Key Points
-
Invariant natural killer T (iNKT) cells are a specialized T cell population that recognizes lipid antigens that are presented by a cell-surface molecule known as CD1d. They have been shown to have important roles in many diverse immune responses.
-
iNKT cells recognize both foreign lipid antigens and self lipid antigens. The T cell receptor (TCR)–lipid–CD1d interaction is similar for both self and foreign lipid antigens, despite the differences that exist in these lipid structures. Strong lipid antigens have a 'lock and key' type of binding, whereas weaker antigens require an 'induced fit' mechanism.
-
The production of lipid self antigens for iNKT cells can be upregulated by antigen-presenting cells (APCs) in response to danger signals, such as Toll-like receptor (TLR) agonists. This provides a mechanism for iNKT cell activation in the absence of foreign lipid antigens.
-
In addition to being activated through their TCRs in response to CD1d-presented lipids, iNKT cells can be activated by indirect stimuli, such as pro-inflammatory cytokines. During many infections, interleukin-12 (IL-12) may have an equally important role to lipid antigens in activating iNKT cells.
-
iNKT cells couple the rapid activation kinetics of innate immune cells with the diverse effector functions of adaptive T cells. Early activation during infection leads to rapid cytokine production in target tissues by polarized iNKT cell subsets.
-
Interactions between iNKT cells and CD1d-expressing APCs lead to bidirectional activation. Cytokines produced by iNKT cells activate and recruit other cell types early during immune responses, while activated APCs direct the ensuing adaptive immune responses. Thus, iNKT cells and their lipid antigens help to orchestrate innate and adaptive immune responses.
Abstract
Invariant natural killer T (iNKT) cells exist in a 'poised effector' state, which enables them to rapidly produce cytokines following activation. Using a nearly monospecific T cell receptor, they recognize self and foreign lipid antigens presented by CD1d in a conserved manner, but their activation can catalyse a spectrum of polarized immune responses. In this Review, we discuss recent advances in our understanding of the innate-like mechanisms underlying iNKT cell activation and describe how lipid antigens, the inflammatory milieu and interactions with other immune cell subsets regulate the functions of iNKT cells in health and disease.
Similar content being viewed by others
Main
Invariant natural killer T (iNKT) cells are a specialized subset of T cells that use their T cell receptors (TCRs) to recognize self and foreign lipids presented by CD1d as cognate antigens. These cells have been shown to have either protective or harmful roles in many pathological states, including microbial infection1,2, autoimmune disease3, allergic disease4 and cancer5. iNKT cells have previously been the subject of informative reviews6,7,8,9,10,11, but crucial questions underlying their basic biology remain. Recently, our understanding of iNKT cell biology has taken several important steps forward, particularly with regard to the lipid antigens recognized by the iNKT cell TCR. There is also now a better appreciation of the dynamic interactions that occur between iNKT cells and other leukocytes.
The profound immunomodulatory potential of iNKT cells stems from the unique way in which they combine both classically innate and classically adaptive immunological features to function as innate–adaptive 'hybrids'. iNKT cells have an αβ TCR that depends on somatic recombination and selection in the thymus12 (Box 1). However, although somatic recombination has become largely synonymous with adaptive immunity, function does not necessarily follow form. iNKT cells exist in a 'poised effector' state13, are able to respond in an innate-like manner to danger signals and pro-inflammatory cytokines14,15,16,17, and exert their effector functions within hours of being activated, all of which are innate characteristics. Furthermore, iNKT cells share extensive transcriptional identity with both innate and adaptive immune cells18. We outline evidence that iNKT cells adopt a largely innate activation scheme that is cleverly coupled to a diverse effector toolbox, utilizing nearly all of the effector capabilities of adaptive MHC-restricted T cells. This Review covers the establishment of the iNKT cell compartment, how the iNKT cell TCR recognizes microbial and self lipid antigens, how TCR signals and pro-inflammatory cytokines synergize for innate-like activation, and how iNKT cell activation is translated into a wide array of effector functions that orchestrate both innate and adaptive immune responses.
Establishment of a poised effector compartment
iNKT cells: the basics. CD1d is a conserved, non-polymorphic MHC class I-like molecule and a member of a larger family that includes the CD1a, CD1b and CD1c molecules, all of which present lipids rather than peptides as antigens to non-MHC-restricted T cells7,8. CD1d is expressed by many cells of haematopoietic origin, including dendritic cells, macrophages, granulocytes and B cells19,20. Around the time that CD1-mediated presentation of lipid antigens was discovered21,22, a distinct population of T cells with an invariant TCR repertoire was identified23,24,25, and these invariant TCR-bearing lymphocytes were subsequently found to be CD1d restricted26,27,28. This CD1d restriction now defines NKT cells. Many studies not cited here contributed to the identification of iNKT cells, and a more detailed timeline of the early discoveries can be found elsewhere29. In 1997, Kawano and colleagues identified the first CD1d-presented lipid antigen for iNKT cells, α-galactosylceramide (αGalCer)30 (Fig. 1). This specific and potent lipid antigen has contributed greatly to our understanding of iNKT cells. Although αGalCer was identified from a marine sponge sample, this lipid may have originated from a bacterium present in the sample rather than from the sponge itself. CD1d tetramers loaded with αGalCer enabled the definitive identification of a population of CD1d-restricted iNKT cells as a subset of T cells that in mice expresses mainly TCRs comprising a Vα14Jα18 chain paired with a limited Vβ chain repertoire (Vβ2, Vβ7, Vβ8.1, Vβ8.2 or Vβ8.3)31,32. An analogous limited TCR repertoire is used by human iNKT cells, for which a Vα24Jα18 chain is paired almost exclusively with a Vβ11 chain. CD1d is highly conserved in mammals33, and there is a large degree of functional and structural similarity between the TCRs that are expressed by human and mouse iNKT cells34. In addition to iNKT cells, which have an invariant TCR α-chain, there is another population of CD1d-restricted T cells, referred to as 'diverse' NKT (dNKT) or 'type II' NKT cells35,36,37, which express diverse TCR α- and β-chains38,39 and typically do not recognize αGalCer. dNKT cells also recognize lipid antigens40,41,233, but we know relatively little about dNKT cells (as compared with our knowledge of iNKT cells), and this Review focuses on iNKT cells.
Shown are the structures of various lipid antigens for invariant natural killer T (iNKT) cells: the prototypical iNKT cell lipid antigen α-galactosylceramide (αGalCer); the lipid self antigens β-glucosylceramide (βGlcCer), plasmalogen lysophosphatidylethanolamine (plasmalogen lysoPE) and isoglobotrihexosylceramide (iGb3); and the microbial lipid antigen α-glucosyldiacyglycerol (αGlcDAG) from Streptococcus pneumoniae. For each lipid antigen, a closely related non-antigenic structure is shown, revealing how small structural changes in distinct regions of these lipids can critically affect lipid antigenicity. C24:1 and C16:0 indicate the N-acyl chain structure. TCR, T cell receptor.
The peripheral iNKT cell compartment. Both mouse and human iNKT cells express a range of chemokine receptors and other homing receptors, features that are likely to regulate their distribution and localization42,43,44. iNKT cells have been best studied in the spleen and liver of C57BL/6 mice, where they represent approximately 1–2% and 20–30% of lymphocytes, respectively31,32. Mature iNKT cells are distributed widely, including in the bone marrow, gastrointestinal tract and skin32,45. Compared with the recirculation of MHC-restricted T cells, iNKT cell recirculation from the tissues is limited. In the liver, for example, iNKT cells are retained as a result of constitutive interactions between lymphocyte function-associated antigen 1 (LFA1; also known as αLβ2 integrin) and intercellular adhesion molecule 1 (ICAM1)46. iNKT cells patrol liver sinusoids, and after activation by either pro-inflammatory cytokines or lipid antigens they arrest to exert effector functions47,48,49. Following the experimental induction of stroke, however, iNKT cells rapidly disappear from the liver, unexpectedly permitting bacteraemia from translocated colonic microflora. In this stroke model, it was not lipid antigens, but adrenergic signals, that regulated the migration of hepatic iNKT cells50. Intravital microscopy of reporter mice that express green fluorescence protein under the control of the CXC-chemokine receptor 6 (Cxcr6) promoter has revealed that iNKT cells are also stationed inside the vasculature of the lungs, and following exposure to αGalCer, rather than arresting, they extravasate and contribute to intraparenchymal inflammation and adaptive immune responses51. iNKT cells are also highly enriched in mouse adipose tissue, where they have a role in metabolic regulation52,53,54,55,56 (Box 2). In the lymph nodes, iNKT cells are present at approximately tenfold lower levels than in the spleen, and they are enriched for a subpopulation of cells that has a NK1.1−CD103+CCR6+ phenotype and produces interleukin-17A (IL-17A)45.
In human peripheral blood, iNKT cells account for 0.1–0.2% of T cells on average, but this proportion is highly variable among individuals, ranging from undetectable to over 1%57,58,59. In humans, iNKT cells are not highly enriched in the liver60,61, but they are enriched in the omentum, where they represent approximately 10% of T cells and, interestingly, are decreased in number in obesity62. In addition, a reduction in iNKT cell numbers in the peripheral blood has been correlated with several autoimmune or inflammatory conditions and cancers63. Whether decreased iNKT cell numbers in human peripheral blood are a cause or consequence of disease, or may in some cases simply reflect the recruitment of iNKT cells to inflamed tissues, is not known. Pathological states other than acute infection have only rarely demonstrated increased iNKT cell numbers, making the marked increase in both peripheral blood and lung iNKT cell populations that is seen in sickle cell disease particularly interesting64,65.
Microbial exposure can shape the iNKT cell compartment in early life. Germ-free mice were found to have increased iNKT cell numbers at mucosal sites, and this was dependent on CXC-chemokine ligand 16 (CXCL16)66. Reconstitution of the intestinal commensal microflora in these mice led to normalization of the iNKT cell compartment, but only when the commensals were introduced in the early neonatal period. Other interesting work has supported a role for commensal flora in shaping the iNKT cell compartment67, and specific lipid antigen-producing bacteria have been proposed to play a part in this process68,69,70. Viral infections that occur in early life may also alter the iNKT cell compartment; indeed, neonatal influenza virus infection leads to iNKT cell-dependent protection from adult airway hyperreactivity in a mouse model of asthma68. A recent study has shown that, compared with adult stem cells, fetal stem cells have a greater propensity to develop into iNKT cells. This provides an interesting possible mechanistic explanation for the distinct effects of early-life exposures on iNKT cells. The increased frequency of iNKT cells generated by fetal stem cells was shown to be mediated by the upregulation of promyelocytic leukaemia zinc finger protein (PLZF) and was dependent on LIN28B, a regulator of microRNAs71.
