Key Points
-
Inflammation is a pathogenic link between obesity and insulin resistance.
-
Adipose tissue macrophages display characteristics ranging from alternative to classical, and these reflect the nutritional state of the organism.
-
Obesity leads to the recruitment of CCR2+LY6C+ monocytes, which give rise to pro-inflammatory, classically activated macrophages in white adipose tissue.
-
Saturated fatty acids, potentially acting via Toll-like receptor 4, drive classical macrophage activation in the obese state.
-
Alternatively activated macrophages populate lean adipose tissue and are associated with insulin sensitivity.
-
Eosinophil-derived interleukin-4 supports alternative activation of adipose tissue macrophages to promote insulin sensitivity.
-
Peroxisome proliferator-activated receptor-γ (PPARγ) and PPARδ, acting as fatty acid sensors in macrophages, cooperate with signal transducer and activator of transcription 6 and Krüppel-like factor 4 to sustain alternative macrophage activation.
-
Chronic overnutrition results in the activation of adaptive immune responses, which synergize with macrophage-mediated inflammation to promote insulin resistance.
Abstract
Metabolism and immunity are two fundamental systems of metazoans. The presence of immune cells, such as macrophages, in metabolic tissues suggests dynamic, ongoing crosstalk between these two regulatory systems. Here, we discuss how changes in the recruitment and activation of macrophages contribute to metabolic homeostasis. In particular, we focus our discussion on the pathogenic and protective functions of classically and alternatively activated macrophages, respectively, in experimental models of obesity and metabolic disease.
Similar content being viewed by others
Main
During the last 25 years, the incidence of obesity has increased dramatically throughout the world. In 2008, it was estimated that there were 1.45 billion overweight adults in the world, of which ∼500 million were obese1. The number of overweight individuals now exceeds the number of malnourished individuals by ∼525 million, a statistic that, in part, reflects the global acceptance of energy-dense foods that are rich in fats but are often lacking in vitamins and other micronutrients2. Consequently, the rise in worldwide obesity has resulted in an explosion of obesity-related health problems, including insulin resistance, type 2 diabetes, coronary artery disease, fatty liver disease and some cancers and degenerative diseases3,4,5. As behavioural and dietary approaches have been ineffective in combating obesity6, a greater emphasis is being placed on understanding the molecular links between obesity and chronic metabolic diseases. In this context, chronic low-grade inflammation, which is primarily mediated by innate and adaptive immune cells, has emerged as a key pathogenic link between obesity and its metabolic sequelae7,8,9,10,11,12.
Almost two decades ago, Spiegelman and colleagues identified the first links between inflammation, obesity and insulin resistance13,14. In these initial reports, the adipose tissue of obese animals was shown to express tumour necrosis factor (TNF), a pro-inflammatory cytokine that was found to promote insulin resistance via serine phosphorylation of insulin receptor substrate 1 (IRS1)13,14. Although these initial descriptions laid the groundwork for how inflammation in adipose tissue mediates insulin resistance, the true nature of this inflammatory process was not explored for another decade. In 2003, two reports by Ferrante and Chen demonstrated that obesity leads to infiltration of adipose tissue by macrophages — immune cells that are primarily responsible for the inflammatory response in this metabolic tissue15,16. Subsequently, there has been an avalanche of reports demonstrating that the activation state of macrophages, along with the infiltration of other innate and adaptive immune cells, contributes to the inflammatory milieu in adipose tissue that mediates insulin resistance. In this Review, we provide a general framework for understanding the dynamic crosstalk between macrophages, metabolic tissues and macronutrient metabolism, and discuss how this crosstalk is altered in the disease state of obesity.
Obesity-induced insulin resistance
The resistance of metabolic tissues — such as adipose tissue, liver and muscle — to the anabolic actions of insulin is termed insulin resistance, a characteristic feature of obesity-induced metabolic dysfunction17. Insulin resistance manifests as impaired glucose disposal in muscle and enhanced triglyceride lipolysis in adipose tissue, and this results in hyperinsulinaemia, hyperglycaemia and hyperlipidaemia18. By contrast, the liver exhibits partial insulin resistance, as evidenced by impaired suppression of glucose production (gluconeogenesis) but not lipogenesis19. Collectively, this peripheral insulin resistance in metabolic tissues causes β-cells in the pancreas to secrete more insulin, a process that is termed compensatory hyperinsulinaemia. However, with the worsening of insulin resistance, β-cell exhaustion often ensues, and this results in sustained hyperglycaemia and type 2 diabetes17,18.
Because insulin resistance has a pivotal role in the pathogenesis of type 2 diabetes, a considerable effort has been made to elucidate the causal factors responsible for obesity-induced insulin resistance. In general, several cell-extrinsic and -intrinsic mechanisms have been identified that exhibit a cause-and-effect relationship between weight gain and peripheral insulin resistance20. The cell-intrinsic pathways include mitochondrial dysfunction, oxidative stress, endoplasmic reticulum (ER) stress and ectopic lipid deposition, whereas alterations in the levels of circulating adipokines and fatty acids and the occurrence of metabolic tissue inflammation are the dominant cell-extrinsic mechanisms that modulate peripheral insulin action. As detailed discussion on these topics is beyond the scope of this Review, readers are referred to other excellent reviews on these related areas7,8,17,20. Here, we focus our discussion on the relationship between macrophage-mediated inflammation and insulin resistance.
Inflammation and insulin resistance
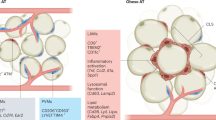
Among the immune cells that infiltrate obese adipose tissue, macrophages are functionally and numerically dominant12,21. In the adipose tissue of lean mice, approximately 10–15% of cells express the macrophage marker F4/80 (also known as EMR1), whereas 45–60% of cells in the adipose tissue of obese animals are F4/80+, indicating that obesity substantially alters the ratio of macrophages to adipocytes15. In addition to the difference in their numbers, adipose tissue macrophages in lean and obese animals exhibit distinct cellular localizations and inflammatory potentials22. Adipose tissue macrophages in lean animals have an alternatively activated (M2) phenotype (ARG1+CD206+CD301+), are less inflammatory than classically activated macrophages and are uniformly dispersed throughout the adipose tissue, whereas adipose tissue macrophages of obese mice have a pro-inflammatory, classical (M1) phenotype (NOS2+TNF+) and are primarily found in 'crown-like' structures around dying adipocytes22,23. As discussed in detail below, alternatively activated macrophages found in lean adipose tissue have a crucial role in maintaining the insulin sensitivity of adipocytes via the secretion of interleukin-10 (IL-10)21,23,24, a regulatory cytokine that potentiates insulin signalling in adipocytes. By contrast, classically activated macrophages in obese adipose tissue secrete pro-inflammatory cytokines8,12,25, which induce insulin resistance via IκB kinase-β (IKKβ)- and JUN N-terminal kinase (JNK)-mediated inhibitory serine phosphorylation of IRS proteins (Box 1). Although segregating macrophages into these two states is experimentally very useful, it also has its limitations, as macrophage phenotypes in vivo exhibit plasticity across the entire spectrum of activation states that are encompassed by the M1 and M2 nomenclature26,27.
