Abstract
Social isolation can exacerbate the negative consequences of stress and increase the risk of developing psychopathology. However, the influence of living alone on experiences generally considered to be beneficial to the brain, such as physical exercise, remains unknown. We report here that individual housing precludes the positive influence of short-term running on adult neurogenesis in the hippocampus of rats and, in the presence of additional stress, suppresses the generation of new neurons. Individual housing also influenced corticosterone levels—runners in both housing conditions had elevated corticosterone during the active phase, but individually housed runners had higher levels of this hormone in response to stress. Moreover, lowering corticosterone levels converted the influence of short-term running on neurogenesis in individually housed rats from negative to positive. These results suggest that, in the absence of social interaction, a normally beneficial experience can exert a potentially deleterious influence on the brain.
Similar content being viewed by others
Main
The physiological consequences of stressful experiences can range dramatically from one person to the next1,2. Differential outcomes can depend on psychological factors related to the stressor itself, such as whether it is predictable or controllable3,4. However, it is clear that even identical perturbations can elicit varying responses from different individuals. Numerous studies indicate that individual differences in stress responses are determined by the environment. In this regard, social context has been shown to influence stress responsiveness. In rats, group housing can buffer the influence of some types of stress on the activity of the hypothalamic pituitary adrenal (HPA) axis5,6,7. Likewise, social support lessens the negative impact of stress on the brain and seems to protect against psychiatric illness in humans8,9.
Exercise is an experience that is associated with health benefits and yet it results in physiological changes that are indicative of stress. Running is known to activate the HPA axis, an effect that persists even after physical fitness is achieved10. Despite large and continual elevations in stress hormones, running is generally linked to positive influences on the brain and behavior. For example, running has been associated with elevated expression of brain-derived neurotrophic factor, increased synaptic plasticity and improved performance on spatial navigation learning tasks11,12,13. Also, running increases the number of new neurons in the dentate gyrus of adult rats12,13,14,15. These results may seem paradoxical as glucocorticoids, elevated during running, suppress adult neurogenesis16,17,18,19,20,21. The majority of studies on running and neurogenesis have examined rats living in groups12,13,14,15. Thus, it is possible that social interaction buffers the brains of runners from the negative effects of elevated glucocorticoids.
Here we report that social isolation precludes the positive effects of short-term exercise on adult neurogenesis in the dentate gyrus. Enhancement of adult neurogenesis eventually emerged in individually housed runners, but only after a considerably longer period of running. Both isolated and group-housed runners showed elevations in glucocorticoids during the active period, but individually housed runners experienced higher levels of corticosterone at a different point in the circadian rhythm as well as in response to stress. Reducing corticosterone levels reversed the effects of running on adult neurogenesis only in rats living alone. These findings suggest that social experience buffers the runner from the negative actions of elevated glucocorticoids by blunting stress reactivity and by preventing corticosterone from suppressing adult neurogenesis. By contrast, isolation rendered the hippocampus more susceptible to the suppressive actions of glucocorticoids on adult neurogenesis and delayed the positive consequences of physical activity.
Results
Effects of short-term running depend on social context
To determine whether social isolation affects the response of adult neurogenesis to physical activity, rats were exposed to 12 d of running or no running, while housed in groups or alone. Rats were injected with 50 mg per kg (body weight) of bromodeoxyuridine (BrdU), a marker of DNA synthesis, once daily for 10 d beginning on day 2 of the experiment. Runners housed in groups showed a substantial increase in the number of BrdU-labeled cells in the dentate gyrus, compared to group-housed controls. By contrast, individually housed runners showed the opposite effect: a significant decrease in the number of BrdU-labeled cells compared to individually housed controls (interaction of housing and physical activity, F1,20 = 16.68, P = 0.006; Figs. 1 and 2). There were no substantial differences in the numbers of BrdU-labeled cells between group-housed and individually housed controls. Similar to the effect seen with BrdU labeling, the number of cells stained for Ki67, an endogenous marker of cell proliferation, was increased in group-housed runners relative to group-housed controls but was decreased in individually housed runners relative to individually housed controls (interaction of housing and physical activity, F1,20 = 36.35, P = 0.001; Figs. 1 and 2).
The top panel outlines the BrdU injection protocol for these studies. The rats received daily injections of BrdU on days 2–11 and were perfused 24 h after the last injection. Left, the number of BrdU-labeled cells was lower in individually housed short-term runners compared to individually housed controls. By contrast, the number of BrdU-labeled cells in group-housed runners was elevated compared to group-housed controls. Right, similar results were obtained with the endogenous marker of cell proliferation, Ki67: individually housed runners had fewer Ki67-labeled cells, whereas group-housed runners had more Ki67-labeled cells, when compared with their respective control conditions. Error bars represent s.e.m. *P < 0.05 in comparison to controls (two-way ANOVA and Tukey HSD post-hoc test).
