Key Points
-
The liver serves as an important filter organ to remove circulating pathogens from the blood. However, certain pathogens — such as hepatitis B virus, hepatitis C virus and malaria-inducing Plasmodium spp. parasites — efficiently target the liver and can establish persistent infections in hepatocytes.
-
Despite the function of the liver in skewing immune responses towards tolerance, hepatic cells are equipped with innate immune sensory receptors to detect infectious microorganisms and mount inflammatory responses.
-
Most infectious microorganisms are cleared from the liver through the combination of innate and adaptive immune defence, despite pathogen-specific immune escape strategies. However, our knowledge on the decisive molecular mechanisms that discriminate acute, resolving infections from chronic infections is still incomplete.
-
Persistent infection of hepatocytes is perpetuated by the immunoregulatory liver microenvironment, the predominant co-inhibitory over co-stimulatory signalling by hepatic antigen-presenting cells and the exhaustion of pathogen-specific T cells.
-
Novel therapeutic strategies to overcome chronic viral infection of hepatocytes will combine measures to lower the level of viral antigens in combination with measures to increase the number of virus-specific T cells and their efficiency to locally exert their effector function in the liver.
Abstract
The liver has vital metabolic and clearance functions that involve the uptake of nutrients, waste products and pathogens from the blood. In addition, its unique immunoregulatory functions mediated by local expression of co-inhibitory receptors and immunosuppressive mediators help to prevent inadvertent organ damage. However, these tolerogenic properties render the liver an attractive target site for pathogens. Although most pathogens that reach the liver via the blood are eliminated or controlled by local innate and adaptive immune responses, some pathogens (such as hepatitis viruses) can escape immune control and persist in hepatocytes, causing substantial morbidity and mortality worldwide. Here, we review our current knowledge of the mechanisms of liver targeting by pathogens and describe the interplay between pathogens and host factors that promote pathogen elimination and maintain organ integrity or that allow pathogen persistence.
Similar content being viewed by others
Main
The liver is a vital organ that fulfils diverse but closely connected functions in the metabolism of carbohydrates, proteins and lipids, the clearance of toxins and pathogens, and the regulation of immune responses. Lipids, peptides, carbohydrates and nutrients (such as iron) are transported to the liver through gut-derived portal venous blood, and then pass through sinusoidal lining cells before finally being taken up and metabolized by hepatocytes. As immune responses against antigens that are metabolized in the liver could cause local organ damage, such responses are regulated in a unique manner in the liver. The liver microenvironment induces immune tolerance towards antigens that are taken up and presented (or cross-presented) by tolerogenic non-parenchymal liver cells or expressed by hepatocytes and directly presented on MHC molecules1. This combination of organ-specific immunobiology and physiological roles in metabolism and toxin removal predisposes the liver to infection by pathogens that circulate in the blood and exploit these functions.
Indeed, several clinically important pathogens specifically target the liver and establish chronic infections in hepatocytes. Targeting of the liver, followed by the presentation of microbial antigens in the liver rather than in lymphoid tissues, may allow pathogens to escape T cell-mediated immunity and to establish hepatic infection. Although most infectious microorganisms that reach the liver via the blood are rapidly cleared, it is evident from the success of certain pathogens — such as the hepatitis B and hepatitis C viruses and malaria-causing Plasmodium spp. (Box 1) — in establishing hepatic infections that the liver provides a favourable environment for escaping immune responses.
Here, we review our current knowledge on the cellular and molecular mechanisms that allow pathogens to reach the liver and establish chronic infections in hepatocytes.
Liver targeting by pathogens
The liver clears blood-borne pathogens through uptake by hepatic scavenger cells, such as liver sinusoidal endothelial cells (LSECs) and Kupffer cells. Despite intensive efforts, liver-specific or hepatocyte-specific receptor molecules that mediate pathogen binding have not been identified. Instead, hepatotropic pathogens attach to and are taken up by hepatocytes following binding to broadly expressed molecules, suggesting that hepatotropism results from functional properties of the liver rather than from the expression of unique receptor molecules. These properties may involve the physiological processes that transport essential nutrients across sinusoidal cells to hepatocytes or a particular cellular milieu that provides hepatocyte-specific transcription and replication factors.
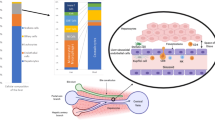
Hepatocytes are shielded from the bloodstream, and therefore from blood-borne pathogens, by Kupffer cells, LSECs and hepatic stellate cells (Fig. 1). Electron microscopy studies indicate that fenestrae (small openings) in LSECs can be up to 100 nm in diameter. However, the passage of gold particles larger than 20 nm was found to be prevented by the presence of electron-lucent material, such as extracellular matrix2, suggesting that passive diffusion of pathogens into the space of Dissé is unlikely. However, this sinusoidal barrier can be overcome either by the mechanical force generated by cells flowing through sinusoids, which has been shown to allow chylomicrons (lipoprotein particles that are 100 nm in diameter and flexible) to squeeze through endothelial cell fenestrae3, or by active transport across sinusoidal cells, as has been reported for the delivery of iron to hepatocytes by transferrin4. Similar active transport processes may explain how gold particles that are larger than 20 nm and coated with ligands for the mannose receptor (a C-type lectin expressed by sinusoidal cells) can access hepatocytes2. Indeed, active transport processes are known to be important for the directed delivery of IgA across mucosal cells and of chemokines across endothelial cells5,6. Given that LSECs and Kupffer cells have extensive receptor-mediated endocytic capacity7, it is likely that transcytosis is more important in liver physiology than is currently appreciated. So, circulating pathogens might infect hepatocytes either directly by squeezing through LSEC fenestrae or following passage through sinusoidal cells (Fig. 1). These pathways are difficult to distinguish experimentally and may function in parallel, but evidence is accumulating that pathogens use the transport properties of sinusoidal cells to increase the efficiency of hepatocyte infection.
Sinusoidal cell populations (Kupffer cells, liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells) form a loose physical barrier between hepatocytes and the blood circulating within the sinusoids. Hepatocyte microvilli make contact with sinusoidal cells and protrude through endothelial cell fenestrae into the sinusoid lumen. Blood-borne pathogens may infect hepatocytes through direct contact with hepatocytes, either after passage through fenestrae (a) or by contacting microvilli that extend into the sinusoidal lumen (b). Pathogens may also first exit the bloodstream by entering Kupffer cells (c) or LSECs (d) before infecting their final target cell, the hepatocyte. Pathogens may escape innate immune sensing by Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I) both in sinusoidal cell populations and after infecting hepatocytes. For example, malarial Plasmodium spp. sporozoites evade immune sensing by remaining in a parasitophorous vacuole, and hepatitis C virus escapes cytosolic recognition by helicases by blocking the signalling molecule IFNB-promoter stimulator 1 (IPS1). IFN, interferon; IRF3, IFN-regulatory factor 3.
Plasmodium spp. initially target Kupffer cells before infecting hepatocytes. Infection by Plasmodium spp. sporozoites has been intensively studied thanks to the availability of well-characterized animal models. Following the delivery of parasites into the skin by a mosquito bite, the rapid migration of sporozoites allows them to escape clearance by phagocytic cells and to enter lymphatics and blood vessels. Once in the circulation, sporozoites rapidly reach the liver and, after gliding on heparan sulphate proteoglycans (HSPGs) in liver sinusoids, they use circumsporozoite protein (CSP) and thrombospondin-related anonymous protein (TRAP) to bind to Kupffer cells8. Interaction with and passage through Kupffer cells is important for hepatocyte infection9,10, indicating that the parasite uses Kupffer cells to overcome the sinusoidal barrier and, ultimately, to infect hepatocytes11. The switch between continued migration and infection is determined by the high level of sulphation of HSPGs that are found on hepatocytes, which promotes proteolytic cleavage of CSP and initiates signalling events in the parasite that promote infection12. The expression of CD81 by hepatocytes is also required for infection by Plasmodium falciparum and Plasmodium yoelii13. Once inside a hepatocyte, the parasites develop into merozoites, which are released into the sinusoid following hepatocyte rupture and are then able to infect erythrocytes14. Taken together, these data show that sporozoites not only use their migratory capacity to escape elimination by phagocytic cells, but also use Kupffer cells to increase their efficiency at infecting hepatocytes.
