Key Points
-
Membrane sorting between secretory and endocytic organelles is predominantly controlled by small carrier vesicles or tubules that have specific protein coats on their cytoplasmic surfaces. The most widely studied of the various membrane-transport intermediates are clathrin-coated vesicles.
-
By positioning crystal structures of domains of clathrin and auxilin (auxilin links clathrin to the 'unfoldase' Hsc70) in cryo-electron-microscopy maps of clathrin cages that were assembled in the presence of auxilin, molecular explanations have been obtained for how clathrin-coat disassembly might proceed.
-
Many clathrin adaptors, which are defined as proteins that link polymerized clathrin to membranes and their embedded proteins, have different sequences and structures, but have a common architecture that consists of folded domains that are linked by extended, structureless linkers.
-
Structural and biochemical studies on the heterotetrameric adaptor protein complexes (APs), which are the most abundant of all the clathrin adaptors, show how they carry out their numerous and often phosphorylation-regulated functions. These functions include binding protein cargo, phospholipids and clathrin, and coordinating the recruitment of many of the other proteins that are involved in assembling and subsequently disassembling clathrin-coated vesicles.
Abstract
Membrane sorting between secretory and endocytic organelles is predominantly controlled by small carrier vesicles or tubules that have specific protein coats on their cytoplasmic surfaces. Clathrin–clathrin-adaptor coats function in many steps of intracellular transport and are the most extensively studied of all transport-vesicle coats. In recent years, the determination of structures of clathrin assemblies by electron microscopy, of domains of clathrin and of its adaptors has improved our understanding of the molecular mechanisms of clathrin-coated-vesicle assembly and disassembly.
Similar content being viewed by others
Main
The transfer of macromolecules in the secretory and endocytic pathways occurs by the trafficking of membrane-bound vesicles between donor and acceptor compartments. During the formation of transport vesicles, the protein and lipid cargoes that are to be transported become concentrated in a patch on the donor membrane. Once a protein coat has stably assembled on this patch1,2, the membrane becomes deformed and is eventually 'pinched off' to form a vesicle that is then targeted to, and ultimately fuses with, an acceptor membrane.
Transport vesicles are classified according to the components of the protein coat that surrounds them during their genesis and early life. One of the most common and probably best-characterized classes of coated vesicle is that comprising three-layered clathrin-coated vesicles (CCVs). CCVs are so-called because the main component of the coat is clathrin, which forms a polymeric mechanical scaffold on the vesicle surface. The inner, membrane layer with its embedded cargo is linked to the outer, clathrin layer by a middle layer that consists of various clathrin-adaptor molecules and other proteins that have accessory/regulatory roles in CCV assembly (Box 1). A clathrin adaptor is a protein that can connect the purely mechanical clathrin scaffold and a component of the membrane, be it a phospholipid, a transmembrane protein or both simultaneously. At least 20 clathrin adaptors have been identified3. They share a common design principle in that they are composed of compact folded domains and long unstructured regions through which they bind to the N-terminal, 330-residue β-propeller terminal domains (TDs) of clathrin and also to each other. These interactions are almost always mediated by short, linear motifs that interact with a folded domain, which results in a moderate-to-weak strength of binding (Kd = 1–100 μM) with a relatively short half life (t1/2 = 1–10 s). Such interactions are frequently found between proteins that are involved in transport-vesicle coat formation4, as well as in other systems, including signal-transduction pathways, for which dynamic interactions (that is, binding partners that can be exchanged easily) are required.
The first clathrin adaptors to be identified were the protein-cargo-binding and phospholipid-binding heterotetrameric adaptor protein complexes (APs) AP1 and AP2, and, to this day, they serve as functional paradigms for all clathrin–membrane/cargo adaptors. This review focuses on how the high-resolution structures of AP1, AP2 and domains of clathrin, in combination with electron microscopy (EM) studies on clathrin assemblies, have brought us to a point where we can understand their functions in CCV formation at a molecular level. A large amount of structural and biochemical work has been carried out on other clathrin–membrane adaptors. These studies have shed light on the mechanical processes of membrane deformation (involving epsins and amphiphysins) and the mechanisms by which specific cargoes are recruited into CCVs (involving clathrin-associated sorting proteins (CLASPs) such as ARH and Dab2, as well as the arrestins). However, these studies do not fall within the scope of this review (for reviews of these topics, see Refs 3,5 and references therein).