Innate-like mechanisms of iNKT cell activation
Lipid antigens for iNKT cells. The lipid antigens for iNKT cells identified so far fall almost exclusively into two basic categories: ceramide-based glycolipids (glycosphingolipids); and glycerol-based lipids (such as membrane phospholipids). For the ceramide-based prototypical iNKT cell lipid antigen αGalCer, the α refers to the orientation of the glycosidic linkage between the carbohydrate head group and the lipid backbone (Fig. 1). As mammals are not known to attach anomeric carbohydrates to lipids in the α-orientation, the α-linkage provides a potential foreign structural motif for antigenicity. Indeed, antigenic glycosphingolipids with α-linked glucuronic or galacturonic acid molecules have been found in Sphingomonas spp.72,73,74, and α-linked glucosyl or galactosyl diacyglycerols have been found in Borrelia burgdorferi75 and Streptococcus pneumoniae76 (Fig. 1). Other foreign lipid antigens have been proposed to be recognized by the iNKT cell TCR, including a cholesterol ester produced by Helicobacter pylori68, lipopeptidophosphoglycans from Leishmania donovani77 and Entamoeba histolytica78, and mycobacterial phosphatidylinositol mannoside79, but the antigenicity of these lipids is less well characterized. Although many investigators in the field had expected that these α-linked microbial lipid antigens might largely explain how iNKT cells are activated during infection, direct evidence for a significant contribution of such lipid antigens has remained elusive. Nevertheless, a contextual role for foreign lipid antigens is likely, and future study is warranted regarding their contribution to iNKT cell activation during infection.
iNKT cells are potently activated by antigen-presenting cells (APCs) that have been stimulated by Toll-like receptor (TLR) agonists in the absence of infection, and thus in the absence of pathogen-associated lipid antigens14,15,16,17,80. This activation still requires a signal from a lipid–CD1d complex, suggesting that lipid self antigens can contribute to iNKT cell activation during infection. Furthermore, iNKT cells are prominently activated during viral infections, cancer and autoimmune diseases, when foreign lipids are not present. These observations — along with the expectation that lipid self antigens are important for the development of iNKT cells in the thymus — led multiple groups to investigate the role of lipid self antigens in the activation of iNKT cells81. An initial approach for identifying lipid antigens that are presented by CD1d involved investigation of the iNKT cell compartment in the presence of genetic lesions that alter lipid metabolism. Through this approach, isoglobotrihexosylceramide (iGb3) (Fig. 1) was identified as a potential lipid self antigen based on the absence of iNKT cells in β-hexosaminidase B-deficient mice, which were predicted to have low iGb3 levels82. However, the significance of iGb3 as a self antigen in mice and humans has been called into question, as iGb3 synthase-deficient mice have a normal iNKT cell compartment83. Instead, the lack of iNKT cells in β-hexosaminidase B-deficient mice may be due to altered lysosomal function, as occurs in many lipid storage diseases84. In addition, iGb3 might not be antigenic for iNKT cells in most humans85, and humans do not express the synthase required to produce iGb3 (Ref. 86).
Another approach used to identify lipid self antigens that are recognized by iNKT cells involved the elution and characterization of the lipids bound to CD1d molecules at the cell surface. The lipids eluted from CD1d included glycosphingolipids, glycerol-based phospholipids and lysophospholipids87,88,89,90. From these experiments, it was clear that most lipids presented by CD1d molecules do not activate iNKT cells. However, one group unexpectedly identified an iNKT cell lipid self antigen among human-CD1d-presented lysophospholipids (which contain a single fatty-acid tail)87. This lipid class can accumulate in human inflammatory conditions in which phospholipases are activated. Among the lysophospholipids, lysophosphatidylcholine (lysoPC) (Fig. 1) was found to be antigenic for a subset of human iNKT cell clones. Indeed, stimulation with lysoPC led to iNKT cell secretion of granulocyte–macrophage colony-stimulating factor (GM-CSF), but not of other cytokines associated with the strong activation of iNKT cells, such as interferon-γ (IFNγ) and IL-4 (Ref. 91). LysoPC does not appear to activate mouse iNKT cells85,92.
As most of the CD1d-eluted lipids that were detected appeared not to be stimulatory for iNKT cells, a more direct approach was undertaken, which involved testing the activity of fractionated cellular lipids for their ability to stimulate iNKT cells. As they are the closest mammalian structural counterparts to αGalCer, β-linked galactose- and glucose-based mammalian glycosphingolipids were tested for their ability to activate iNKT cells. Synthetic βGalCer with a specific non-mammalian fatty-acid tail has been shown to stimulate iNKT cells93,94, but mice deficient in lipids based on β-linked galactose — including βGalCer, sulphatide and the gangliosides of the central nervous system — have no apparent abnormalities in their iNKT cell compartment95. However, cell lines deficient in β-glucose-based lipids had a defect in their ability to stimulate the self-reactivity of iNKT cells, suggesting that a β-glucosyl lipid may indeed be a self antigen95. Partial protection from concanavalin A-induced hepatitis (an iNKT cell-dependent disease model) was reported following the administration of β-glucosylceramide (βGlcCer), suggesting that βGlcCer can alter iNKT cell responses96. The ganglioside GD3, a β-glucosyl glycosphingolipid, was also reported to be antigenic for a small subset of iNKT cells97.
Recently, antigenic activity was directly demonstrated for mammalian βGlcCer, and the activity was found to be critically dependent on the specific fatty-acid chains incorporated (Fig. 1). When injected into mice, some βGlcCer species found in mammalian tissues potently activated iNKT cells. Importantly, antigenic βGlcCer species were shown to accumulate in APCs that were stimulated with TLR agonists, and these cells presented βGlcCer on CD1d molecules, promoting iNKT cell activation85. Thus, βGlcCer can serve as a danger signal that leads to the activation of iNKT cells during infection. This example suggests a theme for the recognition of lipid self antigens by iNKT cells: that simple β-linked self lipids may be recognized in a similar manner to their α-linked microbial counterparts, albeit with a lower potency. In fact, crystal structure analyses of lipid antigens in complex with CD1d and an iNKT cell TCR reveal a remarkable degree of similarity between the complexes formed by α- and β-linked lipids (see below). Whether βGlcCer is also involved in the thymic selection of iNKT cells is not known.
To identify lipid antigens that are involved in iNKT cell thymic selection, a lipid fractionation and activity-screening approach has been applied to thymic lipids. This recently led to the identification of plasmalogen lysophosphatidylethanolamine (plasmalogen lysoPE) (Fig. 1) — a glycerol-based lipid with a single fatty-acid chain attached through a vinyl ether linkage — as a potent iNKT cell antigen. Mice unable to generate lipids that contain vinyl ether linkages (known as plasmalogens) have reduced iNKT cell numbers, suggesting a role for this class of lipids in iNKT cell development98. The lipid self antigens involved in the selection of iNKT cells in the thymus may differ from those involved in iNKT cell activation in the periphery, and it will be interesting to see whether plasmalogens are also involved in the peripheral activation of iNKT cells.
For many of the self and microbial lipid antigens identified, specific structural features in either the head group or the fatty-acid tails distinguish these lipids from other lipids that are non-antigenic but sometimes more abundant (Fig. 1). How these structural features regulate antigenicity is not yet clear in all cases, but antigenicity may rely on efficient loading onto CD1d, a stable lipid–CD1d complex, and a particular topological orientation of CD1d or the lipid head group that results in recognition by the TCR99,100,101,102.
CD1d-presented lipid antigens recognized by the iNKT TCR. CD1d is a transmembrane protein that, like MHC class I, binds non-covalently to β2-microglobulin. The antigen-presenting surface of CD1d comprises the α1 and α2 helices, below which lie two deep hydrophobic channels, termed the A′ and F′ channels. The crystal structure of αGalCer-loaded CD1d revealed the two fatty-acid tails of αGalCer buried in the hydrophobic channels and the head-group moiety at the surface of CD1d, exposed to the aqueous environment and accessible for recognition by the iNKT cell TCR103,104. The trimolecular complex of CD1d, αGalCer and an iNKT cell TCR demonstrated a binding mode that is quite distinct from that adopted by MHC-restricted TCRs105 (Fig. 2a,b). In general, for the recognition of a peptide loaded in an MHC molecule, both the α- and β-chains of the TCR contact MHC α-helices, and the interface with the αβ TCR is situated such that the two hypervariable complementarity-determining region 3 (CDR3) loops of the TCR recognize the presented peptide. For the iNKT cell TCR, the TCR α–β axis is, remarkably, rotated into an orientation that is more parallel with the antigen-binding cleft of the antigen-presenting molecule compared with the alignment of MHC-restricted TCRs. Moreover, the TCR is shifted to the F′ channel end of CD1d, such that only the invariant TCR α-chain is positioned directly over the head group of the lipid antigen and the surrounding regions of CD1d. CDR1α and CDR3α bind to the lipid head-group moiety, and CDR3α also makes extensive contacts with the CD1d antigen-binding cleft, thus explaining the importance of the invariant TCR α-chain in lipid recognition. CDR2 and CDR3 of the TCR β-chain make stabilizing contacts with CD1d at a site distant from the lipid head group105, and the degree of contact between CD1d and the TCR β-chain modulates the affinity of a specific iNKT cell TCR106,107. Although stabilizing interactions provided by the lipid antigen are crucial for the binding of most iNKT cell TCRs, most of the surface area and energy supporting TCR binding depends on contacts between CD1d and the TCR α- and β-chains108. The binding of the iNKT cell TCR to CD1d and the presented αGalCer molecule requires minimal conformational change from the non-TCR-bound state, suggesting a 'lock and key' type of recognition (in which the TCR is the lock and the lipid–CD1d complex is the key)105.
a,b | The space-filling models show the structure of CD1d in complex with α-galactosylceramide (αGalCer) and the structure of an MHC class I molecule with a bound peptide. The footprints of the interacting T cell receptor (TCR) loops are highlighted. Note that for the invariant natural killer T (iNKT) cell TCR, interactions with the lipid–CD1d complex are mediated only through the complementarity-determining region 1 (CDR1) and CDR3 loops in the TCR α-chain. c,d | The ribbon diagrams show the regions of contact between the iNKT cell TCR and αGalCer or βGalCer. They demonstrate that the carbohydrate head groups of both lipids adopt similar orientations following TCR binding. e | The ribbon diagram shows a comparison between the positions of the β-linked carbohydrate head group in a 'free' sulphatide–CD1d complex and a TCR-bound βGalCer–CD1d complex, illustrating how the head group is flattened by the iNKT cell TCR during binding. f,g | The ribbon diagrams show the docking modes of TCRs from an iNKT cell and a diverse NKT (dNKT) cell on lipid-loaded CD1d molecules. The iNKT cell TCR docks on CD1d with a parallel binding mode (parallel to the long axis of the CD1d apical surface), whereas the dNKT cell TCR docks in a perpendicular manner, similarly to the docking of an αβ TCR on an MHC molecule. The images were modified, with permission, from Ref. 105 © (2007) Macmillan Publishers Ltd; Ref. 115 © (2011) Macmillan Publishers Ltd; and Ref. 119 © (2012) Macmillan Publishers Ltd. All rights reserved.
Recently, a relatively small population of iNKT cells expressing an alternative semi-invariant TCR that binds to αGalCer-loaded CD1d tetramers was identified in mice. These cells express Vα10Jα50 rather than Vα14Jα18. Although Vα10Jα50-expressing iNKT cells have a slightly different antigen preference from the more abundant Vα14Jα18-expressing subset, a TCR–αGlcCer–CD1d crystal structure using the Vα10Jα50 iNKT cell TCR revealed a very similar binding mode to that used by the Vα14Jα18 iNKT cell TCR109. Humans also possess iNKT cells that do not express a canonical Vα24Jα18 TCR α-chain110,111, and the binding mode of one such non-canonical TCR to αGalCer-loaded CD1d was again shown to be similar to that seen with the Vα24Jα18 TCR112.