Classically activated macrophages. Temporal analysis of obese adipose tissue has revealed that the recruitment of classically activated macrophages coincides with the appearance of necrotic adipocytes and the onset of insulin resistance28. During obesity, the mass of adipose tissue increases by hyperplasia and hypertrophy, and the latter is associated with the activation of stress signalling pathways in adipocytes that can result in cell death. For example, hypertrophic adipocytes are under constant stress, as evidenced by an increase in ER stress, hypoxic responses, release of free fatty acids and increased production of reactive oxygen species (ROS)7. Interestingly, immunohistochemical studies demonstrate that inflammatory macrophages that express the dendritic cell marker CD11c encircle necrotic adipocytes23,29, thereby forming crown-like structures15. Moreover, these classically activated CD11c+ macrophages phagocytose lipids that are released by the necrotic adipocytes and develop into lipid-engorged, multinucleated giant cells28, which are reminiscent of the inflammatory macrophages found in atherosclerotic plaques30. However, owing to the recent suggestion of a potential dissociation between adipocyte death and macrophage recruitment into obese adipose tissue31, additional work will be required to determine whether ingestion of necrotic debris is sufficient to serve as a separate stimulus for triggering macrophage-mediated inflammation and insulin resistance in adipose tissue.
Four distinct lines of evidence suggest that classically activated, inflammatory macrophages contribute to the pathogenesis of obesity-induced insulin resistance (Fig. 1). First, mice lacking CC-chemokine receptor 2 (CCR2), which is required for the trafficking of inflammatory monocytes and macrophages into tissues, are protected from obesity-induced inflammation and insulin resistance32. Second, selective depletion of the CD11c+ classically activated macrophages in CD11c-DTR mice reduces adipose tissue inflammation and improves insulin action without having a significant impact on diet-induced obesity33. Third, genetic ablation of the pro-inflammatory signalling molecule IKKβ in myeloid cells or reconstitution of mice with JNK-deficient bone marrow reduces myeloid cell-mediated inflammation in adipose tissue, resulting in preservation of insulin sensitivity34,35. Lastly, loss of GPR120, a G protein-coupled receptor that mediates the anti-inflammatory actions of omega-3 unsaturated fatty acids, worsens adipose tissue inflammation and insulin resistance36.
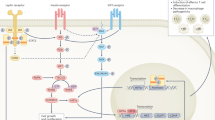
Obesity results in de novo recruitment of monocytes and macrophages into adipose tissue, and this promotes adipose tissue inflammation and insulin resistance. In part, dietary saturated fatty acids activate Toll-like receptor 2 (TLR2) and TLR4 in adipose tissue macrophages, resulting in the activation of the inflammatory signalling cascades mediated by interferon regulatory factor 3 (IRF3), JUN N-terminal kinase (JNK) and nuclear factor-κB (NF-κB). These pathways induce the production of pro-inflammatory cytokines such as tumour necrosis factor (TNF). Production of interleukin-1β (IL-1β) results from activation of the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome, potentially by ceramides, which are synthesized at higher levels during obesity (not shown). The pro-inflammatory cytokines inhibit the insulin sensitivity of adipocytes. Once initiated, these pro-inflammatory cascades are perpetuated by crosstalk between the inflamed adipocytes and classically activated adipose tissue macrophages via the production of various factors. Some of the identified chemotactic factors include CC-chemokine ligand 2 (CCL2) and osteopontin (OPN), the expression of which is induced in adipocytes and macrophages during obesity. Importantly, CCL2 leads to the recruitment of LY6C+CCR2+ inflammatory monocytes, which differentiate into classically activated adipose tissue macrophages to enhance adipose tissue inflammation. In addition, adipose tissue macrophages release CD5-like antigen (CD5L), which promotes lipolysis in adipocytes after being taken up by adipocytes via CD36-mediated endocytosis. In a feed-forward loop, the released fatty acids induce the expression of chemokines, leading to the recruitment of LY6C+CCR2+ inflammatory monocytes and macrophages into adipose tissue. Reciprocally, saturated fatty acids and pro-inflammatory cytokines (TNF and IL-1β) from adipocytes sustain the activation of adipose tissue macrophages. Mineralocorticoid receptor (MR) signalling also contributes to classical activation of adipose tissue macrophages by inhibiting their alternative activation. IL-1R, IL-1 receptor; TNFR, TNF receptor.
Although these distinct genetic manipulations provide strong support for the causative role of macrophage-mediated inflammation in insulin resistance, they raise two important questions: what are the triggers for the inflammatory activation of macrophages in obesity and what signalling pathways control the trafficking of these cells into adipose tissue?
Triggers of macrophage activation in obesity. As dysregulation of fatty acid homeostasis contributes to obesity-induced insulin resistance, fatty acids have been postulated to be a potential trigger of the classical activation of macrophages in obesity (Fig. 1). In support of this, saturated, but not unsaturated, fatty acids promote inflammatory activation of macrophages. This is mediated, in part, by ligation of the pattern-recognition receptor Toll-like receptor 4 (TLR4)37. For instance, acute infusion of lipids is sufficient to promote adipose tissue inflammation and systemic insulin resistance in wild-type but not TLR4-deficient mice38. This is further supported by bone marrow chimaera studies demonstrating that TLR4 expression by haematopoietic cells is necessary for high-fat diet-induced insulin resistance in adipose tissue and liver39. Based on these and other studies, TLR4 seemed an attractive candidate for linking dietary fatty acids with adipose tissue inflammation and insulin resistance40,41.
However, recent reports suggest that the link between dietary fatty acids and insulin resistance might not be a direct consequence of activation of canonical TLR4 signalling pathways42. In support of this, mice lacking myeloid differentiation primary response protein 88 (MYD88), the primary mediator of TLR and IL-1 receptor signalling, have a more severe metabolic disease in response to a high-fat diet than wild-type control mice43, suggesting a protective role for MYD88 signalling in obesity-induced metabolic disease. Similarly, TLR4 deficiency in mice of the C57BL/10 genetic background exacerbates diet-induced obesity, steatosis and insulin resistance44. Thus, additional investigations are necessary to pinpoint the factors and signalling pathways involved in triggering metabolic inflammation in obesity, and to clarify the involvement of the TLR4–MYD88 pathway in adipose tissue inflammation and insulin resistance.
