Key Points
-
Protein–protein interactions represent a large and important class of targets for human therapeutics.
-
However, developing small-molecule antagonists of protein–protein interactions is challenging, owing to issues such as the general lack of small-molecule starting points for drug design, the typical flatness of the interface, the difficulty of distinguishing real from artefactual binding, and the size and character of typical small-molecule libraries.
-
This article uses examples to describe general strategies in the development of small molecule antagonists of protein–protein interactions. Two types of antagonists are described: those that bind directly to the 'hot spot'of a protein–protein interface — a region that has a major contribution to high-affinity binding — and those that bind at allosteric sites distal from the protein–protein interface.
-
Finally, characteristics of programmes that have successfully identified small-molecule antagonists of protein–protein interactions are discussed. A high degree of validation is recommended for antagonists of protein–protein interactions owing to the nature of these targets, and a series of steps for validating and characterizing a new series of antagonists are presented.
Abstract
Protein–protein interactions have a key role in most biological processes, and offer attractive opportunities for therapeutic intervention. Developing small molecules that modulate protein–protein interactions is difficult, owing to issues such as the lack of well-defined binding pockets. Nevertheless, there has been important progress in this endeavour in recent years. Here, we use illustrative examples to discuss general strategies for addressing the challenges inherent in the discovery and characterization of small-molecule inhibitors of protein–protein interactions.
Similar content being viewed by others
Main
We dedicate this manuscript to our revered colleague and friend Andrew Braisted, Ph.D. (1964–2003).
Protein–protein interactions are central to most biological processes — from intercellular communication to programmed cell death — and therefore represent a large and important class of targets for human therapeutics. The current excitement surrounding therapeutic antibodies vividly demonstrates the value of such targets1. In the next year, US $5–7 billion will be spent on antibody-based antagonists, the fastest growing segment of the prescription-drug market2,3. As a compound class, therapeutic antibodies have some excellent properties: they are highly specific for their molecular targets and they tend to be very stable in human serum. On the other hand, antibodies suffer from difficulties in manufacture, high costs of goods and the lack of oral bioavailability. In addition, antibodies are not cell-permeable, and antagonism of intracellular protein–protein systems has so far been limited to antisense therapies, which block the expression of the targeted protein. For these reasons, protein–protein interactions have been of great interest to drug discovery; however, developing small-molecule antagonists has been difficult (for recent reviews see Refs 4–9).
Here, we review some of the issues and challenges associated with finding and characterizing small-molecule antagonists. First, we describe some of the general features of protein–protein interfaces. Next, we consider examples of the discovery of antagonists that bind directly to the 'hot spot' of a protein–protein interface, followed by examples of compounds that bind at allosteric sites distal from the protein–protein interface. In the final section, we discuss some of the general features of successful discovery programmes.
Protein–protein interfaces: the challenges
A number of factors can contribute to the challenge of identifying small, organic compounds that inhibit protein–protein interactions. These include the general lack of small-molecule starting points for drug design, the typical flatness of the interface, the difficulty of distinguishing real from artefactual binding, and the size and character of typical small-molecule libraries.
Natural small molecules known to bind at protein–protein interfaces are rare, whereas drug-friendly enzymes often have small-molecule substrates that can serve as templates for designing antagonists. One approach to getting a small-molecule starting point for a protein–protein interface has been to map the epitope of one of the proteins onto a small peptide or peptidomimetic. This approach has been especially successful for small, continuous peptide epitopes, such as for the integrins GPIIbIIIa, αvβ3 and α4β1 (Refs 10–19). Random screening occasionally identifies compounds that bind and even recruit protein–protein complexes. For example, the vinca alkaloids were discovered as cytotoxic compounds, and were later found to affect the polymerization of tubulin20. Cyclosporins cause the formation of novel protein–protein complexes21; drugs themselves can therefore provide starting points for further small-molecule design on these targets. However, these are rare examples, and a general strategy for tackling protein interfaces is still being sought.
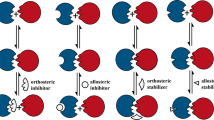
The shape of a typical protein–protein interface adds to the difficulty of drug discovery. Approximately 750–1,500 Å2 of surface area is buried on each side of the interface22, and X-ray structures of protein–protein pairs do not usually show small, deep cavities that look like small-molecule-binding sites. However, it might not be necessary for a small molecule to cover the entire protein-binding surface, because the subset of the interface that contributes to high-affinity binding (the 'hot spot') is often much smaller (Box 1)23,24,25,26. In addition, for many protein–protein interactions, the apparent complementarity between the two surfaces involves a significant degree of protein flexibility and adaptivity27,28. Therefore there might be binding-site conformations that are well-suited to small-molecule binding yet are not visible in a single crystal structure29,30,31. Several studies have reported phage-display selection of small peptides that bind to protein hormones or receptors32,33,34,35,36,37,38,39,40. Strikingly, these randomly selected peptides usually bind at the protein hot spot, even though they were not selected for protein–protein inhibition. These results suggest that hot spots at a protein interface are particularly adept at binding to proteins, peptides and perhaps even small molecules.
Another problem with discovering drug-like small molecules to protein–protein targets is characterizing the stoichiometry and site of binding. Shoichet and co-workers have described a surprisingly common inhibitory mechanism that arises when hydrophobic or amphipathic small molecules form large aggregates, micelles or liposomes41,42,43. Additionally, some compounds act as protein denaturants or covalent inhibitors44,45,46. In these cases, the compounds might inhibit the function of a number of proteins without binding to a discrete site.
Although artefacts might arise for any protein target, they are more difficult to exclude when the target is a protein–protein interaction. These systems are usually screened using an inhibition assay, in which the ratio of compound/protein is very large. In addition, whereas enzyme inhibitors can be characterized for competitive versus ALLOSTERIC binding mechanisms using enzyme kinetics, such experiments are difficult to envision for protein–protein systems. It can even be complex to determine which of the two protein partners binds to the inhibitor, although fitting algorithms are available47. The overall problem is that a plurality of mechanisms might account for the observed inhibition.
Structural and biophysical methods can clarify the binding mechanism. Boehm et al. have demonstrated an efficient use of biophysical methods to characterize the mechanism of inhibitors of DNA gyrase48. Possible inhibitors of this enzyme were identified by in silico screening of the ATP-binding site; 'active' compounds were then screened in a second enzyme assay and analysed qualitatively by methods that address the binding of the compound to protein. These binding methods included analytical ultracentrifugation (AUC), SURFACE PLASMON RESONANCE (SPR), nuclear magnetic resonance (NMR) and X-ray crystallography. Following this battery of tests, seven out of fourteen classes of initial hits were determined to be non-drug-like inhibitors. The same methods have been used qualitatively and quantitatively to define the binding mechanism for protein–protein inhibitors. Additionally, a number of biological experiments, including antibody inhibition and site-directed mutagenesis, have been used to determine whether a series of small-molecule inhibitors of a protein–protein interaction show promise as leads.
Historically, drugs have been highly biased towards a small number of protein classes. It is possible that protein–protein interactions will become more tractable when larger libraries and different types of compounds are tested. This general notion has led to research focused on non-high-throughput-screening (non-HTS) approaches to drug discovery, including structure-based design, in silico screening, and fragment-based discovery. Each of these approaches, including HTS, has been used to identify initial small-molecule antagonists of protein–protein interactions, and these methods have been recently reviewed and evaluated4,8,9. The examples described below focus instead on how compounds are characterized, evaluated and developed into drugs and drug leads.
Competitive antagonists: IL-2 receptor
The cytokine interleukin-2 (IL-2) is a principal mediator of the T-helper immune response. Binding of IL-2 to its trimeric receptor (IL-2R), which contains α, β and γ chains, causes proliferation and differentiation of activated cells49. Clinical data have demonstrated the importance of IL-2 and IL-2Rα in mediating immune disorders; two anti-IL-2Rα antibodies have been approved for use in transplant rejection50,51, and clinical trials for autoimmune diseases are ongoing. The structure of IL-2 is a four-helix bundle. The hot spot for IL-2Rα has been mapped onto the surface of IL-2 by site-directed mutagenesis52,53 (C. Thanos, J. Hyde, J.A.W., personal communication) and NMR54 (Fig. 1a). This binding site has an amphipathic character; one side (to the right in Fig. 1a) is largely hydrophilic and acidic, whereas the other side of the site (to the left in Fig. 1a) is hydrophobic and basic. Recent work indicates that the IL-2Rα binding surface binds to small molecules that complement this amphipathic structure54,55,56,57,58,59.
a | Unliganded interleukin-2 (IL-2)60. b | Ro26-4550 bound to IL-2 (Ref. 56). Hot-spot residues for binding to IL-2Rα are shown in light blue (moderately important) and dark blue (very important), as determined by site-directed mutagenesis52,53. Ro26-4550 is seen to bind at the same hot spot (b), which seems to be highly complementary to the Ro26-4550 structure. On binding of Ro26-4550 and other small-molecule inhibitors, the IL-2 binding surface undergoes a conformational change in the hydrophobic portion of the site (to the left of the structures), while remaining relatively fixed in the guanidine-binding portion of the site (to the right of the structures). The structural mobility of the hydrophobic site has been observed in several structures of IL-2 (Refs 56,58,62).