Liver infection by hepatitis C virus. Compared with parasite infection, infection by hepatotropic viruses involves different processes, as viruses cannot actively move. Moreover, our understanding of how hepatotropic viruses target the liver in vivo is much less detailed, owing to the lack of well-characterized small-animal models. New hepatitis C virus (HCV) particles are released from an infected cell as 'lipoviroparticles'15, which are formed by the binding of the HCV envelope proteins E1 and E2 to host cell lipoproteins. The circulation of lipoviroparticles and their ability to mediate infection implies that lipid transport pathways may be involved in liver targeting by HCV. Furthermore, E2 binds to the C-type lectins DC-specific ICAM3-grabbing non-integrin (DC-SIGN), which is expressed by dendritic cells (DCs) and Kupffer cells, and liver- and lymph node-specific ICAM3-grabbing non-integrin (L-SIGN), which is expressed by LSECs16,17. C-type lectins trap HCV on sinusoidal cells in the liver18,19, but it remains unclear whether the fate of this trapped HCV is lysosomal degradation or infection of hepatocytes in trans. Although there is no evidence for the occurrence of HCV trans-infection in vivo, HIV is known to use DC-SIGN to facilitate its transport by DCs to lymphatic tissue, where it can then infect CD4+ T cells in trans20. The receptors involved in HCV uptake into hepatocytes have been identified using cultured hepatoma cell lines. In a coordinated multistep process, HCV attaches to HSPGs, binds to the low-density lipoprotein (LDL) receptor, scavenger receptor B1 and CD81 on the hepatocyte surface, and then binds to claudin 1 and occludin in tight junctions before being endocytosed21,22,23,24,25,26. The epidermal growth factor receptor (EGFR) and ephrin type A receptor 2 (EPHA2) were the most recent additions to the list of host factors that are involved in HCV infection26. None of these receptors, however, is expressed exclusively by hepatocytes, and it remains unclear how liver targeting by HCV is achieved.
Liver infection by hepatitis B virus. Also for hepatitis B virus (HBV), no hepatocyte-specific receptor has been identified, although cell culture-based HBV infection systems have mapped important determinants of HBV entry27. Myristoylation and the amino-terminal 77 amino acids of the large HBV envelope protein28 and the antigenic loop of the small HBV envelope protein29 are crucial for the infectivity of the virus30. The attachment of HBV to hepatocytes requires interactions with highly sulphated HSPG31, but additional receptor molecules remain unknown. Interestingly, a very low number of HBV particles (<10) is sufficient to establish hepatocyte infection in vivo32,33, indicating that liver targeting by HBV is extremely efficient. This may be enabled by initial scavenging of the virus by LSECs, as described for duck HBV34, or by other sinusoidal cells. The apparent contradiction between high efficiency in liver targeting and rather inefficient uptake of virus by cultured hepatocytes27,31,33 might be explained by transcytosis of the virus across sinusoidal cells34.
Liver infection by hepatitis A and hepatitis E viruses. Hepatitis A virus (HAV) and hepatitis E virus (HEV) are food-borne pathogens that traverse gut epithelial cells to reach the blood and then the liver. The putative attachment receptor for HAV is a mucin-like class I integral-membrane glycoprotein that is ubiquitously expressed35,36,37, and HAV can replicate in different cell types in vitro. However, in vivo, the replication of HAV does not seem to occur outside the liver. It has been proposed that HAV targets the liver through a physiological transport pathway, such as the enterohepatic circulation of IgA. In this model, HAV-specific IgA antibodies produced in the intestinal mucosa bind to circulating HAV and serve as carriers of the virus. Kupffer cells express the Fcα receptor38 and thus may bind to the IgA–HAV complexes and transfer them to hepatocytes, where the virus can be taken up via the asialoglycoprotein receptor. However, the in vivo relevance of this process is not clear, because IgA–HAV complexes may be eliminated before reaching the hepatocytes.
Liver infection by bacteria. Hepatotropism of bacteria has not been described, and most bacteria that reach the liver through the blood are efficiently cleared by immune cells. However, some bacteria, such as mycobacteria and Listeria spp., can establish granulomas in various tissues, including the liver39,40. Granuloma formation by mycobacteria is driven by infected macrophages, which secrete bacterial proteins that induce the expression of matrix metalloproteinase 9 (Ref. 41), leading to tissue remodelling, which is required for the generation of granulomas42. These granulomas can wall off infecting bacteria from non-infected surrounding tissue39, but they have also been shown to contribute to the dissemination of virulent bacteria43. Therefore, although rarely observed, it is possible that granulomas provide a distinct anatomical compartment in the liver that supports the survival of bacteria.
Innate defence in the liver and pathogen evasion
Hepatic innate immune defence. The initiation of immune responses against infecting pathogens requires the detection of pathogens by pattern-recognition receptors (PRRs). Importantly, Toll-like receptors (TLRs) and cytosolic helicases (such as retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5)) are expressed not only by bone marrow-derived immune cells, such as Kupffer cells and hepatic DCs, but also by liver-resident cells, such as hepatocytes, LSECs and hepatic stellate cells44,45,46,47,48. Small differences in PRR expression between hepatic and splenic immune cells have been documented49, but both cell populations are able to sense pathogens50. Kupffer cells and LSECs can detect low concentrations of TLR ligands and produce interleukin-6 (IL-6) and type I interferons (IFNs)46,52. IL-6 induces the expression of innate effector molecules — such as the acute-phase protein C-reactive protein — by hepatocytes51, and type I IFNs have potent antiviral effects, increase natural killer (NK) cell activity and improve antigen presentation. However, the constant exposure of liver cells to the TLR ligand lipopolysaccharide (LPS), which is present in portal venous blood, causes a state of hyporesponsiveness (known as LPS tolerance) towards further pro-inflammatory immune stimulation53. Thus, LSECs and hepatic DCs do not mature into immunogenic antigen-presenting cells (APCs)46,54, and this may impair the local induction of cytotoxic T lymphocyte (CTL) responses55. It is possible that this limits pathogen-specific defence, but no evidence for this idea has been reported.
Other innate immune cells, such as NK cells, are greatly enriched in the liver compared with the circulation and may contribute to viral defence. This is supported by the finding that certain killer cell immunoglobulin-like receptor (KIR) haplotypes are associated with the resolution of acute HCV infection56. Moreover, natural killer T (NKT) cells contribute to antibacterial defence57, and non-classical innate immune cell populations (such as γδ T cells) may also support hepatic immune defence following infection.
Immune evasion strategies of pathogens in the liver. Pathogens that target the liver seem to actively avoid or even overcome local immune sensing. This is important at two stages: first, during entry into the liver; and, second, during productive hepatocyte infection. For example, HBV and HCV capsids are recognized by TLR2, which is expressed by macrophages and Kupffer cells58,59. However, the activation of PRRs by HBV leads to the release of pro-inflammatory cytokines and IL-10, but not type I IFNs, from Kupffer cells and LSECs60. Accordingly, patients with acute HBV infections have high plasma levels of IL-6 and IL-10, which have been shown to exert tissue-protective and immunoregulatory effects61,62, but no increase in antiviral type I IFNs63. This suggests that HBV and HCV may sneak under the 'immune radar' not only by using a limited number of virus particles to efficiently target the liver, but also by avoiding the induction of antiviral IFNs and by initiating cytokine responses that confer tissue protection.
Once an infection is established, pathogens can escape innate immune recognition by adapting their life cycles. For example, HBV is considered to be a stealth virus, as it escapes immune sensing by synthesizing its genome within the viral capsid64. In addition, HBV gene products suppress the response of liver cells to TLR ligands45,60.
HAV and HCV have similar genome structures and share many aspects of their replication strategies. Moreover, both viruses actively interfere with immune sensing65,66. HCV expresses one polyprotein precursor, which activates an unfolded protein response and induces autophagy in the host cell, and this promotes HCV RNA replication and suppresses the induction of type I IFN responses67. Both viruses replicate via a double-stranded RNA intermediate, which is recognized by endosomal TLR3 and the cytosolic immune sensors RIG-I and MDA5 in infected hepatocytes47,68. The HCV protease NS3–NS4A counteracts RIG-I, MDA5 and TLR3 signalling by cleaving the essential mitochondrial signalling molecule IFNB-promoter stimulator 1 (IPS1; also known as MAVS)69,70 and TIR-domain-containing adaptor protein inducing IFNβ (TRIF), thereby disrupting downstream signalling through IFN-regulatory factor 3 (IRF3)69,71. Similarly to HCV, HAV can disrupt the RIG-I, MDA5 and TLR3 signalling pathways by cleaving IPS1 and TRIF using two distinct precursors of the HAV protease72,73. However, in chimpanzees, HCV (but not HAV) induces a strong IFN response in the liver74. This may be explained by the finding that the expression of HCV NS3–NS4A in mice is not sufficient to hinder the induction of type I IFNs or the expression of IFN-responsive genes75, and it suggests that HCV does not interfere with innate immune sensing as successfully as HAV.
