Abstract
Neuroendocrine neoplasms arise in almost every organ of the body and are variably defined according to the site of origin. This Review focuses on neuroendocrine neoplasms of the digestive tract and pancreas. The 2010 WHO classification of tumors of the digestive system introduces grading and staging tools for neuroendocrine neoplasms. A carcinoid is now defined as a grade 1 or 2 neuroendocrine tumor and grade 3, small-cell or large-cell carcinomas are defined as neuroendocrine carcinoma. Epidemiological data show a worldwide increase in the prevalence and incidence of gastroentero-pancreatic neuroendocrine tumors in the past few decades, which is probably due to improved methods of detection of these tumors. The current diagnostic procedures and treatment options for neuroendocrine neoplasms are defined and summarized in the Review, although evidence-based data are lacking. Surgery remains the treatment mainstay and somatostatin analogues the basis for both diagnosis and therapy as the only 'theranostic' tool. Emerging compounds including chemotherapeutic agents, small molecules and biological therapies may provide new hope for patients.
Key Points
-
Two regulated pathways of secretion are found in neuroendocrine cells that share features of secretory pathways found in endocrine cells and nerve cells
-
The WHO 2010 neuroendocrine neoplasm classification has introduced grading and staging; low to intermediate grade tumors are defined as neuroendocrine tumors (previously carcinoids) whereas high-grade carcinomas are termed neuroendocrine carcinomas
-
Gut and pancreas stem cells are fairly well defined, but data are lacking to support the cancer stem cell theory in neuroendocrine neoplasms
-
Therapy mainstays for gut and pancreas neuroendocrine neoplasms include surgery and the use of somatostatin analogues
-
Questions remain concerning both diagnosis and treatment of these neoplasms in current clinical practice because of a lack of evidence-based studies
Similar content being viewed by others
Introduction
'Neuroendocrine tumor' is traditionally an umbrella definition that embraces a broad family of neoplasms that originate in neural and endocrine structures. The location of these neoplasms thus includes the nerve plexuses and extra-adrenal paraganglia (paraganglioma); pure endocrine organs such as the thyroid, parathyroid and adrenal glands (medullary thyroid carcinoma, parathyroid adenomas or carcinomas, and pheochromocytoma, respectively); and specialized structures or elements of the so-called diffuse endocrine system in different organs (islet cell tumors, carcinoids, large-cell and small-cell carcinomas). The neuroendocrine tumor cell may produce and release a large wealth of active molecules, including not only hormones and small mediators but also growth factors and cytokines, which is often the reason for the pleioform presentation of neuroendocrine cancers.
This Review discusses only the neuroendocrine neoplasms of the gastrointestinal tract and pancreas. A rise in the incidence and prevalence of these neoplasms has been observed in the past few decades.1 More worrisome, no improvement in patient survival over the past three decades has been reported for North America—the lack of diagnostic and treatment standards and lack of appropriate research funding being indicated as the probable culprits.2 However, in the past decade, the development of appropriate tools for patient stratification3 and evidence that proves the efficacy of targeted therapy4,5 have opened new horizons for the diagnosis and treatment of these complex and rare cancers.
This Review aims to provide a state-of-the-art discussion of basic and clinical aspects of neuroendocrine neoplasms of the digestive system. The first part of the article is organized to provide instruments to understand the basic aspects, whereas the second part aims to report and discuss diagnostic and therapeutic issues. The Review concludes by outlining how new research has been translated into changes in clinical practice in recent years. Emphasis is given to critically evaluating current diagnostic and therapeutic practices.
Definition and nomenclature
Neuroendocrine tumors are defined as neoplastic lesions, benign or malignant, composed either by cells with a well-developed neuroendocrine phenotype or by cells with poorly developed but still recognizable and prominent neuroendocrine features. By definition, full-blown neuroendocrine features are observed in the endocrine cells dispersed in different organs and belonging to the so-called diffuse endocrine system.6
Cells of the diffuse endocrine system contain two regulated pathways of secretion, which are characterized by two separate storage-release organelles, called large dense-core vesicles and small synaptic-like vesicles (Figure 1). Since both types of vesicles are also observed in neurons, their constituents and relative antigens are defined as neuroendocrine. Classical endocrine cells of the thyroid (except calcitonin-producing C cells), adrenal cortex and gonads do not contain these antigenic constituents.7,8 Similar to some classical endocrine organs, however, cells of the diffuse endocrine system derive from the endodermal primitive tube. On the other hand, and similar to neurons of all visceral organs, the cells of the adrenal medulla and the calcitonin-producing C cells in the thyroid interstitium originate from the neural crest.9 In addition, transcription factors such as neurogenin 3 and neuroD/β2 are shared by gastroentero-pancreatic (GEP) cells of the diffuse endocrine system and nerve elements as key regulators of development and differentiation.10 These antigenic, functional, embryological and developmental properties thus characterize the unique neuroendocrine profile of cells of the diffuse endocrine system. A large variety of hormonal products is observed in the GEP system, with 14 different endocrine cell types producing different hormones identified at different gastrointestinal sites.6
The figure illustrates an open-type cell of the gut with the upper part sensing the gut lumen and secretion toward the basal membrane with access to extracellular stromal structures and vessels for local (paracrine) and distant (endocrine) function. The seven-transmembrane type of somatostatin receptor and the neural cell adhesion molecule are both located on the plasma membrane surface. Two pathways of secretion are shown that are associated with a | large dense-core vesicles and b | small synaptic-like vesicles. a | Ultrastructure of electron-dense granules (arrows) of an enterochromaffin-like cell of the human stomach. b | Ultrastructure of a human lung P/D1 (see typical large dense-core vesicles in the lower-right part of the figure) with small synaptic-like vesicles in the upper left part of the figure (arrowhead), as compared with a true synaptic vesicle (arrow) of a nerve-ending. Abbreviations: n, nucleus; N-CAM, neural cell adhesion molecule; ne, nerve ending; SSTR, somatostatin receptor.
Currently, the term neuroendocrine tumor (NET) encompasses low to intermediate grade, well to moderately differentiated neoplastic lesions, whilst neuroendocrine carcinoma (NEC) refers to high-grade, moderately to poorly differentiated neoplastic lesions. By contrast, the term neuroendocrine neoplasm can be used to describe well, intermediate or poorly differentiated lesions.