(a,b) The number of BrdU-labeled cells in the dentate gyrus of an individually housed runner (a) was lower than that of a group-housed runner (b). (c) High magnification example of BrdU-labeled cells in dentate gyrus of a long-term runner. (d,e) The number of Ki67-labeled cells in an individually housed runner (d) was lower than that of a group-housed runner (e). (f,g) High magnification examples of proliferating cells (labeled with Ki67, f; and phosphorylated histone H3, g). The cell in g appears to be undergoing cytokinesis. (h–k) Immunofluorescence double-labeling for cell type–specific markers (green) and BrdU (red): (h) cell double-labeled with BrdU and a marker of mature neurons, NeuN; (i) cell double-labeled with BrdU and a marker of immature and mature neurons, Tuj1; (j) cell double-labeled with BrdU and a marker of astroglia, GFAP; and (k) cell double-labeled with BrdU and a marker of endothelial cells, von Willebrand factor (vWF). Arrows indicate labeled or double-labeled cells. Scale bars, 10 μm.
We analyzed the phenotype of BrdU-labeled cells and found that most of the new cells expressed the neuronal markers class III β-tubulin (Tuj1; ∼80%) and neuron-specific nuclear protein (NeuN; ∼66%), at similar percentages across conditions. Much smaller percentages of BrdU-labeled cells stained for an astroglial marker, glial fibrillary acidic protein (GFAP; ∼7%), or an endothelial cell marker, von Willebrand factor (∼6%) (Fig. 2). These findings suggest that changes in cell proliferation with running primarily result in changes in adult neurogenesis.
Social housing and physical activity
It is unlikely that the differential effects of running on adult neurogenesis in group- versus individually housed rats were the result of group-housed rats engaging in higher levels of activity: the total amount of running (for group-housed rats, three to a cage, daily km divided by 3) was similar for rats in both social conditions (Supplementary Fig. 1 online). In addition, we observed no differences in the frequency or duration of the running bouts between social conditions (frequency, t8 = 1.866, P = 0.099; duration, t8 = 0.576, P = 0.58). Finally, during the active phase, individually housed rats engaged in slightly more locomotor activity when not in the running wheels than group-housed rats. It is also unlikely that the differential effects of running on adult neurogenesis across social conditions were the result of variation in the frequency of other behaviors, as no differences were observed between these groups during either the inactive or active phase in the amount of time spent eating, drinking, grooming or resting and sleeping.
Social housing alters corticosterone levels
The differential effects of running on adult neurogenesis observed with social conditions may be associated with alterations in the levels of corticosterone. To assess this possibility, we measured corticosterone in trunk blood collected after decapitation at two different times: at the onset of the dark period (7 p.m.), and 4 h after lights-out (11 p.m.). There is a diurnal rhythm in corticosterone—levels are highest around the onset of the dark period. As previously reported10, we observed substantially greater elevations in circulating corticosterone in runners at 7 p.m., the beginning of the active phase (effect of physical activity, F1,20 = 17.21, P = 0.0005, Fig. 3). We found no differences between individual and group-housed runners in this response. In contrast, at the 11 p.m. time point, we observed significant differences in corticosterone levels between social conditions—both controls and runners housed individually had higher levels of corticosterone than those housed in groups (effect of housing, F1,20 = 8.909, P = 0.0073, Fig. 3).
(a) Running increased corticosterone levels in both individually housed and group-housed rats at the beginning of the active phase (7 p.m.), when corticosterone levels were at their circadian peak. *P < 0.05 in comparison to control. (b) Group housing was associated with lower levels of corticosterone at a later time period during the active phase (11 p.m.). *P < 0.05 in comparison to individually housed rats. Note also the overall decline in corticosterone levels between 7 p.m. and 11 p.m. (c) Group housing and running interact to buffer the stress response. Group-housed runners did not show a stress-induced increase in corticosterone when tested during the inactive phase (10 a.m.). A stress-induced increase in corticosterone levels was observed in all other conditions. Error bars represent s.e.m. *P < 0.05 in comparison to baseline.
To determine whether social housing alters the response of the HPA axis to stress in runners, we examined baseline, stress and recovery corticosterone levels during the inactive phase from tail blood samples of controls and runners housed either individually or in groups. In controls, restraint stress elevated corticosterone levels 30 min later—these levels returned to baseline 2 h after the cessation of stress in both social conditions. However, among runners, we observed substantial differences in the corticosterone response to restraint stress between the social conditions. Specifically, group-housed runners showed no elevation in corticosterone levels 30 min after restraint stress (F2,18 = 3.828, P = 0.041; Fig. 3). These data suggested that, compared to individual housing, group housing may reduce the overall exposure to corticosterone, particularly in runners.
Glucocorticoids and socially isolated runners
To investigate whether adrenal steroids have a role in the differential effects of running on adult neurogenesis in group- versus individually housed rats, we repeated the same experiment on rats that received sham operation or adrenalectomy (ADX), and administered low-dose corticosterone in the drinking water (25 μg ml−1 in 0.9% saline). Prevention of the running-induced increase in glucocorticoid levels converted the effect of running on neurogenesis from negative to positive in individually housed rats (effect of glucocorticoid status, F1,38 = 8.89, P = 0.005; interaction of running, glucocorticoid status and housing, F1,38 = 7.75, P = 0.008, Fig. 4). Lowering corticosterone levels did not alter the positive effects on adult neurogenesis in group-housed runners. Thus, both individually housed and group-housed runners showed increased neurogenesis when glucocorticoid levels were clamped. By contrast, sham-operated rats showed the same pattern of results observed with intact rats—for individually housed rats, 12 d of running decreased the number of BrdU-labeled cells whereas for group-housed rats, the same period of running had the opposite effect.
