Key Points
-
Studies published during the past decade have enabled researchers to gain new insights into the diagnosis, physiology and treatment of thyroid disease during pregnancy.
-
The previously recommended TSH cut-offs of 2.5 mU/l or 3.0 mU/l are too low and are likely to lead to overdiagnosis and overtreatment of thyroid disease during pregnancy.
-
The combination of thyroid peroxidase-antibody positivity and a high concentration of TSH seems to synergistically increase the risk of adverse pregnancy outcomes.
-
Substantial new evidence supports the important role of thyroid hormone for fetal neurodevelopment.
-
New studies indicate that in patients treated with levothyroxine, titration to thyroid hormone concentrations in the higher end of the normal range might carry a risk of overtreatment.
-
Particularly during early pregnancy, treatment with methimazole (thiamazole) or propylthiouracil might increase the risk of fetal anomalies, and clinicians should consider the cessation of low-dose regimens.
Abstract
Adequate thyroid hormone availability is important for an uncomplicated pregnancy and optimal fetal growth and development. Overt thyroid disease is associated with a wide range of adverse obstetric and child development outcomes. An increasing number of studies now indicate that milder forms of thyroid dysfunction are also associated with these adverse pregnancy outcomes. The definitions of both overt and subclinical thyroid dysfunction have changed considerably over the past few years, as new data indicate that the commonly used fixed upper limits of 2.5 mU/l or 3.0 mU/l for thyroid-stimulating hormone (TSH) are too low to define an abnormal thyroid function. Furthermore, some studies now show that the reference ranges are not necessarily the best cut-off for identifying pregnancies at high risk of adverse outcomes. In addition, data suggest that thyroid peroxidase autoantibody positivity and high or low concentrations of human chorionic gonadotropin seem to have a more prominent role in the interpretation of thyroid dysfunction than previously thought. Data on the effects of thyroid disease treatment are lacking, but some studies indicate that clinicians should be aware of the potential for overtreatment with levothyroxine. Here, we put studies from the past decade on reference ranges for TSH, determinants of thyroid dysfunction, risks of adverse outcomes and options for treatment into perspective. In addition, we provide an overview of the current views on thyroid physiology during pregnancy and discuss strategies to identify high-risk individuals who might benefit from levothyroxine treatment.
Similar content being viewed by others
Main
Thyroid hormone is essential for a normal pregnancy and fetal development. In the first half of pregnancy, placental and fetal development depend on the supply of maternal thyroid hormone. As a consequence, untreated maternal hypothyroidism is associated with a higher risk of adverse pregnancy outcomes, as well as adverse outcomes for the child1.
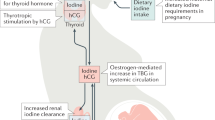
Pregnancy has clear effects on thyroid physiology. The fetal thyroid gland is not functionally mature until weeks 18–20 of gestation, rendering the fetus is dependent on placental transfer of maternal T4 (Fig. 1). The consequent fetal consumption of maternal thyroid hormone, as well as increasing concentrations of thyroxine-binding globulin (TBG), increasing urinary iodide clearance, and increasing thyroid hormone degradation by placental type 3 deiodinase, all necessitate an increase in maternal thyroid hormone production to ensure adequate maternal and fetal availability of thyroid hormone1 (Fig. 1). High concentrations of the pregnancy hormone human chorionic gonadotropin (hCG), a weak agonist of the TSH receptor, increase thyroid hormone production2 (Fig. 1). These pregnancy-specific changes, along with the increased demand for thyroid hormone, might expose pre-existing mild thyroid dysfunction as gestational thyroid disease.
The figure shows the most important changes that occur in thyroid physiology during pregnancy. An increase in thyroxine-binding globulin (TBG), an increase in thyroxine consumption by the fetus and and an increase in placental type 3 deiodinase expression require an upregulation of thyroid hormone production to maintain adequate thyroid hormone availability. This upregulations is largely mediated via increased thyroidal stimulation by human chorionic gonadotropin (hCG), which ultimately leads to a net increase in free T4 concentration and a subsequent decrease in TSH concentration.
Overt maternal hypothyroidism (elevated concentrations of TSH with low concentrations of free T4) occurs in 0.2% to 0.6% of pregnant women1,3,4. Maternal subclinical hypothyroidism (elevated concentrations of TSH with normal concentrations of free T4) is more prevalent, as it occurs in 3.5% to ∼18% of all pregnancies, depending on the definition used5,6,7,8,9. The main risk factor for maternal hypothyroidism is autoimmune thyroid disease, with thyroid peroxidase autoantibody (TPOAb) positivity occurring in roughly one-third of pregnant women with subclinical hypothyroidism10,11. Pathological hyperthyroidism (predominantly Graves disease and toxic nodules or goitres) during pregnancy is less common, with a frequency of 0.4% to 1% before pregnancy and a frequency of approximately 0.2% during pregnancy12. Gestational hyperthyroidism (biochemically defined by elevated concentrations of free T4 and suppressed TSH) is much more frequent and is diagnosed in ∼1% to 3% of pregnancies, with most cases of the disease occurring secondary to high hCG concentrations. In ∼50% of cases, gestational hyperthyroidism co-occurs with hyperemesis gravidarum12.
In the last two decades, evidence has accumulated on the negative consequences of maternal subclinical hypothyroidism, hypothyroxinaemia (low concentrations of free T4 with normal concentrations of TSH) and thyroid autoimmunity (predominantly TPOAb positivity) on pregnancy outcomes and child development. Although the risks of adverse outcomes associated with subclinical diseases are considerably lower than those associated with overt disease, the higher prevalence of subclinical thyroid disease makes it an important public health issue. However, the definitions of the above-mentioned disease entities are under debate, which has limited the potential to compare the results of different studies and has also prevented clinicians from defining treatment cut-offs. In addition, hyperthyroidism has been associated with negative pregnancy outcomes, and data published in 2016 suggest that high concentrations of maternal free T4 might be equally as detrimental as low free T4 concentrations for child neurodevelopment13. Since the publication of studies showing that anti-thyroid drugs are also associated with potential adverse effects, the treatment options for hyperthyroidism are limited14,15,16,17,18. Given these complexities, it remains challenging to determine how and when to assess thyroid dysfunction as well as how and when to treat thyroid disease during pregnancy. Here, we put the results of studies in the field of thyroid disease and pregnancy that were conducted over the past decade into perspective and discuss how these findings might impact clinical decision-making.
Definition of thyroid dysfunction
As a result of the major changes in thyroid physiology that occur during pregnancy, gestational thyroid disease is best defined according to pregnancy-specific reference ranges calculated in a population of pregnant women free of major factors that interfere with thyroid function6,8,19,20. Women with pre-existing thyroid disease (that is, Hashimoto's thyroiditis or Graves disease), thyroid autoimmunity (in particular TPOAb positivity), a history of thyroid-interfering drug use, twin pregnancies or following in vitro fertilization (owing to abnormally high hCG) and iodine deficiency must be excluded from reference populations. The importance of such exclusions is illustrated by a study demonstrating that excluding TPOAb-positive women from analysis changed the upper TSH cut-off limits from 4.15 mU/l to 3.37 mU/l in the first trimester and from 3.77 mU/l to 3.35 mU/l in the second trimester21.
The minimum number of patient samples required for reference range calculations is generally thought to be 120. However, this number is the minimum necessary for calculating a 90% reference range (defined as the range encompassing the 5th to 95th percentiles) of a normally distributed measurement outcome3,22,23. The number of serum samples needed to adequately define reference ranges (ranges encompassing the 2.5th to 97.5th percentiles) for measurements with a skewed distribution (for example, TSH and, to a lesser extent, free T4) is ∼400 (Refs 3,22,23). For centres unable to calculate their own reference ranges, previous international guidelines have provided fixed reference ranges for TSH. These guidelines recommend using fixed upper limits of 2.5 mU/l or 3.0 mU/l for the first and second or third trimesters, respectively6,8,19. However, many studies that were published after the first recommendation of these fixed upper limits in 2011 show that the use of such upper limits results in overdiagnosis of subclinical hypothyroidism. Taken together, these studies show that, in different study populations and different countries, between 8% and 28% of patients have a TSH concentration above these fixed cut-offs24,25. In addition, the TSH concentrations at which lower concentrations of free T4 start to occur (as a proxy for mild thyroid failure) probably lie between 4 mU/l and 5 mU/l25. More than 90% of studies published since 2005 (encompassing >65,000 individuals) that adequately calculated population-based reference ranges report an upper limit for TSH that is considerably higher (ranging from ∼0.13 mU/l to 2.17 mU/l above the fixed cut-offs)3.
Given these novel insights, the 2017 guidelines of the American Thyroid Association still advocate the use of pregnancy-specific, population-based reference ranges26. However, when these are unavailable, the American Thyroid Association now recommends the adoption of population-based reference ranges that have been determined using the same assay and in a population with similar characteristics26. Supplementary information S1 (Table) provides an overview of adequately sized studies from different populations that used various assays to determine TSH reference ranges. Finally, if none of the published studies are generalizable to the population of interest, the 2017 guidelines advocate the use of a fixed upper limit of the non-pregnancy upper limit minus 0.5 mU/l (for most centres, roughly 4 mU/l), which is similar to the upper limits in large studies involving iodine-sufficient populations26.
