Criado, J.R. et al. Mice devoid of prion protein have cognitive deficits that are rescued by reconstitution of PrP in neurons. Neurobiol. Dis. 19, 255–265 (2005).
Collinge, J. et al. Prion protein is necessary for normal synaptic function. Nature 370, 295–297 (1994).
Curtis, J., Errington, M., Bliss, T., Voss, K. & MacLeod, N. Age-dependent loss of PTP and LTP in the hippocampus of PrP-null mice. Neurobiol. Dis. 13, 55–62 (2003).
Katamine, S. et al. Impaired motor coordination in mice lacking prion protein. Cell. Mol. Neurobiol. 18, 731–742 (1998).
Tebano, M.T. et al. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J. Neurochem. 95, 1188–1200 (2005).
Dungo, R. & Deeks, E.D. Istradefylline: first global approval. Drugs 73, 875–882 (2013).
Kachroo, A. & Schwarzschild, M.A. Adenosine A2A receptor gene disruption protects in an α-synuclein model of Parkinson's disease. Ann. Neurol. 71, 278–282 (2012).
Kasai, T. et al. Increased α-synuclein levels in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. J. Neurol. 261, 1203–1209 (2014).
Di Scala, C. et al. Common molecular mechanism of amyloid pore formation by Alzheimer's β-amyloid peptide and α-synuclein. Sci. Rep. 6, 28781 (2016).
Rockenstein, E. et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J. Neurosci. Res. 68, 568–578 (2002).
Büeler, H. et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356, 577–582 (1992).
Morris, R.G., Garrud, P., Rawlins, J.N. & O'Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982).
Laurent, C. et al. A2A adenosine receptor deletion is protective in a mouse model of tauopathy. Mol. Psychiatry 21, 97–107 (2016).
Pedersen, W.A., Wan, R., Zhang, P. & Mattson, M.P. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J. Neurosci. 22, 404–412 (2002).
Valadas, J.S. et al. Neuroprotection afforded by adenosine A2A receptor blockade is modulated by corticotrophin-releasing factor (CRF) in glutamate injured cortical neurons. J. Neurochem. 123, 1030–1040 (2012).
Knot, H.J. et al. Twenty years of calcium imaging: cell physiology to dye for. Mol. Interv. 5, 112–127 (2005).
Barhoumi, R., Qian, Y., Burghardt, R.C. & Tiffany-Castiglioni, E. Image analysis of Ca2+ signals as a basis for neurotoxicity assays: promises and challenges. Neurotoxicol. Teratol. 32, 16–24 (2010).
Garman, R.H. Histology of the central nervous system. Toxicol. Pathol. 39, 22–35 (2011).
Vicente Miranda, H. et al. Heat-mediated enrichment of α-synuclein from cells and tissue for assessing post-translational modifications. J. Neurochem. 126, 673–684 (2013).
Acknowledgements
The authors thank L. Gros for figure layout design and C. Fahlbusch (University Medical Center Göttingen), A. Margarida Nascimento and J. Rino (Instituto de Medicina Molecular (iMM) Bioimaging facility), I. Moreira and J. Marques (iMM Rodent facility), and the iMM Histology and Comparative Pathology laboratory for technical assistance. M.T.F., H.V.M. and J.E.C. were supported by individual grants from Fundação para a Ciência e Tecnologia (FCT) (SFRH/BD/52228/2013; SFRH/BPD/109347/2015; SFRH/BPD/87647/2012); L.V.L. and T.F.O. were supported by a grant from the Fritz Thyssen Stiftung (Az. 10.12.2.165), Germany. L.V.L. received an iMM Lisboa internal fund (BIG – Breakthrough Idea Grant) for part of the project. L.V.L. is an Investigator FCT, Portugal. T.F.O. is supported by the DFG Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB), Germany. LISBOA-01-0145-FEDER-007391, project co-financed by FEDER, POR Lisboa 2020 - Programa Operacional Regional de Lisboa, from PORTUGAL 2020 and by Fundação para a Ciência e a Tecnologia.
Author information
Authors and Affiliations
Contributions
D.G.F. performed most of the experimental work, analyzed data and wrote the manuscript. M.T.-F., J.E.C., V.L.B. and S.H.V. assisted with behavior and calcium experiments. E.M.S. assisted with animal experiments. I.M.-M. performed the immunohistochemistry experiments. M.S., J.S.R. and I.Z. provided the Prnp−/− mice and experimental support. H.V.M. produced and characterized aSyn species. L.V.L. and T.F.O. coordinated the study, designed the experiments and wrote the manuscript. All of the authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Characterization of the aSyn species and biological effects.