The top panel describes the experimental design for this study. Controls or runners were housed in groups or individually and subjected to sham operation (SHAM), or bilateral adrenalectomy (ADX) followed by the administration of 25 μg ml−1 corticosterone (CORT) in the drinking water (ADX + CORT). The BrdU injection protocol was identical to the one described for Figure 1. BrdU labeling in the dentate gyrus of SHAM controls and runners showed the same pattern as that in intact rats. Individually housed runners had fewer labeled cells, whereas group-housed runners had more labeled cells, than controls. Lowering glucocorticoid levels by removing the adrenal glands and providing a low dose of corticosterone in the drinking water (ADX + CORT) permitted the enhancement of neurogenesis in individually housed runners and did not change the effect of running on neurogenesis in the group-housed rats. Error bars represent s.e.m. *P < 0.05 in comparison to controls (2-way ANOVA with Tukey HSD post-hoc tests).
We observed no substantial differences in the amount of running between group- and individually housed rats with or without ADX and corticosterone replacement (Supplementary Fig. 1). Using radioimmunoassay, we verified the reduction of corticosterone levels in adrenalectomized rats across runners and controls housed in groups or individually and observed no marked differences among these conditions.
Longer periods of running in social isolation
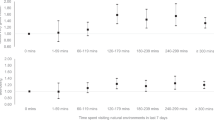
Because the initial period of running in previously sedentary rats is likely to be more demanding physiologically than running over the long term22, we examined whether the influence of exercise on adult neurogenesis in individually housed rats changed over time. To do this, we measured cell proliferation in individually housed rats that ran for 3, 6, 12, 24 or 48 d and received a single injection of BrdU (300 mg per kg body weight) 2 h before perfusion. We found no change in BrdU labeling until the latest time point examined, 48 d (effect of running, F1,47 = 5.32, P = 0.0256; interaction of running and time, F4,47 = 9.85, P = 0.0001; Figs. 2 and 5). Using two endogenous markers of cell proliferation, Ki67 and phospho-histone-H3, we confirmed the increases in cell proliferation following 48 d of running (Ki67, t10 = 4.25, P = 0.0004; phospho-histone-H3, t10 = 3.07, P = 0.0221; Figs. 2 and 5). These results indicated that socially isolated rats ultimately show enhanced cell proliferation but only with relatively long periods of running.
The top panel describes the experimental design for this study: separate cohorts of individually housed rats ran for 3, 6, 12, 24 or 48 d before being injected once with BrdU (300 mg per kg body weight) and perfused 2 h later. Top graph, in socially isolated rats, the number of BrdU-labeled cells in the dentate gyrus was increased by 48 d of running but not at earlier time points examined. Bottom graph, after 48 d of running, similar increases were observed in the numbers of cells that stained for the endogenous markers of cell proliferation Ki67 and phospho-histone-H3 (p-H3). Error bars represent s.e.m. *P < 0.05 in comparison to control (2 × 5 ANOVA and Tukey HSD post-hoc comparisons, or unpaired t-test, comparing Ki67 and phospho-histone-H3 data from runners and controls at the 48-d time point).
Following 48 d of running, the number of BrdU-labeled cells at the 2-week post-BrdU survival time was greater in runners (3,788.0 ± 412.2 (mean ± s.e.m.) for individually housed controls compared to 5,979.0 ± 662.2 for individually housed 48-d runners; t12 = 2.58, P = 0.024). Long-term running also increased the overall volume of the dentate gyrus (effect of running, F1,47 = 6.18, P = 0.0166; Supplementary Fig. 2 online). Most of the BrdU-labeled cells in both groups expressed the neuronal markers Tuj1 (∼82%) and NeuN (∼75%), whereas a much smaller proportion stained for the astroglial marker GFAP (∼6%) and the endothelial marker von Willebrand factor (∼5%). There was no change in the proportion of cells staining for any of the markers, suggesting that differentiation of new cells was not influenced by long-term running. Changes in the amount of running per 24 h are probably not responsible for the increase in adult neurogenesis following 48 d of running, because a plateau in daily running distance was reached considerably before we observed positive effects on neurogenesis (∼21 d of running).
To determine whether the positive influence of long-term running in individually housed rats is comparable to that in group-housed runners, we repeated the 48-d running experiment with rats in both social conditions. We found that 48 d of physical activity stimulated cell proliferation to a similar extent in group- and individually housed rats (effect of running, F1,19 = 9.81, P = 0.005; Supplementary Fig. 3 online). Moreover, long-term running increased the volume of the dentate gyrus in individually housed and group-housed rats (effect of running, F1,19 = 7.844, P = 0.0114; Supplementary Fig. 2). Again, group- and individually housed rats did not differ in the overall amount of running activity.