Chemotactic factors. A key event in the initiation of adipose tissue inflammation is the recruitment of inflammatory LY6ChiCCR2+ monocytes, which differentiate into classically activated macrophages45 (Fig. 1). Although several chemotactic factors have been implicated in the recruitment of inflammatory monocytes and macrophages into adipose tissue, CCR2 and its ligands — such as CC-chemokine ligand 2 (CCL2) — seem to have a dominant role32,46. Absence of CCR2 reduces obesity-induced macrophage infiltration and adipose tissue inflammation, and this results in an improvement in insulin action and hepatic steatosis32. Although transgenic expression of CCL2 in adipose tissue is sufficient to enhance macrophage recruitment and potentiate obesity-induced insulin resistance46,47, it does not seem to be required for the initiation of macrophage-mediated inflammation in adipose tissue48,49, potentially suggesting functional redundancy between CCR2 ligands.
In addition to CCL2 and CCR2, several other factors have been shown to modulate macrophage chemotaxis and adipose tissue inflammation. For example, osteopontin — a secreted matrix glycoprotein that acts as a pro-inflammatory cytokine — promotes adipose tissue inflammation, insulin resistance and hepatic steatosis50,51,52 (Fig. 1). Although osteopontin is not a prototypical chemotactic factor, macrophages lacking osteopontin display impaired chemotaxis towards CCL2, potentially providing an explanation for the reduced numbers of adipose tissue macrophages in obese osteopontin-deficient mice52.
Fatty acids released by adipocytes during lipolysis have also been shown to promote the recruitment of macrophages into adipose tissue. In a weight-loss model, the acute trafficking of macrophages into adipose tissue correlates with lipolysis of stored triglycerides53. Moreover, the recruitment of adipose tissue macrophages is enhanced by treatment with β3-adrenergic agonists, which increase lipolysis of stored triglycerides in adipocytes, whereas genetic disruption of adipose triglyceride lipase (Atgl; also known as Pnpla2), which is required for the breakdown of triglycerides in response to β3-adrenergic signalling, results in decreased recruitment53. Similarly, mice lacking CD5-like antigen (CD5L) — a factor released by macrophages that promotes lipolysis in adipocytes — have reduced macrophage infiltration of their adipose depots after high-fat diet challenge54 (Fig. 1). In this case, at least part of the chemotaxis-promoting activity of CD5L is mediated by the induction of chemokine gene expression in adipocytes; these chemokines include CCL2, CCL5, CCL7 and CCL8.
Inflammasome. The inflammasome is a cytosolic multiprotein complex that consists of an NLRP (NOD-, LRR- and pyrin domain-containing) family member, the inflammasome adaptor molecule ASC and the effector subunit pro-caspase 1. This complex can be activated in myeloid and other cells by a variety of 'danger' signals, including pathogen-derived molecular patterns, noxious foreign substances and endogenous molecules55. Although the composition of the inflammasome depends on the nature of the danger signal, its activation results in the processing of pro-caspase 1 into activated caspase 1, which then cleaves pro-IL-1β and pro-IL-18 into the secreted cytokines IL-1β and IL-18. Interestingly, it was recently reported that obesity leads to activation of the inflammasome in adipose tissue, resulting in IL-1β-mediated inflammation and insulin resistance56,57,58 (Fig. 1). Although there are some discrepancies between the reported results, such as in the relative contributions of adipocytes and macrophages to obesity-induced inflammasome activation, all groups reported improvements in insulin action and glucose disposal in mice lacking NLRP3 or caspase 1. Moreover, Vandanmagsar et al. suggested that ceramides, which are synthesized at higher levels during obesity, might be potential triggers for NLRP3 inflammasome activation57. Alternatively, the NLRP3 inflammasome might be activated by increased ROS production, which is known to occur in obesity as a result of mitochondrial dysfunction59. However, the mechanisms that link ROS to activation of the NLRP3 inflammasome remain controversial60,61. Nonetheless, as targeting of the inflammasome is clinically efficacious in restoring insulin action in obese mice and in humans with type 2 diabetes62, it will be important to identify the pathways that trigger inflammasome activation in obesity.
T cell activation in obese adipose tissue. Although the initial defence against pathogens is innate, the continual persistence of an antigenic stimulus triggers the activation and deployment of the adaptive immune system. Interestingly, the immune response in adipose tissue during obesity follows a parallel course. For example, the initial increase in nutrient intake is accompanied by increased nutrient storage in adipocytes. However, as each adipocyte reaches its maximal capacity for storage, a small proportion of cells undergo necrotic cell death, which is thought to result in the recruitment of macrophages to clear the necrotic debris and remodel the enlarging adipose tissue21,28. In this regard, the infiltrating CD11c+ classically activated macrophages would facilitate the clearance of the dying cells and the remodelling of the extracellular matrix28, processes that ultimately favour the differentiation of new adipocytes to store the excess nutrients. However, with the persistence of increased nutrient intake, more adipocytes undergo cell death, thereby providing the antigenic stimulus for macrophages to activate the adaptive immune system (Fig. 2). Indeed, as discussed below, antigen presentation by CD11c+ adipose tissue macrophages via MHC class I and class II molecules may participate in the activation and maintenance of adaptive immune responses in the adipose tissue of obese mice. These responses collectively amplify adipose tissue inflammation and insulin resistance. In support of this, blockade of CD40 — a co-stimulatory molecule expressed by macrophages that is involved in antigen presentation — or deletion of CD40 ligand (Cd40l) prevented infiltration of adipose tissue by pathogenic T cells and macrophages, and normalized metabolic abnormalities associated with obesity63.
CD4+FOXP3+ regulatory T (TReg) cells and alternatively activated macrophages — which are enriched in the visceral adipose tissue of lean mice — secrete interleukin-10 (IL-10) to enhance insulin action and glucose disposal in adipocytes. With overnutrition, engorged adipocytes undergo necrotic cell death, which is thought to result in the recruitment of classically activated macrophages to clear the debris. In this context, adipose tissue macrophages are potentially capable of presenting antigens to B and T cells to promote adaptive immune responses. This is postulated to promote clonal expansion of CD4+ T helper 1 (TH1) cells and increase infiltration by CD8+ T cells. In a feed-forward loop, interferon-γ (IFNγ) production by CD4+ TH1 cells and the secretion of pro-inflammatory cytokines and chemotactic factors by CD8+ T cells results in increased recruitment and classical activation of macrophages. Concomitant with this, immunosuppressive TReg cell numbers in adipose tissue decrease with obesity, further contributing to the adipose tissue inflammation and insulin resistance. B cells, which infiltrate obese adipose tissue, can present antigens on MHC class I and II molecules to naive T cells. IgG2c autoantibodies produced by mature B cells further amplify adipose tissue inflammation and insulin resistance. TCR, T cell receptor.