Early structures from EM studies gave us views both of clathrin cages6,7,8,9,10 and of the individual trimeric building blocks (known as triskelia) from which they are built, as well as of APs. The resolution of these studies was 50–100 Å and revealed no more than 'fuzzy outlines' of these molecules that could not be interpreted in terms of a molecular mechanism for CCV formation. Since then, many biochemical and functional studies have been carried out (reviewed in Refs 11, 12) and high-resolution X-ray crystallography structures of several clathrin domains have been described13,14,15. Now, the structures of whole clathrin cages at resolutions of up to ∼8 Å in the presence of various interacting proteins have been determined16,17,18,19, into which high-resolution structures of the appropriate proteins can be fitted. High-resolution structures of all of the domains of AP1 and AP2 (with the exception of the AP1 β1-appendage), often in complex with their appropriate peptide ligands, have also been recently elucidated (see later). From these structures, models of whole, intact APs have been built.
We are now in a position to explain much of the existing wealth of functional data in terms of a molecular mechanism for CCV formation. The high-resolution structures also allow structurally non-disruptive point mutations to be designed, which can be used to test functional models in vitro. The ability to remove a wild-type protein efficiently using small interfering RNA (siRNA) in cells that are stably transfected with a gene encoding a mutated version of the protein of interest is making it possible to test the effect of these mutations in vivo. The structures are also predictive, allowing us to propose models for the spatial, temporal and regulatory aspects of this crucial step in membrane trafficking, and to formulate general principles that might apply to the formation of all transport vesicles.
Clathrin
A clathrin coat is a three-dimensional (3D) array of triskelia. Each triskelion is made of three 1,675-residue (approximately 190-kDa) clathrin heavy chains (CHCs) and three 25–29-kDa clathrin light chains (CLCs), and has an approximately three-fold rotational symmetry (Figs 1,2). Purified clathrin can spontaneously assemble into cages or, in the presence of adaptor proteins, into coats in vitro. Clathrin coats come in different shapes and sizes20. The smallest symmetrical form is the hexagonal barrel and studying this barrel has enabled structural biologists to determine the structure of assembled clathrin using cryo-EM and single-particle analysis.
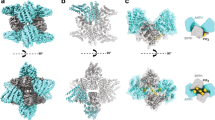
a | Clathrin-cage reconstructions have increased in resolution over time. The first images of clathrin barrels were obtained using negative-stain electron microscopy (EM) in 1969 (Ref. 6). Subsequently, using cryo-EM reconstructions, the resolution increased from approximately 50 Å in 1986 to 8 Å in 2004 (Refs 8, 9, 16, 17). The far left panel is reproduced with permission from Ref. 6 © (1969) The Rockefeller University Press and the far right panel is modified with permission from Ref. 17 © (2004) Macmillan Magazines Ltd. b | An ∼8-Å reconstruction of a clathrin barrel with the light chains highlighted in yellow. Reproduced with permission from Ref. 17 © (2004) Macmillan Magazines Ltd. c | An ∼8-Å reconstruction of a clathrin barrel with one cluster of terminal domains highlighted in red. Modified with permission from Ref. 17 © (2004) Macmilan Magazines Ltd. d | A 12-Å reconstruction of a clathrin barrel that was formed in the presence of a 39-kDa fragment of auxilin. The auxilin J domain is highlighted in purple. Modified with permission from Ref. 18 © (2004) Macmillan Magazines Ltd.