A mutational analysis of iNKT cell TCR 'hotspots' suggested a similar mode of TCR recognition for multiple different α-linked lipid antigens, including a microbial α-linked glycosphingolipid113. Moreover, crystal structures of α-linked glycerol-based microbial lipid antigens from B. burgdorferi and S. pneumoniae in complex with CD1d and an iNKT cell TCR demonstrated a binding mode that is very similar to that observed with αGalCer. A comparison of TCR-bound and unbound lipid–CD1d complexes indicated that TCR binding results in a minor reorientation of the microbial α-linked sugar head group and partial closure of a CD1d α-helix over the F′ pocket, suggesting that a degree of 'induced fit' is required for these microbial antigens to adopt a binding mode similar to that seen with αGalCer99,114. Interestingly, two recent reports demonstrated that the recognition of β-linked lipids by the iNKT cell TCR results from the β-linked head group being flattened into a conformation similar to that naturally assumed by α-linked lipids. This suggests a dramatic induced-fit recognition model for β-linked self lipid antigens115,116. Importantly, independent of whether the lipid component is α-linked or β-linked, the final trimolecular complex of CD1d, lipid and TCR looks nearly identical (Fig. 2c,d,e). The structure of the trimolecular complex containing human CD1d with lysoPC and a reactive human iNKT cell TCR has also been determined, and although there are some differences in how lysoPC is recognized, as for β-linked glycosphingolipids the binding involves repositioning of the lipid head group so that it 'fits' the invariant TCR117. Recently, the crystal structure of a dNKT cell TCR in complex with a lipid antigen and CD1d has been solved and, in contrast to the iNKT cell TCR, this TCR adopts a binding mode similar to that of most MHC-restricted TCRs, with the CDR3β loop of the TCR contacting the lipid antigen118,119 (Fig. 2f,g). This finding demonstrates that the binding mode of the iNKT cell TCR is not dictated by CD1d. More importantly, it suggests that, in addition to facilitating the invariant TCR–lipid–CD1d recognition mode described above, CD1d can present structurally diverse lipids to diverse αβ TCRs for adaptive-type recognition in a manner analogous to peptide–MHC complex recognition. Indeed, dNKT cells have recently been shown to display distinct and partially overlapping reactivities to diverse microbial and self lipid antigens233.
Taken together, the identified lipid self antigens and these structural studies suggest that, rather than using diverse TCR binding strategies to recognize a range of lipid antigens, the iNKT cell TCR uses a single binding strategy with variable degrees of induced fit for different lipid antigens. Not all lipids can be induced to fit the iNKT cell TCR, and studies with an engineered, highly self-reactive TCR β-chain demonstrate that many CD1d-bound lipids, particularly those with bulky head groups, cannot be reoriented and thus block TCR binding106. An antigenic lipid must either fit or be induced to fit the invariant TCR, but the latter process incurs an energetic penalty. Thus, for iNKT cells, the structure of the lipid antigen largely regulates the strength, not the specificity or final binding mode, of the TCR–lipid–CD1d interaction. Rossjohn and colleagues have recently provided an excellent in-depth Review of the structural aspects of CD1d-restricted lipid antigen binding120.
Activation of iNKT cells by non-TCR signals. Cytokine-driven activation may be extremely important for the physiological functions of iNKT cells14,121, and combinations of TCR and cytokine stimuli synergize for robust iNKT cell activation121. Whereas a TCR signal and a co-stimulation signal are the two signals required for the activation of naive MHC-restricted T cells, a TCR signal and a cytokine signal are typically required for the activation of iNKT cells in physiological situations1 (Fig. 3). MHC-restricted T cells can also be influenced by pro-inflammatory cytokines, but their outright activation by cytokine signals generally requires prior TCR-mediated activation. In the steady state, subsets of iNKT cells express the receptors for, and can be activated by, IL-12 (Refs 14, 121), IL-18 (Refs 15, 122), IL-23 (Refs 45, 123) and IL-25 (Refs 124, 125).
Two signals are involved in the physiological activation of invariant natural killer T (iNKT) cells: a T cell receptor (TCR) signal provided by a lipid–CD1d complex; and a cytokine signal that depends on the constitutive expression of certain cytokine receptors by iNKT cells. The left panel shows activation by a strong foreign antigen, which is dominated by the TCR signal and has little dependence on antigen-presenting cell (APC)-derived cytokines that are generated in response to the stimulation of pattern-recognition receptors (PRRs). By contrast, the right panel shows cytokine-dominated iNKT cell activation. In this scenario, PRR-mediated activation of APCs leads to the generation of pro-inflammatory cytokines such as interleukin-12 (IL-12). For cytokine-mediated activation, a TCR signal is still required in most cases and can be provided by a low-affinity microbial lipid antigen or self lipid antigen. The relative contributions of TCR and cytokine signals to iNKT cell activation are likely to be context dependent.
The best-described cytokine mediator of iNKT cell activation is IL-12, and some bacterial and viral infections lead to sufficient production of IL-12 by APCs to activate iNKT cells in vivo, even in the absence of TCR engagement by CD1d14,126,234. The remarkable capacity of a subset of iNKT cells to respond to IL-12 is due to the high-level expression of functional IL-12 receptors at baseline. By contrast, IL-12 receptor expression is induced on MHC-restricted T cells only following TCR-mediated activation. Both in vitro and in vivo, APCs that cannot make IL-12 or recognize microbial patterns are unable to support the productive activation of iNKT cells in response to various bacteria and fungi, including microorganisms for which iNKT cell lipid antigens have been identified. This suggests that even though signalling through the TCR–CD1d interaction is required for efficient iNKT cell activation in most cases, there is a dominant role for cytokine-mediated activation during many infections127,128. Addressing a possible mechanism for the synergy between TCR and cytokine signals, a recent report demonstrated that weak TCR signals may enhance the responsiveness of iNKT cells to cytokines through histone modification at effector cytokine loci129. Taken together, these data support a model in which iNKT cell activation is the result of a combination of TCR and cytokine signals, both of which can be modulated and can contribute to iNKT cell activation during infection.
iNKT cells express multiple receptors that are characteristic of natural killer (NK) cells130, and they were initially named 'NK T cells' owing to their expression of the NK cell receptor NK1.1 (Ref. 131). Although the appropriateness of their name has been debated29, the prominent expression of both activating and inhibitory NK cell receptors on the surface of iNKT cells is likely to play an important part in both their activation and their regulation132. The ligand (or ligands) for NK1.1 is not known, but crosslinking NK1.1 with an antibody is sufficient to activate NK1.1+ T cells133. Most human iNKT cells express the activating NK cell receptor CD161, and a ligand for this receptor — lectin-like transcript 1 (LLT1; also known as CLEC2D) — is upregulated by activated leukocytes, including dendritic cells exposed to TLR agonists134. The activating NK cell receptor NKG2D recognizes multiple stress-induced ligands135, and NKG2D ligation can also contribute directly to iNKT cell activation136. Activating NK cell receptors provide another pathway (in addition to activation by cytokines) through which iNKT cells can respond to inflammation in the absence of a specific activating lipid antigen. Conversely, it is likely that inhibitory NK cell receptors can negatively regulate iNKT cell activation137,138, although the extent of such regulation and its role in disease are not known. iNKT cells also preferentially express other receptors that can mediate or regulate their activation, such as phosphatidylserine receptors that may lead to iNKT cell activation in the presence of apoptotic cells139 and adenosine receptors that have been shown to limit iNKT cell activation in ischaemia–reperfusion injury140,141.
Collectively, the insights gleaned from the study of lipid antigens, TCR binding and non-TCR-driven activation of iNKT cells suggest a dominantly innate strategy for antigen recognition and activation. Irrespective of whether the CD1d-presented lipid antigens are α-linked microbial antigens or danger-induced β-linked self antigens, the mode of recognition by the TCR is essentially the same, and different lipid antigens impart different signal strengths rather than different specificities. During infection, the TCR signal is complemented by what appears to be an equal or even stronger signal from pro-inflammatory cytokines for which iNKT cells constitutively express surface receptors. These cytokines, like lipid self antigens, are induced by danger signals, such as microbial patterns. Thus, iNKT cells are activated by the innate-like recognition of danger or of pro-inflammatory signals (Fig. 3). Importantly, this allows for their activation even in the absence of foreign antigen recognition, by virtually any microorganism or non-infectious state in which pathogen-associated molecular patterns or danger-associated molecular patterns are sensed by APCs.
Determining the outcome of iNKT cell activation
How can a relatively small population of lymphocytes with a nearly monospecific TCR repertoire have so many strong influences on immune responses? The answer to this lies in the contextual regulation of the multiple effector functions of activated iNKT cells and their rapid production of large amounts of many cytokines. The distinct mechanisms of iNKT cell activation can partially control the resulting effector functions. For example, the activation of iNKT cells with a potent lipid antigen leads to the production of both T helper 1 (TH1)- and TH2-type cytokines, whereas activation with IL-12 or TLR agonists leads to the production of IFNγ but not to TH2-type cytokine production14,15. In addition, different lipid antigens can influence not only the magnitude, but also the quality, of iNKT cell activation. αGalCer variants have been generated that skew iNKT cells to produce either TH1- or TH2-type cytokines102,142,143,144, and similar phenomena are likely to occur with endogenous antigens and physiologically relevant foreign antigens. The mechanisms by which different lipids lead to polarized iNKT cell activation involve CD1d-binding kinetics, the subcellular localization of lipid-loaded CD1d molecules and antigen targeting to different APCs101,116,145,146. Besides the differences in how iNKT cells are activated, multiple phenotypically distinct iNKT cell subsets have been identified. In addition, as iNKT cells orchestrate immune responses through their influence on other cell types, their localization and interactions with other cell types critically regulate the outcome of activation. How each of these factors affects the end result of iNKT cell activation is discussed below.
iNKT cell subsets
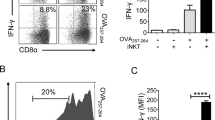
Rapid production of cytokines — including IFNγ, tumour necrosis factor (TNF), IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-13, IL-17, IL-21 and GM-CSF57,147 — is a major outcome of iNKT cell activation. In both humans and mice, there are CD4+ and CD4− iNKT cells, and the relative percentages of these populations vary widely among human individuals57,58,59. The expression of CD4 on human iNKT cells is a useful predictor of iNKT cells with the potential to generate TH2-type cytokines, but both CD4+ and CD4− subsets can make TH1-type cytokines57,58. A substantial portion of TH1-like CD4− human iNKT cells expresses CD8α, and a very small population expresses CD8αβ57,148. CD8α+ iNKT cells produce more IFNγ and are more cytotoxic than the CD4+ or CD4−CD8− subsets149.
