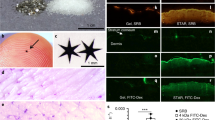
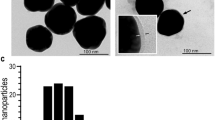
Abstract
Approximately 10% of the population in the USA1,2 suffer from nickel allergy3,4,5, and many are unable to wear jewellery or handle coins and other objects that contain nickel6,7,8,9,10. Many agents have been developed to reduce the penetration of nickel through skin11,12, but few formulations are safe and effective13,14,15. Here, we show that applying a thin layer of glycerine emollient containing nanoparticles of either calcium carbonate or calcium phosphate on an isolated piece of pig skin (in vitro) and on the skin of mice (in vivo) prevents the penetration of nickel ions into the skin. The nanoparticles capture nickel ions by cation exchange, and remain on the surface of the skin, allowing them to be removed by simple washing with water. Approximately 11-fold fewer nanoparticles by mass are required to achieve the same efficacy as the chelating agent ethylenediamine tetraacetic acid. Using nanoparticles with diameters smaller than 500 nm in topical creams may be an effective way to limit the exposure to metal ions that can cause skin irritation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jacob, S. E., Moennich, J. N., McKean, B. A., Zirwas, M. J. & Taylor, J. S. Nickel allergy in the United States: a public health issue in need of a ‘nickel directive’. J. Am. Acad. Dermatol. 60, 1067–1069 (2009).
Marks, J. G. Jr et al. North American Contact Dermatitis Group patch-test results, 1996–1998. Arch. Dermatol. 136, 272–273 (2000).
Hidaka, S., Okamoto, Y. & Abe, K. Elutions of metal ions from dental casting alloys and their effect on calcium phosphate precipitation and transformation. J. Biomed. Mater. Res. 28, 175–180 (1994).
Raison-Peyron, N., Guillard, O., Khalil, Z., Guilhou, J. J. & Guillot, B. Nickel-elicited systemic contact dermatitis from a peripheral intravenous catheter. Contact Dermatitis 53, 222–225 (2005).
Nosbaum, A. et al. Nickel-induced systemic allergic dermatitis from a sacral neurostimulator. Contact Dermatitis 59, 319–320 (2008).
Luo, J. & Bercovitch, L. Cellphone contact dermatitis with nickel allergy. Can. Med. Assoc. J. 178, 23–24 (2008).
Suneja, T., Flanagan, K. H. & Glaser, D. A. Blue-jean button nickel: prevalence and prevention of its release from buttons. Dermatitis 18, 208–211 (2007).
Heim, K. E. & McKean, B. A. Children's clothing fasteners as a potential source of exposure to releasable nickel ions. Contact Dermatitis 60, 100–105 (2009).
Nestle, F. O., Speidel, H. & Speidel, M. O. Metallurgy: high nickel release from 1- and 2-euro coins. Nature 419, 132 (2002).
Bruckner, A. L., Weston, W. L. & Morelli, J. G. Does sensitization to contact allergens begin in infancy? Pediatrics 105, e3 (2000).
Memon, A. A., Molokhia, M. M. & Friedmann, P. S. The inhibitory effects of topical chelating agents and antioxidants on nickel-induced hypersensitivity reactions. J. Am. Acad. Dermatol. 30, 560–565 (1994).
Wohrl, S. et al. A cream containing the chelator DTPA (diethylenetriaminepenta-acetic acid) can prevent contact allergic reactions to metals. Contact Dermatitis 44, 224–228 (2001).
Kaaber, K., Menne, T., Tjell, J. C. & Veien, N. Antabuse treatment of nickel dermatitis. Chelation—a new principle in the treatment of nickel dermatitis. Contact Dermatitis 5, 221–228 (1979).
Christensen, O. B. & Kristensen, M. Treatment with disulfiram in chronic nickel hand dermatitis. Contact Dermatitis 8, 59–63 (1982).
Hopfer, S. M. et al. Increased nickel concentrations in body fluids of patients with chronic alcoholism during disulfiram therapy. Res. Commun. Chem. Pathol. Pharmacol. 55, 101–109 (1987).