Despite the historically dominant role of TSH in defining gestational thyroid dysfunction, the free T4 concentration is necessary to distinguish between overt and subclinical thyroid disease. Commonly used free T4 immunoassays have been reported to be less accurate than other measurement methods using liquid chromatography/mass spectrometry or equilibrium dialysis during pregnancy due to shifts in the concentrations of thyroid hormone-binding proteins, which might interfere with hormone concentration measurements in the third trimester27,28,29,30. Fortunately, the majority of thyroid function tests are carried out at the time of the first clinical presentation, which takes place in the first half of pregnancy in the vast majority of cases.
In addition, immunoassays are a viable alternative to liquid chromatography/mass spectrometry or equilibrium dialysis because of the high correlation in the concentrations of free T4 measured by these methods28,31,32. These high inter-assay correlations enable adequate identification of women with true low or true high concentrations of free T4 and make relevant misclassification unlikely if pregnancy-specific population-based (and thus also assay-specific) reference ranges are calculated33. It has been postulated that raising the lower limit of the non-pregnancy reference range to 150% might enable total T4 concentrations to be used to assess thyroid dysfunction during pregnancy29; however, as more than 99% of T4 is bound to thyroid hormone-binding proteins, this seems to be a crude means of estimating biologically available thyroid hormone. As T4 concentrations are highly dependent on changes in TBG concentrations, T4 concentrations are more variable during early pregnancy than free T4 concentrations34,35. Furthermore, differences in the concentrations of T4 explain much less of the variation in concentrations of TSH than concentrations of free T4 do (2.5% for T4 versus 8.0% for free T4), suggesting that T4 reflects the function of the hypothalamic–pituitary–thyroid axis less accurately than free T4 (Ref. 34). Finally, during early pregnancy, free T4, but not T4, is associated with adverse pregnancy and child outcomes35,36. Taken together, these data suggest that free T4 is a more useful index of thyroid function during early pregnancy than T4 (Ref. 35).
Determinants of thyroid (dys)function
The identification and quantification of determinants of thyroid function not only adds to our knowledge of (patho)physiology but can also improve the interpretation of thyroid function measurements. Moreover, such information can be used to identify individuals at high risk for thyroid dysfunction. Studies on thyroid function determinants published over the last 10 years have further quantified the importance of several well-known risk factors for the development of thyroid disease, such as iodine intake; patient characteristics, including BMI and ethnicity; hCG concentrations; and other placental factors.
Iodine is a major component of thyroid hormone and is essential for its production. Severe maternal iodine deficiency can lead to overt hypothyroidism and cretinism in children37. Although studies have predominantly focused on the consequences of low iodine intake on thyroid function, high iodine intake, and even intake within the normal range, is also associated with reduced thyroid function38. One cross-sectional study of 7,190 pregnant women in China demonstrated that high urinary iodine concentrations (>250 μg/l) are associated with an up to 2.2-fold higher risk of subclinical hypothyroidism and an up to 2.9-fold higher risk of hypothyroxinaemia than urinary iodine concentrations of 150–249 μg/l38. Although low urinary iodine concentrations, namely, urinary iodine concentrations <100 μg/l, were associated with a higher risk of thyroid autoimmunity (both TPOAb positivity and thyroglobulin antibody positivity) and overt hypothyroidism than urinary iodine concentrations of 150–249 μg/l, no associations between low urinary iodine concentrations and subclinical hypothyroidism or hypothyroxinaemia were found, even though some analyses noted U-shaped associations between these parameters38.
Differences in maternal thyroid function between and within different ethnic populations during pregnancy have been described39,40,41,42,43. Ethnicity is determined by a composite of genetic, dietary, environmental and cultural factors; thus, population-based reference ranges for TSH and free T4 differ throughout the world43,44.
Studies consistently show that BMI is also a determinant of thyroid function during pregnancy45,46,47,48,49,50. Higher BMI is associated with higher TSH concentrations46,47,48,49,50, lower free T4 concentrations46,47,48,49,50, higher free T3 concentrations46,48 and a higher T3:T4 ratio. For example, a Finnish study reported that the upper TSH limit for women with a BMI between 20 kg/m2 and 25 kg/m2 is 2.86 mU/l, while the upper TSH limit for women with a BMI >30 kg/m2 is 3.50 mU/l (Ref. 48). Various other clinical characteristics, including maternal age and smoking history, are risk factors for thyroid dysfunction. When analysed in combination, however, these risk factors do not accurately predict the risk of thyroid dysfunction in the general population, indicating that clinical characteristics cannot be used to accurately predict the risk of thyroid dysfunction51.
The rapid rise in hCG concentrations during early pregnancy leads to an increase in the free T4 concentration, which subsequently leads to a decrease in the TSH concentration34,52,53. We have shown that the thyroidal response to hCG stimulation is severely impaired in women with thyroid autoimmunity, as reflected by TPOAb positivity53. Other factors that might also reduce the thyroid gland response to hCG stimulation are a higher BMI and, to a lesser extent, higher parity (specifically ≥2) and male fetal sex11.
In addition to producing hCG, the placenta also produces angiogenic factors, such as anti-angiogenic soluble FMS-like tyrosine kinase and placental growth factor54. The thyroid gland has a high vascular density, and animal studies have demonstrated that changes in the concentrations of the above factors can decrease thyroid vasculature density by up to 68%55,56. The first study examining human pregnancies showed that high soluble FMS-like tyrosine kinase concentrations are associated with a 2.4-fold higher risk of subclinical hypothyroidism and a threefold higher risk of hypothyroxinaemia. In addition, high placental growth factor concentrations were associated with a 1.8-fold higher risk of hypothyroxinaemia57. Furthermore, these two factors also seem to influence the thyroidal response to hCG stimulation57. These data provide novel insights into pregnancy-specific thyroid physiology and illustrate the potential pathways through which the placenta might influence thyroid function other than those associated with its production of hCG.
Although various studies show that clinical characteristics affect reference ranges between, or even within, populations, clinical characteristics are poor predictors of thyroid dysfunction. Therefore, it remains to be elucidated whether the implementation of trimester-, BMI- or ethnicity-based reference ranges can improve the ability of clinicians to identify gestational thyroid disease. Future studies should aim to identify novel determinants of thyroid function and elucidate the contributions of known determinants, such as genetics or endocrine disruptors, to the development of thyroid disease to enable identification of clinically relevant patient subgroups.
Consequences and treatment
Overt hypothyroidism. Overt maternal hypothyroidism is consistently associated with a higher risk of pregnancy complications, including premature delivery, low birth weight, miscarriage and pre-eclampsia58, as well as a higher risk of detrimental effects on fetal neurodevelopment. In 1999, a large case–control study demonstrated that children born to women with untreated hypothyroidism have a 7-point reduction in IQ compared with children whose mothers were euthyroid59. These children also had delays in motor skill development, language development and attention at 7 to 9 years of age59. Interestingly, these deficits were not observed in children born to mothers who reported receiving levothyroxine treatment later in pregnancy59. No data suggest that women with adequately treated hypothyroidism have an increased risk of pregnancy complications compared with women with normal thyroid function, a finding which is in sharp contrast to data pertaining to women with untreated hypothyroidism.
No randomized controlled trials of levothyroxine treatment for overt hypothyroidism during pregnancy have been conducted. It must be noted, however, that given the adverse effects of overt hypothyroidism on pregnancy outcomes, as well as fetal development, the general consensus is that overt hypothyroidism during pregnancy should be treated as early as possible26. As such, performing a placebo-controlled trial involving women with overt hypothyroidism is unethical. The placental transfer of maternal T4 to the fetus is crucial for optimal fetal brain development, and as levothyroxine supplementation is the treatment of choice for overt hypothyroidism, the therapy should be initiated as soon as possible. Patients using levothyroxine and liothyronine combination therapy or desiccated thyroid extracts often have a T4:T3 ratio that is lower than that of patients with normal thyroid function. Therefore, in these patients, the placental transfer of maternal T4 to the fetal brain might be insufficient60,61. These findings indicate that patients using combination therapy or desiccated thyroid extracts who desire to become pregnant should be switched to levothyroxine.
Subclinical hypothyroidism. Similar to overt hypothyroidism, subclinical hypothyroidism is associated with a higher risk of pregnancy loss, placental abruption, premature delivery, pre-eclampsia and neonatal death62,63,64. However, the many studies regarding this topic defined subclinical hypothyroidism differently. Some of the studies used non-pregnancy reference ranges to define subclinical hypothyroidism, while others used fixed TSH cut-offs, and additional studies calculated pregnancy-specific reference ranges63. Subclinical hypothyroidism is associated with various adverse pregnancy outcomes overall, but the largest effect sizes for specific clinical associations were noted when subclinical hypothyroidism was defined according to population-based reference ranges3,63,64. In contrast to its association with various adverse pregnancy outcomes, evidence from prospective cohort studies indicates that subclinical hypothyroidism is not associated with adverse neurobehavioral outcomes in the offspring13,65,66,67.