The terminology for neuroendocrine lesions varies in different organs and includes adenoma or carcinoma in pituitary, parathyroid and thyroid glands (for medullary thyroid carcinoma only), pheochromocytoma and malignant pheochromocytoma in the adrenal glands (paraganglioma and malignant paraganglioma for extra-adrenal paraganglia) and carcinoid and small-cell or large-cell carcinoma in the airway and digestive systems. The definition 'carcinoid' is also used in the thymus, gall bladder, bile ducts, kidney, the urinary system and the gonads.
The largely benign assumption associated with the term carcinoid proved untenable given the poor outcome at follow-up of 40–80% of patients.1,11 In addition, carcinoids with features of small-cell or large-cell carcinomas and marked clinical aggressiveness were identified and labeled as atypical or malignant carcinoid, an effectual oxymoron.12,13 Neuroendocrine neoplasms are also defined according to the specific hormonal hypersecretion syndrome with which they are associated (for example, insulinoma, gastrinoma). Of note, nonfunctioning neuroendocrine neoplasms are indeed more frequent than their functioning counterparts.1,14,15
Classification
Principles
Carcinoid—applied to a neoplasm composed by serotonin-producing, enterochromaffin cells—was the first term used in 1907 to describe a neuroendocrine tumor as defined today.16 Carcinoids are low-grade, well to moderately differentiated epithelial neoplasms that are neuroendocrine in nature (Figure 2a,b). Since they may be composed of cells other than serotonin-producing, enterochromaffin cells, carcinoids are in reality a group of different neoplasms with a specific gastrointestinal site distribution and diverse clinical behavior (Table 1).17
Grade 1 neuroendocrine tumors (NETs), also defined as carcinoid, are characterized by a | trabecular, solid or gyriphorm structures with delicate stroma, abundant vessels and b | mild atypia. Grade 2 NETs, also defined as carcinoid or atypical carcinoid, show c | consolidated trabeculae with d | moderate atypia. Grade 3 neuroendocrine carcinomas all show solid structure with necrosis, with either intermediate to large cells (e,f | large-cell neuroendocrine carcinoma) or small cells (g,h | small-cell carcinoma). Hematoxylin and eosin staining.
Carcinomas composed by small cells or large cells were identified as the most aggressive neuroendocrine neoplasms.18,19,20 Small-cell or large-cell carcinomas are high-grade, clinically aggressive, moderately to poorly differentiated epithelial neoplasms that are neuroendocrine in nature (Figure 2e,h). In terms of histological and clinical aggressiveness of neuroendocrine neoplasms, the carcinoid and the small-cell or large-cell neuroendocrine carcinoma are at opposite ends of the spectrum.
WHO 2010 classification
The WHO 2010 classification adopts the definitions NET for low to intermediate grade (grade 1–2), well to moderately differentiated neuroendocrine neoplasms and NEC for high-grade (grade 3), moderately to poorly differentiated neuroendocrine neoplasms (Table 2).3 These definitions were chosen because they are widely accepted and used because they indicate clinical prognosis.21 By contrast, in the WHO 2000 classification, NETs were defined either as well-differentiated endocrine tumors or well-differentiated endocrine carcinomas, on the basis of stage-pertinent features such as proven invasion or metastasis.22 In the WHO 2010 classification, the malignant potential of neuroendocrine neoplasms is acknowledged and enforced. The fact is that NETs are often malignant because they are metastatic at diagnosis, or at least have the potential to metastasize in a size-dependent fashion.
The WHO 2010 classification uses a proliferation-based grading system together with the classical histopathological diagnostic criteria for this type of neoplasms (Table 2). Staging is adopted as an important instrument for patient stratification and is performed according to site-specific tumor–node–metastasis (TNM) classifications. Grading and staging were introduced and enforced by the WHO 2010 classification as originally proposed by the European Neuroendocrine Tumor Society23,24 and according to the recommendations of the International Union Against Cancer and the American Joint Cancer Committee.25,26 Diagnostic recommendations for pathology reporting of NETs and NECs are also defined (Box 1). The new classification aims to standardize current diagnostic and management procedures and enable systematic and prognostically relevant patient stratification.
Stem cells and neuroendocrine neoplasms
The identification of cancer stem cells is relevant for understanding cancer histogenesis and to unveil potential targets for therapy. However, the definition of stem cell features for neuroendocrine cancer cells remains elusive. Possible clues may be derived from normal stem cell data. In the intestine of rodents, four major transcription factors—protein atonal homolog 1, neurogenin 3, homeobox protein Nkx2.2 and neurogenic differentiation factor 2—regulate gut endocrine cell specification and enteroendocrine cell differentiation.10,27,28,29,30 In the pancreas of rodents, gene tracing experiments indicate islet cell regenerative capacity,31 while pancreas regeneration experiments point to a ductal stem cell niche.32,33 Both sets of data may provide insights into the origin of neuroendocrine cancer. As in the gut, a major role in endocrine cell specification is played by neurogenin 3 in the pancreas of rodents.33
Notably, a key role for sonic hedgehog, Notch signaling and Wnt signaling, together with multiple, mesenchymal growth factor signaling pathways, was demonstrated in both intestine and pancreas of rodents.33 Very little data are available to support the theory that neuroendocrine neoplasms originate from cancer stem cells and the information available mostly refers to classical endocrine organs.34,35 Multipotent (stem-cell-like) cancer cells have been suggested as the origin of pheochromocytomas in Nf1 knockout mice36 and also as the origin of thyroid anaplastic cancer.37 However, expression of some transcription factors relevant for endocrine cell specification has been demonstrated in neuroendocrine neoplasms of the gut and pancreas.38,39,40,41,42 It remains to be seen if the expression of such genes reflects the neuroendocrine cancer stem cell profile.
Epidemiology
During the past few years, the incidence of GEP NETs has been studied globally.11,43,44 The studies point to an annual incidence of ∼2.5–5.0 per 100,000 inhabitants and an estimated 29-years limited-duration prevalence in the USA of 35 per 100,000 individuals, which make GEP NETs the second most common solid tumor and indicate that these tumors are more common than was previously assumed.11,14 The tumor primaries are reported to vary considerably in different regions of the world, with more frequent lesions to the colon or rectum in the Asia-Pacific region as compared to Europe; in Europe, lesions are more commonly found in the stomach and ileum.44,45 However, the observed differences might be explained by hampered data collection and incomplete access to patients. Despite this possible limitation, the incidence of intestinal GEP NETs in the Asia-Pacific region (for example, Japan) seems to be clearly lower than that in the rest of the world. The reason for this difference might be related to differences in diet and/or genetic profiles.