White, B. R., Stackhouse, B. T. & Holcombe, J. A. Magnetic gamma-Fe(2)O(3) nanoparticles coated with poly-L-cysteine for chelation of As(III), Cu(II), Cd(II), Ni(II), Pb(II) and Zn(II). J. Hazard. Mater. 161, 848–853 (2009).
Huhtinen, P. et al. Synthesis, characterization, and application of Eu(III), Tb(III), Sm(III), and Dy(III) lanthanide chelate nanoparticle labels. Anal. Chem. 77, 2643–2648 (2005).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Stone, F. G. A. & West, R. Advances in Organometallic Chemistry Vol. 17 (Academic, 1979).
Pearson, R. G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Educ. 64, 561–567 (1987).
Kuo, T. R. et al. Chemical enhancer induced changes in the mechanisms of transdermal delivery of zinc oxide nanoparticles. Biomaterials 30, 3002–3008 (2009).
Midander, K., Pan, J., Wallinder, I. O., Heim, K. & Leygraf, C. Nickel release from nickel particles in artificial sweat. Contact Dermatitis 56, 325–330 (2007).
Matienzo, L. J., Yin, L. I., Grim, S. O. & Swartz, W. E. Jr X-ray photoelectron spectroscopy of nickel compounds. Inorg. Chem. 12, 2762–2769 (1973).
Briggs, D. & Seah, M. P. Practical Surface Analysis: Auger and X-Ray Photoelectron Spectroscopy 2nd edn, Vol. 1 (Wiley, 1990).
Fullerton, A. & Menne, T. In vitro and in vivo evaluation of the effect of barrier gels in nickel contact allergy. Contact Dermatitis 32, 100–106 (1995).
van Ketel, W. G. & Bruynzeel, D. P. Chelating effect of EDTA on nickel. Contact Dermatitis 11, 311–314 (1984).
Kimura, M. & Kawada, A. Contact dermatitis due to trisodium ethylenediaminetetraacetic acid (EDTA) in a cosmetic lotion. Contact Dermatitis 41, 341 (1999).
Soga, F., Izawa, K., Inoue, T., Katoh, N. & Kishimoto, S. Contact dermatitis due to disodium ethylenediaminetetraacetic acid in cosmetics and shampoo. Contact Dermatitis 49, 105 (2003).
Atrux-Tallau, N. et al. Effects of glycerol on human skin damaged by acute sodium lauryl sulphate treatment. Arch. Dermatol. Res. 302, 435–441 (2010).
Fluhr, J. W. et al. Glycerol accelerates recovery of barrier function in vivo. Acta. Derm. Venereol. 79, 418–421 (1999).
Acknowledgements
The authors thank A. Kimball from the Massachusetts General Hospital for helpful discussions. This work was funded by start-up funds from the Brigham and Women's Hospital (J.M.K.). P.K.V. acknowledges the Ewing Marion Kauffman Foundation for an entrepreneur postdoctoral fellowship. The authors also thank D. Blankschtein and B. Polat for the generous gift of pigskin, as well as the MIT Center for Material Science Engineering Imaging facility and P. Boisvert for assistance, and S. Sonis for valuable discussions.
Author information
Authors and Affiliations
Contributions
P.K.V. and J.M.K. conceived and designed the experiments, analysed the data and co-wrote the manuscript. R.R.A. helped design the experiments and write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1098 kb)
Rights and permissions
About this article
Cite this article
Vemula, P., Anderson, R. & Karp, J. Nanoparticles reduce nickel allergy by capturing metal ions. Nature Nanotech 6, 291–295 (2011). https://doi.org/10.1038/nnano.2011.37
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.37
This article is cited by
-
Amorphous silicon dioxide nanoparticles modulate immune responses in a model of allergic contact dermatitis
Scientific Reports (2019)
-
Immunomodulatory Effects of Nanoparticles on Skin Allergy
Scientific Reports (2017)
-
Histopathology effects of nickel nanoparticles on lungs, liver, and spleen tissues in male mice
International Nano Letters (2014)
-
Reactive Oxygen Species-Mediated DNA Damage and Apoptosis in Human Skin Epidermal Cells After Exposure to Nickel Nanoparticles
Biological Trace Element Research (2014)
-
Animal models for nickel allergy
Nature Nanotechnology (2011)