Thyroid autoimmunity is a major risk factor for subclinical hypothyroidism24. Approximately one-third of women with subclinical hypothyroidism are TPOAb positive10,11. Studies published in the last 3 years show that the combination of subclinical hypothyroidism and TPOAb positivity is associated with a higher risk of adverse pregnancy outcomes, such as miscarriage, gestational diabetes mellitus and premature delivery44,68,69,70. Although the risk of adverse pregnancy outcomes is highest in women with subclinical hypothyroidism who are TPOAb positive, women with subclinical hypothyroidism who are TPOAb positive still have a higher risk of adverse pregnancy outcomes than women who are euthyroid44,68,69,70. Regardless of the presence of subclinical hypothyroidism, the combination of high concentrations of TSH and TPOAb positivity synergistically increases the risk of adverse pregnancy outcomes compared with the presence of only high TSH concentrations or TPOAb positivity. Moreover, the higher risk for women who are TPOAb positive already occurs for TSH concentrations in the high-normal range (in most studies for concentrations of TSH >2.5 mU/l)44,68,69,70.
Data on the effects of treatment for subclinical hypothyroidism are sparse. An observational study published in 2017 found that treatment of subclinical hypothyroidism (defined as concentrations of TSH between 2.5 mU/l and 10 mU/l, concentrations of free T4 >0.8 ng/dl (10.3 pmol/l) and/or concentrations of T4 >7.5 μg/dl (207 nmol/l)) with a median levothyroxine dosage of 50 μg (interquartile range 25.0–62.5) was associated with a lower risk of pregnancy loss (OR 0.62 [95% CI 0.48–0.82]) but a higher risk of premature delivery (OR 1.60 [95% CI 1.14–2.24]), gestational diabetes (OR 1.37 [95% CI 1.05–1.79]) and pre-eclampsia (OR 1.61 [95% CI 1.10–2.37]) than non-treatment71. Interestingly, the beneficial effect of treatment with levothyroxine was dependent on the TSH concentration at presentation71, as was illustrated by a subgroup analysis revealing that the beneficial effects of levothyroxine on pregnancy loss were predominantly present in women with TSH concentrations above 4.0 mU/l (OR 2.5–4.0 mU/l: 0.87 [95% CI 0.62–1.22], versus >4.0 mU/l: OR 0.43 [95% CI 0.29–0.63]; P for difference: <0.01). Unfortunately, no data were available for TPOAbs.
These data suggest that levothyroxine treatment is not beneficial for women whose concentrations of TSH are at the high end of the normal range, defined as concentrations of ∼2.5 mU/l to 4.0 mU/l, regardless of TPOAb status. Further studies are required to identify specific subgroups of women who could benefit from treatment with levothyroxine. Such studies could include investigations into the differences in the risk of adverse pregnancy outcomes associated with different combinations of thyroid function and hCG concentrations, as well as investigations into the threshold TSH concentrations at which treatment with levothyroxine becomes beneficial for women who are TPOAb positive or TPOAb negative. Studies investigating the effects of levothyroxine treatment in women with subclinical hypothyroidism on offspring neurocognition are discussed in the section on hypothyroxinaemia (see below).
Thyroid autoimmunity. TPOAbs, which are a marker of thyroid autoimmunity, are the most important risk factor for thyroid dysfunction during pregnancy. Women who are TPOAb positive have higher TSH concentrations, lower free T4 concentrations and a higher risk of thyroid dysfunction during pregnancy than women who are TPOAb negative24,72. Intriguingly, TPOAb positivity in and of itself is associated with an elevated risk of miscarriage and premature delivery44,72,73. Two major hypotheses regarding the underlying mechanisms through which TPOAb positivity might increase the risk of adverse pregnancy outcomes have been postulated. A higher general susceptibility to autoimmunity might lead to a higher risk of TPOAb positivity74,75 as well as a higher risk of adverse pregnancy outcomes, creating a spurious association for TPOAb positivity74,75. Alternatively, TPOAb positivity could lead to thyroid dysfunction and subsequently result in a higher risk of adverse pregnancy outcomes.
Although various arguments can be applied to the latter hypothesis, two clinical trials have shown that levothyroxine treatment in women who are TPOAb positive markedly decreases the risk of miscarriage as well as that of premature delivery57,58. In one of these trials, 115 women who were TPOAb positive were randomly assigned to receive either levothyroxine at a mean gestational age of 10 weeks at a dosage based on their concentration of TSH (TSH concentration <1 mU/l = 0.5 μg/kg per day; TSH concentration 1–2 mU/l = 0.75 μg/kg per day; TSH concentration >2 mU/l or TPOAbs >1,500 kIU/l = 1 μg/kg per day) or no treatment76. In this study, levothyroxine treatment markedly reduced the rates of miscarriage (13.8% versus 3.4%) and premature delivery (22.4% versus 7.0%)76. The participants' first endocrinology visit took place on average during the 11th week of pregnancy, and 92% of the women enrolled in the study visited the endocrinologist before the 20th week of gestation. Given that the definition of miscarriage is the loss of a pregnancy before the twentieth week of gestation and that roughly 80% of miscarriages occur before the 12th week of gestation, the design of the trial was not adequate for studying the indicated outcome.
In the second trial, which used the same protocol as the first trial, 131 women who were TPOAb positive were randomly assigned to receive levothyroxine or no treatment at a mean gestational age of 11 weeks77. This study also found that levothyroxine reduced the rate of premature delivery (23.7% versus 7.1%) but had no beneficial effect on the rate of miscarriage (3.6% versus 3.4%)77. The treatment and control groups had somewhat high TSH concentrations (3.7 mU/l (interquartile range 2.4–4.8 mU/l) and 3.2 mU/l (2.1–5.2 mU/l)) at presentation, which probably explains the high rate of premature delivery in the non-treated group77. In addition, the authors stratified their analyses based on the TSH concentration at presentation. The data showed that the benefit of levothyroxine treatment in reducing the risk of preterm delivery was mainly present in the group with TSH concentrations >4.0 mU/l (29.4% versus 5.3%, P < 0.01) compared with the women with TSH concentrations <4.0 mU/l (16.7% versus 11.1%, P = 0.69). These findings were consistent with those presented in the section on subclinical hypothyroidism indicating that the risk of adverse pregnancy outcomes is higher in women with both TPOAb positivity and high TSH concentrations than in women with only high TSH concentrations44,68,69,70.
Although both of the above trials had limitations, their findings strongly suggest that the adverse outcomes associated with TPOAb positivity are indeed effectuated through changes in thyroid function76,77; however, data from two on-going randomized controlled trials investigating the effects of levothyroxine treatment in women who are TPOAb positive, namely, the T4LIFE trial78 and the T4LIFE trial79, have yet to be reported.
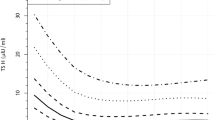
An area that remains unclear is the mechanisms through which thyroid autoimmunity is associated with adverse outcomes. We have shown that women who are TPOAb positive have a severely impaired thyroidal response to hCG stimulation53 (Figs 2,3) compared with women who are TPOAb negative. Furthermore, women with high concentrations of hCG but relatively low free T4 concentrations have a higher risk of premature delivery53; however, women who are TPOAb positive and have hCG and free T4 concentrations similar to those of women who are TPOAb negative do not have a higher risk of premature delivery53. These data indicate that women who are TPOAb positive lack the hCG-mediated increase in thyroid function during early pregnancy and that this leads to a lower availability of thyroid hormone. This might be the mechanism responsible for the association between thyroid autoimmunity and adverse pregnancy outcomes. On the basis of current evidence, we believe that clinicians should consider women who are TPOAb positive a high-risk group for thyroid-related adverse outcomes, especially if TSH concentrations are elevated or in the higher end of the normal range.
Plots showing the association between total hCG concentrations and free T4 in TPOAb positive (a) and TPOAb-negative women (b). The associations are depicted as the mean (dark blue line) and 95% confidence interval of the mean (light blue area). In the original publication, these results were externally replicated (only one of the two cohorts is shown). Adapted from Korevaar, Tim I. M. et al. Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: two population-based prospective cohort studies. J. Clin. Endocrinol. Metab. 102, 69–77 (2016), with permission from Oxford University Press.
Because of a lack of thyroidal stimulation by hCG in thyroid peroxidase antibody (TPOAb)-positive women, the total thyroid hormone availability during pregnancy, which is represented by the area under the curve, is decreased, leading to a relative thyroid hormone shortage during early pregnancy.