Mouse iNKT cells do not seem to express CD8 in most circumstances, and CD4 expression only partially defines distinct iNKT cell subsets in mice147. Other cell-surface markers and transcription factors can, however, identify functionally distinct iNKT cell subsets in mice (Fig. 4). Two particularly useful surface markers that can be used in combination with CD4 to subdivide mouse iNKT cell populations are IL-17 receptor B (IL-17RB; a component of the IL-25 receptor) and NK1.1 (which is expressed by some inbred mouse strains). Just as the iNKT cell subset composition varies among humans, different mouse strains also have different subset compositions150. It should also be noted that there is currently no single group of markers for the identification of iNKT cell subsets and that the subsets described below represent a proposed classification system.
The figure compares three proposed major subsets of invariant natural killer T (iNKT) cells. Crucial transcription factors and surface markers associated with each subset are shown. These subsets are associated with the constitutive expression of cytokine receptors for interleukin-12 (IL-12), IL-23 and/or IL-25. The major cytokines produced by each subset and the tissues in which they are enriched are indicated. GATA3, GATA-binding protein 3; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFNγ, interferon-γ; PLZF, promyelocytic leukaemia zinc finger protein; RORγt, retinoic acid receptor-related orphan receptor-γt; TCR, T cell receptor; TH, T helper.
Subsets of MHC-restricted CD4+ T cells — such as TH1, TH2, TH17, regulatory T (TReg) and follicular helper T (TFH) cells — have been well described. Subpopulations of iNKT cells analogous to most of these subsets have been identified, and some of the same transcription factors that serve as 'master regulators' of MHC-restricted T cell subsets also operate in iNKT cell subsets. The eventual functionality of an individual iNKT cell is likely to be determined by modular transcriptional programmes, including those driven by PLZF in conjunction with T-bet, retinoic acid receptor-related orphan receptor-γt (RORγt), GATA-binding protein 3 (GATA3) and other transcription factors. Interestingly, iNKT cell subsets appear to acquire their identities during thymic development rather than as a result of peripheral experience151, although plasticity in iNKT cell subsets has not been adequately investigated.
'TH1-like' iNKT cells. Constituting the majority of iNKT cells in the mouse liver and spleen, TH1-like iNKT cells produce IFNγ, do not express IL-17RB and can have either a CD4+ or a CD4− phenotype125,151. IFNγ-producing iNKT cells are mostly NK1.1+, although a portion of NK1.1− iNKT cells can also make IFNγ147. These cells can produce some TH2-type cytokines, together with IFNγ, in response to strong TCR stimuli, but they make only TH1-type cytokines in response to IL-12 (Ref. 151). It is possible that under physiological activation conditions in which IL-12 is produced, these cells make only TH1-type cytokines. TH1-like iNKT cells express the TH1 cell-associated transcription factor T-bet. They also express CD122 (the β-chain of the IL-15 receptor) and require IL-15 for development151.
Dedicated 'TH2-like' iNKT cells. TH2-like iNKT cells are marked by the expression of IL-17RB and CD4, and they can produce IL-4, IL-9, IL-10 and IL-13 after activation. These cells can be activated by IL-25 and are enriched in the lungs, where they have been reported to contribute to TH2-type airway hyperreactivity in an IL-25-dependent manner124,125,151. TH2-like iNKT cells do not express T-bet, and they probably correspond to the few iNKT cells present in T-bet-deficient mice that are sufficient to mediate iNKT cell-mediated airway hyperreactivity152. At this point, no specific transcription factor has been uniquely associated with TH2-type cytokine production in iNKT cells. GATA3 — which regulates the production of TH2-type cytokines in MHC-restricted CD4+ T cells — is expressed by all iNKT cells, and is not expressed at higher levels by TH2-like iNKT cells151. Although GATA3 is required for efficient iNKT cell development, the few iNKT cells that develop in GATA3-deficient mice have a profound defect in the production of TH2-type cytokines153, and it is possible that GATA3 expression in the absence of T-bet or RORγt leads to the development of TH2-like iNKT cells. The transcription factor E4 promoter-binding protein 4 (E4BP4; also known as NFIL3) may have a role in the production of TH2-type cytokines by iNKT cells154, but this transcription factor is also required for TH17-type cytokine production by iNKT cells and thus is not specifically associated with TH2-like iNKT cells151.
'TH17-like' iNKT cells. The IL-17A-producing iNKT cells that resemble TH17 cells are mostly contained within the CD4−NK1.1− subpopulation45,123,155. IL-17A-producing iNKT cells have also been reported to have a CD4−IL-17RB− phenotype, and this population overlaps with the TH17-like CD4−NK1.1− subset151. Production of both IL-21 and IL-22 is reported to be limited to this population147,156. Like MHC-restricted TH17 cells, the TH17-like iNKT cell population expresses CC-chemokine receptor 6 (CCR6) and requires RORγt for development, but, in contrast to MHC-restricted TH17 cells, TH17-like iNKT cells develop as a distinct population in the thymus45,151,157. This population also expresses IL-23R and produces IL-17A in response to IL-23-mediated activation45,123,151. TH17-like iNKT cells are enriched in the peripheral lymph nodes, lungs and skin45,147,155. In the lungs, these cells can respond to microbial infection155 and contribute to a neutrophilic type of airway hyperreactivity158. Although IL-17-producing iNKT cells can be induced in vitro from peripheral human iNKT cells, evidence for a naturally occurring human TH17-like iNKT cell population is still limited159,160.
Other iNKT cell subsets. iNKT cell subsets analogous to other MHC-restricted CD4+ T cell populations — including TFH cells and possibly TReg cells — have also been identified. The production of IL-21 by a B cell lymphoma 6 (BCL-6)-dependent 'follicular helper' iNKT cell subset expressing programmed cell death protein 1 (PD1) and CXCR5 has been recently described161,162. Forkhead box P3 (FOXP3)-expressing iNKT cells have only been demonstrated following artificial induction in vitro163, but IL-10-producing iNKT cells (that are presumably FOXP3−) have been identified in various physiological and pathological conditions, including in adipose tissue53,54,55,56 and in the peripheral blood of patients with systemic lupus erythematosis (SLE)164.
iNKT cell interactions with other leukocytes
Cytokines, chemokines and surface molecules expressed by iNKT cells profoundly influence many other cell types. Furthermore, the interactions between iNKT cells and their CD1d-expressing cognate partners are at the core of the ability of iNKT cells to orchestrate immune responses, as outlined below (Fig. 5).
The presentation of self or foreign lipid antigens facilitates cognate interactions between invariant natural killer T (iNKT) cells and CD1d-expressing antigen-presenting cells (APCs), including dendritic cells (DCs), macrophages, neutrophils and B cells. This leads to iNKT cell activation and reciprocal APC activation or modulation. The major iNKT cell-derived cytokines that modulate the functions of the iNKT cell cognate partner during immune activation are shown. Both iNKT cell-derived factors and APC-derived factors can activate other cell types — such as natural killer (NK) cells and MHC-restricted T cells — in a CD1d-independent manner. For example, DCs activated by iNKT cells secrete interleukin-12 (IL-12) to transactivate NK cells, and they can also enhance the ensuing adaptive immune responses mediated by MHC-restricted T cells. CD40L, CD40 ligand; CXCL2, CXC-chemokine ligand 2; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFNγ, interferon-γ; IL-12R, IL-12 receptor; TCR, T cell receptor; TNF, tumour necrosis factor.
The iNKT cell–dendritic cell axis. Dendritic cells express CD1d constitutively and have been shown to be the major splenic population mediating iNKT cell activation in vivo following the intravenous introduction of soluble lipid antigens165,166,167. Similarly, in the lungs following aerosolized αGalCer exposure, both dendritic cells and macrophages can capture lipid antigens and present them to iNKT cells, although it is the pulmonary dendritic cells that carry the antigen to draining lymph nodes, where they can also present protein antigens to MHC-restricted T cells51. The presentation of lipid antigens to iNKT cells by dendritic cells leads to an iNKT cell response characterized by strong IFNγ production as well as NK cell transactivation143,145,166,168. This response is qualitatively different from that induced by antigens presented by other APCs. The phagocytosis of microorganisms or the uptake of lipid-binding proteins (such as low-density lipoprotein particles) can mediate the delivery of lipid antigens to dendritic cells169, and the targeting of lipid antigens to various APCs can critically depend on lipid structure and can alter the biological outcome145.
A major lesson learnt from the study of the interactions between iNKT cells and dendritic cells is that activation is bidirectional. During infection, signals from pattern-recognition receptors (PRRs) activate dendritic cells to produce IL-12 and upregulate their production of stimulatory lipid antigens for iNKT cells16,17,85. After an iNKT cell recognizes a cognate self or foreign lipid antigen displayed by CD1d on the surface of an APC, CD40–CD40 ligand interactions lead to further IL-12 production by the dendritic cell. Resting iNKT cells express the IL-12 receptor127, and its expression is further upregulated in response to dendritic cell-derived IL-12, which together with dendritic cell-expressed ligands for co-stimulatory receptors contributes to iNKT cell activation121,166. This dendritic cell–iNKT cell interaction then leads to NK cell transactivation170, enhanced responses to protein antigens by MHC-restricted CD4+ and CD8+ T cells171,172, and the licensing of dendritic cell cross-presentation173. Thus, through bidirectional interactions, iNKT cells and dendritic cells cooperate to amplify and direct ensuing innate and adaptive immune responses. This ability of activated iNKT cells to promote the development of adaptive T cell responses has important clinical implications. Indeed, lipid antigens recognized by iNKT cells (such as αGalCer) have been shown to perform effectively as vaccine adjuvants for the generation of protective T cell immunity to co-administered protein antigens174,175.
The iNKT cell–B cell axis. iNKT cells are well equipped to influence B cell function, as they can produce IL-4, IL-5, IL-6, IL-13 and IL-21 (Ref. 147) and also express CD40 ligand30. Marginal-zone B cells express the highest CD1d levels of any splenic APC20, but whether these cells contribute to iNKT cell activation in disease remains unclear165,176,177. iNKT cells can provide cognate B cell help178; this occurs when the iNKT cell stimulatory lipid antigen is linked to a specific B cell epitope. The B cell then recognizes the antigen complex and internalizes it, allowing the stimulatory lipid to be presented by CD1d on the same B cell. Recently, a specialized iNKT cell population with characteristics of TFH cells was described that produces IL-21, requires BCL-6 and can provide CD1d-dependent cognate B cell help. Help for B cells from this iNKT cell population results in rapid immunoglobulin production and some affinity maturation, but does not lead to the generation of long-term B cell memory161,162. Interestingly, a human iNKT cell population with surface markers suggestive of follicular helper function was also identified in tonsils161, although whether these cells provide cognate help to B cells remains to be determined. These findings suggest that cognate help from iNKT cells may have a role in enhancing early IgM and IgG responses during infection, but not in generating recall responses.
In the studies cited above, lipid antigens were designed to enable iNKT cells to provide cognate help to antigen-specific B cells. However, when αGalCer is co-administered with (but not directly conjugated to) a protein antigen, so that lipid recognition by iNKT cells can occur separately from antigen recognition by B cells, iNKT cells can still provide efficient B cell help179,180,181. Indeed, when activated in an inflammatory milieu, iNKT cells can provide such non-cognate B cell help182, and this does not require germinal centre localization or SLAM-associated protein (SAP)183. Thus, the possibility of using αGalCer or other iNKT cell-activating lipids184 as vaccine adjuvants aimed at enhancing antibody production is an appealing opportunity. Two informative reviews have recently covered the targeting of iNKT cells in vaccine strategies185,186.