Finally, another evasion strategy is used by Plasmodium spp. sporozoites: they establish a parasitophorous vacuole in Kupffer cells that prevents sporozoite surface molecules from being directly recognized by membrane-bound PRRs8. Sporozoites also inhibit the respiratory burst in Kupffer cells76. Nevertheless, hepatocytes that have been damaged or die following sporozoite transfer can trigger innate immune responses77 and, during the erythrocytic phase of infection, parasite-derived glycosylphosphatidylinositol triggers the activation of immune cells through TLR2- and myeloid differentiation primary-response protein 88 (MYD88)-dependent signalling78. Taken together, these findings suggest that hepatic immune sensing is functional but that certain pathogens have evolved inhibitory and evasion mechanisms to circumvent productive immune responses in liver cells.
Clearance of pathogens from the liver
Bacteria. Blood-borne bacteria are normally cleared rapidly from the liver by phagocytic hepatic immune cells (Fig. 2a). After ingesting bacteria (such as Borrelia spp.), Kupffer cells attract NKT cells in a CXC-chemokine receptor 3 (CXCR3)-dependent manner and present bacterial glycolipid antigens on CD1 molecules to NKT cells57. The concerted action of these sinusoidal immune cell populations induces an intravascular immune response that prevents further bacterial infection57. Rapid initiation of immune defence against circulating pathogens within the hepatic sinusoids strengthens the notion that early pathogen sensing supports successful elimination. NKT cells may be instrumental in this respect, as they can recognize microbial antigens and rapidly exert immune effector functions, thus bridging innate and adaptive immunity79,80. It is likely that, in addition to NKT cells, the sizeable hepatic populations of effector memory T cells and NK cells may participate in rapid local or even systemic induction of immune defence81,82,83,84. Thus, our knowledge of successful antibacterial defence in the liver indicates a functional distinction between the hepatic sinusoidal compartment, where pathogen recognition by immune cells can eliminate pathogens and prevent them from accessing hepatocytes, and the parenchymal compartment, where infection is more difficult to eradicate and may even be facilitated through the tolerogenic properties of the local microenvironment and organ-resident cell populations.
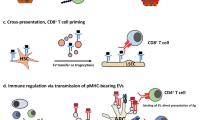
a | Scavenger sinusoidal cell populations, such as Kupffer cells, phagocytose circulating bacteria and crosstalk with natural killer T (NKT) cells to generate strong intravascular pathogen-specific immune responses. Inhibiting the access of pathogens to hepatocytes may have an important role in preventing the development of persistent hepatic infections. b | The death of infected hepatocytes during viral replication may cause the activation of Kupffer cells or dendritic cells (DCs), which in turn promote the killing of other hepatocytes through CD95 (also known as FAS) and the release of pro-inflammatory mediators. Material from dying virus-infected cells increases cross-priming by DCs and thereby augments pathogen-specific adaptive immunity98,175. Combinatorial stimulation by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), such as ATP, may allow immune-mediated control of established hepatic infections. CXCL9, CXC-chemokine ligand 9; CXCR3, CXC-chemokine receptor 3; IFN, interferon; IL, interleukin; LSEC, liver sinusoidal endothelial cell; TCR, T cell receptor; TNF, tumour necrosis factor.
Malaria parasites. Infection by Plasmodium spp. provides an example in which the pathogen is able to overcome the barrier and effector mechanisms of sinusoidal cells to infect hepatocytes. However, Plasmodium spp. sporozoites normally do not persist in the liver, as only their initial maturation and replication stages require hepatocytes and they then actively egress from the liver as merozoites to initiate blood-stage infection85. Natural exposure during repetitive infection with sporozoites in areas endemic for malaria often fails to generate protective immune responses, which are characterized by CTL and antibody responses specific for parasite CSP86. The priming of parasite-specific CTLs occurs in lymph nodes that drain the site of skin inoculation with sporozoites, but not in the liver86,87, and requires CD4+ T helper cells that produce IL-4 (Ref. 88). CSP-specific CTLs that express the IL-4 receptor and receive IL-4 signals differentiate into effector memory CTLs that home to the liver to provide protection against sporozoite infection, whereas CTLs primed in the absence of CD4+ T cell-mediated help fail to do so89,90. These results reveal that even those CTLs that are primed by professional APCs fail to protect against liver infection unless they reside in high numbers in the liver85. It can be assumed, given the low numbers of sporozoites and the short time frame between hepatocyte infection and the release of merozoites, that protection would require high numbers of CTLs to rapidly find and kill the few infected hepatocytes. Key to such escape from hepatic CTLs may therefore be efficient liver targeting and hepatocyte infection in combination with the amplification step, as sparse sporozoite-infected hepatocytes are sufficient to initiate blood-stage infection. The unique microarchitecture of the liver probably assists the escape of the few infected hepatocytes from CSP-specific CTLs, because the extensive hepatic sinusoidal meshwork with irregular blood flow acts like a maze in which CTLs are randomly dispersed. As there is autoregulation of the size of the CSP-specific clonal population through CTL-mediated elimination of APCs presenting CSP, only a sufficiently high antigen concentration can generate protective numbers of CSP-specific CTLs90. As predicted from this assumption, vaccination with high numbers of irradiated sporozoites or genetically modified replication-defective sporozoites and very frequent exposure to sporozoites, leading to prolonged antigen presentation, procures protection91,92.
Viruses. In contrast to Plasmodium spp., hepatitis viruses remain in hepatocytes; their elimination requires the induction of strong adaptive immune responses, which depend on appropriate innate immune stimuli. However, the molecular mechanisms underlying self-limited versus long-term, persistent viral infection of the liver cannot be clearly distinguished. Comparison of HAV and HCV infection may shed light on this issue. As noted above, HAV and HCV both have strategies that allow them to circumvent the induction of type I IFN responses74. However, HAV and HCV infections have different outcomes. HAV never causes chronic hepatitis, although it persists for many weeks in the livers of infected chimpanzees even after clearance of the virus from the serum or faeces74. HCV induces stronger IFN responses and either is cleared more rapidly than HAV74 or establishes a persistent infection in the liver. Although early and strong innate immune responses in HCV-infected individuals are an indicator of subsequent clearance of the infection from the liver93, immune responses fail to clear HCV infection in more than 50 percent of cases. It is possible that the difference in clinical outcomes lies in the unique properties of HAV. In contrast to HCV, HAV is a non-enveloped virus that requires the disruption of host cell membranes to release its progeny. This may provide a distinct immune stimulatory signal, such as a damage-associated molecular pattern (DAMP), that can overcome viral immune escape and liver-intrinsic tolerogenic mechanisms. Support for this assumption comes from the observation that Kupffer cell activation by dying hepatocytes provides a synergistic signal to pathogen-associated molecular pattern (PAMP)-driven immune activation and promotes hepatic inflammation94 (Fig. 2b). Although hepatic inflammation induces the recruitment of neutrophils, which increase local inflammation95, and the production by innate immune cells of type I IFNs, which contribute to the control of hepatic viral infection52,96, the elimination of hepatocellular viral infections requires CD8+ T cells. The uptake of antigens derived from apoptotic virus-infected cells by DCs through the endocytic receptor C-type lectin 9A (CLEC9A) increases the functional maturation of those DCs and the efficiency of CD8+ T cell cross-priming97,98. However, more efforts to identify receptors for DAMPs will be required to unravel the immune-sensing mechanisms that determine the successful induction of strong T cell responses and the elimination of viral infection from hepatocytes.