Hypothyroxinaemia. Initially, thyroidologists considered hypothyroxinaemia a pregnancy-specific disease that reflects a state of mild iodine deficiency; however, hypothyroxinaemia also occurs in iodine-sufficient areas, and concentrations of free T4 and T4 typically do not increase following iodine supplementation80,81,82,83,84,85. Interestingly, the largest study on iodine and thyroid disease enitities performed to date found that a subset of women with low urinary iodine concentrations (<100 or 100–149 μg/l) were not at higher risk of hypothyroxinaemia compared with women with normal urinary iodine concentrations. Women with high urinary iodine concentrations (≥500 μg/l), however, had a 2.9-fold higher risk of hypothyroxinaemia than women with normal urinary iodine concentrations38. Taken together, this suggests that not only iodine deficiency but a multifactorial and pregnancy-specific pathophysiology underlies the development of hypothyroxinaemia. This concept is also in line with the various newly identified risk factors for gestational hypothyroxinaemia, including iron status, placental angiogenic factors and patient characteristics, such as BMI and age38,46,51,57,86. In accordance with the general notion that hypo-thyroxinaemia is a pregnancy-specific disease, in 2017, we reported that the concentration of hCG is a determinant of hypothyroxinaemia11. We also demonstrated that the thyroidal response to hCG in women with hypo-thyroxinaemia was similar to that in euthyroid women, which indicates that hypothyroxinaemia could be a reflection of increased thyroid hormone sensitivity rather than a shortage of thyroid hormone availability11. Further studies will help identify the mechanisms underlying hypothyroxinaemia.
Although subclinical hypothyroidism and hypothyroxinaemia are both considered forms of mild thyroid dysfunction, these entities have been associated with different adverse outcomes. While subclinical hypothyroidism is associated with various adverse pregnancy outcomes, hypothyroxinaemia is predominantly associated with adverse neurobehavioral outcomes in the child44,66,67,87. In 3,659 mother–child pairs from a prospective birth cohort, maternal hypothyroxinaemia (defined as a TSH <2.5 mU/l with free T4 <5th percentile) was associated with a 1.8-fold higher risk of expressive language delay at both 18 and 30 months of age. In addition, the authors reported that maternal hypothyroxinaemia was also associated with a twofold higher risk of nonverbal cognitive delay65. Follow-up data subsequently revealed that maternal hypothyroxinaemia (free T4 <5th percentile and TSH <2.5 mU/l) was associated with a nonverbal IQ that was 4.3 points lower than that associated with maternal euthyroidism in children at 6 years of age88. When various free T4 cut-offs were assessed, maternal free T4 concentrations <10th percentile were associated with a child IQ that was 1.5–3.8 points lower than that associated with maternal free T4 concentrations in the middle 80 percentiles13.
Similar findings were observed in a Spanish prospective birth cohort involving 1,643 mother–child pairs. The authors investigated the association of maternal TSH and free T4 concentrations and the mental score and psychomotor score of infants at 14 months of age67. The data showed that a dose-response relationship existed between low maternal free T4 concentrations and mental score. At 14 months of age, infants whose mothers had gestational free T4 concentrations <10th percentile, <5th percentile and <2.5th percentile had an IQ that was 2.4, 3.4 and 4.2 points lower, respectively, than that of children whose mothers had gestational free T4 concentrations above the indicated cut-offs67.
Although IQ is the most studied clinical outcome, maternal hypothyroxinaemia has also been associated with a higher risk of other neurocognitive child outcomes that are a postnatal marker of intrauterine brain development. One study showed that, compared with maternal euthyroidism, maternal hypothyroxinaemia (defined as a TSH <2.5 mU/l and a free T4 <5th percentile) is associated with a 2.6-fold higher risk of clinical symptoms of autism spectrum disorder in both boys and girls at a median age of 6 years89. Another investigation compared 1,010 patients with schizophrenia with matched controls (1:1). The authors reported that maternal hypothyroxinaemia (defined as a TSH between the 5th and 95th percentiles and a free T4 ≤10th percentile) in early pregnancy was associated with a 1.7-fold higher risk of schizophrenia diagnosed at a mean age of 19.1 years (maximum 26 years; 95% CI 1.13–2.55) compared with euthyroidism90. Maternal hypothyroxinaemia is also associated with other childhood outcomes reflective of suboptimal prenatal brain development, such as attention deficit hyperactivity disorder, slower reaction times, suboptimal school performance and lower grey matter and cortical volumes13,91,92,93.
Two randomized controlled trials have assessed the effects of treatment of mild maternal thyroid dysfunction on child IQ. In the CATS trial, women were randomly assigned to undergo no screening or screening and subsequent treatment with 150 μg levothyroxine if the TSH concentration was above the 97.5th percentile or the free T4 concentration was above the 2.5th percentile (Ref. 7). A total of 390 children of mothers who received treatment and 404 children of mothers who did not receive treatment underwent IQ testing at 3 years of age7. The authors reported no difference between the groups in the mean IQ or the proportion of children with an IQ below 85. The data separately also showed no difference for the low free T4 and high TSH groups comopared with the no screening group7. Various arguments have been proposed for this negative finding. For example, it was suggested that treatment was started too late (median 13 weeks), that IQ cannot be assessed reliably at 3 years of age and that the percentage of patients lost to follow-up was too high (24%). Findings from studies performed in 2016, however, indicate that the high levothyroxine dosage of 150 μg might have contributed to the lack of a net beneficial effect, an idea discussed in more detail below13,94 (Fig. 4).
Graphs showing the association between maternal free T4 concentrations during early pregnancy (8–18 weeks) and child IQ at median age of 6 years (a) or mean cortical volume, as assessed by magnetic resonance imaging, at median age of 8 years (b). The lower two graphs show the optimal treatment scenario (c) and the overtreatment scenario (d). These two graphs depict the concept of overtreatment. The red dots represent the free T4 concentrations of a hypothetical patient before (left red dot) and after treatment (right red dot). The vertical dotted black lines display the upper and lower limit of the population-based, assay-specific free T4 reference range for that particular study. Reprinted from The Lancet Diabetes & Endocrinology, 4, Korevaar, Tim I. M. et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study, 35–43, Copyright (2016), with permission from Elsevier.
Previous clinical studies have predominantly focused on the effects of low maternal thyroid hormone availability on the neurodevelopment of children. In 2016, however, we assessed the effects of the full range of free T4 concentrations on child neurodevelopment and reported that high free T4 concentrations are associated with suboptimal neurodevelopment outcomes to a degree similar to that of low free T4 concentrations. Specifically, we found that high concentrations of maternal free T4 (ranging from the 89th to the 98th percentile) were associated with a 1.4- to 3.7-point lower IQ, lower grey matter volume and a lower cortical volume13. These data indicate that maternal free T4 concentrations above the normal range and even within the higher end of the normal range might have effects on the fetus that are as detrimental as low maternal free T4 concentrations, suggesting that women can be overtreated with levothyroxine during pregnancy. Important follow-up studies involving the patients from the CATS trial are underway, which might further identify the late benefits and potential harms of maternal levothyroxine treatment95.
Another multicentre randomized control trial from the USA included women with hypothyroxinaemia (n = 526) or subclinical hypothyroidism (n = 677) and randomly assigned these patients to receive either levothyroxine treatment (50 μg or 100 μg) or placebo starting at a mean of 17 to 18 weeks of gestation. The children of the patients who completed the treatment regimens underwent IQ testing at 3 or 5 years of age, and 92.3% of the children in the study were assessed at age 5 (Ref. 96). When the study was designed, the sample size was determined based on a previous study showing that children of women with overt hypothyroidism had an IQ that was 7 points lower than that of children of euthyroid women59. The sample size was determined based on the number of women needed to yield a statistically significant difference of 5 IQ points between the two groups97; however, since the start of this trial, prospective cohort studies have shown that mild maternal thyroid dysfunction is associated with a maximum 3–4 point lower IQ13,65,66,67. As such, the design of this trial does not enable the authors to identify the actual expected difference in IQ between the two groups. Therefore, the results of this trial cannot answer the question of whether levothyroxine treatment can reverse the adverse effects of low maternal thyroid function on the neurodevelopment of children.
The results of the trial showed that children of women with subclinical hypothyroidism or hypothyroxinaemia who were treated with levothyroxine had a 3 point higher median IQ (median treated versus untreated: 97 versus 94 and median treated versus untreated: 94 versus 91, respectively) compared with children of untreated women96. This increase in IQ occurred despite the very late start of treatment, which was initiated at a mean of gestational week of 17 in women with subclinical hypothyroidism and in week 18 in women with hypothyroxinaemia. In spite of the late initiation of treatment, the results show that the increase in IQ was equal to the increase expected based on the results of previous observational studies. Due to inadequate statistical power, however, the above result fails to reach statistical significance96. Interestingly, although the sub-analyses performed in the above study were also underpowered, the children of women who were randomly assigned to receive treatment earlier during gestation and the children of women who were TPOAb positive or had the highest TSH or the lowest free T4 concentrations experienced the highest increases in IQ as a result of treatment with levothyroxine96. The median 3 point increase in IQ that was reported in this study indicates that treatment with levothyroxine can lead to considerable public health benefits in a large group of individuals98,99. Additional studies are required to elucidate the effects of levothyroxine treatment in mothers with mild thyroid dysfunction on the neurodevelopment of children.