Recent data suggests that B cells may regulate iNKT cells both during homeostatic conditions and during chronic inflammation. In several autoimmune disorders, patients have decreased numbers of peripheral iNKT cells compared with healthy controls3. The relationship between iNKT cells and B cells has recently been more closely investigated in humans with SLE. Patients with active SLE had low numbers of iNKT cells with reduced levels of IFNγ and TNF production, but increased IL-10 production, compared with healthy controls. These characteristics were correlated with the presence of 'defective' B cells that had altered CD1d expression and a reduced ability to stimulate iNKT cells. Interestingly, the iNKT cell parameters were normalized and 'healthy' B cells re-emerged in patients who responded to therapy. This study suggests the possibility that CD1d-dependent interactions between B cells and iNKT cells have a role in the regulation of iNKT cell homeostasis164.
The iNKT cell–macrophage axis. Some APC populations are involved in lipid antigen presentation in an organ-specific manner. The liver is highly enriched for iNKT cells and is an important site for the recognition and clearance of blood-borne pathogens, including those that have entered from the alimentary system. In the liver, Kupffer cells and stellate cells, both of which are specialized macrophage populations, can present lipid antigens to iNKT cells187,188. Using intravital microscopy, Kupffer cells have been shown to play a key part in iNKT cell activation and bacterial clearance in liver sinusoids following infection with B. burgdorferi, a microorganism known to produce an α-linked lipid antigen for iNKT cells48. In addition to having a crucial role in the liver, macrophages also can mediate iNKT cell activation in lymph nodes, where it was shown that CD169+ subcapsular sinus macrophages — a population known to take up and present particulate antigens — can present lipids derived from particulate antigens to iNKT cells residing in the paracortex189.
In a bidirectional manner, activated iNKT cells can alter macrophage phenotypes in both beneficial and harmful ways. iNKT cell activation can improve the course of pulmonary infections with Pseudomonas aeruginosa or Mycobacterium tuberculosis, in which iNKT cell-derived IFNγ can lead to enhanced phagocytosis and bacterial clearance by pulmonary macrophages190,191. Following common viral infections, human allergic asthma often worsens, and symptoms can persist for weeks. In a mouse model of post-viral airway disease, iNKT cells were shown to contribute to chronic lung disease by inducing the differentiation of M2 macrophages192, providing an example of harmful macrophage regulation. The production of GM-CSF by iNKT cells may alter the functional capacity of blood monocytes, shifting them towards a more dendritic cell-like phenotype193,194. In addition, iNKT cells have been shown to contribute to the low degree of adipose inflammation in the lean state by polarizing macrophages to an M2 phenotype, a function that is lost in obesity52,53,54. Finally, iNKT cells may control tumour growth in part by killing or altering the phenotype of tumour-associated macrophages, a cell population that supports the growth of some neoplasms5,195.
The iNKT cell–granulocyte axis. As rapid responders to infection, iNKT cells and neutrophils share functional duties. It is not surprising therefore that these two cell types also interact. During some infections (including pulmonary infections with S. pneumoniae or P. aeruginosa), iNKT cells have a key role in recruiting neutrophils to the infected tissue, for example by secreting CXCL2 (also known as MIP2)190,196. iNKT cells have also been shown to regulate neutrophil recruitment during ischaemia–reperfusion injury140,197, during ozone exposure158 and following the administration of aerosolized lipid antigens155.
Cognate interactions between iNKT cells and neutrophils lead to both iNKT cell activation and reciprocal neutrophil regulation. The acute-phase reactant protein serum amyloid A1 (SAA1) promotes the development of IL-10-producing immunosuppressive neutrophils. iNKT cells that interact with SAA1-exposed neutrophils can reverse the suppressive phenotype of these neutrophils and promote IL-12 production. This ability of iNKT cells to switch neutrophils from a regulatory to a pro-inflammatory phenotype provides another mechanism whereby iNKT cells may contribute to antitumour responses or to microbial defence198. In some situations, neutrophils may suppress iNKT cell cytokine production through an undefined contact-dependent mechanism, thereby limiting their ongoing contribution to inflammation199.
Summary: putting the pieces back together
In the sections above, we have deconstructed many of the key aspects of iNKT cell activation and their effector functions. During pathological processes, however, these aspects of iNKT cell biology are inextricably interconnected. To reconstruct the aspects of iNKT cell biology discussed above, we take bacterial and viral pulmonary infections as examples to illustrate how iNKT cells can influence disease outcomes.
Bacterial infection. In S. pneumoniae infection of the lungs, iNKT cells have a major role127,196. A summary of the major iNKT cell-regulated control points in S. pneumoniae infection is shown in Fig. 6. Before the onset of infection, the iNKT cell development process results in the generation of 'poised effector' tissue-resident iNKT cells. Both the size and tissue localization of the iNKT cell compartment — including the CXCL16–CXCR6-mediated localization of iNKT cells in the lungs — is regulated in part by the commensal microflora66. During S. pneumoniae infection, inhaled bacteria are engulfed by APCs (such as dendritic cells and macrophages), and this could lead to the presentation of an antigenic microbial lipid, namely α-glucosyldiacylglycerol (αGlcDAG), by CD1d molecules76. PRR-mediated activation of dendritic cells leads to the synthesis of lipid self antigens (such as βGlcCer)85 and to the secretion of IL-12 (Ref. 127). The synergistic effect of IL-12 and TCR-mediated stimulation by self and/or foreign lipid antigens leads to the robust activation of resident and newly recruited iNKT cells, a process that occurs during the first few days of infection127,196. The secretion of IL-12 by dendritic cells in conjunction with IFNγ production by iNKT cells may lead to the transactivation of NK cells145,168,170. Activated iNKT cells, including the TH17-like iNKT cell subset, can recruit neutrophils to the lungs by producing CXCL2 and/or IL-17A155,190,196. iNKT cell-derived IFNγ also activates macrophages, a process that is crucial for microbial clearance190,191. Activated iNKT cells also reciprocally modulate the functions of dendritic cells, which can then promote adaptive MHC-restricted T cell responses171,172. Subsequently, activated MHC-restricted T cells and specialized follicular helper iNKT cells can provide help for B cells in the presence of foreign lipid antigens161,162. Thus, iNKT cell activation contributes to the innate control of S. pneumoniae as well as to protective antibody responses200.
The figure shows a summary of the aspects of invariant natural killer T (iNKT) cell biology that determine the influence of these cells on the immune response during infection with Streptococcus pneumoniae. a | iNKT cells are selected in the thymus by self lipid antigens presented on CD1d by double-positive (DP) thymocytes. As a result of positive selection, iNKT cells express the transcription factor promyelocytic leukaemia zinc finger protein (PLZF) and leave the thymus as 'poised effectors'. b | iNKT cells exhibit subset-specific tissue distribution patterns that contribute to their functional potential. The homing and tissue retention of iNKT cell subsets is controlled in part by adhesion receptors, such as lymphocyte function-associated antigen 1 (LFA1), and also by chemokines, such as CXC-chemokine ligand 16 (CXCL16), and is regulated in part during early life by the commensal microflora. In addition to the existence of tissue-resident iNKT cells, other iNKT cells can be recruited to the tissues during immune responses. c | Following exposure to a microorganism such as S. pneumoniae, an antigen-presenting cell (APC) can present both self and foreign lipid antigens. The activation of APCs by microbial products leads to the production of self lipid antigens and pro-inflammatory cytokines such as interleukin-12 (IL-12). Lipid antigens and pro-inflammatory cytokines mediate the reciprocal activation of iNKT cells and APCs, as described in Fig. 5. d | Activated iNKT cells produce interferon-γ (IFNγ), IL-4, IL-13, IL-17A, other cytokines and chemokines, all of which promote the recruitment and activation of both innate and adaptive immune cell types. Cytokines produced by the reciprocal activation of iNKT cells and dendritic cells can transactivate other innate immune cell types, including macrophages. e | Innate immune cells such as macrophages and neutrophils that have been recruited by iNKT cell-derived cytokines and chemokines promote the early innate clearance of the pathogen. APCs that have been activated through their interactions with iNKT cells, including by CD40–CD40 ligand (CD40L) interactions, initiate antigen-specific adaptive lymphocyte responses and protective immunity. IFNGR, IFNγ receptor; IL-12R, IL-12 receptor; PRR, pattern-recognition receptor; TCR, T cell receptor.
Viral infection. Another instructive example of the role of iNKT cells is influenza virus infection. Studies in CD1d-deficient animals indicate that iNKT cells contribute to the control of influenza virus infection of the lungs201,202, and exogenous iNKT cell activation with αGalCer can improve the outcome of influenza virus infection and promote the generation of protective antibodies203,204. As is the case during bacterial infection of the lungs, iNKT cells may also facilitate the recruitment and activation of other innate and adaptive immune cells that promote viral clearance. However, the crucial cognate partner for iNKT cells during influenza virus infection has been proposed to be the myeloid-derived suppressor cell (MDSC), and iNKT cell activation in this case is probably dependent on CD1d and the PRR-mediated synthesis of stimulatory lipid self antigens. MDSCs are recruited to the lungs early during infection, and they can suppress CD8+ T cells that are crucial for the clearance of influenza virus. Through cognate interactions with MDSCs, iNKT cells prevent the MDSC-mediated suppression of CD8+ T cells201. iNKT cells may also be beneficial during the resolution of infection, and they have been proposed to protect the lungs from inflammatory damage through IL-22 production156 or cytotoxicity205.
iNKT cells can also have harmful effects following viral infection in the lungs. After Sendai virus infection, the pulmonary TH2-like iNKT cell subset can be activated, a process that leads to prolonged airway hyperreactivity as a result of the polarization of macrophages to an M2 phenotype by iNKT cell-derived IL-13 (Ref. 192). Early-life viral infection can also influence the subsequent development of allergen-induced airway hyperreactivity by altering the functional phenotype of the iNKT cell compartment. Although iNKT cells are required for allergen-induced airway hyperreactivity206,207, and αGalCer is sufficient to induce this pathology in adult animals208, early-life influenza virus infection was shown to be associated with iNKT cell-dependent suppression of airway hyperreactivity, possibly through the induction of regulatory T cells68.
iNKT cells have a pivotal role in shaping both innate and adaptive immune responses that occur during infection, autoimmune disease, allergy and cancer. The examples above illustrate how, in different situations, iNKT cells in a single organ can lead to varied pathologies, underscoring how the functional roles of iNKT cells in disease processes rely on several factors, including the inflammatory milieu, the lipid antigen(s) involved, the iNKT cell subset(s) involved and the APC(s) with which the iNKT cells interact. The relative contribution of each of these factors is likely to be context dependent, and defining the disease-specific balance of these factors presents a significant and exciting ongoing challenge. Unravelling the mechanisms of iNKT cell activation is not only crucial for understanding iNKT cell biology; it will also ultimately determine the success of targeting iNKT cells for therapeutic applications.
References
Brigl, M. & Brenner, M. B. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin. Immunol. 22, 79–86 (2010).