One of the first examples of a small-molecule inhibitor of a cytokine–receptor interaction is Ro26-4550 (Ref. 55), shown in Fig. 2. This compound was designed as a peptidomimetic of IL-2 and therefore was expected to bind to IL-2Rα. However, careful enzyme-linked immunosorbent assays (ELISA) and 1H-15N heteronuclear single quantum correlation (HSQC) NMR experiments (Box 2) demonstrated that the compound bound to IL-2 itself, at the IL-2Rα-binding site. Ro26-4550, which has moderate affinity (IC50 = 3–6 μM) for IL-2, was not advanced further, perhaps because it lacked cell-based activity59. Nevertheless, this molecule was the first biophysically characterized inhibitor of a cytokine–receptor interaction and clearly indicated that a small molecule could bind at a protein hot spot and inhibit hormone-receptor binding.
IL-2: structural evidence for binding-site adaptivity. Figure 1 compares the X-ray crystal structures of Ro26-4550 bound to IL-2 and unliganded IL-2 (Refs 56,60). The functionality on Ro26-4550 is complementary to the IL-2Rα hot spot; the positively charged guanidine makes two hydrogen bonds with glutamate 62, whereas the hydrophobic biaryl acetylene moiety fits into a groove on the hydrophobic side of the binding site. Importantly, the Ro26-4550-bound structure shows striking changes in the protein conformation in the hydrophobic region, as no groove is apparent in the unliganded structure of IL-2.
Structural and thermodynamic studies indicate that this portion of the protein is inherently flexible; NMR studies of IL-2 indicate that two loops adjacent to this region are somewhat disordered in solution61; several X-ray crystal structures of IL-2 further support this notion, because the structures vary significantly in the hydrophobic portion of the binding site and the adjacent loops56,58,60,62. Finally, thermodynamic measurements of the Ro26-4550–IL-2 interaction indicate that binding is enthalpically driven, with only moderately unfavourable entropy56. So, the structural and thermodynamic experiments indicate that the conformational freedom of IL-2 is reduced by ligand binding, but that this energetic penalty is not large. Binding probably 'captures' a low-energy conformation rather than 'inducing' a high-energy one. The high degree of adaptivity in the hydrophobic portion of the binding site allows for the creation of pockets and grooves in which small molecules can bind.
IL-2: fragment-based discovery of antagonists. A fragment-based approach was used to develop new compounds from Ro26-4550 (Box 3)57,59. The process was devised in four steps. First, Ro26-4550 was divided into its component fragments, a biaryl acetylene amino acid and a piperidyl guanidine. The aromatic fragment was a weak inhibitor (IC50 = 2 mM) and bound to IL-2 as monitored by SPR; the guanidine moiety, by contrast, showed no binding or inhibition up to 10 mM. Previous studies have shown that weakly binding compounds can be productively utilized as chemical starting points, provided that something is known about their binding mechanism and, preferably, their binding location. So, in the second step, the aromatic amino acid was used as the basis fragment to evolve a new linker and guanidine moiety (Fig. 2, compound 2). In the third step, the optimized linker–guanidine fragment formed the basis for a small compound library from which a novel replacement for the aromatic group was found (compound 3).
At each of these three stages, compounds were characterized by a primary inhibition assay, a second inhibition assay (to test for assay-dependent artefacts) and by a panel of biophysical methods (to ensure stoichiometric binding at the IL-2Rα hot-spot)59. Table 1 compares the data from the biophysical methods for a representative set of compounds. SPR and AUC measurements (Box 4) were done at multiple compound/ protein ratios, allowing calculation of the dissociation constant Kd. 1H-15N HSQC measurements (Box 2) demonstrated that compounds bound in approximately the same site as Ro26-4550, and the degree of signal perturbation was correlated with the binding affinity of the compounds. Importantly, compounds as weak as 300 μM demonstrated the same general behaviour as compounds with 3 μM IC50 values, and the rank-order for the binding was the same as that found by inhibition assays. X-ray crystallography of compound 3 verified that the series bound analogously to Ro26-4550. Chemistry, biophysics and structural biology were therefore used together to advance a fragment with millimolar binding affinity into a validated hit with low micromolar activity.
In the fourth step, compound optimization turned to Tethering (Box 3)57,59. Cysteine mutations were placed in the adaptive region of IL-2, adjacent to the binding site for compound 3, and disulphide-containing fragments were screened for binding. Selected fragments tended to be hydrophobic and acidic; X-ray crystallography demonstrated that these fragments bound in more than one sub-site within the hydrophobic region58. Modelling of the fragments into the co-crystal structure of compound 3 and IL-2 suggested that small aromatic acids could be attached to the tri-cyclic compound via a two-atom spacer. A small number of aromatic acids were appended to an analogue of compound 3, and most of these compounds yielded at least a fivefold improvement in inhibition relative to the unmodified tri-cycle. The strongest inhibitor, containing a furanoic acid (SP-4206; Fig. 2), showed 60–100 nM activity in both a protein inhibition assay and an IL-2-binding assay (SPR). The X-ray crystal structure of SP-4206 bound to IL-2 shows the aromatic acid nestled in a positively charged pocket adjacent to the hydrophobic groove occupied by the tri-cycle62. Again, both this hydrophobic pocket and the basic groove are found to be highly adaptive, with both loop movements and side-chain rotations adjusting for optimal compound–protein complementarity.
In summary, biophysical and structural studies were brought together to characterize the mechanism of action for a series of small molecules, built up from fragments, with affinities ranging from 2 mM to 60 nM. Focusing on biophysics in the early stages of discovery ensured that chemical optimization was on the right track, and demonstrated that even weakly binding compounds can show drug-like binding at a protein–protein interface. Furthermore, due to the highly adaptive nature of the IL-2Rα binding site, it would have been difficult to predict the compound optimization pathway, and fragment-based screening proved to be an efficient tool for discovering new binding elements and increasing binding affinity.
Competitive antagonists: B7/CD28
The cell-surface proteins B7-1 and B7-2, found on ANTIGEN-PRESENTING cells, are important modulators of T-cell activation. Binding of the B7 proteins to CD28, found on the T cell, augments activation, whereas binding of B7 to cytotoxic T lymphocyte antigen-4 (CTLA4) reduces activation63,64. One of the complexities of inhibiting B7 function is the high local concentration of ligands at the cell–cell interface. Although the affinity of the B7/CD28 and B7/CTLA4 interactions are low (0.2–20 μM), the high valency ('Velcro effect') caused by receptor clustering might help stabilize cell–cell interactions65,66,67,68. The crystal structure of B7-1 bound to CTLA4 emphasizes the possible multivalency of this protein–protein interaction69,70. Both B7-1 and CTLA4 are homodimers, and they form a periodic, zipper-like pattern in the crystal lattice. Interestingly, there is less evidence for oligomerization of B7-1/CD28. The structure of B7-1 bound to CTLA4 reveals a highly complementary and hydrophobic protein–protein interface with a relatively small buried-surface area (roughly 600 Å2 for each protein; Fig. 3a)69.
a | Structure of B7-1 (surface) bound to CTLA4 (ribbon)69. Both CTLA4 and CD28 bind to B7-1 using an MYPPPY motif (shown in tubes, coloured by atom type). In the co-crystal structure, these residues are found to bind in a shallow depression in the surface of B7-1, creating a highly complementary and compact binding interface. Mutation of Trp 50 (shown in blue) abolishes binding to CD28, CTLA4, and compounds 8 and 9 (Ref. 71). b | Structure of small-molecule inhibitors identified through high-throughput screening71,72.
Wyeth Research has reported the discovery and characterization of small-molecule ligands that bind to B7-1 (Fig. 3b) at the protein–protein hot spot71,72. Compounds 8 and 9, identified through HTS, are described as reversible inhibitors of B7-1/CD28, with IC50 values in the 4–50 nM range. The mechanism of inhibition was investigated using a number of biochemical assays. Initially, binding reversibility71 was determined by dilution experiments (D. Erbe, personal communication). In these experiments, the compound and protein are incubated at high concentrations; when the compound/protein mixture is diluted, the concentration of compound should be too dilute to inhibit the protein's function. Retention of protein activity in these experiments indicates that the compound binds reversibly, but does not address whether the compound inhibits by forming micelles, liposomes or other aggregates. However, three experiments indicate that the compounds are not aggregators or denaturants: first, they are highly specific for human B7-1; second, their activity is not affected by the addition of detergent43 (D. Erbe, personal communication); and third, the compounds bind to human B7-1, but not to proteins that they do not inhibit.
The strongest evidence in support of bona fide hot-spot binding comes from site-directed mutagenesis. Inhibition assays show that compounds 8 and 9 are selective for human B7-1 over mouse B7-1 and human B7-2. In addition, the binding of the compounds to several proteins was monitored by equilibrium dialysis. In equilibrium dialysis, the protein and small molecule are separated by a membrane with a low-molecular-mass cutoff. If the small molecule binds to the protein, it diffuses through the membrane and becomes concentrated on the side containing protein. Quantitative equilibrium dialysis can be used to obtain stoichiometry and dissociation constants; in the present study, dialysis measurements were done for qualitative and comparative purposes. The authors prepared a number of chimeric B7-1 proteins in which regions of the human (hu) sequence were exchanged for the murine amino acids73. This 'homologue scan' demonstrated that sequences needed for huCD28 binding are also required for compound binding71. In addition, a single amino-acid mutation that results in loss of binding of CTLA4 and CD28 also negated binding of the small molecules. Taken together, these experiments indicate that the small molecules bind to B7-1 with a low stoichiometry at the receptor-binding hot spot.