Social housing buffers the negative effects of stress
Unlike the pattern observed in our initial experiments (Fig. 1), socially isolated rats exposed to 12 d of running in the time course experiment (Fig. 5) did not show decreased cell proliferation. This difference may have been due to the fact that the rats in our initial experiments were subjected to the stress of daily handling and injection23, whereas those in the current experiment were injected only once on the last day of the study. Daily handling and injection significantly elevated baseline levels of circulating corticosterone in socially isolated runners (19.04 ± 2.83 ng ml−1 for undisturbed rats, 32.60 ± 3.40 ng ml −1 for injected rats, t6 = 3.069, P = 0.020). Social isolation in the absence of additional stressors seemed to prevent the positive influence of short-term running on adult neurogenesis, such that the number of BrdU-labeled cells was similar to that of the sedentary controls. However, in the presence of additional stressors (handling and injection), the influence of short-term running was negative, resulting in a net decrease in the number of new cells.
To more directly test the possibility that the decreases in adult neurogenesis observed in individually housed runners exposed to multiple injections were the result of interactions among isolation, running and stress, we repeated the 12-d social housing experiment but replaced the daily BrdU injections with a brief cold-swim stress. This stressor was of comparable duration to that experienced during restraint and BrdU injection (60–90 s). These rats received only a single BrdU injection, on the last day of the experiment. We found that individually housed runners subjected to daily cold-water stress showed decreased cell proliferation compared to individually housed controls. By contrast, group-housed runners exposed to daily cold-water stress had enhanced cell proliferation (Fig. 6). These results indicated that the negative effects of running on neurogenesis in our experiments with multiple BrdU injections were the result of the combined action of individual housing, running and daily stress. Moreover, they suggested that group-housed runners were buffered from the negative effects of stress on cell proliferation.
The top panel represents the experimental design for this study. Rats were subject to brief (60–90 s) daily swim stress in cold water. On the morning of the last day, rats were injected once with BrdU (300 mg per kg body weight) and perfused 2 h later. Daily cold-swim stress resulted in a decrease in cell proliferation in individually housed runners compared to individually housed controls. In contrast, daily cold-swim stress did not prevent the enhancement of cell proliferation in group-housed runners. Error bars represent s.e.m. * P < 0.05 in comparison to control (2-way ANOVA and Tukey HSD post-hoc comparisons).
Duration of isolation may influence effects of running
To determine whether the negative effects of running on adult neurogenesis are dependent on living alone for a certain time before gaining access to the running wheel, we compared rats that were moved directly from group-housed conditions to running wheels with those that spent 1 week living alone before running. We found that the duration of isolation before short-term running (12 d) influenced the results—a decrease in cell proliferation was observed only in the runners with 1 week of isolation before running but not in those with no previous isolation experience (effect of previous isolation, F1,20 = 12.69, P = 0.002; interaction of physical activity and previous isolation, F1,20 = 14.27, P = 0.0012; Fig. 7).
The BrdU injection protocol is identical to the one used in Figures 1 and 4. Before the beginning of the study, some rats were individually housed in standard cages, while others were housed in groups of three until the beginning of the experiment and then housed individually with or without a running wheel. Whereas rats exposed to one week of isolation before running showed a decrease in cell proliferation, those exposed to no previous isolation did not exhibit this effect. This suggested a time-dependent effect of social isolation on adult neurogenesis in response to running. Error bars represent s.e.m. *P < 0.05 in comparison to control, 1 week previous isolation.
Discussion
The results of these experiments demonstrate that the social context in which an experience occurs can influence endocrine and neural responses. When experienced in a group setting, running stimulates adult neurogenesis. However, when running occurs in social isolation, these positive effects are suppressed. Although runners housed in either social condition experienced comparable elevations in corticosterone during one part of the active phase, only those that ran alone were vulnerable to the negative influence of glucocorticoids on neurogenesis in the hippocampus. This may have been due to the cumulative effects of corticosterone exposure over the 24-h period because individually housed rats seemed to experience a greater overall exposure to this hormone, particularly in response to stress. However, the fact that even group-housed runners had elevated corticosterone levels during the active phase but enhanced, rather than suppressed, adult neurogenesis, suggests that these rats were buffered in some unknown way from the negative influences of glucocorticoids. In this regard it is relevant that, unlike individually housed rats, group-housed rats had the same adult neurogenesis response to running regardless of adrenal steroid manipulations. Thus, preventing the elevation in glucocorticoid levels in individually housed runners stimulates neurogenesis, whereas group-housed rats respond to short-term running with enhanced neurogenesis whether or not glucocorticoid levels are elevated.
These paradoxical effects may be the result of an interaction between glucocorticoids, which suppress neurogenesis16,17,18,19,20,21, and an unidentified factor that positively regulates neurogenesis and is altered by both running and social housing. If running and social housing both enhance a system that promotes adult neurogenesis, then these conditions together may result in increased production of new neurons despite the presence of an inhibitory factor such as elevated glucocorticoids. Several candidate mechanisms exist: one interesting possibility is the neurotransmitter serotonin which, through the 5HT1A receptor subtype, enhances neurogenesis24. Release of serotonin in the hippocampus increases with exercise25, whereas expression of the 5HT1A receptor decreases with both social isolation26 and prolonged elevation of corticosterone27. Thus, individual housing could inhibit 5HT1A receptor expression in the hippocampus, a decrease that may be further compounded by overall higher exposure to corticosterone in the running group. Under these conditions, running-induced serotonin release in the hippocampus may be ineffective at stimulating cell proliferation because 5HT1A receptor levels are diminished. In the presence of elevated glucocorticoids, as is observed with additional stress to the running group, a decrease in adult neurogenesis would occur. On the other hand, because group housing elevates 5HT1A receptors, it is possible that the stimulatory effects of running-induced serotonin release on cell proliferation are sufficient to override the negative effects of elevated corticosterone. The extent to which serotonin participates in interactions among social housing, running, glucocorticoids and neurogenesis, however, remains to be determined.