(a) SDS–PAGE separation of the different aSyn species. Monomers (aSyn mon) migrate with monomeric molecular weight (15 kDa) whereas aSyn oligomers (aSyn olig), and fibrils (aSyn fib) display SDS-resistant high-molecular weight species. (b) Changes in fEPSP slope induced by theta-burst stimulation recorded from WT rat hippocampal slices pre-incubated with extracellular aSyn monomers (aSyn mon, 90 min, 500 nM, n = 4), fibrils (aSyn fib, 90 min, 500 nM, n = 3) or in control conditions (CTR, n = 4). (c) Plot of the LTP magnitude represented in b (change in fEPSP slope at 50–60 min after theta-burst stimulation, compared to baseline) (means ± s.e.m., P < 0.01, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test). (d) Plot of the LTP magnitude obtained from WT and Prnp-/- hippocampal slices pre-incubated with extracellular aSyn oligomers (aSyn olig, 90 min, 500 nM, n = 10, 6) or in control conditions (CTR, n = 7, 6) (means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test). (e) Input/Output (I/O) curves corresponding to fEPSP slope evoked by various stimulation intensities (10 – 120 μA) from WT hippocampal slices pre-incubated with or without aSyn oligomers (n = 5, 7; means ± s.e.m., P < 0.001, F-test). (f) I/O curves from Prnp-/- hippocampal slices pre-incubated with (n = 4) or without (n = 4) aSyn oligomers obtained by the same method as in e (means ± s.e.m., P > 0.05, F-test). (g) I/O curves obtained by the same method as in e, from hippocampal slices CTR (n = 6), pre-incubated with aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 7) or in the presence of the anti-PrP 6D11 antibody (6D11 + aSyn olig, 110 min, 100 nM, n = 4) (means ± s.e.m., P < 0.01, F-test). (h) Changes in fEPSP slope, obtained by the same methods as in b, from WT hippocampal slices in control conditions (CTR, n = 4) and in the presence of aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 6) or together with the anti-PrP antibodies, 8B4 (8B4 + aSyn, 110 min, 10 μg, n = 4) or C-20 (C-20 + aSyn olig, 110 min, 10 μg, n = 4) (means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test). (i) Paired Pulse Facilitation (PPF) plotted against 200 ms interpulse intervals in WT slices submitted to the same conditions as in g (n = 3-5; means ± s.e.m., P > 0.05, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test).
Supplementary Figure 2 Src pharmacological blockade prevents aSyn oligomer-induced synaptic impairment.
(a) Plot of the LTP magnitude (change in fEPSP slope at 50–60 min after theta-burst stimulation, compared to baseline) from control WT hippocampal slices (CTR, n = 4), slices pre-incubated with the Src antagonist 1-naphthyl-PP1 (PP1, 110 min, 30 μM, n = 3) and slices pre-incubated with extracellular aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 6) or in the presence of PP1 (PP1 + aSyn olig, n = 3) (means ± s.e.m., P < 0.01, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test). (b) Input/Output (I/O) curves corresponding to fEPSP slope evoked by various stimulation intensities (10 – 120 μA) from control WT hippocampal slices (CTR, n = 5) and slices pre-incubated with extracellular aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 5) or in the presence of PP1 (PP1 + aSyn olig, n = 4) (means ± s.e.m., P < 0.001, F-test). (c) Quantification of the effects of the NMDA receptor antagonist APV (50 μM, 30 min) perfusion on basal fEPSP slope from control WT hippocampal slices (CTR, n = 5), and slices pre-incubated with extracellular aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 4) or in the presence of PP1 (PP1 + aSyn olig, n = 4) (change in slope between baseline and the last 10 min of APV application) (means ± s.e.m., P < 0.01, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test). (d) Effect of NMDA receptor antagonist APV (50 μM, 30 min) perfusion on basal fEPSP slope in WT hippocampal slices in control conditions (n = 3) or in the presence of aSyn oligomers (n = 4). (e) Quantification of the APV perfusion (50 μM, 30 min) effects on basal fEPSP slope (change in slope between baseline and the last 10 min of APV application) from WT and Prnp-/-hippocampal control slices (CTR) or slices pre-incubated with aSyn oligomers (n = 3-4; means ± s.e.m., P < 0.001). (f) Representative immunoblot and quantitation of NMDA receptor subunit 2B (NMDAR2B) and NMDA receptor subunit 1 (NMDAR1) levels in hippocampal slices from WT and Prnp-/- mice in the same conditions as in d (n = 4; means ± s.e.m., P < 0.001, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test). GAPDH was used as a loading control.