Two recent papers have provided evidence for the activation of pathogenic adaptive immune responses in diet-induced obesity64,65. Winer et al. reported that the absolute numbers of CD4+ T cells increased with obesity in mice, and that this was largely due to the accumulation of interferon-γ (IFNγ)-producing TH1 cells65. Moreover, the increased production of IFNγ by these cells contributed to the classical activation of adipose tissue macrophages, resulting in increased inflammation in adipose tissue and progression of insulin resistance (Fig. 2). In agreement with this, depletion of pathogenic TH1 cells using CD3-specific antibodies resulted in a sustained improvement in insulin sensitivity65. Similarly, reconstitution of mice lacking recombination activating gene 1 (Rag1−/− mice; which are devoid of adaptive immune cells) with CD4+ T cells, but not with CD8+ T cells, reduced obesity-induced insulin resistance65. Lastly, as a shift from regulatory T (TReg) cells to pathogenic TH1 cells was observed in humans with body mass index >30, therapy with a CD3-specific antibody might be potentially efficacious in the treatment of insulin resistance and type 2 diabetes in obese humans. Thus, although macrophages are necessary for the initiation of diet-induced inflammation in adipose tissue, the maintenance of inflammation during chronic obesity seems to be also dependent on the adaptive immune system.
In the second study, Nishimura et al. focused on changes in CD8+ T cells, the numbers of which were increased in adipose tissue in dietary and genetic models of obesity64. Similarly to CD11c+ adipose tissue macrophages, CD8+ T cells localized to crown-like structures in obese adipose tissue, suggesting potential crosstalk between CD8+ T cells and macrophages. Depletion of CD8+ T cells by a CD8-specific antibody or genetic CD8a deficiency protected mice from obesity-induced inflammation and insulin resistance, whereas adoptive transfer of CD8+ T cells worsened the local inflammatory response in adipose tissue, as well as insulin sensitivity and glucose disposal. Moreover, as the infiltration by CD8+ T cells preceded the recruitment of macrophages to adipose tissue, the authors suggest that the release of chemotactic factors by CD8+ T cells contributes to macrophage-mediated inflammation in adipose tissue. Taken together, these studies suggest that, in obesity, adipose tissue is an active site for both MHC class I- and MHC class II-mediated antigen presentation by macrophages to CD8+ and CD4+ T cells, respectively, which contribute to adipose tissue inflammation and peripheral insulin resistance.
In the preceding sections, we have discussed how overnutrition results in the recruitment of monocytes into the enlarging adipose tissue, where they differentiate into classically activated macrophages. The production of pro-inflammatory molecules, such as TNF and IL-1β, by infiltrating macrophages contributes to adipose tissue inflammation and insulin resistance (Fig. 1). This inflammatory milieu is further amplified by the crosstalk between pathogenic CD4+ and CD8+ T cells and CD11c+ classically activated macrophages in obese adipose tissue (Fig. 2). However, as in many instances of inflammation, counter-regulatory mechanisms are also in place to prevent excessive inflammation. In adipose tissue, these include TReg cells (Box 2), TH2 cells and alternatively activated macrophages, which are discussed in detail below.
Alternatively activated macrophages
The inherent plasticity of macrophages stems in part from their ability to enact distinct activation programmes. Unlike classically activated macrophages, which exhibit high pro-inflammatory potential, alternatively activated macrophages (which are promoted by the TH2-type cytokines IL-4 and IL-13) are less pro-inflammatory and have distinct secretory and functional capacities66. Alternatively activated macrophages in tissues or at sites of TH2-type inflammation can be identified by their unique functions, including their specific enzymatic (encoded by Arg1), secretory (encoded by Chi3l3, Chi3l4 and Retnla) and phagocytic (encoded by Mrc1, Clec7a and Clec10a) activities66. Indeed, these characteristic features of classically and alternatively activated macrophages have permitted detailed analyses of their roles in metabolic inflammation and insulin resistance. Whereas classically activated macrophages promote metabolic inflammation and insulin resistance8, resident alternatively activated macrophages attenuate inflammation and confer protection against the deleterious effects of diet-induced obesity21. As discussed below, this has been discerned by the identification of transcriptional regulators that sustain alternative macrophage activation in tissues.
PPARs, alternative activation and metabolic disease. Peroxisome proliferator-activated receptors (PPARs) are ligand-dependent transcription factors that function as fatty acid sensors to regulate glucose and lipid metabolism67. Among the three PPAR subtypes (PPARα, PPARγ and PPARδ), PPARγ and PPARδ are abundantly expressed in mouse and human monocytes and macrophages68. Several laboratories have reported that the activation of PPARγ and PPARδ attenuates the expression of pro-inflammatory genes in macrophages, thus implicating PPARs in the negative regulation of classical macrophage activation68,69. However, the importance of PPARγ in alternative macrophage activation was not investigated until recently.
The induction of PPARγ by IL-4 in monocytes and macrophages suggested a potential involvement in alternative macrophage activation70 (Fig. 3). In 2007, Odegaard and colleagues were the first to demonstrate that the transcriptional activity of PPARγ is required for orchestrating the metabolic programmes of alternative activation, including β-oxidation of fatty acids and mitochondrial biogenesis24. In addition, PPARγ was found to directly regulate the expression of arginase 1, a hallmark of alternatively activated macrophages. Congruent with this, myeloid cell-specific disruption of PPARγ impaired alternative activation in vitro and in vivo, and reduced the numbers of arginase 1-expressing macrophages in the adipose tissue of lean mice24. As a consequence, when challenged with a high-fat diet, mice lacking PPARγ in their myeloid cells were predisposed to developing obesity and insulin resistance, in part owing to mitochondrial dysfunction and altered glucose metabolism in adipose tissue24,71. These results thus established that alternatively activated macrophages, which are predominantly found in the adipose tissue of lean mice, have a protective role in obesity-induced metabolic disease.