Other binding and activity data make it unclear whether compounds are behaving in a drug-like manner. First, there is a discrepancy between the extent of inhibition and the extent of binding71. Because dialysis measurements were run with protein concentrations well above the IC50 values, the enrichment of compound on the protein side of the dialysis membrane should have been higher than the two- to fivefold observed. Second, although the compounds seem to bind at the CTLA4 binding site, they inhibit the B7-1–CTLA4 interaction very weakly (∼ 10 μM). The authors propose that the difference between the inhibition of CD28 and CTLA4 could be due to the higher valency of the CTLA4–B7-1 interaction65. This explanation is consistent with the lack of inhibition observed in a cell-adhesion assay, which involves highly avid protein–cell interactions. On the other hand, a B7-1-binding Fab fragment was found to inhibit in the adhesion assay, albeit with a 200-fold reduction in activity71. Last, the binding of inhibitors is time-dependent, which could be due to a slow conformational change in the B7-1 binding site, as the authors suggest, or to a non-standard binding mechanism.
On balance, more than one mechanism of action might be needed to explain the data. A direct determination of stoichiometry of binding, and ideally a structure of the complex, would be very helpful for determining how compounds 8 and 9 inhibit the effects of B7-1.
Competitive antagonists: BCL family
B-cell lymphoma-2 (BCL2) and BCL-XL are anti-APOPTOTIC proteins whose function is regulated by the binding of anti- or pro-apoptotic factors such as BAK74,75,76,77. BAK is a member of the pro-apoptotic proteins known as 'BH3 only' proteins because they share homology with the BCL proteins only in the third homology domain78. The binding of BCL-XL to the 16-residue BH3 domain from BAK has been characterized by NMR (Fig. 4)79. The NMR structure indicates that the BAK-derived peptide forms an α-helix and binds in a hydrophobic groove formed by the seven α-helices of BCL-XL. Several laboratories have suggested that small molecules could bind in this hydrophobic groove and inhibit BCL function. It is noteworthy that this site seems to bind amino naphthalene sulphonic acid (ANS), a hydrophobic dye used to detect exposed hydrophobic areas and partially denatured proteins80. It is possible that ANS binding does not occur in the BAK-binding groove (for example, it does not induce apoptosis). Nevertheless, this observation raises the possibility that molecules developed as drug leads might also have issues with low binding specificity.
The α-helical peptide binds in a long, hydrophobic groove in the surface of BCL-XL; hydrophobic residues in the binding site are shown in yellow, whereas charged residues are shown in red (negative charge) and blue (positive charges). The peptide is largely hydrophobic, but contains charged residues that complement the three charged residues in the binding site. Mutagenesis data indicate that several hydrophobic residues and two of the charge–charge interactions are important for binding affinity. BCL, B-cell lymphoma.
Several laboratories have reported the identification of 100 nM–10 μM small-molecule ligands for BCL2 and/or BCL-XL (Fig. 5)80,81,82,83,84,85,86,87. Discovery methods include virtual screening81,84, HTS82,87, ligand-based design86 and mechanistic analysis of a known compound83. In each case, compounds were shown to inhibit binding of BAK peptide and to induce apoptosis in BCL-expressing cell lines. In addition, several compounds were tested in mechanistic and biophysical assays. Degterev et al. showed that their inhibitors (BH3I-1 and -2) are selective for BCL2 and BCL-XL over unrelated proteins82. Tzung, Kim and co-workers used changes in compound fluorescence and ISOTHERMAL CALORIMETRY to show that antimycin A binds to BCL2 and BCL-XL stoichiometrically, with a Kd that is comparable to the IC5080,83.
Four research teams have monitored the 1H-15N HSQC spectrum of BCL-XL in the presence of their compounds. Enyedy and co-workers found that addition of compound 14 causes small and localized perturbations of residues on one end of the peptide-binding groove (the left side in Fig. 4)84; Kutzki et al. identified the other end of the groove as the binding site for their peptidomimetic compound 15 (Ref. 86). Degterev, Lugovskoy and co-workers extended the HSQC experiment to develop a binding structure–activity relationship (SAR) for related compounds82,85. Coupling multiple HSQC measurements with computational modelling, they propose that compounds BH3I-1 and -2 cause the same conformational change in BCL-XL that is observed on binding of BAK peptide. In a novel application of the technology, Jahnke et al. used a combination of HSQC spectroscopy with spin-label enhanced relaxation to demonstrate the binding orientation of compound 16 on BCL-XL88. Spin labels contain unpaired electrons, which dramatically enhance the relaxation of NMR signals in a distance-dependent manner87. Therefore, HSQC cross-peaks near the spin label are no longer observed in the NMR spectrum, and the binding site and orientation of the spin-labelled compound can be estimated. Jahnke et al. found that compound 16 bound in two sites on BCL-XL; using this information, the authors prepared a dimeric molecule with a 10 μM IC50. Taken together, the NMR and other biophysical data make a strong case for the ability for small molecules to bind in the protein-binding groove and to affect the biological function of BCL2 and BCL-XL.
Similarly to the BCL family, the interaction between the oncoproteins p53 and murine double-minute-2 (MDM2) involves the binding of a single α-helix (from p53) in a hydrophobic groove formed by three α-helices (from MDM2). Recent reports show that this protein–protein interaction is also amenable to inhibition by small molecules89,90,91,92. The allosteric binding site in the I-domain of leukocyte function-associated antigen-1 (LFA1, see below) also bears a structural resemblance to the BCL2 and MDM2 binding sites. Therefore, protein–protein interfaces that utilize an α-helix binding groove might be particularly amenable to small-molecule drug discovery.
Allosteric inhibitors: LFA1
Molecules that undergo large conformational changes offer the possibility of allosteric inhibition. Many cell-surface receptors undergo such conformational changes. The insulin receptor, for example, is activated by a conformational change induced by the binding of insulin93; the extracellular protein LFA1 is elaborately regulated by metal ions outside the cell and by signalling pathways inside the cell94. Furthermore, cell-surface receptors, hormones such as nerve growth factor (NGF)95 and enzymes such as nitric oxide synthase (NOS)96 are regulated by oligomerization. These allosteric mechanisms could provide alternative, even multiple, opportunities for small-molecule antagonism.
LFA1 is a member of the integrin family, a well-studied class of cell-surface proteins found primarily on immune cells. By binding to other cell-surface molecules called cell-adhesion molecules (CAMs), integrins mediate cell–cell adhesion, extravasation and T-cell activation97,98. The integrin/CAM family is so far unique among protein–protein systems in that small-molecule antagonists have been discovered for many members. For integrins lacking a 180-amino-acid domain called the 'inserted domain' (I-domain), these antagonists mimic the CAM-binding epitope and bind to the integrin at the receptor's binding site10,11,12,13,14,15,16,17,18,19. LFA1 contains an I-domain, which serves as the binding site for its ligand, intercellular adhesion molecule-1 (ICAM1) (Refs 99,100). Although no small molecules have been shown to bind to LFA1 at the ICAM1 binding site, two classes of allosteric inhibitors have been identified101. (In addition, Sanfilippo et al. have reported a series of tri-cyclic compounds that inhibit the LFA1/ICAM1 interaction, but the mechanism has not been characterized102.)
Binding of ICAM1 to LFA1 seems to be regulated by conformational changes in LFA1 and by clustering of LFA1 on the surface of activated cells99,103,104. The current model for conformational regulation proposes that changes in the ICAM-binding I-domain are coupled to the rest of the protein through the interaction of the α7 helix in the I-domain with the 'I-like' domain in the β-chain of LFA1 (Fig. 6a)94,105,106,107. One class of allosteric small-molecule inhibitors binds to the inactive conformation of the I-domain, in a deep, hydrophobic cleft next to the α7 helix108,109,110. The other class of allosteric inhibitors seems to bind at the junction between the I-domain and the I-like domain, blocking the interaction of the α7 helix with the I-like domain111,112,113.
a | Models for allosteric inhibition94. The α- and β- chains of LFA1 (αL and CD18, respectively) interact with each other at the headpiece domains. In the active conformation of LFA1, ICAM1 binds to the I-domain, which is held in the active conformation through interactions between the α7 helix with the I-like domain. α/β I-like domain antagonists such as compound 20 (Fig. 7) bind at the junction between the α and β chains, interrupting the α7/I-like domain interaction. I-domain antagonists bind in a hydrophobic groove next to the α7 helix, which also interrupts the α7/I-like domain interaction. b | Structure of lovastatin (yellow tubes, coloured by atom type; Fig. 7) bound to the I-domain of LFA-1 (surface; coloured by atom type)108. Lovastatin is found in a deep, hydrophobic pocket adjacent to the α7 helix. Part a reproduced with permission from Ref. 94 © Macmillan Magazines Ltd (2003).
I-domain allosteric antagonists. Three distinct series of compounds have been shown to bind in the hydrophobic cleft in the I-domain of LFA1 (Fig. 7). A high-throughput screen identified lovastatin as a low-micromolar inhibitor of the LFA1–ICAM interaction108. Optimization of this scaffold and a diazapane scaffold resulted in compounds with IC50 values in the 100 nM range114,115. A hydantoin series, represented by BIRT377, was found through HTS to be a nanomolar inhibitor of LFA1–ICAM116. A third series, represented by compound 19, was initially discovered through HTS and then optimized through medicinal chemistry and a fragment-discovery method called SAR by NMR (Box 3)110,117,118,119,120. Because these compounds did not resemble known integrin antagonists, significant effort went into understanding how they bound to and inhibited LFA1.