Supplementary Figure 3 Extracellular aSyn oligomers induce phosphorylation of SFK kinases and NR2B subunit of NMDA receptors.
(a) Representative image of primary cultures from WT animals at 12 DIV. Mature neurons are labeled with green fluorescence with MAP-2 antibody, astrocytes, in red, are probed with anti-GFAP antibody, and cell nucleus are labeled with Hoechst 33342, in blue fluorescence. Z-stack images were acquired using a confocal microscope at 40x magnification and converted into maximum intensity projections. At the bottom a schematic representation of the aSyn incubation protocol used. (b) Representative immunoblots and quantitation of the aSyn levels in neuronal cultures incubated with extracellular aSyn oligomers over time (n = 4-5; P < 0.01, one-way ANOVA followed by a Dunnett’s Multiple Comparison Test). GAPDH was used as a loading control. (c) Representative immunoblots and quantitation of the Fyn levels in neuronal cultures incubated with extracellular aSyn oligomers over time (n = 4; P > 0.05, one-way ANOVA followed by a Dunnett’s Multiple Comparison Test). GAPDH was used as a loading control. (d) Representative immunoblots and quantitation of the SFK kinases phosphorylation levels, normalized to Fyn immunoreactivity, in primary neuronal cultures incubated with extracellular aSyn oligomers over time (n = 3-10; P < 0.01, one-way ANOVA followed by a Dunnett’s Multiple Comparison Test). (e) Representative immunoblots and quantitation of the PrPC levels in neuronal cultures incubated with extracellular aSyn oligomers over time (n = 6; P > 0.05, one-way ANOVA followed by a Dunnett’s Multiple Comparison Test). GAPDH was used as a loading control. (f) Representative immunoblots and quantitation of the NMDAR2B levels in neuronal cultures incubated with extracellular aSyn oligomers over time (n = 4; P > 0.05, one-way ANOVA followed by a Dunnett’s Multiple Comparison Test). GAPDH was used as a loading control. (g) Representative immunoblots and quantification of NMDA receptors subunit NR2B phosphorylation levels, normalized to NMDA receptor immunoreactivity, in neuronal cultures incubated with extracellular aSyn oligomers over time. (n = 3-7; P < 0.05, one-way ANOVA followed by a Dunnett’s Multiple Comparison Test). (h) Quantitative analysis of IP:aSyn and IP:PrPC (n = 3; means ± s.e.m., P < 0.05, two-sided unpaired t test). (i) Immunohistochemistry in 1.5 μm hippocampal sections from WT and aSyn Tg mice. aSyn is labelled in red and PrPC is labelled in green (scale bar: 25 μm). At the bottom, details from the 63x magnification images are presented (scale bar: 5 μm).
Supplementary Figure 4 mGluR5 mediated aSyn/PrPC long term potentiation impairment.
(a) Plot of the LTP magnitude (change in fEPSP slope at 50–60 min after theta-burst stimulation, compared to baseline) from control WT hippocampal slices (CTR, n = 4), slices pre-incubated with the mGluR5 antagonist MPEP (110 min, 5 μM, n = 3) and slices pre-incubated with extracellular aSyn oligomers alone (aSyn olig, 90 min, 500 nM, n = 6) or in the presence of MPEP (MPEP + aSyn olig, n = 4) (means ± s.e.m., P < 0.05, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test). (b) Changes in fEPSP slope induced by theta-burst stimulation recorded from WT rat hippocampal slices pre-incubated with extracellular aSyn oligomers alone (aSyn mon, 90 min, 500 nM, n = 6), in the presence of the selective A2AR antagonist SCH-58261 (110 min, 50 nM, SCH + aSyn olig, n = 3) and in the presence of SCH-58261 together with the mGluR5 agonist DHPG (110 min, 10 μM; SCH + DHPG + aSyn olig, n = 4). (c) Plot of the LTP magnitude represented in b and in Fig. 4d (change in fEPSP slope at 50–60 min after theta-burst stimulation, compared to baseline) (means ± s.e.m., P < 0.01, one-way ANOVA followed by a Bonferroni’s Multiple Comparison Test).
Supplementary Figure 5 Characterization of Thy1-aSyn (aSyn Tg) overexpressing mice.