In lean animals, adipose tissue macrophages display an alternatively activated phenotype with reduced inflammatory potential and increased production of the insulin-sensitizing cytokine interleukin-10 (IL-10). Eosinophils secrete IL-4 to induce alternative macrophage activation. Activation of signal transducer and activator of transcription 6 (STAT6) by IL-4 induces a transcriptional cascade that involves Krüppel-like factor 4 (KLF4) and the fatty acid sensors peroxisome proliferator-activated receptor-δ (PPARδ) and PPARγ, which synergize with STAT6 to sustain the alternative activation of adipose tissue macrophages. Adiponectin that is released by adipocytes also synergizes with IL-4 signalling through the activation of AMP-activated protein kinase (AMPK) to enhance alternative macrophage activation and reduce macrophage-mediated inflammation. Concurrently, unsaturated fatty acids, such as omega-3 fatty acids, signal via G-protein coupled receptor 120 (GPR120) to dampen nuclear factor-κB activation in adipose tissue macrophages. IL-1R, IL-1 receptor; TLR, Toll-like receptor; TNFR, TNF receptor.
IL-4 also stimulates macrophages to express PPARδ72, which synergizes with signal transducer and activator of transcription 6 (STAT6) to coordinate the function of alternatively activated macrophages72,73. PPARδ is required to amplify and coordinate many facets of alternative activation; for example, it is involved in inducing the expression of signature genes (namely, Arg1, Clec10a, Mgl2, Mrc1, Retnla, Chi3l3 and Pdcd1lg2), transducing the mitogenic action of IL-4 and suppressing the expression of pro-inflammatory genes72,73 (Fig. 3). However, unlike PPARγ, PPARδ is not required for the oxidative metabolism that fuels the alternative activation of macrophages24. Consistent with its role in the maturation of alternatively activated macrophages, disruption of PPARδ in myeloid cells or the generation of PPARδ-deficient bone marrow chimaeras reduces alternative activation of adipose tissue macrophages and liver macrophages, thereby predisposing mice to the metabolic sequelae of obesity (including insulin resistance and hepatic steatosis). These findings thus provide independent verification for the non-redundant roles of PPARγ and PPARδ in alternative macrophage activation. However, the generation of chimaeras with PPARγ- or PPARδ-deficient bone marrow failed to protect C57BL/6J mice from diet-induced insulin resistance74, potentially reflecting strain differences between BALB/cJ and C57BL/6J mice or the relative resistance of resident tissue macrophages to transplantation-induced replacement75. Lastly, whereas the instructive cues for alternative activation are provided by the TH2-type cytokines IL-4 and IL-13, PPARγ and PPARδ primarily function to amplify and sustain this programme of macrophage activation.
KLF4, alternative activation and insulin resistance. Similarly to PPARγ and PPARδ, Krüppel-like factor 4 (KLF4) was found to cooperate with IL-4–STAT6 signalling to promote alternative macrophage activation and ameliorate obesity-induced insulin resistance. Liao et al. found that expression of KLF4 was induced in mouse and human macrophages by IL-4, and overexpression of KLF4 synergized with IL-4 to promote alternative macrophage activation, whereas its deficiency was associated with impairment in alternative polarization of macrophages76 (Fig. 3). In fact, KLF4-deficient macrophages exhibited a profound classical bias that contributed to their enhanced bactericidal activity. Moreover, in the context of metabolic disease, the phenotype of mice lacking KLF4 in myeloid cells mirrored the metabolic phenotype of mice with PPARγ-deficient myeloid cells: both strains were prone to diet-induced obesity and insulin resistance that was associated with impairment in the alternative activation of adipose tissue macrophages.
The mineralocorticoid receptor and alternative activation. In contrast to PPARs and KLF4, mineralocorticoid signalling potentiates classical activation and suppresses alternative activation of macrophages77. For instance, aldosterone, a mineralocorticoid receptor agonist, induces pro-inflammatory gene expression in macrophages and potentiates the lipopolysaccharide-driven programme of classical macrophage activation77,78. The converse was observed in macrophages lacking the mineralocorticoid receptor and after stimulation of wild-type macrophages with RU26752, an antagonist of the mineralocorticoid receptor. So, inhibition of mineralocorticoid signalling enhances expression of alternative activation markers, while suppressing classical macrophage activation. Interestingly, transcriptional profiling revealed that the alternative bias in mineralocorticoid receptor-deficient macrophages was, in part, mediated by the activation of PPARγ signalling, indicating that the mineralocorticoid receptor and PPARγ regulate diametrically opposing facets of macrophage activation. As mineralocorticoid receptor antagonists are already in clinical use for the treatment of congestive heart failure and hypertension79, it will be important to explore the clinical utility of these drugs in obesity-induced inflammation and insulin resistance.
The source of IL-4 in white adipose tissue. Although adipocytes have been implicated in secreting IL-4 and IL-13, a recent study demonstrated that eosinophils are the primary source of IL-4 in adipose tissue. Using 4get reporter mice, Wu and colleagues found that 90% of the cells competent for IL-4 secretion in the adipose tissue of lean mice were eosinophils, and their presence in adipose tissue was inversely correlated with body mass80 (Fig. 3). Importantly, in mice lacking eosinophils, alternative activation of adipose tissue macrophages was severely compromised, indicating that eosinophils are the primary source of IL-4 in adipose tissue80. Accordingly, the presence of eosinophils in adipose tissue displayed a tight correlation with protection from obesity and insulin resistance. For instance, eosinophil-deficient mice gained more weight on a high-fat diet than control mice and were worse off in their metabolic profiles, whereas mice with tissue eosinophilia (such as that which occurs in IL-5-transgenic mice) had lower levels of adiposity and improved glucose tolerance.
Taken together, these findings have established that alternatively activated macrophages and eosinophils protect against the metabolic sequelae of obesity. However, the precise mechanisms by which these cells communicate with metabolic tissues to improve glucose homeostasis remain unknown. Two plausible mechanisms include: the anti-inflammatory effects of alternatively activated macrophages on adipose tissue inflammation; and the secretion by alternatively activated macrophages of insulin-sensitizing factors that improve parenchymal cell glucose homeostasis. The precise nature of the insulin-sensitizing factors and the stimuli that trigger alternative macrophage activation in metabolic tissues are two important areas for future investigations.
Infection, immunity and insulin resistance
As discussed above, evidence gathered over the last decade demonstrates a causal relationship between macrophage-mediated inflammation and insulin resistance. The fact that this crosstalk between the immune and metabolic systems is conserved from flies to humans7 suggests that, under some circumstances, inflammation-mediated insulin resistance might be beneficial. One such scenario is acute bacterial infection, when insulin resistance provides a mechanism for diverting nutrients away from non-essential functions, such as storage, and towards fuelling immunity.