HBV and HCV, despite being important examples of persistent hepatic infections, can also be spontaneously controlled following acute infection. Following the resolution of acute infection, HCV is eliminated by almost all patients99, whereas HBV is controlled but not completely eliminated, and may reactivate under strong immunosuppression100. The initiation of immune responses and the resolution of infection are protracted in HAV, HBV or HCV infections compared with other acute viral infections101. This suggests the occurrence of early viral evasion of immune sensing and immune control that can be successfully overcome in the first few months after infection. Animal models and human studies of acute resolving infections have highlighted the importance of vigorous and multi-specific CTL responses, which develop in the presence of adequate T cell help102. T cell-derived cytokines (such as tumour necrosis factor and IFNγ) limit viral replication in hepatocytes, which ensures an initial reduction in viraemia without significant liver damage103, and CTL-mediated cytotoxicity is required for infection control.
Long-term persistence of infection
If HBV and HCV escape immune-mediated clearance during acute infection, they can live in the liver for many years without inducing disease or they can provoke immune-mediated organ damage that culminates in liver cirrhosis and hepatocellular carcinoma. Treatment with type I IFNs at later stages, when infection has become chronic, is much less efficient than during acute infection. This suggests that distinct mechanisms prevent virus elimination in chronic and acute infection and that the induction of virus-specific immune responses must occur rapidly to prevent viral persistence. The correlates of immune control in the setting of chronic infection, however, are poorly understood.
Evasion of humoral immune responses. Neutralizing antibodies contribute to the immune control of viral infections and to long-lasting protection. The importance of B cell responses in the control of HBV infection has been highlighted recently by the occurrence of disease reactivation induced by the B cell-depleting drug rituximab (Rituxan/MabThera; Biogen Idec/Genentech/Roche)104. However, in individuals with chronic HBV infection, the large amounts of secreted HBV surface antigen (HBsAg) that are present in excess of the levels of infectious virus can capture and saturate circulating HBV-specific antibodies, preventing them from neutralizing the virus. Moreover, in chronic HCV infection, the virus continuously evades neutralizing antibodies owing to the selection of escape variants105. In addition, viruses escape neutralizing antibodies by 'creeping' directly from one hepatocyte to another106.
Depletion and exhaustion of virus-specific CTLs. The most obvious immune deficiency in chronic HBV or HCV infection is the depletion of virus-specific CTLs or their functional inactivation. T cell depletion is partially attributable to the enhanced susceptibility of these cells to apoptosis107,108. This apoptotic propensity may be imposed by tolerogenic hepatic priming109, which induces T cell death through BCL-2-interacting mediator of cell death (BIM)110. BIM is a key pro-apoptotic mediator that contributes to the attrition of virus-specific CTLs during HBV and HCV infection107,111,112. BIM-mediated apoptosis may be promoted by co-inhibitory signals through cytotoxic T lymphocyte antigen 4 (CTLA4) or by T cell-intrinsic transforming growth factor-β (TGFβ)111,113. Suicidal emperipolesis — a recently described phenomenon whereby CTLs that recognize their cognate antigen in the liver invade hepatocytes for subsequent degradation114 — may also contribute to T cell attrition.
The few remaining virus-specific CTLs in chronic infection have functional defects, in keeping with the hierarchical loss of effector functions, termed exhaustion, that has been described for high-dose persistent viral infections115. A main cause of T cell exhaustion is an excess of co-inhibitory signals that outweighs the co-stimulatory signals and results in functional inhibition of T cells. This is best defined for programmed cell death protein 1 (PD1), a co-inhibitory molecule that tightly regulates T cell reactivity to prevent autoimmunity116,117,118. More recent work has revealed that multiple layers of negative co-regulation contribute to T cell exhaustion in chronic infection119. This is supported by studies of HBV and HCV that have shown non-redundant roles for other co-inhibitory molecules, such as CLTA4, T cell immunoglobulin domain and mucin domain protein 3 (TIM3; also known as HAVCR2) and 2B4 (Refs 111, 120, 121). HCV- and HBV-specific CTLs are enriched in the livers of chronically infected patients122,123 and express higher levels of co-inhibitory receptors than their circulating counterparts120,121. The high antigen load in HBV and HCV infection may be an important factor that promotes co-inhibitory receptor expression by virus-specific CTLs. Consistent with this idea, when CTLs are unable to recognize their cognate antigen because of viral epitope mutations, the expression of co-inhibitory receptors is downregulated124. However, antigen load is not the only determinant of PD1 expression by intrahepatic T cells, as expression levels remain high on virus-specific CTLs that reside in the liver after virus titres in the blood have decreased125. The contribution of co-inhibitory pathways to intrahepatic T cell tolerance is further promoted by the high levels of ligands for the co-inhibitory molecules expressed in the liver. Kupffer cells, LSECs, stellate cells and hepatocytes all express PD1 ligand 1 (PDL1)116,126,127,128, and PDL1 expression levels are upregulated in patients with viral hepatitis compared with controls129,130. Kupffer cells also express galectin 9, which is the ligand for TIM3, and its expression is similarly upregulated in HCV infection131. Thus, co-inhibitory pathways that operate to mitigate overzealous responses in the liver117,132 may be induced inappropriately by viruses to subvert effective antiviral immunity.
CTL exhaustion is exacerbated by a lack of adequate CD4+ T cell help115, a situation that is likely to be relevant in the liver, where CD4+ T cells are greatly outnumbered by CTLs133 and where non-professional APCs prime CD8+ T cells in the absence of CD4+ T cell help134. The paucity of CD4+ T cell help observed in the liver in the steady state is accentuated in chronic infection, during which the CD4+ T cell compartment is likely to be affected by inhibitory mechanisms similar to those that affect CTLs135. Compared with normal livers, the livers of patients with HBV or HCV infection contain increased numbers of forkhead box P3 (FOXP3)-expressing CD4+ regulatory T (TReg) cells, which may contribute to the extrinsic regulation of effector T cells136,137. However, these TReg cells also upregulate PD1 expression in HCV-infected livers and are thereby subjected to the constraining effects of PDL1 through inhibition of the phosphorylation of signal transducer and activator of transcription 5 (STAT5)137.
Intrahepatic expression of soluble immunosuppressive factors. In addition to the impaired T cell function caused by membrane-bound inhibitory molecules, other factors of the hepatic milieu can regulate local immune effector functions. The liver is a rich source of immunoregulatory cytokines, such as IL-10 (Ref. 138), which impedes the function of virus-specific T cells and may act synergistically with PD1 (Ref. 139). IL-10 production is induced in acute and chronic HBV infection63,140 and can suppress virus-specific T cells in the HCV-infected liver141. HBV- and HCV-specific CTLs are themselves capable of IL-10 production141,142,143, and can thereby attenuate antiviral immunity in an autocrine feedback loop to prevent excessive immune-mediated liver damage. T cells may also be deprived of the amino acids arginine and tryptophan in the liver, and this results in the induction of stress response pathways in the T cells that lead to their proliferative arrest144,145. Enzymes responsible for the catabolism of these amino acids are released by damaged hepatocytes and other intrahepatic populations and are induced in HBV and HCV infections146,176.
Intriguingly, many of the same soluble immunosuppressive factors and co-inhibitory molecules can be induced in acute, resolving hepatic infections63,129, during which they facilitate the contraction of the immune response and limit organ damage while maintaining viral control. By contrast, when these mechanisms become relentlessly activated during persistent infection, they may perpetuate the disarming of the already diminished antiviral immune responses.
The role of other immune cells in chronic hepatic infection. Innate immune effector cells may be able to substitute for the paralyzed T cell response in hepatic infections. One good candidate might be NK cells, as they are present in large numbers in the liver133,147. Recent findings suggest that NK cells with antigen-specific memory for viral infections are selectively maintained in the liver owing to their expression of CXCR6 (Ref. 84). However, NK cells are also vulnerable to tolerance mechanisms in the liver. In both HBV and HCV infections, NK cells retain cytotoxic potential140,147,148, but they fail to produce IFNγ, an effect that may be mediated by IL-10-producing Kupffer cells140,149,150. In addition, there are large numbers of γδ T cells and T cells that express NK cell markers (such as CD56 and CD161) in the liver81,151; whether these populations are subject to similar tolerance mechanisms or contribute to pathogenesis remains unclear. Classical invariant NKT cells are emerging as potent regulators of hepatic immune responses80, although they are present in much lower numbers in human HCV-infected livers than in mouse livers152.