(a) Representative western blot of independent experiments to evaluate aSyn levels in the hippocampus (HIP) and striatum (STR) of WT and aSyn Tg mice. GAPDH was used as a loading control. At the bottom quantification of aSyn immunoreactivity in relation to WT (n = 2-5; means ± s.e.m., P < 0.05, two-sided unpaired t test). (b) Top panels: compositional images of fluorescence immunohistochemistry of WT and aSyn Tg mice hippocampus (scale bar: 500 μm). aSyn is identified in red fluorescence and cell nuclei are stained with Hoechst in blue fluorescence. Bottom panels: maximum intensity projection images of z-stack taken at 63x magnification in the CA1 area of hippocampus (scale bar: 25 μm). At the right, details from the 63x magnification images are presented (scale bar: 5μm). aSyn is identified in red fluorescence and SNAP25 is labeled in green. (c) Representative western blot of independent experiments to evaluate TH levels in the striatum of WT and aSyn Tg mice. GAPDH was used as a loading control. At the bottom the respective quantification of TH immunoreactivity in relation to WT (n = 4; means ± s.e.m., P > 0.05, two-sided unpaired t test). (d) Representative images of TH immunohistochemistry of WT and aSyn Tg mice coronal brain sections. At the bottom the respective quantitation of TH immunoreactivity in relation to WT (n = 3-4; means ± s.e.m., P > 0.05, two-sided unpaired t test). (e) Representative western blot of 3 independent experiments to evaluate PrP levels in the hippocampus of WT, aSyn Tg, and Prnp-/- mice. GAPDH was used as a loading control. At the bottom the respective quantification of PrP immunoreactivity in relation to WT (n = 5-6; means ± s.e.m., P < 0.05, two-sided unpaired t test).
Supplementary Figure 6 Summary diagram of the mechanism by each aSyn oligomers induce an aberrant PrPC-mGluR5-NMDAR2B signaling at the post-synaptic density.
aSyn oligomers physically interact with PrPC (1) to activate mGluR5 (2)50. This leads to the phosphorylation and activation of the Src kinase Fyn (3) followed by the phosphorylation of NMDAR2B (Y1472) (4)27 and robust increase in intracellular Ca2+ (5). Adenosine A2AR are required for the mGluR5-induced NMDAR2B phosphorylation (6)37.
Supplementary Figure 7 Uncropped gels and blots with molecular weight standards complete images
Uncropped versions of representative western blot images from Fig. 2e, 2f, 2g, 2h, 5g, 6g, and 6h and pre-IP lysates corresponding to IPs shown in Fig. 2g and 2h. Membranes were cut prior to antibody staining to allow for simultaneous detection of proteins running at different sizes on the same membrane without reprobing. Representative membranes with the molecular weight (MW) markers used are shown at the bottom right panels.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1835 kb)
Supplementary Table 1
Complete statistical details (XLSX 14 kb)
aSyn oligomers increase intracellular Ca2+ in neurons
Initial calcium response of FURA-2 AM loaded wildtype neurons to aSyn oligomers (aSyn mon, 500 nM) (AVI 28473 kb)
aSyn oligomers do not increase intracellular Ca2+ in Prnp (-/-) neurons
Initial calcium response of FURA-2 AM loaded Prnp (-/-) neurons to aSyn oligomers (aSyn mon, 500 nM) (AVI 31418 kb)
Rights and permissions
About this article
Cite this article
Ferreira, D., Temido-Ferreira, M., Vicente Miranda, H. et al. α-synuclein interacts with PrPC to induce cognitive impairment through mGluR5 and NMDAR2B. Nat Neurosci 20, 1569–1579 (2017). https://doi.org/10.1038/nn.4648
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4648
This article is cited by
-
In vitro modulation of mTOR and mGlur5 influence α-synuclein accumulation
Molecular Brain (2024)
-
VDR and deubiquitination control neuronal oxidative stress and microglial inflammation in Parkinson’s disease
Cell Death Discovery (2024)
-
α-Synuclein triggers cofilin pathology and dendritic spine impairment via a PrPC-CCR5 dependent pathway
Cell Death & Disease (2024)
-
The alpha-synuclein oligomers activate nuclear factor of activated T-cell (NFAT) modulating synaptic homeostasis and apoptosis
Molecular Medicine (2023)
-
Neuroprotective effects of osmotin in Parkinson’s disease-associated pathology via the AdipoR1/MAPK/AMPK/mTOR signaling pathways
Journal of Biomedical Science (2023)