Diagnosis
Pathology
Several exocrine neoplasms can display low to intermediate grade histology that mimics that of NETs. Distinct NET 'mimickers' can be found at different anatomical sites; for example, the solid pseudopapillary neoplasm, the acinar cell carcinoma and the pancreatoblastoma are found in the pancreas, and the low-grade adenocarcinoma is found in the duodenum.46 High-grade, poorly differentiated carcinomas, which are usually adenocarcinomas in nature, often need to be differentiated from NECs. In these cases, immunohistochemistry with the two robust neuroendocrine markers chromogranin A and synaptophysin is required to reach an accurate diagnosis.47,48
In the case of NETs of unknown primary, immunohistochemical analysis for organ-specific markers is mandatory. Expression of transcription factors such as the homeobox protein CDX-2 and the homeobox protein Nkx2.1 (also known as the thyroid transcription factor) as well as of some hormones (for example, insulin, serotonin and pancreatic polypeptide) may be helpful.
Specific expression of neuroendocrine markers can be observed in some non-neuroendocrine neoplasms, either in minor neoplastic cell subpopulations or in mixed adenoneuroendocrine carcinomas (MANEC), in which at least 30% of neoplastic cells are neuroendocrine in nature as arbitrarily defined by the WHO 2010 classification.21 A minor neuroendocrine cell subpopulation in a tumor probably reflects the multidirectional differentiation capacity of cancer stem cells. This feature bears no clinical relevance at most sites, with the exception of the prostate for which it is an unfavorable prognostic indicator.49 MANEC are quite rare and they result either from two independent lesions that merge together (in which case they are defined as combined) or are unique lesions with different cell populations intermingled (defined as composite). Rare hybrid cell carcinomas with both exocrine and neuroendocrine antigenic features, known as amphicrine carcinomas, can also occur. The clinical course of MANEC and amphicrine carcinomas is in line with the most aggressive component of the tumor, either the adenocarcinoma or the NEC.
Molecular genetics
Abnormalities in several cell cycle regulatory pathways have been described in neuroendocrine neoplastic cells and are probably relevant for both transformation and maintenance of the transformation. A specific genetic signature, involving abnormalities in chromosome 11 often at the MEN1 gene locus, has been identified in pancreatic and gastroduodenal neoplasms in both NETs and NECs.50,51,52,53,54,55 By contrast, abnormalities in chromosome 18 are observed in DNA from tumors in the small or large intestine.56,57,58 In low to intermediate grade tumors, malignancy is associated with increased chromosomal abnormalities, and multiple and severe abnormalities are a constant feature in high-grade carcinomas.51,52,56,59,60 Molecular genetic tests are, however, not useful in the diagnostic process. They do have clinical relevance in case of suspicion of familiar conditions such as multiple endocrine neoplasia syndromes, the Von Hippel Lindau syndrome and other rarer conditions such as neurofibromatosis.61 For all heritable conditions, target gene sequencing of genomic DNA obtained from peripheral blood lymphocytes is the test of choice, the tumor DNA analysis being not necessarily informative. High-grade NECs share a large fraction of gene abnormalities with conventional cancers, the most frequent abnormality being in the cell-cycle key regulatory gene TP53.51,52 Again, in this case, genetic testing has scientific but not diagnostic relevance.
Clinical chemistry
Only about one third of patients with neuroendocrine neoplasms develop a neuroendocrine syndrome. The most frequent neuroendocrine syndromes are hyperinsulinemic hypoglycemia in the case of insulinoma when the primary tumor is in the pancreas, the Zollinger-Ellison syndrome in the case of gastrinoma, with primary tumors either in the pancreas or in the duodenum, and the so-called carcinoid syndrome, with primary tumors either in the intestine or in the lung.1,14,15,43,44,45 Rarely, these syndromes originate from other sites such as the thymus. Well-defined criteria for laboratory test investigation of endocrine syndromes are widely available.62,63,64 Rare functioning tumor syndromes include the sum of symptoms related to the hypersecretion of glucagon, vasoactive intestinal polypeptide, somatostatin, growth-hormone releasing hormone or adrenocorticotropic hormone, and mostly originate in the pancreas.65 The biochemical work-up for investigation of these syndromes strictly depends on the specific syndrome.
Currently, the ideal tumor marker for the screening and follow-up of GEP NETs, as is also true for other tumor entities, is yet to be identified (Table 3). Nonetheless, 5-hydroxylindoleacetic acid (5-HIAA) and chromogranin A are widely used for screening and follow-up in the absence or presence of treatment (Table 3).66 Several commercially available kits with variable sensitivity are in use for marker determination. Care, therefore, has to be taken when results with different kits are compared.
5-HIAA is only useful for serotonin-producing, mostly metastatic NETs (namely, intestinal and bronchial NETs). By contrast, chromogranin A is widely expressed and secreted from many different primary tumors and is considered the common denominator of all NET markers. As yet, no robust prospective study has evaluated the effectiveness of these two markers in a well-defined, large patient population, although a study on the therapeutic efficacy of everolimus in a prospective setting showed that subtle chromogranin A differences could be used to indicate response or nonresponse to treatment.67 Additionally, medical treatment with somatostatin analogues or interferon-α may inhibit secretion more than tumor growth, thereby leading to a misinterpretation of reduced or normalized chromogranin A circulating levels.68 Finally, NECs often lack chromogranin A or 5-HIAA production, which makes these markers poor indicators of therapeutic response in patients with grade 3 neoplasms.
Radiology
During the past two decades, imaging techniques have considerably improved with regards to local resolution.69,70,71 The current approach for visualization of primary tumors (Table 4) entails the use of CT at increasingly higher slice resolutions (64, 128 and even higher slice resolution); MRI at the standard 1.5 Tesla (T), 3.0 T and even, experimentally, 7.0 T for resolution in the low millimeter range; and abdominal ultrasonography with new contrast agents (harmonic imaging, which is the analysis of 'harmonic' waves generated at higher frequencies within the body by the probe wave) together with power Doppler imaging, which is of special interest in the imaging of highly vascularized NETs.