Prolonged exercise ultimately enhanced neurogenesis in the dentate gyrus of rats living in social isolation. This change occurred some time between 24 d and 48 d of running and was accompanied by a significant increase in the volume of this brain region. The basis for these changes in adult neurogenesis and dentate gyrus volume are unknown but may involve alterations in the vascular structure. Angiogenesis supports cell proliferation in the subgranular zone, possibly through the increased expression of neurotrophic factors28,29, and physical activity promotes the extension of the vasculature30. Together, these events may contribute to the increased volume of this brain region over time.
It is noteworthy that socially isolated runners who received multiple BrdU injections, but not those who received a single BrdU injection, showed decreased levels of adult neurogenesis. This differential response of socially isolated runners to short-term physical activity may be related to the added stress of daily injections23. Indeed, we found that daily handling and injection significantly elevated the levels of circulating corticosterone. Moreover, replacing the injections with cold water stress resulted in virtually identical effects on cell proliferation. Thus, in the presence of additional stressors, socially isolated runners showed decreased neurogenesis compared to controls. In the absence of additional stressors, socially isolated runners had levels of adult neurogenesis that were similar to those in sedentary controls. These results are consistent with a study showing that footshock-induced decreases in neurogenesis are only evident in socially isolated rats31. Our data suggest that social housing alters the response of the HPA axis to stress. Whereas isolated runners experienced robust elevations in corticosterone in response to stress, group-housed runners showed no such increase in corticosterone. Collectively, these results suggest that socially isolated runners are exposed to higher levels of corticosterone under certain conditions and, furthermore, that elevations in adrenal steroids are involved in both the negative effects and the prevention of the positive effects on neurogenesis, as blocking glucocorticoid elevations not only eliminated the negative effects but also enabled the positive effects to emerge.
Numerous studies have reported enhanced neurogenesis following voluntary running in rats housed in groups12,13,14,15,32. Similar results have been obtained using forced-running protocols33,34 with group-housed rats (J. Trejo, personal communication; C.-J. Kim, personal communication). Other studies, however, have examined the effects of running on adult neurogenesis in socially isolated rats; most of these findings are consistent with our data in that the duration of running used to enhance adult neurogenesis was relatively long35,36,37. However, there are a few reports of increased cell proliferation in socially isolated adult rats following short bouts of running. Two of these studies used individually housed spontaneously hypertensive rats (SHR)38,39. Because these experiments were conducted in female rats, which show estrous cycle–dependent fluctuations in neurogenesis40 and running activity, and this rat strain differs from the Sprague-Dawley in the rate of hippocampal cell proliferation under basal conditions41, it is not surprising that the physiological response to running would also differ. In contrast to our results, a previous study42 reported increased cell proliferation in the dentate gyrus of individually housed adult male Sprague-Dawley rats following ∼17 d of running, considerably earlier than when we observed an increase in neurogenesis. In this study, rats were housed in isolation for 3 d before the initiation of running as opposed to the 7-d acclimation period we used; the findings therefore suggest that the duration of living in social isolation may be an important determinant of the effect. Our finding that the duration of individual housing alters the influence of running on cell proliferation suggests that this might be the case.
Running is a natural rodent behavior that seems to have a hedonic component. Naïve laboratory-reared rodents will readily engage in wheel running, even without additional incentives, and can be trained to bar-press for wheel access43. Conversely, rodents accustomed to running may experience physiological and behavioral signs of withdrawal when suddenly denied access to a wheel44,45. Our results suggest that engaging in an internally motivated, apparently rewarding activity can exert radically different influences on neural plasticity depending on the context in which this activity occurs. In a socially deprived environment, the enhancement of neurogenesis by running is delayed and in the presence of additional stressors, neurogenesis is actually suppressed. By contrast, running in a group setting elevates neurogenesis—despite activation of the HPA axis and the presence of additional stressors, such as handling and injection or cold-water stress. Although it is possible that different social contexts may alter the hedonic value of running, our behavioral data indicated no differences across groups in the duration or frequency of running or the distance run. Collectively, these results indicate that social isolation can both delay the enhancement of plasticity by physical exercise and exacerbate the consequences of stressful stimuli. Structural plasticity has been linked to anxiety regulation and the therapeutic actions of antidepressants46,47. Our findings present a potential mechanism whereby social isolation may predispose the organism to a negative outcome in the presence of physiologically ambiguous experiences.
Methods
Rats and housing conditions.
All rat experiments conformed to guidelines of the US National Institutes of Health and were approved by the Princeton University Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats from Taconic (250–300 g, n = 6 for group-housing studies, n = 5–8 for time course studies) were housed either individually or in groups of three, with or without access to a running wheel. All rats received unlimited food and water and were maintained on a 12-h light-dark schedule (lights on at 7 a.m.). After arrival, all rats were group-housed and acclimated to the colony for at least 1 week before any manipulations were started.