a | A clathrin barrel with a single triskelion highlighted in blue. Modified with permission from Ref. 97 © (2005) Blackwell Publishing and from Ref. 17 © (2005) Macmillan Magazines Ltd. b | A schematic representation of a clathrin triskelion, which highlights the various domains using different colours (see the key). The clathrin-heavy-chain-repeat modules (CHCRs) that are involved in each domain are listed. c | A Cα trace of a single clathrin-heavy-chain molecule with the domains coloured as in part b. A potential HSC70 (70-kDa heat-shock cognate protein)-binding motif (QLMLT) near the C terminus is labelled. This structure was created using coordinates from the Protein Data Bank accession file 1XI4. Beneath this trace are linear representations of auxilin and HSC70 that illustrate their domain structure, the interactions these domains make and the position of a clathrin-box motif in auxilin. The domains of auxilin are phosphatase-and-tensin-homologue domain (PTEN), clathrin-binding domain (CBD) and J domain (JD). The domains of bovine HSC70 are nucleotide-binding domain (NBD), substrate-binding domain (SBD) and C-terminal domain (CTD). Interactions are shown using arrows — biochemically defined interactions are shown using solid lines, whereas proposed interactions are shown using dashed lines.
CCVs were first visualized by negative-stain EM6, and clathrin triskelia were first visualized using rotary shadowing7. These studies were followed by an analysis21 of the assembly and packing of cage fragments, which again used negative-stain EM. However, the first 3D map of a clathrin cage, both in the presence and absence of adaptor proteins, was obtained in 1986 using cryo-EM8,9 (Fig. 1a). This was one of the first examples of the use of cryo-EM methods to determine protein structure, and was a significant milestone in the development of the technique. It was not until 1998 that the resolution was improved to 21 Å (Ref. 16), which allowed the main parts of the triskelia to be traced and the TDs to be roughly positioned (Fig. 1a). Fotin and colleagues have now extended the resolution even further by obtaining two 3D maps of assembled clathrin using cryo-EM17,18. The first is a 7.9–11-Å-resolution map of clathrin alone, both with and without light chains17 (Fig. 1b,c), and the second is a 12-Å map of clathrin bound to auxilin18, an integral coat protein that is required by the chaperone HSC70 (70-kDa heat-shock cognate protein) during its uncoating of CCVs (Fig. 1d). The resolution of all of these maps is significantly higher than has previously been published and many new insights into the structure and function of assembled clathrin have been exposed. In particular, the nature and location of the CLCs has been confirmed (yellow in Fig. 1b), the shape adopted by the TDs (red in Fig.1c) and linker has been defined more fully, and a whole new level of detail has become available on the nature of the cage vertices, including the trimerization domain and the area beneath it.
Model of clathrin density to residue 1,630. The 7.9-Å-resolution EM map of clathrin that was determined by Fotin et al. was obtained using clathrin enriched in the form of hexagonal barrel cages through the addition of substoichiometric amounts of APs. They used existing knowledge of the repeating units of stacked α-helical hairpins that are present in clathrin13, together with EM data, to try to model the structures of the sections of clathrin that had not been determined by X-ray crystallography. The resulting structural model showed that the α-helical hairpins do indeed repeat throughout a clathrin molecule (Fig. 2c), with the only deviations being at the N and C termini. At the N terminus, the increased resolution allowed a more accurate fit of the TD than had been previously possible22 and the map confirmed the maintenance of the helical hairpin repeating structure throughout the linker region that joins the TD to the ankle segment of the triskelia (Fig. 2c).
The study by Fotin et al. also provided a difference map of clathrin with and without CLCs, which confirmed the nature and location of CLCs on CHCs. The density corresponded to a single α-helix, 71 residues in length, that runs along the outer edge of the cage (yellow in Fig. 1b). A model of such a helix that corresponded to the CLC sequence was successfully docked into the electron-density map using information from mutagenesis studies, and confirmed earlier modelling studies that were carried out by Chen et al. before these structural data were available. These earlier studies had revealed two contacts (CHC-K1326–CLC-W108 and CHC-K1514–CLC-W130)23. In both cases, docking that included one interaction showed that the model was in the correct position for the second interaction, which confirmed the accuracy of the fit. The role of CLCs in vivo is still unclear. It has been shown24,25 that, as well as modulating clathrin assembly, they bind Huntingtin-interacting protein-1 (HIP1) and HIP1-related protein (HIP1R). In view of this, it seems probable that they function in a regulatory capacity. It is hoped that this new structural information will direct future mutagenesis studies that are designed to study this potential role further.