Overall, observational studies show that subclinical hypothyroidism is associated with various adverse pregnancy outcomes, but not with child neurocognitive development, while hypothyroxinaemia is associated with child neurocognitive development but not with adverse pregnancy outcomes13,66,67. The results of studies on the association of hypothyroxinaemia with adverse pregnancy outcomes, such as premature delivery or pre-eclampsia, are less consistent44,100,101. These data suggest that subclinical hypothyroidism or maternal TSH concentrations better reflect maternal thyroid status, whereas maternal free T4 concentrations better reflect thyroid hormone availability for the fetus, independent of TSH concentrations. The negative association of maternal free T4 concentrations with birth weight as well as the lack of an association between maternal TSH concentrations and adverse childhood outcomes noted by the majority of studies regarding these outcomes support this view13,65,67,87,90,92,102,103,104.
Treatment considerations. The field is currently lacking proper randomized studies on the effects of levothyroxine treatment for symptoms associated with mild thyroid hypofunction, such as subclinical hypothyroidism, TPOAb positivity or hypothyroxinaemia. Currently, making evidence-based recommendations regarding indications for levothyroxine treatment for mild thyroid hypofunction is not possible; however, new data indicate that levothyroxine treatment might come with the potential risk of overtreatment13,94. In addition, women with mild thyroid hypofunction might be more prone to overtreatment than women with overt thyroid disease because the thyroid gland still has residual functional capacity and can be stimulated by hCG, regardless of the levothyroxine dosage. Therefore, in contrast to overt hypothyroidism, mild thyroid hypofunction should not be treated with a full, weight-based levothyroxine dosage. We believe that it is more appropriate to start treatment with levothyroxine at a dose of 25–50 μg per day and then re-assess thyroid function after 2 to 3 weeks. Alternatively, some studies have used a weight-based levothyroxine regimen whose starting dose is determined by assessing a patient's TSH concentration at inclusion. For patients with concentrations of TSH <1.0 mU/l, the levothyroxine dosage was 0.5 μg/kg per day. For patients with concentrations of TSH ranging from 1.0 to 2.0 mU/l, the levothyroxine dosage was 0.75 μg/kg per day, and for patients with concentrations of TSH >2.0 mU/l, the levothyroxine dosage was 1 μg/kg per day76. Regarding treatment aims, in our opinion, reducing the concentration of TSH to <2.5 mU/l, the TSH cut-off that is most widely used, seems reasonable. Furthermore, the median population TSH concentration, which in large studies ranges between 0.74 mU/l and 2.0 mU/l during the first and second trimester, could also be taken into account3,26.
Subclinical hyperthyroidism. The majority of cases of subclinical hyperthyroidism that occur during pregnancy result from the transient peak in the concentration of free T4 that occurs under the influence of hCG11. As most cases of gestational subclinical hyperthyroidism are of a transient and physiological nature, there is a lack of data showing an association between subclinical hyperthyroidism and adverse outcomes, and some studies even indicate that transient subclinical hyperthyroidism has protective effects with respect to pregnancy outcomes10,44,93,105,106,107. Alternatively, subclinical hyperthyroidism might represent a subclinical form of Graves hyperthyroidism, in which case the suppressed TSH and high-normal free T4 characteristic of the disease probably persist throughout pregnancy; however, free T4 concentrations that remain above the upper limit of the normal range might have unfavourable effects, as studies have shown that higher free T4 concentrations are associated with reduced birth weight and reduced child neurocognition13,87,102. Although women with suppressed TSH production and free T4 concentrations within the higher end of the normal range might benefit from further follow-up, it is unlikely that lowering free T4 concentrations with anti-thyroid drugs is beneficial given the harms associated with such drugs (see below).
Overt hyperthyroidism. During pregnancy, two major subtypes of overt hyperthyroidism can occur. One is a pathological form that predominantly affects women with Graves disease or diseases characterized by autonomous thyroid hormone production, for example, multi-nodular toxic goitre or toxic adenomas. Pathological hyperthyroidism during pregnancy is rare, with estimated prevalence rates in Western countries of 0.5% to 1.3% for pre-existing Graves disease, 0.05% for new-onset Graves disease and 0.1% for autonomous thyroid hormone production12,108. The disease often presents with clear biochemical abnormalities (suppressed TSH concentrations with high free T4 concentrations typically above 1.5 times the upper limit of normal), symptoms such as palpitations, tremor or anxiety and is associated with a high risk of adverse pregnancy outcomes. The other form is characterized by transient elevations in thyroid function due to high hCG concentrations that typically peak around the 10th week of pregnancy (Fig. 1). As defined by population-based reference ranges, that is, a TSH concentration <2.5th percentile and a free T4 concentration above the 97.5th percentile, gestational hyperthyroidism occurs in 0.3–1.0% of all pregnant women10,44,109. Some of these women do not have symptoms of hyperthyroidism, whereas others are classified as being transiently thyrotoxic and require treatment with therapeutics such as propranolol to relieve their symptoms.
Pathological forms of overt hyperthyroidism. Data on the consequences and effects of treatment of non-hCG-induced hyperthyroidism during pregnancy are sparse, and the studies regarding this issue predominantly include women with Graves disease. Various studies have linked the pathological forms of gestational hyperthyroidism to a higher risk of pre-eclampsia, preterm birth, low birth weight and maternal heart failure12,110,111,112,113,114,115,116; however, the majority of these studies either lack data on the effects of treatment or included only women who received anti-thyroid drugs. Differences in the ability of thyroid-related factors and drugs to cross the placenta (Fig. 5) make it difficult to identify whether these pregnancy complications are caused by maternal hyperthyroidism, fetal hyperthyroidism (caused by the transplacental passage of TSH receptor antibodies) or the adverse effects of anti-thyroid drug treatment, including fetal hypothyroidism or treatment toxicity leading to developmental anomalies or maternal liver dysfunction.
The figure graphically depicts thyroid-related drugs and substances capable of affecting the function of the fetal thyroid gland as well as other tissues crossing the placenta. Of note, the fetus cannot escape the Wolff–Chaikow effect, that is, a phenomenon in which low iodine concentrations lead to decreased thyroid hormone production and high thyroid hormone concentrations lead to increased thyroid hormone production*, until after roughly the 36th week of pregnancy, at which time high iodine concentrations are likely to cause low thyroid hormone production. TSHR-Ab, TSH receptor antibody; PTU, propylthiouracil.
A large American record linkage study compared 417 women diagnosed with hyperthyroidism during pregnancy with ∼217,000 controls and found that women diagnosed with hyperthyroidism had a 1.8-fold higher risk of pre-eclampsia compared with control participants. In line with this higher risk of pre-eclampsia, women diagnosed with hyperthyroidism also had a 1.2-fold higher risk of threatened preterm birth, a 1.8-fold higher risk of late preterm birth and a 3.7-fold higher risk of maternal admission to intensive care compared with control participants117. A similar study from Denmark showed that women diagnosed with hyperthyroidism have higher risks of miscarriage, stillbirth, preterm birth, lower birth weight and having a child with attention deficit hyperactivity disorder than control participants118,119,120.
Although the abovementioned record linkage studies are prone to various types of misclassification bias, such bias was less of an issue for a recent case–control study investigating 208 women with hyperthyroidism (89.4% were diagnosed before pregnancy, and 95% received treatment) and 403 matched control participents116. Overall, women with hyperthyroidism had a 3.9-fold higher risk of pre-eclampsia, a 2.2-fold higher risk of fetal growth restriction, a 1.7-fold higher risk of preterm birth and a 3.6-fold higher risk of induction of labour than control participants116. Interestingly, apart from the risk of fetal growth restriction, these risks did not differ between women diagnosed before (n = 186) or during pregnancy (n = 22).
The risk of the aforementioned adverse outcomes, however, were substantially higher in women with uncontrolled disease (n = 45) than in those with controlled disease (that is, women with free T4 concentrations within or slightly above the reference range and without thyrotoxic symptoms) (n = 163). This study, along with other observational studies, shows that treatment has a beneficial effect on the risk of adverse outcomes113,114, which is in line with studies that did not identify a higher risk of adverse outcomes in women receiving adequate antenatal care115. These findings suggest that high concentrations of thyroid hormone cause adverse pregnancy outcomes rather than anti-thyroid drugs, although the possibility that disease severity or higher TSH receptor antibody concentrations have confounding effects in studies on pregnancy outcomes cannot be excluded116.
Anti-thyroid drug treatment, however, is still associated with potential harms, particularly when used during early pregnancy. The use of propylthiouracil during pregnancy is predominantly associated with maternal liver injury14, as well as with an increased risk of birth defects, such as preauricular sinuses or cysts and hydronephrosis15,16. The use of methimazole (thiamazole) or carbimazole is associated with cases of agranulocytosis17,18 and a higher risk of birth defects such as choanal atresia, aplasia cutis and omphalocele15,16. Clinicians can limit the adverse outcomes associated with anti-thyroid drugs by applying a pregnancy-specific anti-thyroid drug strategy. First, clinicians should discuss the option of definitive treatment with all women of childbearing age with hyperthyroidism before pregnancy. If physicians or their patients choose radioactive iodine as a definitive treatment, then clinicians should monitor the subsequent flare-up of TSH receptor antibodies for up to 1 year after treatment, as TSH receptor antibodies can cross the placenta and stimulate the fetal thyroid. TSH receptor antibody titres generally remain higher after the use of radioactive iodine than after other treatment modalities121.