Structural biology and I-domain antagonists. The isolated I-domain of LFA1 has been recombinantly expressed and characterized by NMR121 and X-ray crystallography122. 1H-15N HSQC NMR spectroscopy of the I-domain in the presence and absence of lovastatin shows that the residues near the ICAM-binding metal-ion binding site (MIDAS) are not affected by lovastatin, whereas the residues in the hydrophobic crevice between helix 1 and helix 7 are strongly affected108. The X-ray crystal structure of the lovastatin–I-domain complex was also solved, verifying the NMR results and providing a detailed description of the small-molecule-binding site (Fig. 6b). A compound analogous to BIRT377 was also crystallized and was shown to bind in the same region of the I-domain109. By analogy to other I-domains, it was proposed that this hydrophobic pocket is filled by α7 in the active, ICAM-binding conformation of LFA1, and that the presence of the compound locks the I-domain — and LFA1 — into the inactive conformation108,110,113. This hypothesis is consistent with the rigid-body motion of the α7 helix observed by NMR and crystallography105,121,123. The hydrophobic groove created by movement of the α7 helix is, like IL-2, an example of an adventitious small-molecule-binding site resulting from the protein's conformational flexibility.
Mapping the binding of I-domain antagonists. For many small-molecule–protein interactions, high-resolution structural data are difficult to obtain. The research team at Boehringer Ingelheim has developed mass spectrometry (MS)-based methods to obtain binding-site information for hydantoin antagonists of LFA1 in the absence of NMR or crystallography109,124. A BIRT377 analogue with a photo-affinity label was synthesized and reacted with the LFA1 I-domain under ultraviolet light. The protein was then digested with trypsin and analysed by MS; careful studies indicated that the probe specifically labelled the I-domain at proline 281. This information led to a binding hypothesis that was refined by computational modelling and later verified by X-ray crystallography109.
An MS method was also used to measure the noncovalent binding of molecules to the I-domain in the gas phase124. The observed mass spectrum correlated with 1:1 binding of the compound and protein, but the ratio of bound: unbound protein did not correlate with binding affinity. This result is not surprising, because gas-phase stability is often very different from solution-phase affinity. An interesting observation from these studies was the change in charge distributions in the MS signal. When a protein is ionized by electrospray ionization, a series of charge states, corresponding to different protonation states of the protein, are observed. Changes in this distribution are indicative of changes in protein structure due to denaturation, changes in conformation or masking of ionizable groups. The binding of hydantoin compounds reduced the amount of denatured and oligomerized I-domains, and reduced the yield of the +9 charged state in favour of the +8. Taken together, these changes suggest that the compounds rigidify the I-domain structure, consistent with their function as allosteric inhibitors.
Antibodies have also been used to assess the allosteric effects of small molecules on the conformation of full-length LFA1. The binding of antibodies to the I-like domain, the I-domain and other regions of LFA1 were monitored in the presence of a series of lovastatin derivatives113 and BIRT377 (Refs 116,125,126). These compounds were found to inhibit antibody binding to certain epitopes on the I-domain, but to have no effect on other portions of the protein. One of the lovastatin derivatives inhibited the binding of certain I-like domain antibodies, suggesting that this compound accesses new binding pockets that are closely linked to the I-like domain113. Using antibodies that recognize the active conformation of LFA1, Woska et al. demonstrated that the I-domain antagonists maintain LFA1 in an inactive state, even in the presence of activating metal ions125. In summary, antibody mapping and MS studies demonstrate that valuable information about the binding of small-molecules can be obtained in the absence of high-resolution structures.
Mapping the binding of I-domain antagonists: α/β I-like allosteric antagonists. The series of compounds represented by compound 20 (Fig. 7)111,127 has a different mechanism of action from the I-domain ligands described above. It was suggested that these compounds mimic the binding epitope of ICAM1 by mapping side-chain functionality onto a small molecule scaffold111. However, biochemical and biophysical measurements do not support the proposal that the compounds bind at the ICAM1-binding site112,113. For example, 1H-15N HSQC NMR113 and SPR measurements112 do not show direct binding of the compounds to the I-domain. Furthermore, compounds bind to a version of LFA1 in which the I-domain has been deleted. Nevertheless, these compounds are highly potent inhibitors, and it is therefore interesting to determine their mechanism of action.
A combination of biochemical measurements indicate that compounds in this series bind at the junction between the I- and I-like domains of LFA1 and function as allosteric antagonists112. Ligand-like antagonists of integrins lacking an I-domain128 typically stabilize the integrin heterodimer towards dissociation in sodium dodecyl (lauryl) sulphate (SDS) solution. When compound-20-like molecules were tested in this assay, gel electrophoresis showed that LFA1 was similarly stabilized, suggesting that compounds bound to a site at the dimer interface112. Next, Shimaoka et al. prepared disulphide mutations that lock the I-domain into the active or inactive conformation. Compounds bound to both conformations but did not block ICAM1 binding to the activated mutant, suggesting that inhibition occurs by an allosteric mechanism. Finally, the binding of LFA1 antibodies in the presence of compound 20 and its analogues was monitored112,113. In contrast to the I-domain ligands, these compounds did not inhibit binding of I-domain antibodies, but did inhibit binding of I-like domain antibodies. Taken together, these data suggest that compound 20 and its analogues bind at the bridge between the α- and β-chains, blocking the interaction between the I-like domain and the α7 helix in the I-domain (Fig. 6a). This binding site is analogous to the ligand-mimetic antagonists of integrins not containing an I-domain.
Drug-development studies with I-domain and α/β I-like domain antagonists demonstrate the potential for small-molecule inhibition through allosteric regulation. The fact that three binding sites (the active site and two allosteric sites) have been found for LFA1 indicates that there might even be several ways to inhibit complex protein signalling systems. In addition, research into the mechanisms of action of these inhibitors underscores the power of combining biochemistry, biophysics and structural biology. Several of the molecular series were identified through functional screens; nevertheless, uncovering the mechanism of binding was important for advancing the initial compounds. Even when the structure of a compound suggests a binding hypothesis, it is important to validate the actual binding site through biophysical and structural means. Finally, both classes of allosteric compounds provide useful drug leads for LFA1, and have furthermore served as probes for understanding the allosteric regulation of this important class of adhesion proteins.
Allosteric inhibitors: inducible NOS
Nitric oxide synthase (NOS) enzymes are multidomain, haem-containing proteins that generate NO from L-arginine96. Two isoforms of NOS are constitutively expressed in endothelium, where NO radical plays a positive role in angiogenesis and vascular health129, and in neurons, where both positive and negative effects have been observed130,131. NO produced by inducible NOS (iNOS), on the other hand, is implicated in tissue damage during inflammation132,133, prompting researchers to look for inhibitors that are selective for iNOS134,135,136. Two laboratories have reported the identification of molecules (Fig. 8) that allosterically inhibit the function of iNOS by binding to the haem cofactor in the protein active site and disrupting protein dimerization137,138,139,140.
a | BBS-2 and PPA250 are potent inhibitors of inducible nitric oxide synthase (iNOS) dimerization during protein synthesis137,138. b | Structure of BBS-2 bound to iNOS, viewed from the dimerization face137. Monomeric iNOS (white ribbon/surface) was made by deletion mutagenesis. BBS-2 (yellow tubes, coloured by atom type) binds to the iron atom in the haem cofactor (green tubes coloured by atom type) and causes helices at the dimerization face to become disordered.
PPA250, BBS-2 and related compounds were found to inhibit dimerization during protein synthesis with inhibition constants (Ki) in the low nanomolar range, but not to disrupt preformed iNOS dimers137,138. The unusual mechanism of BBS-2 activity was further characterized by radioligand binding, absorbance spectroscopy and X-ray crystallography. First, radiolabelled BBS-2 was shown to bind to haem-containing iNOS monomers stoichiometrically and reversibly, with a dissociation rate of 200 minutes at 22 °C139. Absorbance spectroscopy indicated that the imidazole moiety of BBS-2 bound directly to the haem cofactor, as expected. The X-ray crystal structure of BBS-2 bound to the monomeric oxygenase domain of iNOS (Fig. 8) was consistent with the proposed mechanism, and provided clues as to how ligand binding could disrupt dimerization by disrupting the structure of α-helices near the protein–protein interface137. Both BBS-2 and PPA250 were shown to be active in animal models of disease138,140,141,142. Again, mechanistic, structural and biological studies worked together to validate the allosteric inhibition of this protein–protein interaction.
Allosteric inhibitors: nerve growth factor
Nerve-growth factor (NGF) is a 27-kDa homodimer whose binding to neuronal cells can cause neurite growth or cell death, depending on the expression of two cell-surface receptors, TRKA (pro-growth) and p75NTR (pro-apoptotic)95,143. The hot spots for binding to the two receptors are distinct, and the two receptors can bind simultaneously144,145,146. Niederhauser et al. have published the characterization of a small molecule, Ro 028-2750 (Fig. 9), which binds to NGF (IC50 ∼ 1 μM) and inhibits binding to p75NTR selectively over TRKA147. This selectivity was then used to dissect the functions of p75NTR and TRKA in neuronal differentiation, apoptosis and neurite outgrowth.
Small-molecule antagonist of nerve growth factor147.