For all experiments except one, individually housed rats were moved from the standard group-housed condition 1 week before the start of the experiment. In one additional experiment, rats were transferred directly from group to individual housing with or without a running wheel—these rats were compared to similar groups of runners and controls that had experienced a 1-week period of individual housing.
Physical activity and behavioral monitoring.
Distance was recorded daily, between 8 and 10 a.m., from counters attached to the running wheels. Wheel circumference (111.76 cm) was converted to kilometers. For group-housed rats, the total distance run every 24 h was divided by the number of rats in each cage (n = 3). This number may underestimate the total distance run by each rat because rats were occasionally observed to be running in pairs. Additionally, one cohort of group- and individually housed runners was videotaped to evaluate possible differences in the duration and frequency of running bouts. Group-housed rats were marked with black ink for identification. Behavioral analysis was carried out for 10 min at 7 a.m. and 7 p.m., on days 3, 6, 9 and 11 of the experiment. 7 a.m. and 7 p.m. were selected because they were the times of lights on and lights off, respectively. 7 p.m. marks an extremely active phase, when rats are highly likely to engage in running. Digital videos were scored for the duration and frequency of running bouts as well as for behavior when not in the wheel, including rearing, eating, drinking, grooming, resting or sleeping and nonwheel locomotion.
BrdU administration and perfusion.
All rats received injections of the DNA synthesis marker BrdU between 9 and 11 a.m. For multiple injection experiments, we administered 50 mg of BrdU per kg body weight intraperitoneally (i.p.), daily for 10 consecutive days. These rats were perfused 24 h after the last injection. This dose and treatment regimen was modeled after the initial studies of running-enhanced neurogenesis13,14. For experiments using single injections, rats were administered BrdU at a higher dose of 300 mg per kg (i.p.) and perfused 2 h or 2 weeks later. For experiments with a 2-week postinjection survival, the rats continued to have access to a running wheel during this period.
Group-housed rats were removed from the cage sequentially for injection; typically, rats were restrained for BrdU administration for 60–90 s. For perfusion, all rats were deeply anaesthetized with Nembutal (pentobarbital, 100 mg per kg, i.p.) and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer.
Cold-water swim stress.
To assess the effects of a daily stressor on the response of individually housed rats to running, some rats received brief daily cold-water swim stress. These rats were removed from the cage and placed in a bucket of ice water for 60 s before being towel-dried and returned to the home cage. Group-housed rats were administered swim stress in sequence. The total duration of stress (beginning with removal from the home cage until return to the home cage) was ∼90 s.
Corticosterone sampling and radioimmunoassay.
To assess the influence of running on corticosterone levels in individually and group-housed rats, we obtained blood samples from separate groups of unanesthetized rats either from the tail vein or by decapitation. For individually and group-housed runners and controls, we assessed corticosterone levels at two points during the dark phase—7 p.m. and 11 p.m. We examined two time points during the dark phase because a previous study showed a circadian effect of running on corticosterone levels with elevations observed only at the time of lights out (7 p.m.) but not several hours later (11 p.m.) when corticosterone levels naturally decline10.
To assess the response of the HPA axis to stress in runners and controls housed in different social conditions, we obtained corticosterone levels at baseline, during restraint stress and during recovery in the inactive phase, around the time when BrdU injections were normally given. Beginning at 10 a.m., rats were rapidly restrained and blood was obtained in less than 3 min from the tail vein. After 30 min, we obtained a stress blood sample from restrained rats. The rats were then returned to their home cages where they remained for 2 h; then they were rapidly captured and a blood sample was collected in less than 3 min. We obtained plasma from these blood samples and determined the levels of corticosterone using an RIA kit (Diagnostic).
Adrenalectomy and corticosterone replacement.
To determine whether glucocorticoids are involved in the differential effects of running on neurogenesis in individually and socially housed rats, rats were subjected to bilateral adrenalectomy or sham operation. Adrenalectomized rats received low-dose corticosterone replacement (25 μg ml−1 in 0.9% saline; Sigma) in the drinking water to both lower and normalize glucocorticoid levels. Providing corticosterone in the drinking water maintains the diurnal rhythm of glucocorticoids and is sufficient to maintain the survival of mature hippocampal granule neurons17. At the time of perfusion, we obtained blood samples from anesthetized rats to verify the efficacy of adrenalectomy. As indicated above, we used radioimmunoassay to determine corticosterone levels from these samples.
Immunohistochemistry.
Brain sections, 40 μm thick, were cut on a Vibratome into a bath of phosphate-buffered saline (PBS). For BrdU, slide-mounted or free-floating sections were heated in 0.1 M citric acid followed by incubation in trypsin and then 2 M HCl:PBS. For endogenous markers of cell proliferation, the HCl step was omitted. The tissue was incubated overnight with one of the following primary antibodies: mouse monoclonal antibody to BrdU (anti-BrdU, 1:200), mouse monoclonal antibody to Ki67 (1:750, Novocastra) or rabbit polyclonal antibody to phosphorylated histone H3 (1:200, Santa Cruz Biotech). The tissue was then rinsed and incubated with biotinylated anti-mouse or anti-rabbit antibodies (1:200, Vector) for 1 h, rinsed, incubated in avidin-biotin–hydrogen peroxidase complex and then reacted with 0.01% diaminobenzidine (DAB, Sigma). Following this, the slides were counterstained with cresyl violet and coverslipped with Permount (Fisher Scientific).