Taken together, these findings suggest that chronic viral infection in the liver is perpetuated by the inhibition of virus-specific T cell and NK cell responses through several mechanisms (Fig. 3), which may have evolved to protect the liver from immune-mediated damage.
There are several layers of immunoregulatory and co-inhibitory signalling processes in the liver. Indoleamine 2,3-dioxygenase (IDO) and arginase are expressed at high levels in the liver and metabolize amino acids that are essential for immune cell proliferation and function, thus attenuating natural killer (NK) cell and T cell immune reactivity. Immunoregulatory cell populations such as regulatory T (TReg) cells, interleukin-10 (IL-10)-producing cells and myeloid-derived suppressor cells (MDSCs) prevent local expansion of effector T cell populations and restrict the function of the few NK and T cells present in the infected liver. Co-inhibitory signalling through the binding of programmed cell death protein 1 (PD1) on T cells to PD1 ligand 1 (PDL1) on Kupffer cells, liver sinusoidal endothelial cells (LSECs), stellate cells and hepatic dendritic cells (DCs) restricts hepatic immune responses. This inhibition occurs both at the level of T cell priming, by generating anergic or tolerant T cells, and at the level of the cytotoxic recall response, by limiting the effector function of CD8+ T cells in the liver. T cells that express BCL-2-interacting mediator of cell death (BIM) undergo apoptosis. Suicidal emperipolesis in hepatocytes also contributes to the elimination of T cells. CTLA4, cytotoxic T lymphocyte antigen 4; TDO, tryptophan 2,3-dioxygenase; TGFβ, transforming growth factor-β; TIM3, T cell immunoglobulin domain and mucin domain protein 3.
Overcoming persistent viral infection in the liver
The combination of immune escape strategies used by HBV and HCV153, the depletion or exhaustion of CTLs and the tolerogenic hepatic microenvironment that suppresses virus-specific T cell effector functions together contribute to the persistence of HBV and HCV infections (Tables 1,2). Differences in treatment modalities and outcomes are related to the different viral replication strategies. Current therapies for HCV infection that involve IFNα in combination with ribavirin and novel antiviral drugs154 can eliminate the virus by preventing viral replication, which is required for HCV persistence. By contrast, antiviral therapy with reverse transcriptase inhibitors controls but does not eliminate HBV, because the covalently closed circular DNA form of HBV DNA remains unaffected155. The finding that genetic polymorphisms in the IL28B locus correlate with a good response to IFNα treatment of HCV infection156,157,158 provides the rational for developing the IL28B gene product, IFNλ, as an alternative therapy. Moreover, novel approaches are being investigated that combine stimulation of RIG-I, to induce type I IFN production in the liver, with the lowering of viral antigen levels by gene silencing48,159. Finally, immunotherapy approaches could be developed to prevent the attrition of local hepatic T cell-mediated immunity. Such approaches would need to increase both the number and potency of pathogen-specific CTLs and the number of CD4+ T helper cells. To meet these requirements, four different strategies could be envisaged. First, viral antigen levels could be lowered to prevent the exhaustion of virus-specific CTLs. Second, extrahepatic priming could be augmented using a therapeutic vaccine to generate fully functional pathogen-specific CTLs. Third, local regulatory signals that impede effector function and reduce the number of CTLs in the liver could be overridden. And, fourth, functional T cells that have been redirected to recognize viral antigens could be adoptively transferred.
With each of these immunotherapeutic approaches, it is important to appreciate that both cytolytic and non-cytolytic functions of T cells can contribute to viral control but might also exacerbate liver damage through direct hepatocyte cytotoxicity or by driving inflammatory cell infiltration160. Accumulating data support the idea that antiviral T cell responses can be boosted without exacerbating the nonspecific lymphocytic infiltrate that causes liver damage123,147,161,162. This provides the rationale to pursue the development of therapeutic vaccines. Prophylactic vaccination relies on rapid neutralization of the invading pathogen by antibodies, whereas successful therapeutic vaccination depends on the induction of broad and polyfunctional T cell responses against key viral antigens163. Such polyfunctional T cell responses should involve both cytolytic and non-cytolytic clearance of HBV-infected hepatocytes. To counteract the T cell exhaustion caused by high antigen levels, the induction of T cells should be preceded by the activation of a humoral immune response that reduces antigen levels and limits virus spread. New vaccine protocols therefore use prime–boost strategies, in which an adjuvanted protein primes and induces neutralizing antibody responses and a vector-based vaccine then boosts T cell responses164. Alternatively, viral antigen levels may be reduced by gene silencing techniques165,166. Future therapeutic vaccinations may also incorporate, or be combined with, measures that are designed to enhance co-stimulation or override the inhibition of T cells. However, overriding regulatory signals from the liver microenvironment — for example, by blocking co-inhibitory molecules such as PD1 — may pose a risk of further immunopathology in the liver, as the absence of PDL1 has been shown to cause severe autoimmune liver damage in animal models116,117,132. Targeting the downstream master transcriptional regulators of T cell exhaustion may be a more subtle approach and might allow for the heterogeneity of non-redundant pathways that function in patients.
The observation that two-thirds of HBV-infected patients who receive allogeneic stem cell transplants from individuals with immunity to HBV clear HBV infection167,168 is encouraging for the development of adoptive cell therapy strategies. T cell-based therapies are a valid alternative for vaccination strategies, as the numbers and effector functions of the transferred T cells can be defined. Given the paucity of HBV-specific T cells found in HBV-infected patients, attempts at expanding these populations in vitro to create sufficient numbers for T cell therapy would be challenging. One approach being developed for the reconstitution of virus-specific CTLs involves redirecting the specificity of T cells towards key HBV epitopes. In principle, this may be achieved by antibody-mediated redirection or by adoptive transfer of receptor-modified T cells169,170. HBV specificity can be genetically introduced into T cells from patients with chronic HBV infection by transfecting the cells with either cloned T cell receptor α- and β-chain genes or artificial chimeric antigen receptor (CAR) genes171,172,173,174. The use of CARs has the advantage that the T cells recognize native viral antigens on the surface of infected cells, and therefore function independently of the patient's MHC haplotype and do not require antigen processing and presentation. Although immunotherapeutic approaches to HBV infection are progressing, considerable efforts will be required to develop safe and successful treatment strategies.
Concluding remarks
Infections of the liver do not inevitably lead to pathogen persistence. Indeed, hepatic cell populations can sense infection, mount strong innate immune responses and allow for sufficient local adaptive immune surveillance to clear infectious microorganisms. Based on the outcome of their interaction with the immune system, hepatic infections can be classified into three groups. First, there are infections by microorganisms, such as bacteria, that are rapidly cleared through the induction of immediate immune effector functions within liver sinusoids. Second, there are infections by hepatocyte-targeting pathogens that are cleared slowly once innate immune activation and pathogen-specific T cell responses predominate over pathogen immune escape mechanisms (for example, as occurs in HAV infections, and in cases of HBV or HCV infections that resolve after acute infection). Third, there are infections by pathogens that persist in hepatocytes by escaping innate and adaptive immune responses, as occurs in chronic HBV or HCV infections. Viral persistence is sustained as a result of T cell exhaustion — which is caused by high viral antigen loads and increased co-inhibitory signalling in the inflamed infected liver — and immune evasion strategies used by the virus. Although the exact combination of host and viral factors that leads to persistent infection rather than viral clearance remains to be established, our current knowledge supports the development of therapeutic strategies that lower viral antigen load and increase the numbers of functionally active virus-specific T cells in the infected liver.
References
Thomson, A. W. & Knolle, P. A. Antigen-presenting cell function in the tolerogenic liver environment. Nature Rev. Immunol. 10, 753–766 (2010).
Schlepper-Schafer, J. et al. Endocytosis via galactose receptors in vivo. Ligand size directs uptake by hepatocytes and/or liver macrophages. Exp. Cell Res. 165, 494–506 (1986).
Wisse, E., De Zanger, R. B., Charels, K., Van Der Smissen, P. & McCuskey, R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 5, 683–692 (1985).
Tavassoli, M., Kishimoto, T., Soda, R., Kataoka, M. & Harjes, K. Liver endothelium mediates the uptake of iron–transferrin complex by hepatocytes. Exp. Cell. Res. 165, 369–379 (1986).