The authors propose that Ro 028-2750 causes a conformational change in NGF that abolishes binding selectively to p75NTR at submicromolar concentrations and to both p75NTR and TRKA at higher concentrations147. AUC and fluorescence quenching measurements demonstrated ∼1:1 binding between the small molecule and the NGF dimer. Inhibition of NGF–p75NTR binding was measured by crosslinking 125I-NGF to cells expressing p75NTR followed by gel electrophoresis. Inhibition of the hormone–receptor interaction was found to be time-dependent, leading the authors to conclude that Ro 028-2750 causes a conformational change in NGF over time. No direct evidence is provided for a conformational change or for binding at the dimer interface.
The interesting biological effects of Ro 028-2750 would be augmented by further clarification of the molecule's mechanism of action. To use the compound as a tool for understanding biology, it would be relevant to understand the selectivity of Ro 028-2750 for NGF over other proteins. In addition, time-dependent inhibition can be due to conformational change, but can also be a symptom of covalent binding. Ro 028-2750 contains an aldehyde, which can covalently modify proteins148,149; it would therefore be valuable to demonstrate that Ro 028-2750 binds to NGF reversibly45. Evidence for binding to the dimer interface would also be of interest. Finally, as it is known that the two receptors for NGF have different binding epitopes146, site-directed mutagenesis might further clarify the small-molecule binding site.
Conclusions
There are now a number of reports of small-molecule inhibitors of protein–protein interactions. Have patterns emerged? It seems that the approaches for initial compound discovery — HTS, computational screening, fragment discovery — have many of the same advantages, and encounter the same hurdles, for protein–protein systems as for more traditional therapeutic targets. It is likely that, as with traditional targets, a variety of discovery approaches will be needed. Given the general difficulty of this class, selection of a tractable protein–protein system is also important. On the basis of the data so far, good targets for small-molecule inhibition are those that have small hot spots that can be covered by a drug-sized molecule, and perhaps those hot spots that have demonstrated binding to small peptides. Furthermore, although it seems obvious that proteins must have cavities for molecules to bind, the cavities themselves might not be obvious from an initial inspection. They might be found away from the binding interface in an allosteric site, or they might be found in cryptic sites within the adaptive regions of the protein hot spot.
Drug discovery is also crucially augmented by the availability of orthogonal methods of characterization; such methods include biophysics, mutagenesis, epitope mapping and structural biology. Box 5 and Table 2outline some of these experiments, their uses and their limitations. In general, a novel molecule can be described as 'validated' when it has been shown to bind noncovalently with 1:1 binding stoichiometry to the target of interest. In the cases in which the data are unexpected — as when the compound inhibits in cells but not in vitro, or vice versa — added emphasis should be placed on understanding the mechanism of inhibition. Knowledge of the precise binding site is also advantageous, and therefore NMR and X-ray crystallography have key roles in several of the examples described in this review. In some cases, crystallography is used to identify the binding site or to generate hypotheses for structure-based design. In other cases, high-resolution structural data back up results from other methods. Such confidence building is important, especially when the methods are new and the drug targets novel, but it should be noted that a combination of chemical and biological approaches do provide valuable information. Often, it is the combination of methods, rather than any one experiment, that propel a drug-discovery project forward.
Protein–protein interactions are clearly more challenging than drug targets that naturally bind small molecules. However, there have been clear inroads into these targets. The drug discovery community understands more about what kinds of binding sites might be more tractable than others. We have developed better ways of screening diversity space (both empirically and computationally) for new compounds that interact with these sites. We are also improving screening and characterization protocols that allow more accurate selection of advance-able molecules. This field is still in its infancy, but given the progress that has been made and the importance of this target class, it is likely to receive increased attention in the future.
References
Stockwin, L. & Holmes, S. Antibodies as therapeutic agents: vive la renaissance! Expert Opin. Biol. Ther. 3, 1133–1152 (2003).
Das, R. C. & Morrow, K. J. J. in Antibody Engineering Report (Drug and Market Development Publications, 2002).
Monoclonal Antibodies Report 2003: Meeting Clinical and Financial Expectations (Visiongain, 2003).
Berg, T. Modulation of protein–protein interactions with small organic molecules. Angew. Chem. Int. Ed. Engl. 42, 2462–2481 (2003).
Cochran, A. G. Protein–protein interfaces: mimics and inhibitors. Curr. Opin. Chem. Biol. 5, 654–659 (2001).
Gadek, T. R. & Nicholas, J. B. Small molecule antagonists of proteins. Biochem. Pharmacol. 65, 1–8 (2003).
Ockey, D. A. & Gadek, T. R. Inhibitors of protein–protein interactions. Expert Opin. Ther. Pat. 12, 393–400 (2002).
Sharma, S. K., Ramsey, T. M. & Bair, K. W. Protein–protein interactions: lessons learned. Curr. Med. Chem. Anti-Canc. Agents 2, 311–330 (2002).
Toogood, P. L. Inhibition of protein–protein association by small molecules: approaches and progress. J. Med. Chem. 45, 1–16 (2002).
Sulyok, G. A. et al. Solid-phase synthesis of a nonpeptide RGD mimetic library: new selective αvβ3 integrin antagonists. J. Med. Chem. 44, 1938–1950 (2001).
Goodman, S. L., Holzemann, G., Sulyok, G. A. & Kessler, H. Nanomolar small molecule inhibitors for αv(β)6, αv(β)5, and αv(β)3 integrins. J. Med. Chem. 45, 1045–1051 (2002).
Gibson, C. et al. Nonpeptidic α(v)β(3) Integrin antagonist libraries: on-bead screening and mass spectrometric identification without tagging. Angew. Chem. Int. Ed. Engl. 40, 165–169 (2001).
Hoekstra, W. J. & Poulter, B. L. Combinatorial chemistry techniques applied to nonpeptide integrin antagonists. Curr. Med. Chem. 5, 195–204 (1998).
Mousa, S. A. Anti-integrin as novel drug-discovery targets: potential therapeutic and diagnostic implications. Curr. Opin. Chem. Biol. 6, 534–541 (2002).
Scarborough, R. M. & Gretler, D. D. Platelet glycoprotein IIb-IIIa antagonists as prototypical integrin blockers: novel parenteral and potential oral antithrombotic agents. J. Med. Chem. 43, 3453–3473 (2000).
Eldred, C. D. & Judkins, B. D. Fibrinogen receptor antagonists: design and clinical applications. Prog. Med. Chem. 36, 29–90 (1999).
Ojima, I., Chakravarty, S. & Dong, Q. Antithrombotic agents: from RGD to peptide mimetics. Bioorg. Med. Chem. 3, 337–360 (1995).
Gadek, T. R. & McDowell, R. S. Discovery of small molecule leads in a biotechnology datastream. Drug Discov. Today 8, 545–550 (2003).
Jackson, D. Y. α4 integrin antagonists. Curr. Pharm. Des. 8, 1229–1253 (2002).
Jordan, M. A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anti-Canc. Agents 2, 1–17 (2002).
Schreiber, S. L. & Crabtree, G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today 13, 136–142 (1992).
Lo Conte, L., Chothia, C. & Janin, J. The atomic structure of protein–protein recognition sites. J. Mol. Biol. 285, 2177–2198 (1999).
Bogan, A. A. & Thorn, K. S. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 280, 1–9 (1998).
Clackson, T. & Wells, J. A. A hot spot of binding energy in a hormone-receptor interface. Science 267, 383–386 (1995).
DeLano, W. L. Unraveling hot spots in binding interfaces: progress and challenges. Curr. Opin. Struct. Biol. 12, 14–20 (2002).
Ma, B., Elkayam, T., Wolfson, H. & Nussinov, R. Protein–protein interactions: structurally conserved residues distinguish between binding sites and exposed protein surfaces. Proc. Natl Acad. Sci. USA 100, 5772–5777 (2003).
Sundberg, E. J. & Mariuzza, R. A. Luxury accommodations: the expanding role of structural plasticity in protein–protein interactions. Structure Fold. Des. 8, R137–R142 (2000).
DeLano, W. L., Ultsch, M. H., de Vos, A. M. & Wells, J. A. Convergent solutions to binding at a protein–protein interface. Science 287, 1279–1283 (2000). The protein–protein hot spot on the Fc domain of immunoglobulin G is characterized by comparing several structures of the protein bound to its natural ligands and to a synthetic peptide. The hot spot is found to be more involuted and hydrophobic than most of the surface; in addition, the conformation of the binding site varies to complement each protein partner.
Teague, S. J. Implications of protein flexibility for drug discovery. Nature Rev. Drug Discov. 2, 527–541 (2003).
Luque, I. & Freire, E. Structural stability of binding sites: consequences for binding affinity and allosteric effects. Proteins S4, 63–71 (2000).
Ma, B., Shatsky, M., Wolfson, H. J. & Nussinov, R. Multiple diverse ligands binding at a single protein site: a matter of pre-existing populations. Protein Sci. 11, 184–197 (2002).
Sidhu, S. S., Fairbrother, W. J. & Deshayes, K. Exploring protein–protein interactions with phage display. Chembiochem. 4, 14–25 (2003). Recent review describing how phage display has been used to probe protein hot spots and identify novel peptide agonists/antagonists of protein–protein interactions.
Pillutla, R. C. et al. Peptides identify the critical hotspots involved in the biological activation of the insulin receptor. J. Biol. Chem. 277, 22590–22594 (2002).