Given the new developments in imaging, existing data on the cost-effectiveness of different imaging techniques need to be re-assessed. This re-assessment might lead to recommending the replacement of the previously recommended imaging combinations with a single procedure; for example, use of PET-CT or even PET-MRI instead of somatostatin receptor scintigraphy plus CT or MRI separately might be advantageous. Despite these advances and the existing standards of the European Neuroendocrine Tumor Society practice guidelines,72,73 the use of the various imaging devices varies considerably worldwide in terms of the quantity and quality of data collection after the intravenous administration of the contrast agents. Furthermore, it remains to be determined if extremely high resolution will automatically lead to improved patient outcomes, as current therapeutic algorithms are derived from imaging data with lower resolution. So far, no prospective multicenter study has been performed that compares the sensitivity and specificity of all existing imaging modalities.
Diagnostic and interventional endoscopy
Complete access to the gastrointestinal tract can now be achieved via the luminal route by modern endoscopic means. Advances in endoscopic imaging enable resolution of hollow organs even at a subcellular level (so-called cytoendoscopy or endomicroscopy).74 The neoplastic involvement of the gastrointestinal wall will probably be best measured in the future by a combination of endoscopic ultrasonography and endomicroscopy, which will enable even submillimeter local resolution.75
The use of the endocapsule is a noninvasive technique for the visualization of small intestinal neuroendocrine neoplasms.76 However, this technique is limited by its high cost, the extensive time consumption for the investigator and the lack of the possibility to obtain biopsies. In addition, higher miss rates as compared to conventional endoscopy can be expected, as patients with NET often have increased motility. Finally, until now, no robust, prospective, multicenter data exist on the specificity, sensitivity and predictive value of the above endoscopic procedures as compared to newer radiological techniques.77
Nuclear medicine
During the past two decades, imaging techniques have dramatically improved, especially in the field of nuclear medicine.78 This statement holds especially true for somatostatin receptor imaging; three different radiotracers are now in use: 111In, 68Ga and 99Tc. These radiotracers are coupled via a chemical bridge (tetraazacyclododecane tetraacetic acid [DOTA] or diethylenetriamine pentaacetic acid [DTPA]) to a somatostatin analogue.79 For 111In and 99Tc, scintigraphic detection is necessary, whereas for 68Ga, PET detection is required. Scintigraphy, however, requires at least four scans in a period of 48 h while PET requires one scan only 1 h after injection of the radioligand, leading to patients' demands for PET rather than scintigraphy. Since a high percentage of NETs express vesicular monoamine transporters as well as somatostatin receptors, it is not surprising that L-dihydroxyphenylalanine (DOPA)-PET appears to be as sensitive as 68Ga DOTA-PET.80 Only one, single-center prospective study has compared the sensitivity and specificity of DOPA-PET and 68Ga DOTA-PET for patients with neuroendocrine tumors.81 Also lacking are comparative studies on different radioligands and cost-effectiveness. Finally, for most procedures, no internationally accepted standardization exists for the extent of radioactivity, specific radioligand and scanning time to be used.
Therapy
Patients with NETs survive longer than patients with adenocarcinomas;14 therefore, end-points of clinical trials can only be progression-free survival (PFS) and time-to-progression (TTP) but not survival. No final prove exists that new treatments lead to improved survival, as TTP and PFS findings only indirectly suggest an effect on survival. Furthermore, NETs have low incidence, so most studies are hampered by low levels of evidence. As a result, therapeutic recommendations often do not exceed the level of expert opinions. Nonetheless, treatment targets (Figure 3) and related treatment options have drastically increased in the past decade for some primary tumors, especially pancreatic NETs.5,82,83 To a lesser degree, this advance also holds true for ileo-cecal primary tumors,84 whereas no convincing therapeutic advances exist for colorectal primary tumors.
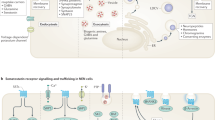
Targeted therapy exerts its effect on both tumor cells and their inter-relationship with stromal, vessel and immune cells. The figure illustrates a neoplastic cell with disordered neuroendocrine features including large dense-core vesicles (pink), small synaptic-like vesicles (orange) and drugable membrane receptors and cell signaling pathways. Bioactive substances are released by the neoplastic cell in the extracellular space to exert their unregulated effect on the cell itself and on extracellular stromal structures and vessels, as indicated by curved arrows on the right. Targets for current therapy are listed and sketched as specific binding sites on the plasma membrane and the two arrows in a circle signify specific signaling pathways in the cell cytoplasm. Out of the many pathways affected by currently available medical agents, the PI3K-AKT-mTor pathway is indicated as one of the many examples. Therapeutic agents are sketched as incomplete circles docking to specific targets sketched as triangles in both the right and left part of the drawing.
Esophageal and colonic neuroendocrine neoplasms are often NEC grade 3, whilst pancreatic and ileocecal neoplasms are often NET grade 1 or 2.85,86 Depending on the primary site of the tumor, different functionalities can also be observed. Accordingly, treatment has to be adjusted to the primary tumor location, functionality, tumor grade and stage. Finally, ileal primary tumors have to be considered separately, since they tend to metastasize via lymphatic or hematic vessels (Figure 4).
a | The L type shows almost exclusively lymphatic metastasis, with bulky lymph-node conglomerates in the mesenteric root, in the absence of liver metastasis (arrowheads). b | On the contrary, the H type shows mostly hematogenous liver metastases in the absence of synchronous lymph-node deposits (arrows, Rinke scale grade A84).
Surgery
Surgery is the treatment of choice for grade 1–2 NETs of almost all stages, except stages with advanced distant metastases and extensive local invasion.87,88 In the case of de-bulking surgery of liver metastasis, intraoperative ablation may be an adjunctive treatment. However, no prospective studies exist to support this new, more aggressive surgical concept. Also missing are studies demonstrating the postulated need for primary resection in extensive metastatic disease. Although surgeons argue for the need of de-bulking surgery to control symptoms, modern anti-secretory medical treatments have excluded this requirement in almost all cases.89 However, interesting insights could be gained by comparing the long-term outcome with extensive, de-bulking surgery or long-term, extensive medical therapy with regards to patient outcome and costs. Established standards of care in surgery are extensive surgical 'cleansing' of the mesenteric root in the case of midgut tumors and tricuspid valve replacement in advanced carcinoid heart disease.90,91 In the latter case, however, it remains to be determined at which stage of the disease open heart surgery or minimally invasive procedures are preferable.