Perhaps the most interesting feature of the new EM map is the extra detail that is now available on the architecture of the clathrin vertices. Figure 3 shows the different features by focusing on one vertex. Travelling towards the centre of a cage, starting at a corner vertex, the first structure encountered is a so-called hub that is composed of three CHC proximal legs (blue in Figs 2,3a). These three legs are mainly held together by the 'helical-tripod' trimerization domain (purple in Figs 2,3a), which is made from the C-terminal 100 residues of each clathrin molecule. This domain follows each proximal leg (blue in Figs 2,3a) and is found directly beneath the place where the three proximal legs meet. As we travel down towards the centre of the cage, we encounter a triangular structure that is composed of three distal leg segments (green in Figs 2,3a) that come from three separate neighbouring triskelia, and below them are three ankle segments (yellow–orange in Figs 2,3a) from three further triskelia. Beneath these, there are three TDs (red in Figs 2,3a), which each come from three separate triskelia that are not connected to the distal leg segments or the ankle segments above them. These TDs curve round towards each other and almost create an enclosure underneath the distal and trimerization domains. Travelling further into the centre of the cage, if it were fully present, there would be the AP layer and, finally, if this was a native coated vesicle, there would be the membrane and cargo.
a | Layers of clathrin molecules can be seen by taking a slice through a clathrin coat. The various domains of the clathrin molecule are coloured as shown in Fig. 2b,c. Moving from the outside towards the membrane (top to bottom), the outer layer is composed of proximal domains (blue) and trimerization domains (purple). Three trimerization domains, each a long single helix, come together to form a 'helical tripod' (purple). The next layer is formed primarily by distal domains (green), and the inner layer is composed of linker and ankle domains (yellow–orange) and terminal domains (TDs; red). In each layer, the clathrin domains involved come from different clathrin triskelia. The grey sphere indicates the approximate position of the auxilin J domain that was proposed by Fotin and co-workers18. b | A cross-section through a clathrin barrel. This shows the array of TDs (red) that extend from the scaffold, which is made up of the various leg domains (blue), towards the membrane to form a multivalent structure that can bind the extended, motif-rich portions of clathrin adaptors. The structures shown in parts a and b were created using coordinates from the Protein Data Bank accession file 1XI4. c | The interactions between a TD and two peptide motifs — a W-box motif (the carbon atoms of this motif are coloured cyan) and a clathrin-box motif (the carbon atoms of this motif are coloured green) — that are found in the extended regions of clathrin adaptors. The enlargements show the molecular details of these interactions. Specificity is the result of side chains fitting into chemically compatible pockets, with further strength coming from hydrogen bonds that are formed between the TD and the backbones of the peptides. Reproduced with permission from Ref. 3 © (2004) Annual Reviews.
The high-resolution EM map goes a long way towards defining the molecular architecture of the 'hub/trimerization assemblies'. Up to residue 1,597 of the CHC sequence, the density fits with the helical hairpin model. The final hairpins stack against each other forming the top of the trimerization domain (blue in Fig. 3a). From residues 1,598 to 1,630, the density moves into the cage as a 50-Å-long rod-like structure, probably an α-helix (purple in Fig. 3a). Three rods combine to form a tripod shape beneath the hub and end close to the triangle of distal domains that lies beneath the vertex. Finally, density that probably arises from the C-terminal residues 1,631–1,675 merges with the density of the ankle regions (yellow–orange in Fig. 3a) that lie below the distal domains.