Second, if a patient prefers anti-thyroid drugs or is already pregnant while on anti-thyroid drugs, 'block and replace therapy', that is, therapy consisting of high-dose anti-thyroid drugs and titration of levothyroxine, is not an option. For these patients, therapy should consist of anti-thyroid drug monotherapy administered at the lowest possible dose. Patient follow-up is required to monitor anti-thyroid drug therapy effects, and it should be noted that the dose of anti-thyroid drugs would typically need to be reduced during later pregnancy due to the immune tolerance of the fetus and subsequent pregnancy-related decreases in the maternal immune response122,123. Large population studies have indicated that the use of propylthiouracil during early pregnancy is associated with a slightly lower risk of adverse reactions or outcomes, as well as less severe fetal anomalies, compared with thiamazole15,16. Therefore, clinicians are advised to switch women who are receiving thiamazole and are in need of continuing therapy during pregnancy to propylthiouracil as early as possible and remain treated with propylthiouracil up until the 16th week of pregnancy. After this time point, the critical window for fetal organogenesis has passed. Interestingly, data from one study show that a novel approach in which potassium iodide is used to control Graves hyperthyroidism during the first trimester might reduce the incidence of congenital anomalies124.
Third, following anti-thyroid drug therapy, the rate of relapse in women with Graves disease is 30–50%; however, women whose Graves disease is biochemically controlled with a stable low dose of thiamazole (5–10 mg) or propylthiouracil (100–200 mg) before pregnancy have a lower chance of relapse than women with uncontrolled disease. If relapse does occur upon anti-thyroid drug cessation this is likely to occur after a few months in susceptible patients125. Therefore, in order to prevent the potential harmful effects of anti-thyroid drugs on the fetus, clinicians can discuss the possibility of stopping treatment with their patients when they wish to become pregnant or at the time of the first positive pregnancy test in the case of non-planned pregnancies. Upon anti-thyroid drug cessation, we advise clinicians to monitor thyroid function closely at regular intervals, for example, every 1 to 2 weeks26.
Finally, clinicians should assess the concentrations of TSH receptor antibodies in patients with Graves hyperthyroidism at the time of the first presentation. If the concentrations of TSH receptor antibodies are elevated, the fetus should be monitored every 4–6 weeks by assessing the fetal heart rate, fetal growth and/or fetal thyroid appearance via ultrasonography from mid-pregnancy until birth. Together with maternal thyroid function, these measures are a reflection of fetal thyroid hormone status126,127.
Gestational hyperthyroidism. Gestational hyperthyroidism is typically considered a non-pathological condition because the biochemical disorder is driven by the physiological peak in hCG. Despite the fact that gestational hyperthyroidism generally occurs as a result of physiological changes, some87,102,105,128, but not all101,111,113,117,129,130, studies on the association of gestational hyperthyroidism with adverse pregnancy and/or child neurodevelopmental outcomes have shown that patients with gestational hyperthyroidism have a higher risk of low birth weight and a higher risk of pre-eclampsia than patients with euthyroid pregnancies. These studies, however, lacked measurement of TSH receptor antibody levels; therefore, Graves disease could have been classified as gestational hyperthyroidism. To overcome misclassification, we studied the combination of thyroid function and hCG on pregnancy outcomes. We reported that women with a high hCG concentration and high thyroid function do not have a higher risk of pre-eclampsia. By contrast, women with high thyroid function despite low hCG concentrations, presumably due to TSH receptor antibodies or autonomous thyroid hormone secretion, have up to an 11-fold higher risk of pre-eclampsia than euthyroid patients128. These findings indicate that an additional hCG concentration measurement could help clinicians distinguish physiological from pathological forms of hyperthyroidism during pregnancy. These results warrant further studies on the different subtypes of biochemical hyperthyroidism.
Conclusions
Studies published over the past 15 years have aided our interpretation of the definitions of normal and abnormal thyroid function during pregnancy. The most specific way to define reference ranges is still a population-based approach. Fortunately, the wide range of available studies measuring thyroid function does now allow the possibility of adopting population-based reference ranges and evidence-based recommendations regarding fixed cut-off values. Some studies, however, indicate that the risks of thyroid function-related adverse pregnancy outcomes are on a continuous spectrum as opposed to being binary, that is, defined only through reference ranges. Furthermore, the risk of adverse pregnancy and child development outcomes also seems to be dependent on thyroid functional determinants, such as TPOAb positivity and hCG concentrations, and differs according to combinations of thyroid function-related measurements, including the combination of TPOAb and TSH concentrations.
Further elucidation of the conditional risks and risk thresholds will enable us to identify patients who are at risk of thyroid dysfunction-related adverse pregnancy or child developmental outcomes and might benefit from treatment, which could help prevent unnecessary treatment. Future studies are needed to identify more specific percentile-based disease-risk thresholds, and collaborative efforts intended to fulfil these objectives are underway131. Further studies on the determinants of thyroid function, such as thyroid autoimmunity, hCG, endocrine-disrupting chemicals and iodine, could prove invaluable in improving our clinical interpretation of individuals at risk and our understanding of understudied diseases, such as hypothyroxinaemia and hyperthyroidism. The field currently lacks clinical trial data. Such trials are necessary to prove that treatments have beneficial effects; however, the potential risks associated with treatment or overtreatment with levothyroxine during pregnancy require further investigation.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Krassas, G. E., Poppe, K. & Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 31, 702–755 (2010).
Hershman, J. M. The role of human chorionic gonadotropin as a thyroid stimulator in normal pregnancy. J. Clin. Endocrinol. Metab. 93, 3305–3306 (2008).
Medici, M., Korevaar, T. I., Visser, W. E., Visser, T. J. & Peeters, R. P. Thyroid function in pregnancy: what is normal? Clin. Chem. 61, 704–713 (2015).
Pop, V., Broeren, M. & Wiersinga, W. The attitude toward hypothyroidism during early gestation: time for a change of mind? Thyroid 24, 1541–1546 (2014).
Abalovich, M. et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 92, S1–S47 (2007).
Lazarus, J. et al. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur. Thyroid J. 3, 76–94 (2014).
Lazarus, J. H. et al. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med. 366, 493–501 (2012).
Stagnaro-Green, A. et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21, 1081–1125 (2011).
Blatt, A. J., Nakamoto, J. M. & Kaufman, H. W. National status of testing for hypothyroidism during pregnancy and postpartum. J. Clin. Endocrinol. Metab. 97, 777–784 (2012).
Casey, B. M. et al. Subclinical hyperthyroidism and pregnancy outcomes. Obstet. Gynecol. 107, 337–341 (2006).
Korevaar, T. et al. Stimulation of thyroid function by hCG during pregnancy: a risk factor for thyroid disease and a mechanism for known risk factors. Thyroid 27, 440–450 (2017).
Cooper, D. S. & Laurberg, P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 1, 238–249 (2013).
Korevaar, T. I. et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 35–43 (2016).
Cooper, D. S. & Rivkees, S. A. Putting propylthiouracil in perspective. J. Clin. Endocrinol. Metab. 94, 1881–1882 (2009).
Laurberg, P. & Andersen, S. L. Antithyroid drug use in pregnancy and birth defects: why some studies find clear associations, and some studies report none. Thyroid 25, 1185–1190 (2015).
Andersen, S. L., Olsen, J., Wu, C. S. & Laurberg, P. Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J. Clin. Endocrinol. Metab. 98, 4373–4381 (2013).
Takata, K. et al. Methimazole-induced agranulocytosis in patients with Graves' disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid 19, 559–563 (2009).
Taylor, P. N. & Vaidya, B. Side effects of anti-thyroid drugs and their impact on the choice of treatment for thyrotoxicosis in pregnancy. Eur. Thyroid J. 1, 176–185 (2012).
De Groot, L. et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 97, 2543–2565 (2012).
Dashe, J. S. et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet. Gynecol. 106, 753–757 (2005).
Lambert-Messerlian, G. et al. First- and second-trimester thyroid hormone reference data in pregnant women: a FaSTER (First- and Second-Trimester Evaluation of Risk for aneuploidy) Research Consortium study. Am. J. Obstet. Gynecol. 199, 62e1–62e6 (2008).
Harris, E. K. & Boyd, J. C. Statistical Bases of Reference Values in Laboratory Medicine (Marcel Dekker, 1995).
Geffre, A. et al. Reference values: a review. Vet. Clin. Pathol. 38, 288–298 (2009).
Medici, M. et al. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R study. J. Clin. Endocrinol. Metab. 97, 646–652 (2012).
Li, C. et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J. Clin. Endocrinol. Metab. 99, 73–79 (2014).
Alexander, E. K. et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease during Pregnancy and the Postpartum. Thyroid 27, 315–389 (2017).
Anckaert, E. et al. FT4 immunoassays may display a pattern during pregnancy similar to the equilibrium dialysis ID-LC/tandem MS candidate reference measurement procedure in spite of susceptibility towards binding protein alterations. Clin. Chim. Acta 411, 1348–1353 (2010).
Berta, E. et al. Evaluation of the thyroid function of healthy pregnant women by five different hormone assays. Pharmazie 65, 436–439 (2010).