Mostov, K. E. Transepithelial transport of immunoglobulins. Annu. Rev. Immunol. 12, 63–84 (1994).
Middleton, J. et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91, 385–395 (1997).
Smedsrod, B. Clearance function of scavenger endothelial cells. Comp. Hepatol. 3, S22 (2004).
Pradel, G. & Frevert, U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology 33, 1154–1165 (2001). The first report showing that sporozoites target Kupffer cells to overcome the sinusoidal barrier.
Ishino, T., Yano, K., Chinzei, Y. & Yuda, M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2, e4 (2004).
Baer, K. et al. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 9, 397–412 (2007).
Mota, M. M., Hafalla, J. C. & Rodriguez, A. Migration through host cells activates Plasmodium sporozoites for infection. Nature Med. 8, 1318–1322 (2002).
Coppi, A. et al. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J. Exp. Med. 208, 341–356 (2011).
Silvie, O. et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nature Med. 9, 93–96 (2003).
Sturm, A. et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313, 1287–1290 (2006).
Bartenschlager, R., Penin, F., Lohmann, V. & Andre, P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 19, 95–103 (2011).
Bashirova, A. A. et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193, 671–678 (2001).
Lai, W. K. et al. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Am. J. Pathol. 169, 200–208 (2006).
Gardner, J. P. et al. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc. Natl Acad. Sci. USA 100, 4498–4503 (2003).
Pohlmann, S. et al. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77, 4070–4080 (2003). References 18 and 19 were the first two reports on the potential role of L-SIGN and DC-SIGN in liver infection by HCV.
Geijtenbeek, T. B. et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100, 587–597 (2000).
Pileri, P. et al. Binding of hepatitis C virus to CD81. Science 282, 938–941 (1998).
Agnello, V., Abel, G., Elfahal, M., Knight, G. B. & Zhang, Q. X. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl Acad. Sci. USA 96, 12766–12771 (1999).
Scarselli, E. et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21, 5017–5025 (2002).
Evans, M. J. et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446, 801–805 (2007).
Ploss, A. et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886 (2009).
Lupberger, J. et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nature Med. 17, 589–595 (2011).
Gripon, P. et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl Acad. Sci. USA 99, 15655–15660 (2002).
Blanchet, M. & Sureau, C. Analysis of the cytosolic domains of the hepatitis B virus envelope proteins for their function in viral particle assembly and infectivity. J. Virol. 80, 11935–11945 (2006).
Salisse, J. & Sureau, C. A function essential to viral entry underlies the hepatitis B virus “a” determinant. J. Virol. 83, 9321–9328 (2009).
Petersen, J. et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nature Biotech. 26, 335–341 (2008).
Schulze, A., Gripon, P. & Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46, 1759–1768 (2007).
Jilbert, A. R., Miller, D. S., Scougall, C. A., Turnbull, H. & Burrell, C. J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226, 338–345 (1996).
Asabe, S. et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J. Virol. 83, 9652–9662 (2009).
Breiner, K. M., Schaller, H. & Knolle, P. A. Endothelial cell-mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms. Hepatology 34, 803–808 (2001).
Ashida, M. & Hamada, C. Molecular cloning of the hepatitis A virus receptor from a simian cell line. J. Gen. Virol. 78, 1565–1569 (1997).
Kaplan, G. et al. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 15, 4282–4296 (1996).
Feigelstock, D., Thompson, P., Mattoo, P., Zhang, Y. & Kaplan, G. G. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J. Virol. 72, 6621–6628 (1998).
van Egmond, M. et al. FcαRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nature Med. 6, 680–685 (2000).
Popov, A. et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J. Clin. Invest. 116, 3160–3170 (2006).
Egen, J. G. et al. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28, 271–284 (2008).
Volkman, H. E. et al. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327, 466–469 (2010).
Taylor, J. L. et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect. Immun. 74, 6135–6144 (2006).
Davis, J. M. & Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 (2009).
Wang, B. et al. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J. Hepatol. 51, 1037–1045 (2009).
Wu, J. et al. Hepatitis B virus suppresses Toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 49, 1132–1140 (2009).
Kern, M. et al. Virally infected mouse liver endothelial cells trigger CD8+ T-cell immunity. Gastroenterology 138, 336–346 (2010).
Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M. Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 (2008).
Ebert, G. et al. 5′ triphosphorylated small interfering RNAs control replication of hepatitis B virus and induce an interferon response in human liver cells and mice. Gastroenterology 141, 696–706 (2011).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nature Med. 13, 1324–1332 (2007).
Gao, B., Jeong, W. I. & Tian, Z. Liver: an organ with predominant innate immunity. Hepatology 47, 729–736 (2008).
Baumann, H. & Gauldie, J. The acute phase response. Immunol. Today 15, 74–80 (1994).
Wu, J. et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology 46, 1769–1778 (2007).
Biswas, S. K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009).
De Creus, A. et al. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J. Immunol. 174, 2037–2045 (2005).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nature Med. 6, 1348–1354 (2000).
Khakoo, S. I. et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874 (2004).
Lee, W. Y. et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nature Immunol. 11, 295–302 (2010). A seminal paper demonstrating the efficient intravascular immune response in the liver against bacteria.
Dolganiuc, A. et al. Hepatitis C core and nonstructural 3 proteins trigger Toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology 127, 1513–1524 (2004).
Cooper, A., Tal, G., Lider, O. & Shaul, Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J. Immunol. 175, 3165–3176 (2005).
Hosel, M. et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology 50, 1773–1782 (2009).
Klein, C. et al. The IL-6–gp130–STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J. Clin. Invest. 115, 860–869 (2005).
Gehring, S. et al. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology 130, 810–822 (2006).
Dunn, C. et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology 137, 1289–1300 (2009).
Wieland, S., Thimme, R., Purcell, R. H. & Chisari, F. V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl Acad. Sci. USA 101, 6669–6674 (2004).
Gale, M. Jr & Foy, E. M. Evasion of intracellular host defence by hepatitis C virus. Nature 436, 939–945 (2005).
Qu, L. & Lemon, S. M. Hepatitis A and hepatitis C viruses: divergent infection outcomes marked by similarities in induction and evasion of interferon responses. Semin. Liver Dis. 30, 319–332 (2010).
Ke, P. Y. & Chen, S. S. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 121, 37–56 (2011).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Li, X. D., Sun, L., Seth, R. B., Pineda, G. & Chen, Z. J. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl Acad. Sci. USA 102, 17717–17722 (2005).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Foy, E. et al. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300, 1145–1148 (2003). The first report on HCV immune escape blocking IRF3 function.
Yang, Y. et al. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc. Natl Acad. Sci. USA 104, 7253–7258 (2007).
Qu, L. et al. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS Pathog. 7, e1002169 (2011).
Lanford, R. E. et al. Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA. Proc. Natl Acad. Sci. USA 108, 11223–11228 (2011).
Desai, M. M. et al. Differential, type I interferon-mediated autophagic trafficking of hepatitis C virus proteins in mouse liver. Gastroenterology 141, 674–685 (2011).
Usynin, I., Klotz, C. & Frevert, U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell. Microbiol. 9, 2610–2628 (2007).
Torgler, R. et al. Sporozoite-mediated hepatocyte wounding limits Plasmodium parasite development via MyD88-mediated NF-κB activation and inducible NO synthase expression. J. Immunol. 180, 3990–3999 (2008).
Gowda, D. C. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 23, 596–604 (2007).
Taniguchi, M., Seino, K. & Nakayama, T. The NKT cell system: bridging innate and acquired immunity. Nature Immunol. 4, 1164–1165 (2003).
Swain, M. G. Natural killer T cells within the liver: conductors of the hepatic immune orchestra. Dig. Dis. 28, 7–13 (2010).
Klugewitz, K., Adams, D. H., Emoto, M., Eulenburg, K. & Hamann, A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 25, 590–594 (2004).
Polakos, N. K. et al. Early intrahepatic accumulation of CD8+ T cells provides a source of effectors for nonhepatic immune responses. J. Immunol. 179, 201–210 (2007).
Keating, R. et al. Virus-specific CD8+ T cells in the liver: armed and ready to kill. J. Immunol. 178, 2737–2745 (2007).
Paust, S. et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nature Immunol. 11, 1127–1135 (2010). A demonstration of a population of CXCR6-expressing intrahepatic NK cells able to mediate antigen-specific memory.