Ablative therapy
Numerous ablative therapies have been used in neuroendocrine neoplasms metastatic to the liver.92 Among these, radiofrequency ablation and trans-arterial (chemo) embolization are most frequently used. So far, no prospective, multicenter controlled trials exist that demonstrate the superiority of one ablative therapy over the other. Radioablative therapy has also been put forward as a promising new procedure.93 However, it remains to be determined in a prospective multicenter study if this very costly method (which is associated with the rare though disabling adverse effect of extensive gastroduodenal necrosis) is superior to other ablative measures. Clearly, for intra-operative purposes, only radiofrequency ablation is a viable option. This method is limited by factors such as high number of lesions, deep intra-hepatic location and presence of synchronous metastasis at other sites, in most cases rendering this procedure solely palliative. Of note, an anecdotal report of an ablative treatment attempt in the lung exists.94
Medical treatment
Regardless of the primary tumor, grade 1–2 NETs tend to grow slowly, if at all. A watch and wait approach that involves patient follow-up for 3–6 months before treatment should be considered. Clearly, this approach should be different for patients with fast-growing NECs. Currently, medical treatment options are good for grade 1–2 primary NETs of the pancreas,95 moderate for primary tumors of ileocecal origin and poor for those of the colorectum. Limited data exist for bronchial and other extra-digestive neoplasms.96
For NECs, standard chemotherapeutic options apply, similar to those for small-cell lung cancer. The combination of oxaliplatin with either 5-fluorouracil/folinic acid or capecitabine has been suggested in the past few years.97 However, high levels of thymidilate synthase, a negative predictor of response to 5-fluorouracil therapy, have been reported in both lung and GEP series.98,99
No adjuvant therapies exist for NETs or NECs. This situation is best explained by the fact that the required number of patients for a robust prospective multicenter trial cannot be recruited and clinical studies with potentially interesting agents lack sponsorship. This also holds true for neo-adjuvant approaches.
Medical treatment for symptom control and for progressive disease is only applicable in the presence of distant metastasis when curative surgery or other ablative procedures are not possible. Most patients for whom medical treatment is indicated have liver metastasis and their prognosis is limited by the extent of liver involvement. Therefore, the logical step is to subdivide patients by the extent of hepatic metastasis into four subgroups according to Rinke et al.84 and adapt the treatment choice consequently. The volume of tumor load in the liver can be established by analysis of radiograms with specific software programs or rough estimation by experienced radiologists as grade A (up to 10% liver involvement), B (10–25% involvement), C (25–50%) or D (>50%).
Current medical treatments include four classes of pharmacological agents: biotherapeutics, which include somatostatin analogues and interferon-α; chemotherapeutics, which include streptozotocin, temozolomide and platinum-containing compounds; small molecules (for example, sunitinib100 and everolimus4); and biologicals (bevacizumab and other antibodies).
The specific profiles of response, adverse effects and costs of available medical agents have not been properly evaluated in patients with NETs or NECs in a comparative prospective fashion. However, a comparison of efficacy, quality of life and adverse effects of these compounds is absolutely essential, especially to guide the long-term treatment expected for patients with grade 1–2 NETs. Long-term treatment with somatostatin analogues represents the gold standard in this setting, because of its minor adverse effects combined with effective, long-term (>5 years) control of symptoms and tumor growth.
Although we lack information on long-term use and adverse effects of medical treatments in patients with NETs, especially for newer agents such as sunitinib, everolimus and temozolomide, data already exist in other settings. Examples include the use of everolimus and sunitinib for the treatment of gastrointestinal stromal tumors and renal cell carcinoma, and temozolomide for the treatment of glioblastoma. For everolimus, long-term treatment data in the transplant field exist; however, the concentrations of everolimus used in this setting are tenfold lower. Therefore, if these new agents are to be used for long-term therapy, special care will have to be taken. Until now, all medical treatments for NETs are limited by response rates lower than 30%. In addition, no valid biomarkers exist to predict response to treatment. Aside from somatostatin receptor subtype 2, methylguanine-DNA methyltransferase may represent one of the first predictive NET biomarkers for patients considered for treatment with temozolomide.101 However, currently, no convincing data exist that enable treatment based solely on expression of methylguanine-DNA methyltransferase. On the basis of current information, available medical agents, with the exception of interferon-α and somatostatin, reach multiple targets and affect not only one but multiple signal transduction pathways. A single biomarker or even a few will, therefore, probably not fulfill the requested criteria as predictors of therapeutic response. The ideal single biomarker for NETs, therefore, remains elusive.
Radiotherapy and PRRT
Conventional radiotherapy plays a rather limited role in the treatment of these tumors that is only applicable to cases with pain caused by bone metastasis or preventative cranial radiation in grade 3 neoplasms.102 In the past 20 years, peptide-receptor radionuclide therapy (PRRT) has evolved as a new treatment option.103 A prospective study evaluated the symptomatic response to 90Y as a radioligand in patients with highly advanced, refractory carcinoid syndrome.104 A second prospective study evaluated TTP in a rather heterogeneous group of patients with different primary tumor locations, tumor extent and functional activity who were treated with 177Lu as a radioligand.105 In both studies (to a lesser extent with 177Lu) major adverse effects were observed, including variable renal and myelopoietic impairment. Rather disappointing results were also reported for TTP and PFS, with therapeutic success comparable to that with other treatment options. Such findings could possibly be explained by the fact that grade 1 and 2 NETs normally display a high degree of radio-resistance. The major advantage of this treatment modality is that somatostatin receptor subtypes represent both a diagnostic as well as a therapeutic target and thereby represent the only bona fide 'theranostic' (therapeutic and diagnostic) approach in daily clinical practice.