Tupin, E., Kinjo, Y. & Kronenberg, M. The unique role of natural killer T cells in the response to microorganisms. Nature Rev. Microbiol. 5, 405–417 (2007).
Novak, J. & Lehuen, A. Mechanism of regulation of autoimmunity by iNKT cells. Cytokine 53, 263–270 (2011).
Meyer, E. H. DeKruyff, R. H. & Umetsu, D. T. iNKT cells in allergic disease. Curr. Top. Microbiol. Immunol. 314, 269–291 (2007).
Vivier, E., Ugolini, S., Blaise, D., Chabannon, C. & Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nature Rev. Immunol. 12, 239–252 (2012).
Bendelac, A., Savage, P. B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Brigl, M. & Brenner, M. B. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 (2004).
Cohen, N. R., Garg, S. & Brenner, M. B. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv. Immunol. 102, 1–94 (2009).
Godfrey, D. I. & Kronenberg, M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 (2005).
Matsuda, J. L., Mallevaey, T., Scott-Browne, J. & Gapin, L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr. Opin. Immunol. 20, 358–368 (2008).
Godfrey, D. I. & Berzins, S. P. Control points in NKT-cell development. Nature Rev. Immunol. 7, 505–518 (2007).
Stetson, D. B. et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 (2003). This study shows that the constitutive production of cytokine-encoding mRNAs enables iNKT cells to rapidly secrete large amounts of cytokines following activation.
Brigl, M., Bry, L., Kent, S. C., Gumperz, J. E. & Brenner, M. B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nature Immunol. 4, 1230–1237 (2003). This study defined how iNKT cell reactivity to self lipids combined with the release of pro-inflammatory cytokines from APCs results in iNKT cell activation during infection.
Nagarajan, N. A. & Kronenberg, M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 178, 2706–2713 (2007).
Paget, C. et al. Activation of invariant NKT cells by Toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 (2007).
Salio, M. et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl Acad. Sci. USA 104, 20490–20495 (2007). References 16 and 17 show that the enhanced presentation of stimulatory self lipid antigens by APCs in response to microbial stimulation contributes to iNKT cell activation.
Cohen, N. R. et al. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nature Immunol. 14, 90–99 (2013).
Brossay, L. et al. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J. Immunol. 159, 1216–1224 (1997).
Roark, J. H. et al. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J. Immunol. 160, 3121–3127 (1998).
Beckman, E. M. et al. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372, 691–694 (1994). This study was the first to show that CD1 molecules present lipids as antigens.
Porcelli, S., Morita, C. T. & Brenner, M. B. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature 360, 593–597 (1992).
Dellabona, P., Padovan, E., Casorati, G., Brockhaus, M. & Lanzavecchia, A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J. Exp. Med. 180, 1171–1176 (1994).
Lantz, O. & Bendelac, A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180, 1097–1106 (1994).
Porcelli, S., Yockey, C. E., Brenner, M. B. & Balk, S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- α/β T cells demonstrates preferential use of several V β genes and an invariant TCR α chain. J. Exp. Med. 178, 1–16 (1993).
Bendelac, A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995).
Bendelac, A. et al. CD1 recognition by mouse NK1+ T lymphocytes. Science 268, 863–865 (1995).
Exley, M., Garcia, J., Balk, S. P. & Porcelli, S. Requirements for CD1d recognition by human invariant Vα24+ CD4-CD8- T cells. J. Exp. Med. 186, 109–120 (1997).
Godfrey, D. I., MacDonald, H. R., Kronenberg, M., Smyth, M. J. & Van Kaer, L. NKT cells: what's in a name? Nature Rev. Immunol. 4, 231–237 (2004). This Review provides an excellent summary and timeline of the early discoveries that defined the iNKT cell field and describes the different NKT cell subsets.
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997). This report identified α GalCer as the first lipid antigen for iNKT cells.
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Matsuda, J. L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000). References 31 and 32 first described the use of α GalCer-loaded CD1d tetramers for the specific identification of mouse iNKT cells.
Dascher, C. C. & Brenner, M. B. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol. 24, 412–418 (2003).
Brossay, L. et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188, 1521–1528 (1998).
Behar, S. M., Podrebarac, T. A., Roy, C. J., Wang, C. R. & Brenner, M. B. Diverse TCRs recognize murine CD1. J. Immunol. 162, 161–167 (1999).
Cardell, S. et al. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 182, 993–1004 (1995).
Park, S. H., Roark, J. H. & Bendelac, A. Tissue-specific recognition of mouse CD1 molecules. J. Immunol. 160, 3128–3134 (1998).
Arrenberg, P., Halder, R., Dai, Y., Maricic, I. & Kumar, V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a β-linked self-glycolipid. Proc. Natl Acad. Sci. USA 107, 10984–10989 (2010).
Park, S. H. et al. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 193, 893–904 (2001).
Blomqvist, M. et al. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur. J. Immunol. 39, 1726–1735 (2009).
Jahng, A. et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199, 947–957 (2004).
Johnston, B., Kim, C. H., Soler, D., Emoto, M. & Butcher, E. C. Differential chemokine responses and homing patterns of murine TCR αβ NKT cell subsets. J. Immunol. 171, 2960–2969 (2003).
Kim, C. H., Johnston, B. & Butcher, E. C. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+Vβ11+ NKT cell subsets with distinct cytokine-producing capacity. Blood 100, 11–16 (2002).
Thomas, S. Y. et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J. Immunol. 171, 2571–2580 (2003).
Doisne, J. M. et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor γt+ and respond preferentially under inflammatory conditions. J. Immunol. 183, 2142–2149 (2009).
Thomas, S. Y. et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1–ICAM-1 interactions. J. Exp. Med. 208, 1179–1188 (2011). This study shows that peripheral iNKT cells are mainly tissue resident and are retained locally owing to constitutive LFA1–ICAM1 interactions as a result of PLZF expression.
Geissmann, F. et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3, e113 (2005).
Lee, W. Y. et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nature Immunol. 11, 295–302 (2010).
Velazquez, P. et al. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J. Immunol. 180, 2024–2028 (2008).
Wong, C. H., Jenne, C. N., Lee, W. Y., Leger, C. & Kubes, P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105 (2011). This study shows that the activation of iNKT cells by noradrenergic transmitters contributes to systemic immunosuppression following stroke.
Scanlon, S. T. et al. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J. Exp. Med. 208, 2113–2124 (2011).
Ji, Y. et al. Short-term high-fat-diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J. Biol. Chem. 287, 24378–24386 (2012).
Ji, Y. et al. Activation of natural killer T cells promotes M2 macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J. Biol. Chem. 287, 13561–13571 (2012).
Lynch, L. et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37, 574–587 (2012).
Schipper, H. S. et al. Natural killer T cells in adipose tissue prevent insulin resistance. J. Clin. Invest. 122, 3343–3354 (2012).
Wu, L. et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl Acad. Sci. USA 109, e1143–e1152 (2012). References 52–56 describe the role of iNKT cells in diet-induced obesity and the metabolic syndrome.
Gumperz, J. E., Miyake, S., Yamamura, T. & Brenner, M. B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195, 625–636 (2002).
Lee, P. T., Benlagha, K., Teyton, L. & Bendelac, A. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 195, 637–641 (2002). References 57 and 58 describe the use of α GalCer-loaded CD1d tetramers for the characterization of human iNKT cells.
Montoya, C. J. et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 122, 1–14 (2007).
Kenna, T. et al. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J. Immunol. 171, 1775–1779 (2003).
Kita, H. et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology 123, 1031–1043 (2002).
Lynch, L. et al. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 39, 1893–1901 (2009).
Berzins, S. P., Smyth, M. J. & Baxter, A. G. Presumed guilty: natural killer T cell defects and human disease. Nature Rev. Immunol. 11, 131–142 (2011).
Field, J. J., Nathan, D. G. & Linden, J. Targeting iNKT cells for the treatment of sickle cell disease. Clin. Immunol. 140, 177–183 (2011).
Wallace, K. L. et al. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-γ and CXCR3 chemokines. Blood 114, 667–676 (2009).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012). This study describes a prominent role for the intestinal microbiota in iNKT cell development and function.
Hansen, C. H. et al. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE 7, e34043 (2012).
Chang, Y. J. et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J. Clin. Invest. 121, 57–69 (2011).
Wei, B. et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 184, 1218–1226 (2010).
Wingender, G. et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 143, 293–296 (2012).
Yuan, J., Nguyen, C. K., Liu, X., Kanellopoulou, C. & Muljo, S. A. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335, 1195–1200 (2012).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Sriram, V., Du, W., Gervay-Hague, J. & Brutkiewicz, R. R. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J. Immunol. 35, 1692–1701 (2005).
Kinjo, Y. et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nature Immunol. 7, 978–986 (2006).
Kinjo, Y. et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nature Immunol. 12, 966–974 (2011). References 72–76 describe microbial glycolipid antigens that stimulate most (if not all) iNKT cells.
Amprey, J. L. et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200, 895–904 (2004).
Lotter, H. et al. Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoeba histolytica are critically important to control amebic liver abscess. PLoS Pathog. 5, e1000434 (2009).
Fischer, K. et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl Acad. Sci. USA 101, 10685–10690 (2004).
Darmoise, A. et al. Lysosomal α-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity 33, 216–228 (2010).
Gapin, L. iNKT cell autoreactivity: what is 'self' and how is it recognized? Nature Rev. Immunol. 10, 272–277 (2010).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Porubsky, S. et al. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl Acad. Sci. USA 104, 5977–5982 (2007).
Gadola, S. D. et al. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J. Exp. Med. 203, 2293–2303 (2006).
Brennan, P. J. et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nature Immunol. 12, 1202–1211 (2011). This report characterized β GlcCer as a danger-induced self lipid antigen for iNKT cells.
Christiansen, D. et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 6, e172 (2008).
Cox, D. et al. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE 4, e5325 (2009).
Haig, N. A. et al. Identification of self-lipids presented by CD1c and CD1d proteins. J. Biol. Chem. 286, 37692–37701 (2011).
Muindi, K. et al. Activation state and intracellular trafficking contribute to the repertoire of endogenous glycosphingolipids presented by CD1d. Proc. Natl Acad. Sci. USA 107, 3052–3057 (2010).
Yuan, W., Kang, S. J., Evans, J. E. & Cresswell, P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J. Immunol. 182, 4784–4791 (2009).
Fox, L. M. et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 7, e1000228 (2009).
Pei, B. et al. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J. Immunol. 186, 1348–1360 (2011).
Ortaldo, J. R. et al. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J. Immunol. 172, 943–953 (2004).
Parekh, V. V. et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J. Immunol. 173, 3693–3706 (2004).
Stanic, A. K. et al. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by β-D-glucosylceramide synthase deficiency. Proc. Natl Acad. Sci. USA 100, 1849–1854 (2003).
Margalit, M. et al. Glucocerebroside treatment ameliorates ConA hepatitis by inhibition of NKT lymphocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G917–G925 (2005).
Wu, D. Y., Segal, N. H., Sidobre, S., Kronenberg, M. & Chapman, P. B. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198, 173–181 (2003).