Schmidt, N. W. et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl Acad. Sci. USA 105, 14017–14022 (2008).
Kumar, K. A. et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444, 937–940 (2006).
Chakravarty, S. et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nature Med. 13, 1035–1041 (2007). A classical paper reporting extrahepatic priming of sporozoite-specific T cells.
Carvalho, L. H. et al. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nature Med. 8, 166–170 (2002).
Morrot, A., Hafalla, J. C., Cockburn, I. A., Carvalho, L. H. & Zavala, F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J. Exp. Med. 202, 551–560 (2005).
Overstreet, M. G., Cockburn, I. A., Chen, Y. C. & Zavala, F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an 'unnatural' immune response. Immunol. Rev. 225, 272–283 (2008).
Cockburn, I. A. et al. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 6, e1000877 (2010).
Good, M. F. & Doolan, D. L. Malaria vaccine design: immunological considerations. Immunity 33, 555–566 (2010).
Amadei, B. et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology 138, 1536–1545 (2010).
Canbay, A. et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 38, 1188–1198 (2003).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Lang, P. A. et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology 52, 25–32 (2010).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
Sancho, D. et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 458, 899–903 (2009).
Veerapu, N. S., Raghuraman, S., Liang, T. J., Heller, T. & Rehermann, B. Sporadic reappearance of minute amounts of hepatitis C virus RNA after successful therapy stimulates cellular immune responses. Gastroenterology 140, 676–685 (2011).
Rehermann, B., Ferrari, C., Pasquinelli, C. & Chisari, F. V. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nature Med. 2, 1104–1108 (1996). A landmark paper demonstrating that HBV is controlled but not eliminated.
Bertoletti, A. & Ferrari, C. Kinetics of the immune response during HBV and HCV infection. Hepatology 38, 4–13 (2003).
Rehermann, B. & Nascimbeni, M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Rev. Immunol. 5, 215–229 (2005).
Guidotti, L. G. & Chisari, F. V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19, 65–91 (2001). A key review on the non-cytolytic antiviral activity of cytokines in HBV-infected transgenic mice.
Garcia-Rodriguez, M. J., Canales, M. A., Hernandez-Maraver, D. & Hernandez-Navarro, F. Late reactivation of resolved hepatitis B virus infection: an increasing complication post rituximab-based regimens treatment? Am. J. Hematol. 83, 673–675 (2008).
von Hahn, T. et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 132, 667–678 (2007).
Brimacombe, C. L. et al. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J. Virol. 85, 596–605 (2011).
Lopes, A. R. et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J. Clin. Invest. 118, 1835–1845 (2008).
Radziewicz, H. et al. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J. Virol. 82, 9808–9822 (2008).
Bowen, D. G. et al. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J. Clin. Invest. 114, 701–712 (2004). A key paper describing that initial priming of T cells in the liver determines the development of peripheral immune tolerance.
Holz, L. E. et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology 135, 989–997 (2008).
Schurich, A. et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 53, 1494–1503 (2011).
Larrubia, J. R. et al. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1+/CD127− HCV-specific CD8+ cells targeting the virus in chronic hepatitis C virus infection. Cell. Immunol. 269, 104–114 (2011).
Tinoco, R., Alcalde, V., Yang, Y., Sauer, K. & Zuniga, E. I. Cell-intrinsic transforming growth factor-β signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31, 145–157 (2009).
Benseler, V. et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc. Natl Acad. Sci. USA 108, 16735–16740 (2011).
Wherry, E. J. T cell exhaustion. Nature Immunol. 12, 492–499 (2011).
Iwai, Y., Terawaki, S., Ikegawa, M., Okazaki, T. & Honjo, T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 198, 39–50 (2003). This paper reports the seminal discovery of the essential protective role of PDL1 for the liver.
Dong, H. et al. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 20, 327–336 (2004).
Isogawa, M., Furuichi, Y. & Chisari, F. V. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity 23, 53–63 (2005).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunol. 10, 29–37 (2009). An elegant demonstration of multiple co-inhibitory pathways driving T cell exhaustion.
Klenerman, P. & Thimme, R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut 28 Aug 2011 (doi:10.1136/gutjnl-2011-300620).
Fisicaro, P. et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 138, 682–693 (2010).
He, X. S. et al. Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide–MHC tetramers. Proc. Natl Acad. Sci. USA 96, 5692–5697 (1999).
Maini, M. K. et al. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191, 1269–1280 (2000).
Bengsch, B. et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6, e1000947 (2010).
Blackburn, S. D. et al. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J. Virol. 84, 2078–2089 (2010).
Diehl, L. et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 47, 296–305 (2008).
Yu, M. C. et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 40, 1312–1321 (2004).
Mühlbauer, M. et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and -γ and mediates T cell apoptosis. J. Hepatol. 45, 520–528 (2006).
Zhang, Z. et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology 134, 1938–1949 (2008).
Kassel, R. et al. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology 50, 1625–1637 (2009).
Mengshol, J. A. et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS ONE 5, e9504 (2010).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). A seminal report on the role of PD1 in T cell exhaustion during viral infection.
Doherty, D. G. et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J. Immunol. 163, 2314–2321 (1999).
Wuensch, S. A., Spahn, J. & Crispe, I. N. Direct, help-independent priming of CD8+ T cells by adeno-associated virus-transduced hepatocytes. Hepatology 52, 1068–1077 (2010).
Raziorrouh, B. et al. Inhibitory molecules that regulate expansion and restoration of HCV-specific CD4+ T cells in patients with chronic infection. Gastroenterology 141, 1422–1431 (2011).
Manigold, T. & Racanelli, V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infect. Dis. 7, 804–813 (2007).
Franceschini, D. et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Invest. 119, 551–564 (2009).
Knolle, P. et al. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J. Hepatol. 22, 226–229 (1995).
Ha, S. J., West, E. E., Araki, K., Smith, K. A. & Ahmed, R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol. Rev. 223, 317–333 (2008).
Peppa, D. et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 6, e1001227 (2010).
Accapezzato, D. et al. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J. Clin. Invest. 113, 963–972 (2004).
Abel, M. et al. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology 44, 1607–1616 (2006).
Chang, J. J. et al. The phenotype of hepatitis B virus-specific T cells differ in the liver and blood in chronic hepatitis B virus infection. Hepatology 46, 1332–1340 (2007).
Chisari, F. V. et al. Production of two distinct and independent hepatic immunoregulatory molecules by the perfused rat liver. Hepatology 5, 735–743 (1985).
Munn, D. H. & Mellor, A. L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117, 1147–1154 (2007).
Larrea, E. et al. Upregulation of indoleamine 2,3-dioxygenase in hepatitis C virus infection. J. Virol. 81, 3662–3666 (2007).
Dunn, C. et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J. Exp. Med. 204, 667–680 (2007).
Oliviero, B. et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 137, 1151–1160 (2009).
Tu, Z. et al. TLR-dependent cross talk between human Kupffer cells and NK cells. J. Exp. Med. 205, 233–244 (2008).
Sene, D. et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 6, e1001184 (2010).
Billerbeck, E. et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc. Natl Acad. Sci. USA 107, 3006–3011 (2010).
de Lalla, C. et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J. Immunol. 173, 1417–1425 (2004).
Finlay, B. B. & McFadden, G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124, 767–782 (2006).
Rosen, H. R. Clinical practice. Chronic hepatitis C infection. N. Engl. J. Med. 364, 2429–2438 (2011).
Werle-Lapostolle, B. et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126, 1750–1758 (2004).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nature Genet. 41, 1105–1109 (2009).
Suppiah, V. et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nature Genet. 41, 1100–1104 (2009).
Han, Q., Zhang, C., Zhang, J. & Tian, Z. Reversal of hepatitis B virus-induced immune tolerance by an immunostimulatory 3p-HBx-siRNAs in a retinoic acid inducible gene I-dependent manner. Hepatology 54, 1179–1189 (2011).
Bertoletti, A. & Maini, M. K. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr. Opin. Immunol. 12, 403–408 (2000).
Kakimi, K., Guidotti, L. G., Koezuka, Y. & Chisari, F. V. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192, 921–930 (2000).
Loggi, E. et al. Anti-HBs re-seroconversion after liver transplantation in a patient with past HBV infection receiving a HBsAg positive graft. J. Hepatol. 50, 625–630 (2009).