Conclusions
Great steps have been made towards a more standardized approach to the diagnosis and treatment of neuroendocrine neoplasms of the gastrointestinal tract and pancreas. The introduction of grading and staging within the WHO 2010 classification will have an important effect on patient stratification and management. Many intriguing and important questions remain. Little is known about the cancer stem cell theory in neuroendocrine neoplasms. The use of immunosuppressive agents, such as platinum compounds or everolimus, demands improved knowledge of immune function in patients with neuroendocrine neoplasms. In terms of functional properties, given the similarities in cell biology between neuroendocrine and neuronal cells, more needs to be learned about the secretory machinery of the synapse in order to better understand the NET (hyper)secretory setting. As for NET cell growth, lessons from the adenocarcinoma field may be helpful to dissect and better understand the complex signaling involved in NET cell proliferation and differentiation.106
In the past few years, demand for biomarkers that can predict therapeutic outcome has been increasing. Ideally, a predictive biomarker could be visualized under in vivo conditions by molecular imaging. So far, only somatostatin receptor scintigraphy has fulfilled this expectation and represents the first valuable example of theranostics in daily clinical practice. Targets other than somatostatin receptor subtype 2, however, remain elusive. Methylguanine-DNA methyltransferase was proposed as a means to predict response to temozolomide,101 and chromogranin A and neuron-specific enolase proved useful predictors of therapeutic response to everolimus.67 In all these settings, however, no robust, prospective data exist that demonstrate a powerful predictive value for these markers.
Personalized treatment based on molecular genetics, tumor biology, grading and TNM classifications is the ideal situation. It remains to be seen if detection and control of microtumors or micrometastases with the current therapeutic armamentarium may indeed generate clinical benefit. Many patients with NETs have stable disease over long periods; therefore, care should be taken in providing treatment. This implies that a watch and wait or even a watch and worry philosophy represents a better and more reasonable approach than early overtreatment.
Review criteria
A search for original articles published between 1960 and 2010 focusing on neuroendocrine neoplasms was performed in MEDLINE and PubMed. The search terms used were “carcinoids”, “endocrine tumors”, “neuroendocrine tumours”, “neuroendocrine carcinoma” AND “gastrointestinal tract”, “pancreas”, “digestive system”, “lung and thymus” as well as AND “prognostic factors”, “genetics”, “molecular biology”, “imaging”, “medical therapy”, “surgery”, “PRRT”, “guidelines”. Also a specific search was done on “gastrointestinal tract”, “pancreas” AND “stem cell” and “cancer stem cell” AND “carcinoids”, “endocrine tumors”, “neuroendocrine tumors”, “neuroendocrine carcinoma”. All papers identified were English-language, full-text papers. We also searched the reference lists of identified articles for further papers.
Change history
13 December 2011
In the version of this article initially published online, in Table 2, the value for the Ki-67 index (%) for NET Grade 1 should have read ≤2 instead of <2. The error has been corrected for the HTML and PDF versions of the article.
References
Yao, J. C. et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 26, 3063–3072 (2008).
Modlin, I. M., Moss, S. F., Chung, D. C., Jensen, R. T. & Snyderwine, E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J. Natl Cancer Inst. 100, 1282–1289 (2008).
Bosman, F. T., Carneiro, F., Hruban, R. H. & Theise, N. D. (Eds) WHO Classification of Tumours of the Digestive System 4th edn Vol. 3 (IARC Press, Lyon, 2010).
Yao, J. C. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 (2011).
Raymond, E., Faivre, S., Hammel, P. & Ruszniewski, P. Sunitinib paves the way for targeted therapies in neuroendocrine tumors. Target. Oncol. 4, 253–254 (2009).
Solcia, E., Rindi, G. & Capella, C. in Histochemistry in Pathology 2nd edn (eds Felipe, M. I. & Lake, B. D.) 397–409 (Churchill-Livingstone, Edinburgh, 1990).
Wiedenmann, B. & Huttner, W. B. Synaptophysin and chromogranins/secretogranins—widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 58, 95–121 (1989).
Wiedenmann, B. et al. Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology 95, 1364–1374 (1988).
Adams, M. S. & Bronner-Fraser, M. Review: the role of neural crest cells in the endocrine system. Endocr. Pathol. 20, 92–100 (2009).
Schonhoff, S. E., Giel-Moloney, M. & Leiter, A. B. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 145, 2639–2644 (2004).
Lepage, C., Rachet, B. & Coleman, M. P. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology 132, 899–904 (2007).
Soga, J. & Tazawa, K. Pathologic analysis of carcinoids. Histologic reevaluation of 62 cases. Cancer 28, 990–998 (1971).
Arrigoni, M. G., Woolner, L. B. & Bernatz, P. E. Atypical carcinoid tumors of the lung. J. Thorac. Cardiovasc. Surg. 64, 413–421 (1972).
Modlin, I. M. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 9, 61–72 (2008).
Zerbi, A. et al. Clinicopathological features of pancreatic endocrine tumors: a prospective multicenter study in Italy of 297 sporadic cases. Am. J. Gastroenterol. 105, 1421–1429 (2010).
Oberndorfer, S. Karzinoide Tumoren des Du¨nndarms. Frankf Z. Pathol. Int. 1, 425–432 (1907).
Rindi, G., Capella, C. & Solcia, E. in Recent Advances in the Pathophysiology and Management of Inflammatory Bowel Diseases and Digestive Endocrine Tumors (eds Mignon, M. & Colombel, J. F.) 177–191 (John Libbey Eurotext, Montrouge, 1999).
Gould, V. E. Neuroendocrinomas and neuroendocrine carcinomas: APUD cell system neoplasms and their aberrant secretory activities. Pathol. Annu. 12, 33–62 (1977).
Gould, V. E. & Chejfec, G. Ultrastructural and biochemical analysis of “undifferentiated” pulmonary carcinomas. Hum. Pathol. 9, 377–384 (1978).
Travis, W. D. et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am. J. Surg. Pathol. 15, 529–553 (1991).
Rindi, G. et al. in WHO Classification of Tumours of the Digestive System 4th edn Vol. 3 (eds Bosman, F. T., Carneiro, F., Hruban, R. H. & Theise, N. D.) 10–12 (IARC Press, Lyon, 2010).
Solcia, E., Klöppel, G. & Sobin, L. H. World Health Organization International Histological Classification of Tumours: Histological Typing of Endocrine Tumours 2nd edn (Springer-Verlag, New York, 2000).
Rindi, G. et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 449, 395–401 (2006).
Rindi, G. et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 451, 757–762 (2007).
Sobin, L. H., Gospodarowicz, M. K. & Wittekind, C. (eds) TNM Classification of Malignant Tumours 7th edn (Wiley-Blackwell, Chichester, 2009).
Edge, S. B. et al. (eds) AJCC Cancer Staging Manual 7th edn (Springer, New York, 2010).
Yang, Q., Bermingham, N. A., Finegold, M. J. & Zoghbi, H. Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158 (2001).
Jenny, M. et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21, 6338–6347 (2002).
Desai, S. et al. Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev. Biol. 313, 58–66 (2008).