Lee, R. H. et al. Free T4 immunoassays are flawed during pregnancy. Am. J. Obstet. Gynecol. 200, 260e1–260e6 (2009).
Kahric-Janicic, N. et al. Tandem mass spectrometry improves the accuracy of free thyroxine measurements during pregnancy. Thyroid 17, 303–311 (2007).
Christofides, N. D., Wilkinson, E., Stoddart, M., Ray, D. C. & Beckett, G. J. Assessment of serum thyroxine binding capacity-dependent biases in free thyroxine assays. Clin. Chem. 45, 520–525 (1999).
Sapin, R. & d'Herbomez, M. Free thyroxine measured by equilibrium dialysis and nine immunoassays in sera with various serum thyroxine-binding capacities. Clin. Chem. 49, 1531–1535 (2003).
Bliddal, S. et al. Gestational age-specific reference ranges from different laboratories misclassify pregnant women's thyroid status: comparison of two longitudinal prospective cohort studies. Eur. J. Endocrinol. 170, 329–339 (2014).
Glinoer, D. et al. Regulation of maternal thyroid during pregnancy. J. Clin. Endocrinol. Metab. 71, 276–287 (1990).
Korevaar, T. I. et al. Maternal total T4 during the first half of pregnancy: physiologic aspects and the risk of adverse outcomes in comparison with free T4. Clin. Endocrinol. (Oxf.) 85, 757–763 (2016).
Oken, E. et al. Neonatal thyroxine, maternal thyroid function, and child cognition. J. Clin. Endocrinol. Metab. 94, 497–503 (2009).
Zimmermann, M. B. The effects of iodine deficiency in pregnancy and infancy. Paediatr. Perinat Epidemiol. 26 (Suppl. 1), 108–117 (2012).
Shi, X. et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. J. Clin. Endocrinol. Metab. 100, 1630–1638 (2015).
Benhadi, N. et al. Ethnic differences in TSH but not in free T4 concentrations or TPO antibodies during pregnancy. Clin. Endocrinol. (Oxf.) 66, 765–770 (2007).
La'ulu, S. L. & Roberts, W. L. Second-trimester reference intervals for thyroid tests: the role of ethnicity. Clin. Chem. 53, 1658–1664 (2007).
La'ulu, S. L. & Roberts, W. L. Ethnic differences in first-trimester thyroid reference intervals. Clin. Chem. 57, 913–915 (2011).
Walker, J. A., Illions, E. H., Huddleston, J. F. & Smallridge, R. C. Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstet. Gynecol. 106, 1365–1371 (2005).
Dhatt, G. S. et al. Thyrotrophin and free thyroxine trimester-specific reference intervals in a mixed ethnic pregnant population in the United Arab Emirates. Clin. Chim. Acta 370, 147–151 (2006).
Korevaar, T. I. et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J. Clin. Endocrinol. Metab. 98, 4382–4390 (2013).
Pop, V. J., Biondi, B., Wijnen, H. A., Kuppens, S. M. & L. Vader, H. Maternal thyroid parameters, body mass index and subsequent weight gain during pregnancy in healthy euthyroid women. Clin. Endocrinol. 79, 577–583 (2013).
Knight, B. A., Shields, B. M., Hattersley, A. T. & Vaidya, B. Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur. J. Endocrinol. 174, 51–57 (2016).
Han, C. et al. High body mass index is an indicator of maternal hypothyroidism, hypothyroxinemia, and thyroid-peroxidase antibody positivity during early pregnancy. Biomed. Res. Int. 2015, 321831 (2015).
Mannisto, T. et al. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid 21, 291–298 (2011).
Laurberg, P., Andersen, S. L., Hindersson, P., Nohr, E. A. & Olsen, J. Dynamics and predictors of serum TSH and fT4 reference limits in early pregnancy: a study within the Danish national birth cohort. J. Clin. Endocrinol. Metab. 101, 2484–2492 (2016).
Mosso, L. et al. Early pregnancy thyroid hormone reference ranges in Chilean women: the influence of body mass index. Clin. Endocrinol. (Oxf.) 85, 942–948 (2016).
Korevaar, T. I. et al. Risk factors and a clinical prediction model for low maternal thyroid function during early pregnancy: two population-based prospective cohort studies. Clin. Endocrinol. (Oxf.) 85, 902–909 (2016).
Haddow, J. E. et al. Variability in thyroid-stimulating hormone suppression by human chorionic [corrected] gonadotropin during early pregnancy. J. Clin. Endocrinol. Metab. 93, 3341–3347 (2008).
Korevaar, T. I. et al. Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: two population-based prospective cohort studies. J. Clin. Endocrinol. Metab. 102, 69–77 (2017).
Romero, R. et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J. Matern Fetal Neonatal Med. 21, 9–23 (2008).
Kamba, T. et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H560–H576 (2006).
Yang, Y. et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc. Natl Acad. Sci. USA 110, 12018–12023 (2013).
Korevaar, T. I. et al. Placental angiogenic factors are associated with maternal thyroid function and modify hCG-mediated FT4 stimulation. J. Clin. Endocrinol. Metab. 100, E1328–E2334 (2015).
van den Boogaard, E. et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum. Reprod. Update 17, 605–619 (2011).
Haddow, J. E. et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 341, 549–555 (1999).
de Escobar, G. M., Obregon, M. J. & del Rey, F. E. Role of thyroid hormone during early brain development. Eur. J. Endocrinol. 151, U25–U37 (2004).
Calvo, R., Obregon, M. J., Deona, C. R., Delrey, F. E. & Deescobar, G. M. Congenital hypothyroidism, as studied in rats - crucial role of maternal thyroxine but not of 3,5,3′-Triiodothyronine in the protection of the fetal brain. J. Clin. Invest. 86, 889–899 (1990).
Sheehan, P. M., Nankervis, A., Araujo Junior, E. & Da Silva Costa, F. Maternal thyroid disease and preterm birth: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 100, 4325–4331 (2015).
Maraka, S. et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 26, 580–590 (2016).
Negro, R. & Stagnaro-Green, A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 349, g4929 (2014).
Henrichs, J. et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J. Clin. Endocrinol. Metab. 95, 4227–4234 (2010).
Henrichs, J., Ghassabian, A., Peeters, R. P. & Tiemeier, H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin. Endocrinol. (Oxf.) 79, 152–162 (2013).
Julvez, J. et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 24, 150–157 (2013).
Karakosta, P. et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J. Clin. Endocrinol. Metab. 97, 4464–4472 (2012).
Liu, H. et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid 24, 1642–1649 (2014).
Ying, H. et al. Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocr 54, 742–750 (2016).
Maraka, S. et al. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ 356, i6865 (2017).
Thangaratinam, S. et al. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 342, d2616 (2011).
He, X. et al. Thyroid antibodies and risk of preterm delivery: a meta-analysis of prospective cohort studies. Eur. J. Endocrinol. 167, 455–464 (2012).
Biro, E. et al. Association of systemic and thyroid autoimmune diseases. Clin. Rheumatol 25, 240–245 (2006).
Nakamura, H. et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J. Endocrinol. Invest. 31, 861–865 (2008).
Negro, R. et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J. Clin. Endocrinol. Metab. 91, 2587–2591 (2006).
Nazarpour, S. et al. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur. J. Endocrinol. 176, 253–265 (2016).
ISRCTNregistry Thyroid AntiBodies and LEvoThyroxine study (TABLET) trial registration. ISRCTNregistry http://www.isrctn.com/ISRCTN15948785 (2017).
Nederlands Trialregister T4LIFE trial registration. Trialregister http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3364 (2017).
Berbel, P. et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid 19, 511–519 (2009).
Pop, V. J. & Vulsma, T. Maternal hypothyroxinaemia during (early) gestation. Lancet 365, 1604–1606 (2005).
Zimmermann, M. B. The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: a review. Thyroid 17, 829–835 (2007).
Zimmermann, M. B. & Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 3, 286–295 (2015).
Pedersen, K. M. et al. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J. Clin. Endocrinol. Metab. 77, 1078–1083 (1993).
Negro, R., Soldin, O. P., Obregon, M. J. & Stagnaro-Green, A. Hypothyroxinemia and pregnancy. Endocr. Pract. 17, 422–429 (2011).
Yu, X. et al. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J. Clin. Endocrinol. Metab. 100, 1594–1601 (2015).
Medici, M. et al. Maternal thyroid hormone parameters during early pregnancy and birth weight: the Generation R Study. J. Clin. Endocrinol. Metab. 98, 59–66 (2013).
Ghassabian, A. et al. Downstream effects of maternal hypothyroxinemia in early pregnancy: nonverbal IQ and brain morphology in school-age children. J. Clin. Endocrinol. Metab. 99, 2383–2390 (2014).
Roman, G. C. et al. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann. Neurol. 74, 733–742 (2013).
Gyllenberg, D. et al. Hypothyroxinemia during gestation and offspring schizophrenia in a national birth cohort. Biol. Psychiatry 79, 962–970 (2016).
Finken, M. J., van Eijsden, M., Loomans, E. M., Vrijkotte, T. G. & Rotteveel, J. Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J. Clin. Endocrinol. Metab. 98, 1417–1426 (2013).