Additional tissue sections were processed for double-labeling immunofluorescence for BrdU and cell type markers. Brain sections were treated with 2M HCl:TBS (tris-buffered saline) and incubated overnight with rat anti-BrdU (1:250, with 0.5% Tween-20; Accurate Chemical). Following rinses, the tissue was incubated for 1 h in biotinylated goat antibody to rat (1:250, Vector), rinsed and then incubated with streptavidin Alexa 568 (1:1,000, Molecular Probes). Sections were then incubated with one of the following primary antibodies: mouse antibody to NeuN (1:500, Chemicon), mouse antibody to TuJ1 (1:500, Covance), guinea pig antibody to GFAP (1:250, Advanced Immunochemical) or rabbit antibody to von Willebrand's factor (1:200, Sigma) for 24 h at 4 °C. Alexa 488 secondary antibodies conjugated to the appropriate species were applied for 1 h (Molecular Probes). Following rinses, sections were mounted and coverslipped with 90% glycerol:TBS.
Microscopic data analysis.
Slides were coded until completion of the data analysis. Stereological estimates of the total number of labeled cells were determined from peroxidase-stained tissue for BrdU, Ki67 or phospho-histone-H3 on a 1:12 series of brain sections throughout the dentate gyrus. The volume of the dentate gyrus was estimated using Cavalieri's principle and Image-Pro Plus software (Media Cybernetics).
A Zeiss Axiovert 510 LSM confocal microscope was used to examine tissue processed for immunofluorescence (HeNe and Argon lasers). A series of 1-μm-thick sections throughout the extent of each labeled cell were analyzed. For each marker in each brain, 25 BrdU-labeled cells were examined thoughout the rostrocaudal extent of the dentate gyrus.
Statistical analysis.
For all studies with more than two groups, analysis of variance (ANOVA) was performed with Tukey HSD post-hoc tests. For the separate studies of 48-d runners and controls, we used two-tailed t-tests to analyze cells labeled with BrdU, Ki67 and phospho-histone-H3. In addition, we calculated the mean distance run over each experiment for rats in each group and analyzed the results with either an analysis of variance or two-tailed t-tests, depending on the number of groups.
Note: Supplementary information is available on the Nature Neuroscience website.
References
Selye, H. The Stress of Life. (McGraw-Hill, New York, 1976).
Roy, M.P., Steptoe, A. & Kirschbaum, C. Life events and social support as moderators of individual differences in cardiovascular and cortisol reactivity. J. Pers. Soc. Psychol. 75, 1273–1281 (1998).
Weiss, J.M. Effects of coping behavior with and without a feedback signal on stress pathology in rats. J. Comp. Physiol. Psychol. 77, 22–30 (1971).
Amat, J. et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 8, 365–371 (2005).
Weiss, I.C., Pryce, C.R., Jongen-Relo, A.L., Nanz-Bah, N.I. & Feldon, J. Effect of social isolation on stress-related behavioral and neuroendocrine state in the rat. Behav. Brain. Res 152, 279–295 (2004).
Bartolomucci, A. et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 28, 540–558 (2003).
Ruis, M.A. et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioral and physiological effects of social defeat. Psychoneuroendocrinology 24, 285–300 (1999).
Seeman, T.E. & McEwen, B.S. Impact of social environment characteristics on neuroendocrine regulation. Psychosom. Med. 58, 459–471 (1996).
McEwen, B.S. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 22, 108–124 (2000).
Droste, S.K. et al. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology 144, 3012–3023 (2003).
Neeper, S.A., Gomez-Pinilla, F., Choi, J. & Cotman, C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 726, 49–56 (1996).
Farmer, J., Zhao, X., Gage, F.H. & Chistie, B.R. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124, 71–79 (2004).
van Praag, H., Chistie, B.R., Sejnowski, T.J. & Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl. Acad. Sci. USA 96, 13427–13431 (1999).
van Praag, H., Kempermann, G. & Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 (1999).
Fabel, K., Fabel, K. & Palmer, T.D. VEGF is necessary for exercise-induced neurogenesis. Eur. J. Neurosci. 18, 2803–2812 (2003).
Gould, E., Cameron, H.A., Daniels, D.C., Woolley, C.S. & McEwen, B.S. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 12, 3642–3650 (1992).
Cameron, H.A. & Gould, E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61, 203–209 (1994).
Tanapat, P., Hastings, N.B., Rydel, T.A., Galea, L.A. & Gould, E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. 437, 496–504 (2001).
Montaron, M.F. et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol. Aging published online June 13 2005 (doi: 10.1016/j.neurobiolaging.2005.02.014).
Cameron, H.A. & McKay, R.D. Restoring production of hippocampal neurons in old age. Nat. Neurosci. 2, 894–897 (1999).
Ambrogini, P. et al. Persistently high corticosterone levels but not normal circadian fluctuations of the hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology 76, 366–372 (2002).