Fairbrother, W. J. et al. Novel peptides selected to bind vascular endothelial growth factor target the receptor-binding site. Biochemistry 37, 17754–17764 (1998).
Lowman, H. B. Bacteriophage display and discovery of peptide leads for drug development. Annu. Rev. Biophys. Biomol. Struct. 26, 401–424 (1997).
Schaffer, M. L., Deshayes, K., Nakamura, G., Sidhu, S. & Skelton, N. J. Complex with a phage display-derived peptide provides insight into the function of insulin-like growth factor I. Biochemistry 42, 9324–9334 (2003).
Nakamura, G. R., Reynolds, M. E., Chen, Y. M., Starovasnik, M. A. & Lowman, H. B. Stable 'zeta' peptides that act as potent antagonists of the high-affinity IgE receptor. Proc. Natl Acad. Sci. USA 99, 1303–1308 (2002).
Wrighton, N. C. et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science 273, 458–464 (1996).
Cwirla, S. E. et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science 276, 1696–1699 (1997).
Scott, J. K. et al. Evidence that a protein–protein interaction 'hot spot' on heterotrimeric G protein βγ subunits is used for recognition of a subclass of effectors. EMBO J. 20, 767–776 (2001).
McGovern, S. L., Helfand, B. T., Feng, B. & Shoichet, B. K. A specific mechanism of nonspecific inhibition. J. Med. Chem. 46, 4265–4272 (2003).
Seidler, J., McGovern, S. L., Doman, T. N. & Shoichet, B. K. Identification and prediction of promiscuous aggregating inhibitors among known drugs. J. Med. Chem. 46, 4477–4786 (2003).
McGovern, S. L., Caselli, E., Grigorieff, N. & Shoichet, B. K. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J. Med. Chem. 45, 1712–1722 (2002). References 41–43 describe methods for identifying complexes that cause artefactual inhibition by an aggregating mechanism. This mechanism seems to be common at compound concentrations in the mid-micromolar range.
Carter, P. H. et al. Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF-α. Proc. Natl Acad. Sci. USA 98, 11879–11884 (2001).
Wiekowski, M. et al. Characterization of potential antagonists of human interleukin 5 demonstrates their cross-reactivity with receptors for interleukin 3 and granulocyte–macrophage colony-stimulating factor. Eur. J. Biochem. 246, 625–632 (1997). References 44 and 45 describe the characterization of covalent inhibitors of protein–protein interactions. Interestingly, neither compound was initially intended for use as a covalent modifier, but this mechanism was identified through careful analysis.
Way, J. C. Covalent modification as a strategy to block protein–protein interactions with small-molecule drugs. Curr. Opin. Chem. Biol. 4, 40–46 (2000).
Woska, J. R. Jr, Morelock, M. M., Jeanfavre, D. D. & Bormann, B. J. Characterization of molecular interactions between intercellular adhesion molecule-1 and leukocyte function-associated antigen-1. J. Immunol. 156, 4680–4685 (1996).
Boehm, H. J. et al. Novel inhibitors of DNA gyrase: 3D structure based biased needle screening, hit validation by biophysical methods, and 3D guided optimization. A promising alternative to random screening. J. Med. Chem. 43, 2664–2664 (2000). This paper describes the efficient use of biophysical screens to assign priority to a series of early-stage compounds; half of the initial series are determined to inhibit by an acceptable mechanism.
Nelson, B. H. & Willerford, D. M. Biology of the interleukin-2 receptor. Adv. Immunol. 70, 1–81 (1998).
Berard, J. L., Velez, R. L., Freeman, R. B. & Tsunoda, S. M. A review of interleukin-2 receptor antagonists in solid organ transplantation. Pharmacotherapy 19, 1127–1137 (1999).
Waldmann, T. A. & O'Shea, J. The use of antibodies against the IL-2 receptor in transplantation. Curr. Opin. Immunol. 10, 507–512 (1998).
Zurawski, S. M. et al. Definition and spatial location of mouse interleukin-2 residues that interact with its heterotrimeric receptor. EMBO J. 12, 5113–5119 (1993).
Sauve, K. et al. Localization in human interleukin 2 of the binding site to the α chain (p55) of the interleukin 2 receptor. Proc. Natl Acad. Sci. USA 88, 4636–4640 (1991).
Emerson, S. D. et al. NMR characterization of interleukin-2 in complexes with the IL-2Rα receptor component, and with low molecular weight compounds that inhibit the IL-2/IL-Rα interaction. Protein Sci. 12, 811–822 (2003).
Tilley, J. W. et al. Identification of a small molecule inhibitor of the IL-2/IL-2Rα receptor interaction which binds to IL-2. J. Am. Chem. Soc. 119, 7589–7590 (1997). References 54 and 55 describe an early and well-characterized small-molecule inhibitor of a protein–protein interaction.
Arkin, M. R. et al. Binding of small molecules to an adaptive protein:protein interface. Proc. Natl Acad. Sci. USA 100, 1603–1608 (2003).
Braisted, A. C. et al. Discovery of a potent small molecule IL-2 inhibitor through fragment assembly. J. Am. Chem. Soc. 125, 3714–3715 (2003).
Hyde, J., Braisted, A. C., Randal, M. & Arkin, M. R. Discovery and characterization of cooperative ligand binding in the adaptive region of interleukin-2. Biochemistry 42, 6475–6483 (2003).
Raimundo, B. C. et al. Discovery of small-molecule inhibitors of interleukin-2 using fragment assembly. J. Med. Chem. (in the press). References 56–59 (and 62 below) detail an interdisciplinary approach, integrating fragment-based discovery, medicinal chemistry, and structural biology, to discovering inhibitors of the IL-2/IL-2R interaction.
McKay, D. B. Unraveling the structure of interleukin-2: reply. Science 257, 412–413 (1992).
Mott, H. R. et al. The solution structure of the F42A mutant of human interleukin 2. J. Mol. Biol. 247, 979–994 (1995).
Thanos, C., Randal, M. & Wells, J. A. Potent small-molecule binding to a dynamic hot spot on IL-2. J. Am. Chem. Soc. 125, 15280–15281 (2003).
Harding, F. A., McArthur, J. G., Gross, J. A., Raulet, D. H. & Allison, J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356, 607–609 (1992).
Walunas, T. L. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413 (1994).
van der Merwe, P. A. & Davis, S. J. Molecular interactions mediating T cell antigen recognition. Annu. Rev. Immunol. 21, 659–684 (2003).
Collins, A. V. et al. The interaction properties of costimulatory molecules revisited. Immunity 17, 201–210 (2002).
Egen, J. G., Kuhns, M. S. & Allison, J. P. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature Immunol. 3, 611–618 (2002).
Darlington, P. J. et al. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J. Exp. Med. 195, 1337–1347 (2002).
Stamper, C. C. et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 410, 608–611 (2001).
Ikemizu, S. et al. Structure and dimerization of a soluble form of B7-1. Immunity 12, 51–60 (2000).
Erbe, D. V., Wang, S., Xing, Y. & Tobin, J. F. Small molecule ligands define a binding site on the immune regulatory protein B7. 1. J. Biol. Chem. 277, 7363–7368 (2002).
Green, N. J. et al. Structure-activity studies of a series of dipyrazolo[3,4-b:3',4'-d]pyridin-3-ones binding to the immune regulatory protein B7.1. Bioorg. Med. Chem. 11, 2991–3013 (2003). References 71 and 72 report the chemical SAR and biochemical characterization of small-molecule inhibitors of B7-1.
Wang, S. et al. Antibodies to B7. 1 define the GFCC'C' face of the N-terminal domain as critical for co-stimulatory interactions. Immunol. Lett. 83, 77–83 (2002).
Huang, Z. The chemical biology of apoptosis. Exploring protein–protein interactions and the life and death of cells with small molecules. Chem. Biol. 9, 1059–1072 (2002). Review of small-molecule inhibitors of the Bcls and other targets in the apoptotic pathway.
Burlacu, A. Regulation of apoptosis by Bcl-2 family proteins. J. Cell. Mol. Med. 7, 249–257 (2003).
Gross, A., McDonnell, J. M. & Korsmeyer, S. J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899–1911 (1999).
Cory, S. & Adams, J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nature Rev. Cancer 2, 647–656 (2002).
Huang, D. C. & Strasser, A. BH3-only proteins — essential initiators of apoptotic cell death. Cell 103, 839–842 (2000).
Sattler, M. et al. Structure of Bcl-XL-Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997).
Kim, K. M. et al. Biophysical characterization of recombinant human Bcl-2 and its interactions with an inhibitory ligand, antimycin A. Biochemistry 40, 4911–4922 (2001).
Wang, J. L. et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl Acad. Sci. USA 97, 7124–7129 (2000).
Degterev, A. et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-XL . Nature Cell Biol. 3, 173–182 (2001).
Tzung, S. P. et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nature Cell Biol. 3, 183–191 (2001).
Enyedy, I. J. et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J. Med. Chem. 44, 4313–4124 (2001).
Lugovskoy, A. A. et al. A novel approach for characterizing protein ligand complexes: molecular basis for specificity of small-molecule Bcl-2 inhibitors. J. Am. Chem. Soc. 124, 1234–1240 (2002).
Kutzki, O. et al. Development of a potent Bcl-XL antagonist based on α-helix mimicry. J. Am. Chem. Soc. 124, 11838–11839 (2002).
Jahnke, W. et al. Second-site NMR screening and linker design. Curr. Top. Med. Chem. 3, 69–80 (2003).