Rindi, G. et al. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development 126, 4149–4156 (1999).
Dor, Y., Brown, J., Martinez, O. I. & Melton, D. A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004).
Xu, X. et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 (2008).
Puri, S. & Hebrok, M. Cellular plasticity within the pancreas–lessons learned from development. Dev. Cell 18, 342–356 (2010).
Lichtenauer, U. D. & Beuschlein, F. The tumor stem cell concept-implications for endocrine tumors? Mol. Cell. Endocrinol. 300, 158–163 (2009).
Thomas, D., Friedman, S. & Lin, R. Y. Thyroid stem cells: lessons from normal development and thyroid cancer. Endocr. Relat. Cancer 15, 51–58 (2008).
Powers, J. F., Evinger, M. J., Zhi, J., Picard, K. L. & Tischler, A. S. Pheochromocytomas in Nf1 knockout mice express a neural progenitor gene expression profile. Neuroscience 147, 928–937 (2007).
Zhang, P., Zuo, H., Ozaki, T., Nakagomi, N. & Kakudo, K. Cancer stem cell hypothesis in thyroid cancer. Pathol. Int. 56, 485–489 (2006).
Heiskala, K., Arola, J., Heiskala, M. & Andersson, L. C. Expression of Reg IV and Hath1 in neuroendocrine neoplasms. Histol. Histopathol. 25, 63–72 (2010).
van Eeden, S. et al. Goblet cell carcinoid of the appendix: a specific type of carcinoma. Histopathology 51, 763–773 (2007).
Westerman, B. A. et al. Basic helix-loop-helix transcription factor profiling of lung tumors shows aberrant expression of the proneural gene atonal homolog 1 (ATOH1, HATH1, MATH1) in neuroendocrine tumors. Int. J. Biol. Markers 22, 114–123 (2007).
Schmitt, A. M. et al. Islet 1 (Isl1) expression is a reliable marker for pancreatic endocrine tumors and their metastases. Am. J. Surg. Pathol. 32, 420–425 (2008).
Lejonklou, M. H., Edfeldt, K., Johansson, T. A., Stålberg, P. & Skogseid, B. Neurogenin 3 and neurogenic differentiation 1 are retained in the cytoplasm of multiple endocrine neoplasia type 1 islet and pancreatic endocrine tumor cells. Pancreas 38, 259–266 (2009).
Garcia-Carbonero, R. et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. 21, 1794–1803 (2010).
Ito, T. et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J. Gastroenterol. 45, 234–243 (2010).
Niederle, M. B., Hackl, M., Kaserer, K. & Niederle, B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr. Relat. Cancer 17, 909–918 (2010).
Hruban, R. H., Bishop Pitman, M. & Klimstra, D. S. Tumors of the Pancreas 6th edn (American Registry of Pathology Press, Washington DC, 2007).
Klöppel, G. et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology 90, 162–166 (2009).
Klimstra, D. S. et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am. J. Surg. Pathol. 34, 300–313 (2010).
Volante, M., Rindi, G. & Papotti, M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 449, 499–506 (2006).
Tönnies, H. et al. Analysis of sporadic neuroendocrine tumours of the enteropancreatic system by comparative genomic hybridisation. Gut 48, 536–541 (2001).
Pizzi, S. et al. Genetic alterations in poorly differentiated endocrine carcinomas of the gastrointestinal tract. Cancer 98, 1273–1282 (2003).
Furlan, D. et al. Different molecular profiles characterize well-differentiated endocrine tumors and poorly differentiated endocrine carcinomas of the gastroenteropancreatic tract. Clin. Cancer Res. 10, 947–957 (2004).
Kim do, H. et al. Allelic alterations in well-differentiated neuroendocrine tumors (carcinoid tumors) identified by genome-wide single nucleotide polymorphism analysis and comparison with pancreatic endocrine tumors. Genes Chromosomes Cancer 47, 84–92 (2008).
Missiaglia, E. et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J. Clin. Oncol. 28, 245–255 (2010).
Jiao, Y. et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331, 1199–1203 (2011).
Löllgen, R. M., Hessman, O., Szabo, E., Westin, G. & Akerström, G. Chromosome 18 deletions are common events in classical midgut carcinoid tumors. Int. J. Cancer 92, 812–815 (2001).
Wang, G. G. et al. Comparison of genetic alterations in neuroendocrine tumors: frequent loss of chromosome 18 in ileal carcinoid tumors. Mod. Pathol. 18, 1079–1087 (2005).
Cunningham, J. L. et al. Common pathogenetic mechanism involving human chromosome 18 in familial and sporadic ileal carcinoid tumors. Genes Chromosomes Cancer 50, 82–94 (2011).
Rigaud, G. et al. High resolution allelotype of nonfunctional pancreatic endocrine tumors: identification of two molecular subgroups with clinical implications. Cancer Res. 61, 285–292 (2001).
Jonkers, Y. M. et al. DNA copy number status is a powerful predictor of poor survival in endocrine pancreatic tumor patients. Endocrine Relat. Cancer 14, 769–779 (2007).
Calender, A. in Handbook of Neuroendocrine Tumours (eds Caplin, M. & Kvols, L.) 55–81 (Bioscientifica, Bristol, 2006).
de Herder, W. W. et al. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology 84, 183–188 (2006).
Jensen, R. T. et al. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 84, 173–182 (2006).
Eriksson, B. et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors–well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology 87, 8–19 (2008).
Jensen, R. T. in Recent Advances in the Pathophysiology and Management of Inflammatory Bowel Diseases and Digestive Endocrine Tumours (eds Mignon, M. & Colombel, J. F.) 192–219 (John Libbey Eurotext, Paris, 1999).
Modlin, I. M. et al. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med. J. Aust. 193, 46–52 (2010).
Yao, J. C. et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J. Clin. Oncol. 28, 69–76 (2010).
Massironi, S. et al. Plasma chromogranin A response to octreotide test: prognostic value for clinical outcome in endocrine digestive tumors. Am. J. Gastroenterol. 105, 2072–2078 (2010).
Rufini, V., Calcagni, M. L. & Baum, R. P. Imaging of neuroendocrine tumors. Semin. Nucl. Med. 36, 228–247 (2006).
Scarsbrook, A. F. et al. Anatomic and functional imaging of metastatic carcinoid tumors. Radiographics 27, 455–477 (2007).