Modesto, T. et al. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/Hyperactivity disorder symptoms in children. JAMA Pediatr. 169, 838–845 (2015).
Pakkila, F. et al. Maternal and child's thyroid function and child's intellect and scholastic performance. Thyroid 25, 1363–1374 (2015).
European Thyroid Association Abstracts: Hales, C. et al. (p59) and Taylor, P. et al. (p128) Eur. Thyroid J. 5 (Suppl. 1), 57–176 (2016).
Hales, C. et al. The second wave of the Controlled Antenatal Thyroid Screening (CATS II) study: the cognitive assessment protocol. BMC Endocr. Disord. 14, 95 (2014).
Casey, B. M. et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N. Engl. J. Med. 376, 815–825 (2017).
ClinicalTrials Thyroid therapy for mild thyroid deficiency in pregnancy (TSH). ClinicalTrials https://clinicaltrials.gov/ct2/show/NCT00388297 (2017).
Attina, T. M. et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 4, 996–1003 (2016).
Power, C., Kuh, D. & Morton, S. From developmental origins of adult disease to life course research on adult disease and aging: insights from birth cohort studies. Annu. Rev. Publ. Health 34, 7–28 (2013).
Casey, B. M. et al. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet. Gynecol. 109, 1129–1135 (2007).
Mannisto, T. et al. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J. Clin. Endocrinol. Metab. 95, 1084–1094 (2010).
Haddow, J. E. et al. Implications of High Free Thyroxine (FT4) concentrations in euthyroid pregnancies: the FaSTER trial. J. Clin. Endocrinol. Metab. 99, 2038–2044 (2014).
Pakkila, F. et al. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J. Clin. Endocrinol. Metab. 99, E1–E8 (2014).
Dosiou, C. & Medici, M. Isolated maternal hypothyroxinemia during pregnancy: knowns and unknowns. Eur. J. Endocrinol. 176, R21–R38 (2016).
Medici, M. et al. Maternal early-pregnancy thyroid function is associated with subsequent hypertensive disorders of pregnancy: the generation R study. J. Clin. Endocrinol. Metab. 99, E2591–2598 (2014).
Tudela, C. M., Casey, B. M., McIntire, D. D. & Cunningham, F. G. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet. Gynecol. 119, 983–988 (2012).
Tong, Z. et al. The effect of subclinical maternal thyroid dysfunction and autoimmunity on intrauterine growth restriction: a systematic review and meta-analysis. Med. (Baltimore) 95, e3677 (2016).
Carle, A. et al. Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study. Eur. J. Endocrinol. 164, 801–809 (2011).
Springer, D., Zima, T. & Limanova, Z. Reference intervals in evaluation of maternal thyroid function during the first trimester of pregnancy. Eur. J. Endocrinol. 160, 791–797 (2009).
Sheffield, J. S. & Cunningham, F. G. Thyrotoxicosis and heart failure that complicate pregnancy. Am. J. Obstet. Gynecol. 190, 211–217 (2004).
Sahu, M. T., Das, V., Mittal, S., Agarwal, A. & Sahu, M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch. Gynecol. Obstet. 281, 215–220 (2010).
Luewan, S., Chakkabut, P. & Tongsong, T. Outcomes of pregnancy complicated with hyperthyroidism: a cohort study. Arch. Gynecol. Obstet. 283, 243–247 (2011).
Millar, L. K. et al. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet. Gynecol. 84, 946–949 (1994).
Davis, L. E., Lucas, M. J., Hankins, G. D., Roark, M. L. & Cunningham, F. G. Thyrotoxicosis complicating pregnancy. Am. J. Obstet. Gynecol. 160, 63–70 (1989).
Pillar, N., Levy, A., Holcberg, G. & Sheiner, E. Pregnancy and perinatal outcome in women with hyperthyroidism. Int. J. Gynaecol. Obstet. 108, 61–64 (2010).
Aggarawal, N. et al. Pregnancy outcome in hyperthyroidism: a case control study. Gynecol. Obstet. Invest. 77, 94–99 (2014).
Mannisto, T. et al. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J. Clin. Endocrinol. Metab. 98, 2725–2733 (2013).
Andersen, S. L., Laurberg, P., Wu, C. S. & Olsen, J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 121, 1365–1374 (2014).
Andersen, S. L., Olsen, J., Wu, C. S. & Laurberg, P. Low birth weight in children born to mothers with hyperthyroidism and high birth weight in hypothyroidism, whereas preterm birth is common in both conditions: A danish national hospital register study. Eur. Thyroid J. 2, 135–144 (2013).
Andersen, S. L., Olsen, J., Wu, C. S. & Laurberg, P. Spontaneous abortion, stillbirth and hyperthyroidism: a danish population-based study. Eur. Thyroid J. 3, 164–172 (2014).
Laurberg, P. et al. TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur. J. Endocrinol. 158, 69–75 (2008).
Zakarija, M. & McKenzie, J. M. Pregnancy-associated changes in the thyroid-stimulating antibody of Graves' disease and the relationship to neonatal hyperthyroidism. J. Clin. Endocrinol. Metab. 57, 1036–1040 (1983).
Barbesino, G. & Tomer, Y. Clinical review: Clinical utility of TSH receptor antibodies. J. Clin. Endocrinol. Metab. 98, 2247–2255 (2013).
Yoshihara, A. et al. Substituting potassium iodide for methimazole as the treatment for graves' disease during the first trimester may reduce the incidence of congenital anomalies: a retrospective study at a single medical institution in Japan. Thyroid 25, 1155–1161 (2015).
Nedrebo, B. G. et al. Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with Graves' disease. Eur. J. Endocrinol. 147, 583–589 (2002).
Momotani, N., Noh, J., Oyanagi, H., Ishikawa, N. & Ito, K. Antithyroid drug therapy for Graves' disease during pregnancy. Optimal regimen for fetal thyroid status. N. Engl. J. Med. 315, 24–28 (1986).
Korevaar, T. I. et al. Maternal and birth characteristics are determinants of offspring thyroid function. J. Clin. Endocrinol. Metab. 101, 206–213 (2016).
Korevaar, T. I. et al. The risk of pre-eclampsia according to high thyroid function in pregnancy differs by hCG concentration. J. Clin. Endocrinol. Metab. 101, 5037–5043 (2016).
Ashoor, G., Maiz, N., Rotas, M., Kametas, N. A. & Nicolaides, K. H. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent development of preeclampsia. Prenat Diagn. 30, 1032–1038 (2010).
Wilson, K. L., Casey, B. M., McIntire, D. D., Halvorson, L. M. & Cunningham, F. G. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet. Gynecol. 119, 315–320 (2012).
Korevaar, T. I., Taylor, P. N., Dayan, C. M. & Peeters, R. P. An invitation to join the consortium on thyroid and pregnancy. Obstet. Gynecol. 128, 913 (2016).
Acknowledgements
The authors' research work was supported by a clinical fellowship from The Netherlands Organisation for Health Research and Development (ZonMw), project number 90700412 to R.P. Peeters.
Author information
Authors and Affiliations
Contributions
T.I.M.K. and R.P.P. wrote the first draft of the manuscript, after which all authors contributed equally to researching the data for the article, discussion of content, writing the article and reviewing and/or editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.I.M.K. has received lecture fees from Berlin-Chemie, Goodlife Healthcare and Excemed. R.P.P. has received lecture fees from Goodlife Healthcare. The other authors declare no competing interests.
Supplementary information
Supplementary information S1 (table)
Reference ranges for TSH and FT4 during early pregnancy worldwide. (PDF 148 kb)
Glossary
- Subclinical hypothyroidism
-
Defined as a high TSH concentration (>97.5th percentile) with a normal free T4 concentration (2.5th to 97.5th percentiles).
- Hypothyroxinaemia
-
Defined as a normal TSH concentration (2.5th to 97.5th percentiles) with a low free T4 (<2.5th percentile; but in some cases <5th percentile).
- Mental score
-
Determined by the outcome of standardized neurodevelopment tests, such as the WISC, and is a reflection of child IQ.
- Psychomotor score
-
The outcome of standardized neurodevelopment tests, such as the WISC, and reflects the psychomotor development of the child, including the child's achievement of developmental milestones.
Rights and permissions
About this article
Cite this article
Korevaar, T., Medici, M., Visser, T. et al. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 13, 610–622 (2017). https://doi.org/10.1038/nrendo.2017.93
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2017.93
This article is cited by
-
Effects of altitude on thyroid disorders according to Chinese three-rung, ladder-like topography: national cross-sectional study
BMC Public Health (2024)
-
Mediation by Thyroid Hormone in the Relationships Between Gestational Exposure to Methylmercury and Birth Size
Exposure and Health (2024)
-
Verhoog de levothyroxinedosering bij zwangeren met hypothyreoïdie
Huisarts en wetenschap (2024)
-
EndoBridge 2023: highlights and pearls
Hormones (2024)
-
Maternal preconception thyroid autoimmunity is associated with neonatal birth weight conceived by PCOS women undergoing their first in vitro fertilization/intracytoplasmic sperm injection
Journal of Ovarian Research (2023)