Jahnke, W. et al. Second-site NMR screening with a spin-labeled first ligand. J. Am. Chem. Soc. 122, 7394–7395 (2000). References 84–88 outline a range of NMR-based methods to characterize the binding of small-molecule inhibitors of BCL-X L.
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004). This report describes the activity of 'Nutlins,' small molecules that bind in the p53-helix-binding groove of MDM2. The X-ray crystal structure of Nutlin-2 bound to MDM2 shows the compound bound analogously to p53; compounds were also shown to be active in cell-based and xenograft models of cancer.
Zhao, J. et al. The initial evaluation of non-peptidic small-molecule HDM2 inhibitors based on p53-HDM2 complex structure. Cancer Lett. 183, 69–77 (2002).
Stoll, R. et al. Chalcone derivatives antagonize interactions between the human oncoprotein MDM2 and p53. Biochemistry 40, 336–344 (2001).
Duncan, S. J. et al. Isolation and structure elucidation of Chlorofusin, a novel p53-MDM2 antagonist from a Fusarium sp. J. Am. Chem. Soc. 123, 554–560 (2001).
Yip, C. C. & Ottensmeyer, P. Three-dimensional structural interactions of insulin and its receptor. J. Biol. Chem. 278, 27329–27332 (2003).
Shimaoka, M. & Springer, T. A. Therapeutic antagonists and conformational regulation of integrin function. Nature Rev. Drug Discov. 2, 703–716 (2003). Reviews the small-molecule inhibitors of integrins. Describes how I-domain antagonists regulate ICAM1 binding and provides a clear summary of the authors' model for the mechanism of 'α/β-allosteric inhibitors' (see reference 112).
Wiesmann, C. & de Vos, A. M. Nerve growth factor: structure and function. Cell. Mol. Life Sci. 58, 748–759 (2001).
Alderton, W. K., Cooper, C. E. & Knowles, R. G. Nitric oxide synthases: structure, function and inhibition. Biochem J. 357, 593–615 (2001).
Humphries, M. J. Integrin structure. Biochem. Soc. Trans. 28, 311–339 (2000).
Yusuf-Makagiansar, H., Anderson, M. E., Yakovleva, T. V., Murray, J. S. & Siahaan, T. J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 22, 146–167 (2002).
Shimaoka, M. et al. Structures of the α L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99–111 (2003).
Randi, A. M. & Hogg, N. I domain of β 2 integrin lymphocyte function-associated antigen-1 contains a binding site for ligand intercellular adhesion molecule-1. J. Biol. Chem. 269, 12395–12398 (1994).
Liu, G. Small molecule antagonists of the LFA-1/ICAM-1 interaction as potential therapeutic agents. Expert Opin. Ther. Patents 11, 1383–1393 (2001).
Sanfilippo, P. J. et al. Novel thiazole based heterocycles as inhibitors of LFA-1/ICAM-1 mediated cell adhesion. J. Med. Chem. 38, 1057–1059 (1995).
Hogg, N., Laschinger, M., Giles, K. & McDowall, A. T-cell integrins: more than just sticking points. J. Cell Sci. 116, 4695–4705 (2003).
Carman, C. V. & Springer, T. A. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 15, 547–556 (2003).
Huth, J. R. et al. NMR and mutagenesis evidence for an I domain allosteric site that regulates lymphocyte function-associated antigen 1 ligand binding. Proc. Natl Acad. Sci. USA 97, 5231–5236 (2000).
Lupher, M. L. Jr et al. Cellular activation of leukocyte function-associated antigen-1 and its affinity are regulated at the I domain allosteric site. J. Immunol. 167, 1431–1439 (2001).
Lu, C., Shimaoka, M., Zang, Q., Takagi, J. & Springer, T. A. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc. Natl Acad. Sci. USA 98, 2393–2398 (2001).
Kallen, J. et al. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J. Mol. Biol. 292, 1–9 (1999). Presents the only published X-ray crystal structure of an LFA1 antagonist bound to the I-domain. This research group has used a variety of methods to demonstrate how I-domain inhibitors function and to show their biological relevance.
Last-Barney, K. et al. Binding site elucidation of hydantoin-based antagonists of LFA-1 using multidisciplinary technologies: evidence for the allosteric inhibition of a protein–protein interaction. J. Am. Chem. Soc. 123, 5643–5650 (2001).
Liu, G. et al. Novel p-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction. 2. Mechanism of inhibition and structure-based improvement of pharmaceutical properties. J. Med. Chem. 44, 1202–1010 (2001).
Gadek, T. R. et al. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science 295, 1086–1089 (2002).
Shimaoka, M., Salas, A., Yang, W., Weitz-Schmidt, G. & Springer, T. A. Small molecule integrin antagonists that bind to the β2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity 19, 391–402 (2003).
Welzenbach, K., Hommel, U. & Weitz-Schmidt, G. Small molecule inhibitors induce conformational changes in the I domain and the I-like domain of lymphocyte function-associated antigen-1. Molecular insights into integrin inhibition. J. Biol. Chem. 277, 10590–10598 (2002).
Weitz-Schmidt, G. et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Med. 7, 687–692 (2001).
Wattanasin, S. et al. 1,4-Diazepane-2-ones as novel inhibitors of LFA-1. Bioorg. Med. Chem. Lett 13, 499–502 (2003).
Kelly, T. A. et al. Cutting edge: a small molecule antagonist of LFA-1-mediated cell adhesion. J. Immunol. 163, 5173–5177 (1999). The series of compounds first described in this paper have been used to elucidate mechanisms of LFA1 function and have led to drug development candidates.
Liu, G. et al. Discovery of novel p-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction. 1. Identification of an additional binding pocket based on an anilino diaryl sulfide lead. J. Med. Chem. 43, 4025–4040 (2000).
Pei, Z. et al. Discovery of potent antagonists of leukocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. 3. Amide (C-ring) structure-activity relationship and improvement of overall properties of arylthio cinnamides. J. Med. Chem. 44, 2913–2920 (2001).
Winn, M. et al. Discovery of novel p-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. 4. Structure-activity relationship of substituents on the benzene ring of the cinnamide. J. Med. Chem. 44, 4393–4403 (2001). The compound series described in this paper and previous papers in this series (references 117 and 118) led to a clinical candidate for psoriasis.
Link, J. T. et al. Discovery and SAR of diarylsulfide cyclopropylamide LFA-1/ICAM-1 interaction antagonists. Bioorg. Med. Chem. Lett. 11, 973–976 (2001).
Legge, G. B. et al. NMR solution structure of the inserted domain of human leukocyte function associated antigen-1. J. Mol. Biol. 295, 1251–1264 (2000).
Qu, A. & Leahy, D. J. Crystal structure of the I-domain from the CD11a/CD18 (LFA-1, α L β 2) integrin. Proc. Natl Acad. Sci. USA 92, 10277–10281 (1995).
Qu, A. & Leahy, D. J. The role of the divalent cation in the structure of the I domain from the CD11a/CD18 integrin. Structure 4, 931–942 (1996).
Davidson, W. et al. Characterization of the allosteric inhibition of a protein–protein interaction by mass spectrometry. J. Am. Soc. Mass. Spectrom. 14, 8–13 (2003).
Woska, J. R. Jr et al. A small-molecule antagonist of LFA-1 blocks a conformational change important for LFA-1 function. J. Leukoc. Biol. 70, 329–334 (2001).
Woska, J. R. Jr et al. Small molecule LFA-1 antagonists compete with an anti-LFA-1 monoclonal antibody for binding to the CD11a I domain: development of a flow-cytometry-based receptor occupancy assay. J. Immunol. Methods 277, 101–115 (2003).
Burdick, D. J. et al. N-Benzoyl amino acids as LFA-1/ICAM inhibitors 1: amino acid structure-activity relationship. Bioorg. Med. Chem. Lett 13, 1015–1018 (2003).
Thibault, G. Sodium dodecyl sulfate-stable complexes of echistatin and RGD-dependent integrins: a novel approach to study integrins. Mol. Pharmacol. 58, 1137–1145 (2000).
Ignarro, L. J., Cirino, G., Casini, A. & Napoli, C. Nitric oxide as a signaling molecule in the vascular system: an overview. J. Cardiovasc. Pharmacol. 34, 879–886 (1999).
Gyurko, R., Leupen, S. & Huang, P. L. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143, 2767–2774 (2002).
Huang, Z. et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 265, 1883–1885 (1994).
Nathan, C. Inducible nitric oxide synthase: what difference does it make? J. Clin. Invest. 100, 2417–2423 (1997).
Bogdan, C. Nitric oxide and the immune response. Nature Immunol. 2, 907–916 (2001).
Vallance, P. & Leiper, J. Blocking NO synthesis: how, where and why? Nature Rev. Drug Discov. 1, 939–950 (2002).
Mete, A. & Connolly, S. Inhibitors of the NOS enzymes: a patent review. IDrugs 6, 57–65 (2003).
Lirk, P., Hoffmann, G. & Rieder, J. Inducible nitric oxide synthase — time for reappraisal. Curr. Drug. Targets Inflamm. Allergy 1, 89–108 (2002).
McMillan, K. et al. Allosteric inhibitors of inducible nitric oxide synthase dimerization discovered via combinatorial chemistry. Proc. Natl Acad. Sci. USA 97, 1506–1511 (2000). Presents the biochemical and structural characterization of a potent inhibitor of iNOS dimerization. Pyridines are known ligands for the haem cofactor in iNOS, but previous members have not been characterized as allosteric dimerization inhibitors.