Insulin resistance: an adaptive mechanism (bacterial infections). In mammals, innate immune cells (such as neutrophils, macrophages and dendritic cells) and adaptive immune cells (B and T cells) utilize high levels of glucose following activation81,82,83,84. In the case of B and T cells, aerobic glycolysis fuels their clonal proliferation85,86,87, whereas, for macrophages, aerobic glycolysis provides the building blocks for their secretory and respiratory bursts83. For example, the production of ROS by the NADPH oxidase complex in macrophages requires continual generation of NADPH88, which is mainly produced by the pentose phosphate pathway, a pathway that branches off from the glycolytic pathway89. Interestingly, this pathway generates pentose sugars including ribose — a building block that is necessary for DNA and RNA synthesis, which sustains cellular activation and proliferation89,90. Thus, the bioenergetic demands of the activated immune system are fuelled primarily by glucose. In this circumstance, peripheral insulin resistance functions as an adaptive mechanism that allows the organism to mobilize fuel from sites of storage to combat infection (Fig. 4). For example, in liver and adipose tissue, insulin resistance drives gluconeogenesis and lipolysis to provide glucose and fatty acids to support immune activation and peripheral metabolism, respectively17,18. Similarly, insulin resistance in skeletal muscle, the primary site of glucose disposal, reduces the storage of glucose as glycogen, thereby providing the activated immune system with unfettered access to nutrients (Fig. 4). Therefore, infection-induced insulin resistance is an adaptive strategy that supports the bioenergetic demands of the activated immune system to ensure survival of the organism.
Bacterial infection of the host activates innate immune cells, resulting in the release of pro-inflammatory cytokines that mediate insulin resistance in metabolic tissues. Insulin resistance in the liver increases gluconeogenesis, whereas in muscle it decreases glucose disposal and increases the breakdown of stored glycogen (a process termed glycogenolysis). This has the net effect of increasing circulating levels of glucose, a nutrient that is preferentially used by innate and adaptive immune cells to fuel their activation. In parallel, insulin resistance in adipose tissue increases lipolysis and decreases lipogenesis. The free fatty acids released by adipocytes are used to support the metabolic demands of immune and non-immune cells.
An important corollary of these observations is that, in response to infection or inflammation, organisms ranging from flies to humans are 'hard-wired' to develop insulin resistance. This suggests that this adaptive strategy in the setting of infection has the potential of being maladaptive if inflammation is prolonged and chronic. This is indeed the case in obesity, during which excess nutrient intake leads to chronic macrophage-mediated inflammation and insulin resistance7,8,9,10,12. As discussed above, cellular and molecular pathways used in sensing and eradicating bacterial pathogens also contribute to obesity-induced inflammation and insulin resistance.
TH2-type immunity enhances insulin action (helminth infections). In contrast to bacterial infections, parasitic infections with extracellular helminths are chronic and, to a large extent, do not acutely affect survival91. However, this chronic parasitism poses a metabolic challenge for the host, as the helminths continually use host nutrients for their own growth. This raises the important question of whether immunity against helminths is somehow coupled to the regulation of peripheral metabolism.
In humans and experimental models, helminths are potent inducers of TH2-type immune responses, which involve the infiltration of tissues by eosinophils, alternatively activated macrophages and TH2 cells, and increased production of TH2-type cytokines (such as IL-4, IL-5 and IL-13)91. Interestingly, these aspects of the TH2-type immune response have been demonstrated to enhance insulin action and promote nutrient storage (Fig. 5). First, the TH2-type cytokine IL-4 confers protection against diet-induced obesity and insulin resistance in mice92. Second, mice lacking the TH2 cell-associated signalling molecule STAT6 are more prone to diet-induced insulin resistance and completely refractory to the glucose-lowering effects of IL-4 (Ref. 92). Third, polarization of the immune response towards the TH2 axis in a model of allergic inflammation improves glucose tolerance and insulin resistance92. Fourth, infection with the migratory helminth Nippostrongylus brasiliensis confers long-term protection from obesity-induced glucose intolerance and insulin resistance80. Lastly, as discussed above, alternatively activated macrophages and adipose tissue eosinophils are necessary for maintaining glucose homeostasis during diet-induced obesity24,72,73,76,80. Collectively, these findings indicate that the TH2-type immune response invoked by helminths sequesters nutrients for long-term storage, a strategy that is probably advantageous to the host, as it prevents the growth of parasites.
Parasitic helminths induce the prototypical T helper 2 (TH2)-type immune responses characterized by tissue eosinophilia, alternative macrophage activation and increased production of TH2-type cytokines (such as interleukin-4 (IL-4) and IL-13), as well as IL-10. Each aspect of this anti-helminth immune response enhances insulin action in the liver, adipose tissue and, potentially, muscle. Lean adipose tissue contains abundant numbers of eosinophils (which are the primary producers of IL-4 and IL-13), and these cells sustain the alternative activation of adipose tissue macrophages. In a paracrine manner, alternatively activated macrophages protect against insulin resistance by directly suppressing the clonal expansion of TH1 cells and dampening the inflammation mediated by classically activated macrophages. Although local production of IL-4 and IL-10 enhances insulin-stimulated glucose disposal in adipose tissue, IL-4 stimulates the anabolic actions of insulin in liver. The net effect of TH2-type immunity is to enhance nutrient storage by potentiating the anabolic actions of insulin in tissues. TNF, tumour necrosis factor; TReg, regulatory T.
Conclusion
In all metazoans, immunity and metabolism are two fundamental systems. The former provides an effective defence against invading pathogens and permits colonization by commensals, whereas the latter allows the organism to adapt to changes in nutrient availability. Interestingly, studies over the last decade suggest that macrophages — the sentinels of innate immunity — mediate dynamic crosstalk between these two essential systems.
The functions of macrophages in the regulation of metabolism have principally been investigated in white adipose tissue, the primary site for storage of excess nutrients. Although macrophages take residence in the white adipose tissue of both lean and obese mice, their activation phenotypes and functional roles vary with the metabolic status of the animal. In lean mice, alternatively activated macrophages predominate and enhance the anabolic actions of insulin. In striking contrast, obese adipose tissue is largely infiltrated with classically activated macrophages, which promote insulin resistance via the secretion of pro-inflammatory cytokines. Although adipose tissue macrophages clearly have an important role in the pathogenesis of metabolic disease, the crosstalk between macrophages and metabolic tissues probably evolved to modulate insulin action and nutrient availability during times of infection. Lastly, as innate and adaptive immune cells normally reside in adipose tissue, the enlarging adipose tissue is not only a site for the storage of fats or the secretion of adipokines, but also a tertiary lymphoid organ for macrophage-mediated antigen presentation and lymphocyte activation.
References
Flegal, K. M., Carroll, M. D., Ogden, C. L. & Curtin, L. R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 (2010).