Allen, D.L. et al. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 90, 1900–1908 (2001).
Cassano, W.J. Jr . & D'mello, A.P. Acute stress-induced facilitation of the hypothalamic-pituitary-adrenal axis: evidence for the roles of stressor duration and serotonin. Neuroendocrinology 74, 167–177 (2001).
Banasr, M., Hery, M., Printemps, R. & Daszuta, A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated though different and common 5HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29, 450–460 (2004).
Gomez-Merino, D., Bequet, F., Berthelot, M., Chennaoui, M. & Guezennec, C.Y. Site-dependent effects of an acute intensive exercise on extracellular 5-HT and 5-HIAA levels in rat brain. Neurosci. Lett. 301, 143–146 (2001).
Schiller, L., Jahkel, M., Kretzschmar, M., Brust, P. & Oehler, J. Autoradiographic analyses of 5HT1A and 5HT2A receptors after social isolation in mice. Brain Res. 980, 169–178 (2003).
Chalmers, D.T., Kwak, S.P., Mansour, A., Akil, H. & Watson, S.J. Corticosteroids regulate brain hippocampal 5HT1A receptor mRNA expression. J. Neurosci. 13, 914–923 (1993).
Palmer, T.D., Willhoite, A.R. & Gage, F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479–494 (2000).
Leventhal, C., Rafii, S., Rafii, D., Shahar, A. & Goldman, S.A. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell. Neurosci. 13, 450–464 (1999).
Lopez-Lopez, C., LeRoith, D. & Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. USA 101, 9833–9838 (2004).
Westenbroek, C., Den Boer, J.A., Veenhuis, M. & Ter Horst, G. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res. Bull. 64, 303–308 (2004).
Brown, J. et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–2046 (2003).
Trejo, J.L., Carro, E. & Torres-Aleman, I. Circulating IGF-1 mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 (2001).
Kim, Y.-P. et al. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci. Lett. 355, 152–154 (2004).
Rhodes, J.S., van Praag, H., Garland, T. & Gage, F.H. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel-running. Behav. Neurosci. 117, 1006–1016 (2003).
Holmes, M.M., Galea, L.A., Mistlberger, R.E. & Kempermann, G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J. Neurosci. Res. 76, 216–222 (2004).
Bjornebekk, A., Mathe, A.A. & Brene, S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int. J. Neuropsychopharmacol. 8, 357–368 (2005).
Persson, A.I. et al. Differential regulation of hippocampal cell proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur. J. Neurosci. 19, 1847–1855 (2004).
Naylor, A.S., Persson, A.I., Erikkson, P.S., Jonsdottir, I.H. & Thorlin, T. Extended voluntary running inhibits exercise induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J. Neurophysiol. 93, 2406–2414 (2004).
Tanapat, P., Hastings, N.B., Reeves, A.J. & Gould, E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 19, 5792–5801 (1999).
Perfilieva, E., Risedal, A., Nyberg, J., Johansson, B.B. & Eriksson, P.S. Gender and strain influence on neurogenesis in dentate gyrus of young rats. J. Cereb. Blood Flow Metab. 21, 211–217 (2001).
Eadie, B.D., Redila, V.A. & Chistie, B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 486, 39–47 (2005).
Iversen, I.H. Techniques for establishing schedules with wheel running as reinforcement in rats. J. Exp. Anal. Behav. 60, 219–238 (1993).
Hoffmann, P., Thorén, P. & Ely, D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR). Behav. Neural Biol. 47, 346–355 (1987).
Widenfalk, J., Olson, L. & Thoren, P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci. Res. 34, 125–132 (1999).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Malberg, J.E., Eisch, A.J., Nestler, E.J. & Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 (2000).
Acknowledgements
The authors acknowledge the assistance of C. Gross, Y. Kozorovitskiy, B. Leuner and C. Mirescu in the preparation of this manuscript. This work was supported by a National Research Service Award predoctoral fellowship to A.S. and a National Institutes of Mental Health grant to E.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Differences in the amount of running do not explain differences in neurogenesis across housing conditions or glucocorticoid status. (PDF 18 kb)
Supplementary Fig. 2
The volume of the dentate gyrus increases with prolonged physical activity. (PDF 64 kb)
Supplementary Fig. 3
The increase in neurogenesis with short-term running in group-housed animals is sustained over longer periods of activity. (PDF 106 kb)
Rights and permissions
About this article
Cite this article
Stranahan, A., Khalil, D. & Gould, E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci 9, 526–533 (2006). https://doi.org/10.1038/nn1668
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1668
This article is cited by
-
Validation of Wistar-Kyoto rats kept in solitary housing as an animal model for depression using voxel-based morphometry
Scientific Reports (2024)
-
Voluntary running-induced activation of ventral hippocampal GABAergic interneurons contributes to exercise-induced hypoalgesia in neuropathic pain model mice
Scientific Reports (2023)
-
Social isolation and the brain: effects and mechanisms
Molecular Psychiatry (2023)
-
Neuroprotective effects of strength training in a neuroinflammatory animal model
BMC Neuroscience (2022)
-
Amylin-Calcitonin receptor signaling in the medial preoptic area mediates affiliative social behaviors in female mice
Nature Communications (2022)