Ohtsuka, M. et al. PPA250 [3-(2,4-difluorophenyl)-6-[2-[4-(1H-imidazol-1-ylmethyl) phenoxy]ethoxy]-2-phenylpyridine], a novel orally effective inhibitor of the dimerization of inducible nitric-oxide synthase, exhibits an anti-inflammatory effect in animal models of chronic arthritis. J. Pharmacol. Exp. Ther. 303, 52–57 (2002).
Blasko, E. et al. Mechanistic studies with potent and selective inducible nitric-oxide synthase dimerization inhibitors. J. Biol. Chem. 277, 295–302 (2002).
Enkhbaatar, P. et al. Inducible nitric oxide synthase dimerization inhibitor prevents cardiovascular and renal morbidity in sheep with combined burn and smoke inhalation injury. Am. J. Physiol. Heart Circ. Physiol. 285, H2430–H2436 (2003).
Enkhbaatar, P. et al. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am. J. Respir. Crit. Care Med. 167, 1021–1026 (2003).
Szabolcs, M. J. et al. Effects of selective inhibitors of nitric oxide synthase-2 dimerization on acute cardiac allograft rejection. Circulation 106, 2392–2396 (2002).
Chao, M. et al. Neurotrophin receptors: mediators of life and death. Brain Res. Brain Res. Rev. 26, 295–301 (1998).
Ibanez, C. F. et al. Disruption of the low affinity receptor-binding site in NGF allows neuronal survival and differentiation by binding to the trk gene product. Cell 69, 329–341 (1992).
Ryden, M. & Ibanez, C. F. A second determinant of binding to the p75 neurotrophin receptor revealed by alanine-scanning mutagenesis of a conserved loop in nerve growth factor. J. Biol. Chem. 272, 33085–33091 (1997).
Wiesmann, C., Ultsch, M. H., Bass, S. H. & de Vos, A. M. Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature 401, 184–188 (1999).
Niederhauser, O. et al. NGF ligand alters NGF signaling via p75(NTR) and trkA. J. Neurosci. Res. 61, 263–272 (2000). In characterizing an inhibitor of nerve growth factor, the authors use analytical ultracentrifugation to determine the binding partner and to estimate binding stoichiometry.
Lee, A., Huang, L. & Ellman, J. A. General solid-phase method for the preparation of mechanism-based cysteine protease inhibitors. J. Am. Chem. Soc. 121, 9907–9914 (1999).
Otto, H. -H. & Schirmeister, T. Cysteine proteases and their inhibitors. Chem. Rev. 97, 133–172 (1997).
Tsai, C. J., Lin, S. L., Wolfson, H. J. & Nussinov, R. Protein–protein interfaces: architectures and interactions in protein–protein interfaces and in protein cores. Their similarities and differences. Crit. Rev. Biochem. Mol. Biol. 31, 127–152 (1996).
Stites, W. E. Protein–protein interactions: interface structure, binding thermodynamics, and mutational analysis. Chem. Rev. 97, 1233–1250 (1997).
Hu, Z., Ma, B., Wolfson, H. & Nussinov, R. Conservation of polar residues as hot spots at protein interfaces. Proteins 39, 331–342 (2000).
Atwell, S., Ultsch, M., De Vos, A. M. & Wells, J. A. Structural plasticity in a remodeled protein–protein interface. Science 278, 1125–1128 (1997).
Shuker, S. B., Hajduk, P. J., Meadows, R. P. & Fesik, S. W. Discovering high-affinity ligands for proteins: SAR by NMR. Science 274, 1531–1534 (1996). The first paper describing the SAR by NMR technique used by this laboratory and one of the first demonstrations of fragment-based drug discovery.
Erlanson, D. A. et al. Site-directed ligand discovery. Proc. Natl Acad. Sci. USA 97, 9367–9372 (2000).
Hann, M. M., Leach, A. R. & Harper, G. Molecular complexity and its impact on the probability of finding leads for drug discovery. J. Chem. Inf. Comput. Sci. 41, 856–864 (2001).
Oprea, T. I., Davis, A. M., Teague, S. J. & Leeson, P. D. Is there a difference between leads and drugs? A historical perspective. J. Chem. Inf. Comput. Sci. 41, 1308–1315 (2001).
Lepre, C. A. et al. Applications of SHAPES screening in drug discovery. Comb. Chem. High. Throughput Screen. 5, 583–590 (2002).
Fejzo, J. et al. The SHAPES strategy: an NMR-based approach for lead generation in drug discovery. Chem. Biol. 6, 755–769 (1999).
Hajduk, P. J., Meadows, R. P. & Fesik, S. W. NMR-based screening in drug discovery. Q. Rev. Biophys. 32, 211–240 (1999).
Hajduk, P. J. et al. High-throughput nuclear magnetic resonance-based screening. J. Med. Chem. 42, 2315–2317 (1999).
Hajduk, P. J. et al. Design of adenosine kinase inhibitors from the NMR-based screening of fragments. J. Med. Chem. 43, 4781–4786 (2000).
Nienaber, V. L. et al. Discovering novel ligands for macromolecules using X-ray crystallographic screening. Nature Biotechnol. 18, 1105–1108 (2000).
Carr, R. & Jhoti, H. Structure-based screening of low-affinity compounds. Drug Discov. Today 7, 522–527 (2002).
Erlanson, D. A. et al. Discovery of a new phosphotyrosine mimetic for PTP1B using breakaway tethering. J. Am. Chem. Soc. 125, 5602–5603 (2003).
Erlanson, D. A. et al. In situ assembly of enzyme inhibitors using extended tethering. Nature Biotechnol. 21, 308–314 (2003).
Lebowitz, J., Lewis, M. S. & Schuck, P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein. Sci. 11, 2067–2079 (2002).
Philo, J. S. Sedimentation equilibrium analysis of mixed associations using numberical contraints to impose mass or signal conservation. Meth. Enzymol. 321, 100–120 (2000).
Arkin, M. & Lear, J. D. A new data analysis method to determine binding constants of small molecules to proteins using equilibrium analytical ultracentrifugation with absorption optics. Anal. Biochem. 299, 98–107 (2001).
Mayer, M. & Meyer, B. Group epitope mapping by saturation transfer difference NMR to identify segments of a ligand in direct contact with a protein receptor. J. Am. Chem. Soc. 123, 6108–6117 (2001).
Hajduk, P. J., Olejniczak, E. T. & Fesik, S. W. One-Dimensional relaxtion- and diffusion-edited NMR methods for screening compounds that bind to macromolecules. J. Am. Chem. Soc. 119, 12257–12261 (1997).
Campbell, A. P. & Sykes, B. D. The two-dimensional transferred nuclear Overhauser effect: theory and practice. Annu. Rev. Biophys. Biomol. Struct. 22, 99–122 (1993).
Day, Y. S. & Myszka, D. G. Characterizing a drug's primary binding site on albumin. J. Pharm. Sci. 92, 333–343 (2003).
Rich, R. L. & Myszka, D. G. Survey of the year 2000 commercial optical biosensor literature. J. Mol. Recognit. 14, 273–294 (2001).
Leavitt, S. & Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 11, 560–566 (2001).
Hajduk, P. J. et al. High-throughput nuclear magnetic resonance-based screening. J. Med. Chem. 42, 2315–2317 (1999).
Blundell, T. L., Jhoti, H. & Abell, C. High-throughput crystallography for lead discovery in drug design. Nature Rev. Drug Discov. 1, 45–54 (2002).
Acknowledgements
All images derived from X-ray crystallography data were prepared using Pymol (Delano Scientific, Belmont, California, USA).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
M.R.A. and J.A.W. are employed by Sunesis Pharmaceuticals, which uses proprietary target-directed fragment discovery technologies.
Glossary
- ALLOSTERIC PROTEIN
-
A protein containing two or more topologically distinct binding sites that interact functionally with each other.
- SURFACE PLASMON RESONANCE
-
A method for measuring binding interactions between a surface-immobilized molecule and a solution-phase analyte. The technology measures changes in refractive index caused by a change in mass at the surface.
- ANTIGEN-PRESENTING CELL
-
An immune system cell that takes up antigens, processes them, and presents them to T cells, which are then stimulated to mount an immune response.
- APOPTOSIS
-
Programmed cell death.
- ISOTHERMAL CALORIMETRY
-
A method for measuring the change in heat that occurs when a protein–ligand complex is formed. From these data, the thermodynamic parameters for the interaction can be determined.
Rights and permissions
About this article
Cite this article
Arkin, M., Wells, J. Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nat Rev Drug Discov 3, 301–317 (2004). https://doi.org/10.1038/nrd1343
Issue Date:
DOI: https://doi.org/10.1038/nrd1343
This article is cited by
-
Structure-based drug design and molecular dynamics studies of an allosteric modulator targeting the protein–protein interaction site of PDK1
Journal of Molecular Modeling (2024)
-
Single-molecule fingerprinting of protein-drug interaction using a funneled biological nanopore
Nature Communications (2023)
-
A cryptic pocket in Ebola VP35 allosterically controls RNA binding
Nature Communications (2022)
-
Comprehensive exploration of chemical space using trisubstituted carboranes
Scientific Reports (2021)
-
SARS-CoV-2 nucleocapsid and Nsp3 binding: an in silico study
Archives of Microbiology (2021)