Facciotti, F. et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nature Immunol. 13, 474–480 (2012). This study describes peroxisome-derived plasmalogens as self antigens that stimulate iNKT cells in the thymus and thereby contribute to iNKT cell development.
Girardi, E. et al. Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cells. PLoS Biol. 9, e1001189 (2011).
McCarthy, C. et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J. Exp. Med. 204, 1131–1144 (2007).
Wun, K. S. et al. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity 34, 327–339 (2011).
Yu, K. O. et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc. Natl Acad. Sci. USA 102, 3383–3388 (2005).
Koch, M. et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nature Immunol. 6, 819–826 (2005).
Zajonc, D. M. et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nature Immunol. 6, 810–818 (2005).
Borg, N. A. et al. CD1d–lipid–antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448, 44–49 (2007). This study describes the structure of the trimolecular TCR– α GalCer–CD1d complex and defines the unique mode of antigen recognition by iNKT cells, which is distinct from the recognition of peptide–MHC complexes.
Mallevaey, T. et al. A molecular basis for NKT cell recognition of CD1d–self-antigen. Immunity 34, 315–326 (2011).
Matulis, G. et al. Innate-like control of human iNKT cell autoreactivity via the hypervariable CDR3β loop. PLoS Biol. 8, e1000402 (2010).
Wun, K. S. et al. A minimal binding footprint on CD1d–glycolipid is a basis for selection of the unique human NKT TCR. J. Exp. Med. 205, 939–949 (2008).
Uldrich, A. P. et al. A semi-invariant Vα10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nature Immunol. 12, 616–623 (2011).
Brigl, M. et al. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J. Immunol. 176, 3625–3634 (2006).
Gadola, S. D., Dulphy, N., Salio, M. & Cerundolo, V. Vα24-JαQ-independent, CD1d-restricted recognition of α-galactosylceramide by human CD4+ and CD8αβ+ T lymphocytes. J. Immunol. 168, 5514–5520 (2002).
Lopez-Sagaseta, J., Kung, J. E., Savage, P. B., Gumperz, J. & Adams, E. J. The molecular basis for recognition of CD1d/α-galactosylceramide by a human non-Vα24 T cell receptor. PLoS Biol. 10, e1001412 (2012).
Scott-Browne, J. P. et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nature Immunol. 8, 1105–1113 (2007).
Li, Y. et al. The Vα14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J. Exp. Med. 207, 2383–2393 (2010).
Pellicci, D. G. et al. Recognition of β-linked self glycolipids mediated by natural killer T cell antigen receptors. Nature Immunol. 12, 827–833 (2011).
Yu, E. D., Girardi, E., Wang, J. & Zajonc, D. M. Cutting edge: structural basis for the recognition of β-linked glycolipid antigens by invariant NKT cells. J. Immunol. 187, 2079–2083 (2011). References 115 and 116 show how β -linked self lipids can be recognized by the iNKT cell TCR.
Lopez-Sagaseta, J., Sibener, L. V., Kung, J. E., Gumperz, J. & Adams, E. J. Lysophospholipid presentation by CD1d and recognition by a human natural killer T-cell receptor. EMBO J. 31, 2047–2059 (2012).
Girardi, E. et al. Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nature Immunol. 13, 851–856 (2012).
Patel, O. et al. Recognition of CD1d–sulfatide mediated by a type II natural killer T cell antigen receptor. Nature Immunol. 13, 857–863 (2012).
Rossjohn, J., Pellicci, D. G., Patel, O., Gapin, L. & Godfrey, D. I. Recognition of CD1d-restricted antigens by natural killer T cells. Nature Rev. Immunol. 12, 845–857 (2012).
Kitamura, H. et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189, 1121–1128 (1999). This study demonstrated bidirectional activation between iNKT cells and APCs.
Leite-De-Moraes, M. C. et al. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J. Immunol. 163, 5871–5876 (1999).
Rachitskaya, A. V. et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180, 5167–5171 (2008).
Stock, P., Lombardi, V., Kohlrautz, V. & Akbari, O. Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J. Immunol. 182, 5116–5122 (2009).
Terashima, A. et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 205, 2727–2733 (2008).
Wesley, J. D., Tessmer, M. S., Chaukos, D. & Brossay, L. NK cell-like behavior of Vα14i NK T cells during MCMV infection. PLoS Pathog. 4, e1000106 (2008).
Brigl, M. et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208, 1163–1177 (2011).
Cohen, N. R. et al. Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe 10, 437–450 (2011).
Wang, X. et al. Human invariant natural killer T cells acquire transient innate responsiveness via histone H4 acetylation induced by weak TCR stimulation. J. Exp. Med. 209, 987–1000 (2012). This study describes how stimulation with self lipid antigens alters the acetylation of cytokine gene loci, enabling iNKT cell responsiveness to stimulation with IL-12 and IL-18.
Bendelac, A. Mouse NK1+ T cells. Curr. Opin. Immunol. 7, 367–374 (1995).
Makino, Y., Kanno, R., Ito, T., Higashino, K. & Taniguchi, M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int. Immunol. 7, 1157–1161 (1995).
Skold, M. & Cardell, S. Differential regulation of Ly49 expression on CD4+ and CD4−CD8− (double negative) NK1.1+ T cells. Eur. J. Immunol. 30, 2488–2496 (2000).
Arase, H., Arase, N. & Saito, T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 183, 2391–2396 (1996).
Germain, C. et al. Induction of lectin-like transcript 1 (LLT1) protein cell surface expression by pathogens and interferon-γ contributes to modulate immune responses. J. Biol. Chem. 286, 37964–37975 (2011).
Champsaur, M. & Lanier, L. L. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 235, 267–285 (2010).
Kuylenstierna, C. et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur. J. Immunol. 41, 1913–1923 (2011).
Maeda, M., Lohwasser, S., Yamamura, T. & Takei, F. Regulation of NKT cells by Ly49: analysis of primary NKT cells and generation of NKT cell line. J. Immunol. 167, 4180–4186 (2001).
Skold, M. et al. MHC-dependent and -independent modulation of endogenous Ly49 receptors on NK1.1+ T lymphocytes directed by T-cell receptor type. Immunology 110, 313–321 (2003).
Lee, H. H. et al. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyperreactivity. J. Immunol. 185, 5225–5235 (2010).
Lappas, C. M., Day, Y. J., Marshall, M. A., Engelhard, V. H. & Linden, J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J. Exp. Med. 203, 2639–2648 (2006).
Nowak, M. et al. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur. J. Immunol. 40, 682–687 (2010).
Miyamoto, K., Miyake, S. & Yamamura, T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534 (2001).
Schmieg, J., Yang, G., Franck, R. W. & Tsuji, M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J. Exp. Med. 198, 1631–1641 (2003).
Sullivan, B. A. et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J. Immunol. 184, 141–153 (2010).
Bai, L. et al. Distinct APCs explain the cytokine bias of α-galactosylceramide variants in vivo. J. Immunol. 188, 3053–3061 (2012).
Im, J. S. et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity 30, 888–898 (2009). This study shows that the manner in which different lipids are loaded onto CD1d can determine the outcome of iNKT cell activation.
Coquet, J. M. et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4−NK1.1− NKT cell population. Proc. Natl Acad. Sci. USA 105, 11287–11292 (2008). This study provided important information on the diversity and extent of cytokine production by iNKT cell subsets.
Takahashi, T. et al. Cutting edge: analysis of human Vα24+CD8+ NK T cells activated by α-galactosylceramide-pulsed monocyte-derived dendritic cells. J. Immunol. 168, 3140–3144 (2002).
O'Reilly, V. et al. Distinct and overlapping effector functions of expanded human CD4+, CD8α+ and CD4−CD8α− invariant natural killer T cells. PLoS ONE 6, e28648 (2011).
Hammond, K. J. et al. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 167, 1164–1173 (2001).
Watarai, H. et al. Development and function of invariant natural killer T cells producing Th2- and Th17-cytokines. PLoS Biol. 10, e1001255 (2012). This report is a comprehensive analysis of mouse iNKT cell functional subsets.
Kim, H. Y. et al. The development of airway hyperreactivity in T-bet-deficient mice requires CD1d-restricted NKT cells. J. Immunol. 182, 3252–3261 (2009).
Kim, P. J. et al. GATA-3 regulates the development and function of invariant NKT cells. J. Immunol. 177, 6650–6659 (2006).
Motomura, Y. et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nature Immunol. 12, 450–459 (2011).
Michel, M. L. et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001 (2007).
Paget, C. et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J. Biol. Chem. 287, 8816–8829 (2012).
Michel, M. L. et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc. Natl Acad. Sci. USA 105, 19845–19850 (2008).
Pichavant, M. et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 205, 385–393 (2008).
Moreira-Teixeira, L. et al. Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells. J. Immunol. 186, 5758–5765 (2011).
Snyder-Cappione, J. E. et al. A comprehensive ex vivo functional analysis of human NKT cells reveals production of MIP1-α and MIP1-β, a lack of IL-17, and a Th1-bias in males. PLoS ONE 5, e15412 (2010).
Chang, P. P. et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nature Immunol. 13, 35–43 (2012).
King, I. L. et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nature Immunol. 13, 44–50 (2012).
Monteiro, M. et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-β. J. Immunol. 185, 2157–2163 (2010).
Bosma, A., Abdel-Gadir, A., Isenberg, D. A., Jury, E. C. & Mauri, C. Lipid-antigen presentation by CD1d+ B cells is essential for the maintenance of invariant natural killer T cells. Immunity 36, 477–490 (2012).
Barral, P., Sanchez-Nino, M. D., van Rooijen, N., Cerundolo, V. & Batista, F. D. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 31, 2378–2390 (2012).
Fujii, S., Liu, K., Smith, C., Bonito, A. J. & Steinman, R. M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199, 1607–1618 (2004).
Fujii, S., Shimizu, K., Kronenberg, M. & Steinman, R. M. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nature Immunol. 3, 867–874 (2002).
Bezbradica, J. S. et al. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J. Immunol. 174, 4696–4705 (2005).
van den Elzen, P. et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437, 906–910 (2005).
Carnaud, C. et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163, 4647–4650 (1999). This study defined the transactivation of NK cells and B cells following iNKT cell activation.
Fujii, S., Shimizu, K., Smith, C., Bonifaz, L. & Steinman, R. M. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198, 267–279 (2003).
Hermans, I. F. et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 171, 5140–5147 (2003).
Semmling, V. et al. Alternative cross-priming through CCL17–CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nature Immunol. 11, 313–320 (2010).
Gonzalez-Aseguinolaza, G. et al. Natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 195, 617–624 (2002).
Silk, J. D. et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J. Clin. Invest. 114, 1800–1811 (2004).
Bialecki, E. et al. Role of marginal zone B lymphocytes in invariant NKT cell activation. J. Immunol. 182, 6105–6113 (2009).
Zietara, N., Lyszkiewicz, M., Krueger, A. & Weiss, S. ICOS-dependent stimulation of NKT cells by marginal zone B cells. Eur. J. Immunol. 41, 3125–3134 (2011).
Leadbetter, E. A. et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl Acad. Sci. USA 105, 8339–8344 (2008).
Galli, G. et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 197, 1051–1057 (2003).
Galli, G. et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl Acad. Sci. USA 104, 3984–3989 (2007).