Elsayes, K. M. et al. Imaging of carcinoid tumors: spectrum of findings with pathologic and clinical correlation. J. Comput. Assist. Tomogr. 35, 72–80 (2011).
Kwekkeboom, D. J. et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: somatostatin receptor imaging with (111)In-pentetreotide. Neuroendocrinology 90, 184–189 (2009).
Sundin, A., Vullierme, M. P., Kaltsas, G. & Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology 90, 167–183 (2009).
Kiesslich, R. et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 133, 1769–1778 (2007).
May, A. et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut 53, 634–640 (2004).
Swain, P. & Fritscher-Ravens, A. Role of video endoscopy in managing small bowel disease. Gut 53, 1866–1875 (2004).
Moglia, A., Menciassi, A., Dario, P. & Cuschieri, A. Capsule endoscopy: progress update and challenges ahead. Nat. Rev. Gastroenterol. Hepatol. 6, 353–362 (2009).
Carrasquillo, J. A. & Chen, C. C. Molecular imaging of neuroendocrine tumors. Semin. Oncol. 37, 662–679 (2010).
Kwekkeboom, D. J. et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 17, R53–R73 (2010).
Ambrosini, V., Tomassetti, P., Franchi, R. & Fanti, S. Imaging of NETs with PET radiopharmaceuticals. Q. J. Nucl. Med. Mol. Imaging 54, 16–23 (2010).
Putzer, D. et al. Comparison of (68)Ga-DOTA-Tyr(3)-octreotide and (18)F-fluoro-L-dihydroxyphenylalanine positron emission tomography in neuroendocrine tumor patients. Q. J. Nucl. Med. Mol. Imaging 54, 68–75 (2010).
Yao, J. C. Neuroendocrine tumors. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 21, 163–172 (2007).
Strosberg, J. R. et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 117, 268–275 (2011).
Rinke, A. et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J. Clin. Oncol. 27, 4656–4663 (2009).
Pape, U. F. et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 113, 256–265 (2008).
Jann, H. et al. Neuroendocrine tumors of midgut and hindgut origin: Tumor-node-metastasis classification determines clinical outcome. Cancer 117, 3332–3341 (2011).
de Herder, W. W., O'Toole, D., Rindi, G. & Wiedenmann, B. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors Part 1—Stomach, Duodenum and Pancreas. Neuroendocrinology 84, 151–216 (2006).
de Herder, W. W., O'Toole, D., Rindi, G. & Wiedenmann, B. ENETS Consensus Guidelines for the Diagnosis and Treatment of Neuroendocrine Gastrointestinal Tumors Part 2—Midgut and Hindgut Tumors. Neuroendocrinology 87, 1–63 (2008).
Oberg, K. E., Reubi, J. C., Kwekkeboom, D. J. & Krenning, E. P. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 139, 742–753 (2010).
Roberts, W. C. A unique heart disease associated with a unique cancer: carcinoid heart disease. Am. J. Cardiol. 80, 251–256 (1997).
Dumoulein, M. et al. Carcinoid heart disease: case and literature review. Acta Cardiol. 65, 261–264 (2010).
Schmidt, C., Bloomston, M. & Shah, M. H. Well-differentiated neuroendocrine tumors: a review covering basic principles to loco-regional and targeted therapies. Oncogene 30, 1497–1505 (2011).
Ahmadzadehfar, H., Biersack, H. J. & Ezziddin, S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin. Nucl. Med. 40, 105–121 (2010).
Crocetti, L. & Lencioni, R. Radiofrequency ablation of pulmonary tumors. Eur. J. Radiol. 75, 23–27 (2010).
Wiedenmann, B., Pavel, M. & Kos-Kudla, B. From targets to treatments—a review of molecular targets in pancreatic neuroendocrine tumors. Neuroendocrinology (in press).
Bertino, E. M., Confer, P. D., Colonna, J. E., Ross, P. & Otterson, G. A. Pulmonary neuroendocrine/carcinoid tumors: a review article. Cancer 115, 4434–4441 (2009).
Bajetta, E. et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother. Pharmacol. 59, 637–642 (2007).
Ceppi, P. et al. Thymidylate synthase expression in gastroenteropancreatic and pulmonary neuroendocrine tumors. Clin. Cancer Res. 14, 1059–1064 (2008).
O'Toole, D. et al. Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 17, 847–856 (2010).
Raymond, E. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 501–513 (2011).
Kulke, M. H. et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin. Cancer Res. 15, 338–345 (2009).
Hlatky, R., Suki, D. & Sawaya, R. Carcinoid metastasis to the brain. Cancer 101, 2605–2613 (2004).
Pool, S. E. et al. Preclinical and clinical studies of peptide receptor radionuclide therapy. Semin. Nucl. Med. 40, 209–218 (2010).
Bushnell, D. L. Jr et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J. Clin. Oncol. 28, 1652–1659 (2010).
Kwekkeboom, D. J. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 26, 2124–2130 (2008).
Nilsson, O., Arvidsson, Y., Johanson, V., Forssell-Aronsson, E. & Ahlman, H. New medical strategies for midgut carcinoids. Anticancer Agents Med. Chem. 10, 250–269 (2010).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, provided substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
G. Rindi declares associations with the following companies: Ipsen (consultant), Novartis (speakers bureau), Pfizer (speakers bureau). B. Wiedenmann declares associations with the following companies: Ipsen (consultant, speakers bureau), Lexicon (consultant), Novartis (consultant, speakers bureau), Pfizer (consultant, speakers bureau).
Rights and permissions
About this article
Cite this article
Rindi, G., Wiedenmann, B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol 8, 54–64 (2012). https://doi.org/10.1038/nrendo.2011.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.120
This article is cited by
-
Radiomics analysis from magnetic resonance imaging in predicting the grade of nonfunctioning pancreatic neuroendocrine tumors: a multicenter study
European Radiology (2023)
-
Immunoreactivity of HOXB13 in Neuroendocrine Neoplasms Is a Sensitive and Specific Marker of Rectal Well-Differentiated Neuroendocrine Tumors
Endocrine Pathology (2023)
-
The Spectrum of Endocrine Pathology
Endocrine Pathology (2023)
-
Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms
Endocrine Pathology (2022)
-
Pancreatic adenocarcinoma and pancreatic high-grade neuroendocrine carcinoma: two sides of the moon
Medical Oncology (2022)