Maini, M. K. & Schurich, A. The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J. Hepatol. 52, 616–619 (2010).
Kutscher, S., Bauer, T., Dembek, C., Sprinzl, M. & Protzer, U. Design of therapeutic vaccines: hepatitis B as an example. Microb. Biotechnol. 29 Sep 2011 (doi:10.1111/j.1751-7915.2011.00303.x).
McCaffrey, A. P. et al. Inhibition of hepatitis B virus in mice by RNA interference. Nature Biotech. 21, 639–644 (2003).
Klein, C. et al. Inhibition of hepatitis B virus replication in vivo by nucleoside analogues and siRNA. Gastroenterology 125, 9–18 (2003).
Ilan, Y. et al. Ablation of persistent hepatitis B by bone marrow transplantation from a hepatitis B-immune donor. Gastroenterology 104, 1818–1821 (1993).
Hui, C. K. et al. A long-term follow-up study on hepatitis B surface antigen-positive patients undergoing allogeneic hematopoietic stem cell transplantation. Blood 106, 464–469 (2005).
Hawkins, R. E. et al. Development of adoptive cell therapy for cancer: a clinical perspective. Hum. Gene Ther. 21, 665–672 (2010).
Protzer, U. & Abken, H. Can engineered “designer” T cells outsmart chronic hepatitis B? Hepat. Res. Treat. 2010, 901216 (2010).
Gehring, A. J. et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J. Hepatol. 55, 103–110 (2011).
Bohne, F. et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology 134, 239–247 (2008).
Abken, H., Hombach, A. & Heuser, C. Immune response manipulation: recombinant immunoreceptors endow T-cells with predefined specificity. Curr. Pharm. Des. 9, 1992–2001 (2003).
Riddell, S. R. & Protzer, U. Carving the CAR. Gene Ther. 17, 1191–1192 (2010).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nature Med. 15, 1170–1178 (2009).
Das, A. et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 205, 2111–2124 (2008).
Thomas, D. L. et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801 (2009).
Dazert, E. et al. Loss of viral fitness and cross-recognition by CD8+ T cells limit HCV escape from a protective HLA-B27-restricted human immune response. J. Clin. Invest. 119, 376–386 (2009). An important report demonstrating the link between viral fitness and HLA-B27-restricted T cell immunity.
Chisari, F. V. & Ferrari, C. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13, 29–60 (1995).
Kurts, C., Robinson, B. W. & Knolle, P. A. Cross-priming in health and disease. Nature Rev. Immunol. 10, 403–414 (2010).
Wieland, S. F., Spangenberg, H. C., Thimme, R., Purcell, R. H. & Chisari, F. V. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc. Natl Acad. Sci. USA 101, 2129–2134 (2004).
Murray, J. M., Wieland, S. F., Purcell, R. H. & Chisari, F. V. Dynamics of hepatitis B virus clearance in chimpanzees. Proc. Natl Acad. Sci. USA 102, 17780–17785 (2005).
Summers, J. & Mason, W. S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 29, 403–415 (1982).
Acknowledgements
We acknowledge financial support by the German Research Foundation (DFG; SFB 704, SFB 670, SFB 576, SFB TR57), the Helmholtz Alliance on Immunotherapy of Cancer and the UK Medical Research Council (G0801213). We apologize to all colleagues whose excellent work could not be cited owing to space restrictions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Liver sinusoidal endothelial cells
-
(LSECs). Cells that line the hepatic sinusoids and take up molecules from the blood. LSECs function as liver-resident antigen-presenting cells and contribute to the induction of local tolerance.
- Kupffer cells
-
Specialized macrophages in the liver that line the sinusoidal vessels. They present antigens, regulate local immune responses and remove microbial particles, endotoxins and other noxious substances that are present in portal venous blood.
- Hepatic stellate cells
-
A perisinusoidal cell population within the space of Dissé that controls sinusoidal diameter and hepatic blood flow. Stellate cells are the main reservoir of retinol in the liver and contribute to the development of liver fibrosis following inflammation. They have antigen-presenting cell function and contribute to local hepatic immune tolerance.
- Space of Dissé
-
The space between liver sinusoidal endothelial cells (LSECs) and hepatocytes that is filled with extracellular matrix and is populated by hepatic stellate cells. Access to the space of Dissé is provided through fenestrae in LSECs or following transcytotic transport through LSECs.
- Granulomas
-
Structures that are orchestrated by macrophages at different stages of activation and are usually surrounded by a layer of lymphocytes. These macrophages resemble epithelial cells and often include multinucleated giant cells. Granuloma formation is a chronic inflammatory response that can be initiated by various infectious and non-infectious agents.
- Pattern-recognition receptors
-
Immune sensory receptors — such as membrane-bound Toll-like receptors or cytosolic RIG-I-like helicases — that recognize conserved structures of pathogens.
- LPS tolerance
-
A state of hyporesponsiveness to various pro-inflammatory stimuli that results from continuous exposure to low-level lipopolysaccharide derived from the gut. It was described first for Kupffer cells.
- Unfolded protein response
-
A response that increases the ability of the endoplasmic reticulum to fold and translocate proteins, decreases the synthesis of proteins and causes cell cycle arrest and apoptosis.
- Autophagy
-
An evolutionarily conserved process, in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation and recycling, through fusion with lysosomes.
- Damage-associated molecular pattern
-
(DAMP). A molecule that is produced by or released from host cells following cellular stress, damage or non-physiological cell death. DAMPs are thought to be responsible for the initiation and perpetuation of inflammatory responses and tissue repair under non-infectious conditions. Examples include: hyaluronan (which is released from the degraded stroma); HMGB1 (which is released from the nucleus); and ATP, uric acid, S100 calcium-binding proteins and heat-shock proteins (which are released from the cytosol). They can induce tolerogenic or immunogenic myeloid dendritic cells, depending on the nature of other signals that are present.
- Pathogen-associated molecular pattern
-
(PAMP). A conserved microbial structure that is not found in mammalian cells and is recognized by non-variable pattern-recognition receptors. PAMPs include microbial components such as bacterial lipopolysaccharide, hypomethylated DNA, flagellin, double-stranded RNA and other cytosolic nucleic acids.
- Suicidal emperipolesis
-
A recently described process during which CD8+ T cells invade hepatocytes, leading to CD8+ T cell death.
- Ribavirin
-
A drug that interferes with RNA metabolism and blocks viral replication. Ribavirin is used in combination with interferon-α to treat hepatitis C.
- IFNλ
-
(Interferon-λ; also known as type III IFNs). There are three IFNλ cytokines, interleukin-28A (IL-28A), IL-28B and IL-29, which can be produced by almost every cell type after infection and bind to a heterodimeric receptor (comprised of the IL-10 receptor β-chain and the IL-28 receptor α-chain) that is expressed mainly by epithelial cells. They have direct antiviral properties, induce IFN-responsive gene expression and increase antigen presentation.
- Chimeric antigen receptor
-
An artificial T cell receptor construct that consists of an extracellular single-chain antibody fragment that functions as the antigen-binding domain, together with transmembrane and intracellular signalling domains from the T cell receptor CD3ζ chain and/or from a co-stimulatory molecule such as CD28.
Rights and permissions
About this article
Cite this article
Protzer, U., Maini, M. & Knolle, P. Living in the liver: hepatic infections. Nat Rev Immunol 12, 201–213 (2012). https://doi.org/10.1038/nri3169
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3169
This article is cited by
-
Effect of infiltrating immune cells in tumor microenvironment on metastasis of hepatocellular carcinoma
Cellular Oncology (2023)
-
In Vivo and in Vitro Biocompatibility Studies of Pt Based Nanoparticles: a New Agent for Chemoradiation Therapy
Journal of Cluster Science (2023)
-
The gut–liver axis in sepsis: interaction mechanisms and therapeutic potential
Critical Care (2022)
-
Ultra-thin layered double hydroxide-mediated photothermal therapy combine with asynchronous blockade of PD-L1 and NR2F6 inhibit hepatocellular carcinoma
Journal of Nanobiotechnology (2022)
-
What’s happening where when SARS-CoV-2 infects: are TLR7 and MAFB sufficient to explain patient vulnerability?
Immunity & Ageing (2022)