Lang, G. A., Devera, T. S. & Lang, M. L. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood 111, 2158–2162 (2008).
Tonti, E. et al. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood 113, 370–376 (2009).
Detre, C. et al. SAP expression in invariant NKT cells is required for cognate help to support B-cell responses. Blood 120, 122–129 (2012).
Li, X. et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc. Natl Acad. Sci. USA 107, 13010–13015 (2010).
Cerundolo, V., Silk, J. D., Masri, S. H. & Salio, M. Harnessing invariant NKT cells in vaccination strategies. Nature Rev. Immunol. 9, 28–38 (2009).
Vasan, S. & Tsuji, M. A double-edged sword: the role of NKT cells in malaria and HIV infection and immunity. Semin. Immunol. 22, 87–96 (2010). References 185 and 186 are review articles that describe the potential of using pharmacological iNKT cell activation in vaccine strategies.
Schmieg, J., Yang, G., Franck, R. W., Van Rooijen, N. & Tsuji, M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc. Natl Acad. Sci. USA 102, 1127–1132 (2005).
Winau, F. et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 26, 117–129 (2007).
Barral, P. et al. CD169+ macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nature Immunol. 11, 303–312 (2010).
Nieuwenhuis, E. E. et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nature Med. 8, 588–593 (2002). This report provides an important example of the tissue-specific effector functions of iNKT cells during infection.
Sada-Ovalle, I., Chiba, A., Gonzales, A., Brenner, M. B. & Behar, S. M. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-γ, and kill intracellular bacteria. PLoS Pathog. 4, e1000239 (2008).
Kim, E. Y. et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nature Med. 14, 633–640 (2008).
Hegde, S. et al. NKT cells direct monocytes into a DC differentiation pathway. J. Leukoc. Biol. 81, 1224–1235 (2007).
Kotsianidis, I. et al. Regulation of hematopoiesis in vitro and in vivo by invariant NKT cells. Blood 107, 3138–3144 (2006).
Metelitsa, L. S. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin. Immunol. 140, 119–129 (2011).
Kawakami, K. et al. Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur. J. Immunol. 33, 3322–3330 (2003). This study demonstrated a prominent role for iNKT cells during infection.
Li, L. et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia–reperfusion injury. J. Immunol. 178, 5899–5911 (2007).
De Santo, C. et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nature Immunol. 11, 1039–1046 (2010).
Wingender, G. et al. Neutrophilic granulocytes modulate invariant NKT cell function in mice and humans. J. Immunol. 188, 3000–3008 (2012).
Kobrynski, L. J., Sousa, A. O., Nahmias, A. J. & Lee, F. K. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J. Immunol. 174, 1787–1790 (2005).
De Santo, C. et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J. Clin. Invest. 118, 4036–4048 (2008).
Paget, C. et al. Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J. Immunol. 186, 5590–5602 (2011).
Guillonneau, C. et al. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc. Natl Acad. Sci. USA 106, 3330–3335 (2009).
Ho, L. P. et al. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur. J. Immunol. 38, 1913–1922 (2008).
Kok, W. L. et al. Pivotal advance: invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. J. Leukoc. Biol. 91, 357–368 (2012).
Akbari, O. et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Med. 9, 582–588 (2003). This report demonstrated an important role for iNKT cells in airway hyperresponsiveness.
Lisbonne, M. et al. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171, 1637–1641 (2003).
Meyer, E. H. et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl Acad. Sci. USA 103, 2782–2787 (2006).
D'Cruz, L. M., Yang, C. Y. & Goldrath, A. W. Transcriptional regulation of NKT cell development and homeostasis. Curr. Opin. Immunol. 22, 199–205 (2010).
Godfrey, D. I., Stankovic, S. & Baxter, A. G. Raising the NKT cell family. Nature Immunol. 11, 197–206 (2010).
Dao, T. et al. Development of CD1d-restricted NKT cells in the mouse thymus. Eur. J. Immunol. 34, 3542–3552 (2004).
Egawa, T. et al. Genetic evidence supporting selection of the Vα14i NKT cell lineage from double-positive thymocyte precursors. Immunity 22, 705–716 (2005).
Gapin, L., Matsuda, J. L., Surh, C. D. & Kronenberg, M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nature Immunol. 2, 971–978 (2001).
Coles, M. C. & Raulet, D. H. Class I dependence of the development of CD4+ CD8− NK1.1+ thymocytes. J. Exp. Med. 180, 395–399 (1994).
Ohteki, T. & MacDonald, H. R. Major histocompatibility complex class I related molecules control the development of CD4+8− and CD4−8− subsets of natural killer 1.1+ T cell receptor-α/β+ cells in the liver of mice. J. Exp. Med. 180, 699–704 (1994).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Seiler, M. P. et al. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nature Immunol. 13, 264–271 (2012).
Kovalovsky, D. et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nature Immunol. 9, 1055–1064 (2008).
Savage, A. K. et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 29, 391–403 (2008). References 218 and 219 describe the crucial role of the transcription factor PLZF in iNKT cell lineage determination.
Mathew, R. et al. BTB-ZF factors recruit the E3 ligase cullin 3 to regulate lymphoid effector programs. Nature 491, 618–621 (2012).
Kovalovsky, D. et al. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J. Immunol. 184, 6746–6755 (2010).
Raberger, J. et al. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc. Natl Acad. Sci. USA 105, 17919–17924 (2008).
Savage, A. K., Constantinides, M. G. & Bendelac, A. Promyelocytic leukemia zinc finger turns on the effector T cell program without requirement for agonist TCR signaling. J. Immunol. 186, 5801–5806 (2011).
Griewank, K. et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity 27, 751–762 (2007).
Kageyama, R. et al. The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development. Immunity 36, 986–1002 (2012).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Benlagha, K., Wei, D. G., Veiga, J., Teyton, L. & Bendelac, A. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202, 485–492 (2005).
Gadue, P. & Stein, P. L. NKT cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J. Immunol. 169, 2397–2406 (2002).
Berzins, S. P., McNab, F. W., Jones, C. M., Smyth, M. J. & Godfrey, D. I. Long-term retention of mature NK1.1+ NKT cells in the thymus. J. Immunol. 176, 4059–4065 (2006).
Matangkasombut, P., Pichavant, M., Dekruyff, R. H. & Umetsu, D. T. Natural killer T cells and the regulation of asthma. Mucosal Immunol. 2, 383–392 (2009).
Lukens, J. R. & Kanneganti, T. D. Fat chance: not much against NKT cells. Immunity 37, 447–449 (2012).
Hammond, K. J. & Kronenberg, M. Natural killer T cells: natural or unnatural regulators of autoimmunity? Curr. Opin. Immunol. 15, 683–689 (2003).
Tatituri, R. V. et al. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc. Natl Acad. Sci. USA (in the press).
Tyznik, A. J. et al. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 181, 4452–4456 (2008).
Acknowledgements
P.J.B. is supported by a career development award from the American Academy of Allergy, Asthma and Immunology ARTrust. M.B. is supported by the US National Institutes of Health (grant AI077795). M.B.B. is supported by research grants from the US National Institutes of Health (AI063428, AI028973 and DK057521) and the American Diabetes Association (7-12-IN-07). We thank R. Tatituri, E. Kim and L. Lynch for helpful discussions during the preparation of this manuscript. We also thank J. Rossjohn for providing the protein crystal structures shown in Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Somatic recombination
-
(Also known as V(D)J recombination). The somatic rearrangement of variable (V), diversity (D) and joining (J) regions of the genes that encode antigen receptors, leading to repertoire diversity of both T cell and B cell receptors.
- Omentum
-
The folds of peritoneum between the stomach and abdomen that contain lymphoid aggregates known as 'milky spots'.
- Sickle cell disease
-
An inherited disorder of erythrocytes, with a high prevalence in African and African American populations, that is caused by a mutation in the β-globin gene. A single nucleotide substitution (and the resultant amino-acid substitution) leads to the polymerization of haemoglobin when it is deoxygenated, ultimately resulting in the occlusion of small blood vessels. Disease manifestations include chronic anaemia, multiple painful crises, organ damage and increased susceptibility to bacterial infections.
- MicroRNAs
-
Small RNA molecules that regulate the expression of genes by binding to the 3′-untranslated regions of specific mRNAs.
- Gangliosides
-
A group of glycosphingolipids that are prominent components of nerve cell membranes.
- β2-microglobulin
-
A protein comprising a single immunoglobulin-like domain that non-covalently associates with the main polypeptide chain of MHC class I molecules. In the absence of β2-microglobulin, MHC class I molecules are unstable and are therefore found at very low levels on the cell surface.
- Complementarity-determining region 3
-
(CDR3). The CDRs are the amino-acid sequences of the B cell receptor and the T cell receptor that physically contact the antigen and are the most variable parts of the receptors. There are three such regions — CDR1, CDR2 and CDR3 — in each receptor chain. CDR3 arises from recombination of the variable (V), diversity (D) and joining (J) regions of each receptor chain and is the most variable CDR.
- Ischaemia–reperfusion injury
-
An injury in which the tissue first suffers from hypoxia as a result of severely decreased, or completely arrested, blood flow. The restoration of normal blood flow then triggers inflammation, which exacerbates the tissue damage.
- NK cell transactivation
-
The secondary activation of a natural killer (NK) cell by interleukin-12 (IL-12), which leads to the production of interferon-γ. This process occurs following the primary activation of an IL-12- producing cell type, such as an activated dendritic cell.
- Cross-presentation
-
The ability of certain antigen-presenting cells to load peptides that are derived from exogenous antigens onto MHC class I molecules. This property is atypical, because most cells exclusively present peptides from their endogenous proteins on MHC class I molecules. Cross-presentation is essential for the initiation of immune responses to viruses that do not infect antigen-presenting cells.
- M2 macrophages
-
Macrophages that differentiate in response to interleukin-4 (IL-4) or IL-13 and are thought to mediate T helper 2-type immune responses, such as protection from parasites and wound healing. M2 macrophages are typically defined by their expression of arginase 1, the mannose receptor CD206 and the IL-4 receptor -chain, and they can produce large amounts of IL-10.
- Myeloid-derived suppressor cell
-
(MDSC). A member of a heterogeneous population of immature myeloid cells with immunosuppressive functions. MDSCs can accumulate in tissues during inflammation or in response to tumour-derived cytokines.
Rights and permissions
About this article
Cite this article
Brennan, P., Brigl, M. & Brenner, M. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13, 101–117 (2013). https://doi.org/10.1038/nri3369
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3369
This article is cited by
-
The gut-liver axis in hepatobiliary diseases
Inflammation and Regeneration (2024)
-
Motility and tumor infiltration are key aspects of invariant natural killer T cell anti-tumor function
Nature Communications (2024)
-
IL-12 reprograms CAR-expressing natural killer T cells to long-lived Th1-polarized cells with potent antitumor activity
Nature Communications (2024)
-
Identification of SPRYD4 as a tumour suppressor predicts prognosis and correlates with immune infiltration in cholangiocarcinoma
BMC Cancer (2023)
-
Reprogramming T cell differentiation and exhaustion in CAR-T cell therapy
Journal of Hematology & Oncology (2023)