Finucane, M. M. et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377, 557–567 (2011).
Berrington de Gonzalez, A. et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 363, 2211–2219 (2010).
Flegal, K. M., Graubard, B. I., Williamson, D. F. & Gail, M. H. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298, 2028–2037 (2007).
Zheng, W. et al. Association between body-mass index and risk of death in more than 1 million Asians. N. Engl. J. Med. 364, 719–729 (2011).
Leibel, R. L. Molecular physiology of weight regulation in mice and humans. Int. J. Obes. 32, S98–S108 (2008).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Olefsky, J. & Glass, C. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 1–28 (2010).
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Odegaard, J. I. & Chawla, A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nature Clin. Pract. Endocrinol. Metab. 4, 619–626 (2008).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Ferrante, A. W. Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J. Intern. Med. 262, 408–414 (2007).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Hotamisligil, G. S. et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271, 665–668 (1996). This was the first evidence that serine phosphorylation of IRS proteins underlies the inhibition of insulin signalling by pro-inflammatory cytokines.
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003). References 15 and 16 provided the initial evidence for an involvement of macrophages in obesity-induced adipose tissue inflammation and insulin resistance.
Kahn, B. B. & Flier, J. S. Obesity and insulin resistance. J. Clin. Invest. 106, 473–481 (2000).
Shulman, G. I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 (2000).
Brown, M. S. & Goldstein, J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008).
Qatanani, M. & Lazar, M. A. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 21, 1443–1455 (2007). An assessment of various cellular pathways and molecular mechanisms that contribute to obesity-associated insulin resistance.
Odegaard, J. I. & Chawla, A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 6, 275–297 (2011).
Lumeng, C. N., DelProposto, J. B., Westcott, D. J. & Saltiel, A. R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246 (2008).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007). This paper demonstrates that the adipose tissues of lean and obese mice are preferentially populated by alternatively and classically activated macrophages, respectively.
Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007). This was the first demonstration that PPARγ regulates alternative activation of adipose tissue macrophages, which ameliorates obesity-induced insulin resistance.
Lumeng, C. N., Deyoung, S. M. & Saltiel, A. R. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am. J. Physiol. Endocrinol. Metab. 292, e166–e174 (2007).
Stout, R. D. & Suttles, J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 76, 509–513 (2004).
Shaul, M. E., Bennett, G., Strissel, K. J., Greenberg, A. S. & Obin, M. S. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet-induced obesity in mice. Diabetes 59, 1171–1181 (2010).
Strissel, K. J. et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56, 2910–2918 (2007).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Cho, H. J. et al. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol. Genomics 29, 149–160 (2007).
Feng, D. et al. High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes 60, 2134–2143 (2011).
Weisberg, S. P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 (2006).
Patsouris, D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 (2008).
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nature Med. 11, 191–198 (2005). This paper shows that IKKβ deficiency in myeloid cells confers protection against obesity-induced insulin resistance, thereby implicating classically activated macrophages in the pathogenesis of metabolic disease.
Solinas, G. et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 (2007).
Oh, D. Y. et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010).
Konner, A. C. & Bruning, J. C. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol. Metab. 22, 16–23 (2011).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Saberi, M. et al. Hematopoietic cell-specific deletion of Toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Poggi, M. et al. C3H/HeJ mice carrying a Toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50, 1267–1276 (2007).
Tsukumo, D. M. et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56, 1986–1998 (2007).
Erridge, C. & Samani, N. J. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler. Thromb. Vasc. Biol. 29, 1944–1949 (2009).
Hosoi, T., Yokoyama, S., Matsuo, S., Akira, S. & Ozawa, K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS ONE 5, e12537 (2010).
Vijay-Kumar, M. et al. Loss of function mutation in Toll-like receptor-4 does not offer protection against obesity and insulin resistance induced by a diet high in trans fat in mice. J. Inflamm. 8, 2 (2011). References 43 and 44 describe the paradoxical worsening of metabolic disease in mice lacking TLR4 or MYD88.
Gordon, S. & Taylor, P. R. Monocyte and macrophage heterogeneity. Nature Rev. Immunol. 5, 953–964 (2005).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Kamei, N. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281, 26602–26614 (2006).
Inouye, K. E. et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56, 2242–2250 (2007).
Kirk, E. A., Sagawa, Z. K., McDonald, T. O., O'Brien, K. D. & Heinecke, J. W. Macrophage chemoattractant protein-1 deficiency fails to restrain macrophage infiltration into adipose tissue. Diabetes 57, 1254–1261 (2008).
Chapman, J. et al. Osteopontin is required for the early onset of high fat diet-induced insulin resistance in mice. PLoS ONE 5, e13959 (2010).
Kiefer, F. W. et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia 54, 2132–2142 (2011).
Nomiyama, T. et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Invest. 117, 2877–2888 (2007).
Kosteli, A. et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120, 3466–3479 (2010).
Kurokawa, J. et al. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc. Natl Acad. Sci. USA 108, 12072–12077 (2011).
Petrilli, V., Dostert, C., Muruve, D. A. & Tschopp, J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19, 615–622 (2007).
Stienstra, R. et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 12, 593–605 (2010).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Med. 17, 179–188 (2011).
Wen, H. et al. Fatty acid-induced NLRP3–ASC inflammasome activation interferes with insulin signaling. Nature Immunol. 12, 408–415 (2011). References 56–58 describe the links between inflammasome activation, obesity-induced inflammation and insulin resistance.
Chen, G. Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nature Rev. Immunol. 10, 826–837 (2010).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunol. 11, 136–140 (2010).
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nature Immunol. 11, 897–904 (2010).
De Nardo, D. & Latz, E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 32, 373–379 (2011).
Poggi, M. et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler. Thromb. Vasc. Biol. 31, 2251–2260 (2011).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Med. 15, 914–920 (2009).
Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nature Med. 15, 921–929 (2009). References 64 and 65 show the involvement of adaptive immune responses in the modulation of obesity-induced metabolic disease.
Gordon, S. Alternative activation of macrophages. Nature Rev. Immunol. 3, 23–35 (2003).
Evans, R. M., Barish, G. D. & Wang, Y.-X. PPARs and the complex journey to obesity. Nature Med. 10, 355–361 (2004).
Chawla, A. Control of macrophage activation and function by PPARs. Circ. Res. 106, 1559–1569 (2010).
Glass, C. K. & Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature Rev. Immunol. 10, 365–376 (2010).
Huang, J. T. et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 400, 378–382 (1999).
Hevener, A. L. et al. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J. Clin. Invest. 117, 1658–1669 (2007).
Kang, K. et al. Adipocyte-derived TH2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. Cell Metab. 7, 485–495 (2008).
Odegaard, J. I. et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 (2008).
Marathe, C. et al. Preserved glucose tolerance in high-fat-fed C57BL/6 mice transplanted with PPARγ−/−, PPARδ−/−, PPARγδ−/−, or LXRαβ−/− bone marrow. J. Lipid Res. 50, 214–224 (2009).
Kennedy, D. W. & Abkowitz, J. L. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood 90, 986–993 (1997).
Liao, X. et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 (2011).
Usher, M. G. et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J. Clin. Invest. 120, 3350–3364 (2010).
Gilbert, K. C. & Brown, N. J. Aldosterone and inflammation. Curr. Opin. Endocrinol. Diabetes Obes. 17, 199–204 (2010).
McManus, F., McInnes, G. T. & Connell, J. M. Drug insight: eplerenone, a mineralocorticoid-receptor antagonist. Nature Clin. Pract. Endocrinol. Metab. 4, 44–52 (2008).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011). This paper demonstrates the protective effects of eosinophils and T H 2-type parasitic inflammation in metabolic disease.
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Fox, C. J., Hammerman, P. S. & Thompson, C. B. Fuel feeds function: energy metabolism and the T-cell response. Nature Rev. Immunol. 5, 844–852 (2005).
Newsholme, P., Curi, R., Gordon, S. & Newsholme, E. A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 239, 121–125 (1986).
Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 (2010).
Doughty, C. A. et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 107, 4458–4465 (2006).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Hume, D. A., Radik, J. L., Ferber, E. & Weidemann, M. J. Aerobic glycolysis and lymphocyte transformation. Biochem. J. 174, 703–709 (1978).
El-Benna, J., Dang, P. M., Gougerot-Pocidalo, M. A. & Elbim, C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch. Immunol. Ther. Exp. 53, 199–206 (2005).
Wamelink, M. M., Struys, E. A. & Jakobs, C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J. Inherit. Metab. Dis. 31, 703–717 (2008).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 (2008).
Maizels, R. M. & Yazdanbakhsh, M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Rev. Immunol. 3, 733–744 (2003).
Ricardo-Gonzalez, R. R. et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl Acad. Sci. USA 107, 22617–22622 (2010).
Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nature Med. 11, 183–190 (2005).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Yuan, M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293, 1673–1677 (2001).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Med. 15, 930–939 (2009).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature Med. 17, 610–617 (2011).
Acknowledgements
We thank members of the Chawla laboratory and A. Loh for valuable comments on the manuscript, and K. Vicari and R. Levinson for assistance with illustrations. The authors' work was supported by grants from the US National Institutes of Health (NIH; DK076760 and HL076746), a Larry L. Hillblom Foundation Network Grant and an NIH Director's Pioneer Award (DP1OD006415) to A.C. Support was also provided by a Stanford Graduate Fellowship to K.D.N. and an A-STAR Fellowship to Y.P.G. Owing to space limitations, we regret that we are unable to cite all relevant publications on this topic from our colleagues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Insulin resistance
-
A physiological or pathophysiological state in which insulin becomes less effective at lowering serum glucose owing to decreased responsiveness of insulin target tissues, such as adipose tissue, skeletal muscle and liver.
- Adipokines
-
Hormones and/or cytokines secreted by adipose tissue, such as leptin and adiponectin.
- Alternatively activated (M2) phenotype
-
A macrophage phenotype that is stimulated by the TH2-type cytokines interleukin-4 (IL-4) and IL-13. These macrophages express arginase 1, the mannose receptor (CD206) and CD301. Chronic states of helminth infections are associated with alternatively activated macrophages.
- Classical (M1) phenotype
-
A pro-inflammatory, antimicrobial programme of macrophage activation induced by interferon-γ and Toll-like receptor ligands.
- CD11c-DTR mice
-
Transgenic mice that express the diphtheria toxin receptor (DTR) under the control of the Cd11c promoter. Injection of diphtheria toxin allows for specific ablation of CD11c+ cells from these mice.
- CD4+ T cells
-
A T cell subset that expresses the glycoprotein CD4, which assists the T cell receptor in the recognition of antigens presented on MHC class II molecules by antigen-presenting cells.
- TH1 cells
-
(T helper 1 cells). TH1 cells secrete interferon-γ and tumour necrosis factor to promote cell-mediated immunity by supporting the classical activation of macrophages and the proliferation of cytotoxic CD8+ T cells.
- CD8+ T cells
-
CD8+ T cells express the co-receptor CD8, which together with the T cell receptor recognizes antigens bound to MHC class I molecules. Activated CD8+ T cells induce the death of virus-infected or damaged cells.
- TH2 cells
-
(T helper 2 cells). TH2 cells secrete the cytokines interleukin-4 (IL-4), IL-5 and IL-13 to stimulate humoral immunity (B cells) and alternative macrophage activation.
- B2 cells
-
Conventional bone marrow-derived B cells that make up the bulk of splenic B cells. They express high levels of B220 and membrane-bound IgD.
- Mineralocorticoid
-
A steroid hormone that regulates the concentration of minerals, such as sodium and potassium, in extracellular fluids. These hormones bind and activate the mineralcorticoid receptor to mediate their transcriptional effects.
- 4get reporter mice
-
Knock-in mice that express enhanced green fluorescent protein from the interleukin-4 (Il4) locus, allowing for the detection of cells competent for IL-4 production.
- Pentose phosphate pathway
-
This pathway uses glucose to generate NADPH and pentose sugars (such as ribose). The first (oxidative) phase converts glucose-6-phosphate to ribulose-5-phosphate and generates NADPH. The second (non-oxidative) phase synthesizes other sugars from ribulose-5-phosphate.
Rights and permissions
About this article
Cite this article
Chawla, A., Nguyen, K. & Goh, Y. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 11, 738–749 (2011). https://doi.org/10.1038/nri3071
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3071
This article is cited by
-
M2 macrophages-derived exosomes regulate osteoclast differentiation by the CSF2/TNF-α axis
BMC Oral Health (2024)
-
Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the National Health and Nutrition Examination Survey 2011–2016
Journal of Translational Medicine (2023)
-
Leptin/obR signaling exacerbates obesity-related neutrophilic airway inflammation through inflammatory M1 macrophages
Molecular Medicine (2023)
-
Mendelian randomization indicates that atopic dermatitis contributes to the occurrence of diabetes
BMC Medical Genomics (2023)
-
A cytotoxic T cell inspired oncolytic nanosystem promotes lytic cell death by lipid peroxidation and elicits antitumor immune responses
Nature Communications (2023)