Maps of clathrin with auxilin bound. When a fragment of auxilin, including its J domain, was bound to clathrin, the whole shape of the cage altered, and became more square and less well ordered (Fig. 1d). Density that related to this 39-kDa fragment of auxilin was clearly seen in the space beneath each clathrin vertex, which is created by a hub and a tripod assembly that is surrounded by triskelion ankles and TDs (Fig. 3a). Docking a five-helix model for auxilin (residues 800–910), in which three of the helices correspond to the known structure of the auxilin J domain26, into this density accounted for approximately a third of the observed electron density that was not accounted for by clathrin. This is consistent with this fragment representing about a third of the molecular weight of the 39-kDa auxilin fragment that was bound to the cages. The fact that other parts of this fragment can bind to clathrin and therefore possess visible electron density is consistent with pulldown data from Scheele and colleagues27,28, which showed that auxilin has several binding sites for clathrin that are characterized by a range of motifs including DLL, DPW, WDW and NWQ. The motifs between residues 547–715 are believed to bind mainly to TDs, whereas motifs between residues 716–910 are believed to bind mainly to the distal and proximal legs of clathrin, and some of these regions of clathrin border the volume occupied by the auxilin electron density (Fig. 3a).
HSC70 is recruited to clathrin coats through the auxilin J domain, which also stimulates the ATPase activity of the HSC70 chaperone. It has always been intriguing how HSC70, which usually carries out its unfolding/disassembly function by binding to unfolded polypeptides, might function on clathrin, which presumably remains folded during CCV uncoating. Now we can see that the location of the auxilin J domain means that HSC70 could be recruited into the space beneath the vertex where a large number of inter-triskelion contacts are made. Crucially, the trimerization domains and potentially structureless C termini of clathrin meet with the distal domains and ankle segments of other triskelia at this position. The map from Fotin et al. indicates that the C terminus of a CHC merges with the density of the ankle segments. Interestingly, the C terminus is proline rich and is therefore probably unfolded. Fotin et al. also noted a possible HSC70-binding motif in the proline-rich domain — QLMLT (Fig. 2c) — which is similar to a known HSC70-binding peptide motif (FYQLALT)29. The authors propose that auxilin introduces a change to the ankle-crossing contacts, which could release the C-terminal segments from their interaction with the ankles, priming these segments for HSC70 binding. HSC70 binding to these C-terminal segments could destabilize the numerous interactions that link several individual triskelia together and, in this way, promote triskelion release from the cage. This would initiate the cascade of triskelion dissociation from the clathrin coat that results in the uncoating of the CCV — that is, the 'death' of the coat. The predicted location of HSC70 at the vertex is consistent with early studies, which indicated that up to three HSC70 molecules could bind to each clathrin vertex30.
The model proposed by Fotin et al. for HSC70 binding and function is an attractive one because of the undeniable logic of achieving uncoating by disrupting a site where so many contacts are present in such a small space. However, a recent, albeit much lower resolution, 28-Å map of HSC70 bound to clathrin cages might pose a challenge to this model. Heymann et al.19 obtained a map of clathrin cages that were assembled in the presence of a recombinant fragment containing the auxilin J domain joined to part of the AP180 clathrin-binding domain. The proposed location of HSC70 that was derived from this map differs from the location predicted by Fotin and colleagues. However, it should be noted that the samples used had different components and that both publications agree that the J domains, to which HSC70 binds, lie close to the clathrin TDs. What is clear is that each map poses interesting questions as to the mechanism of action of HSC70, which should be investigated using biophysical techniques as well as by striving for a map of HSC70 bound to clathrin at a much higher resolution.
The results of Fotin et al.18 are consistent with the proposal of Smith et al.31 that the N-terminal PTEN (phosphatase and tensin homologue) domain of auxilin might contact the membrane (Fig. 2c). The structure of Fotin et al. contains only residues 547–910 of auxilin, whereas the structure of Smith et al. contains the intact protein. The key difference between the two is the presence in the work of Smith et al. of density close to where the membrane would be in a native coated vesicle. Placing the PTEN domain innermost on the membrane would allow a clathrin-box motif at residue 495, which was identified by Smith et al., to bind nearby TDs. The 547–910 segment, which includes the J domain at its C terminus, would then be able to interact at several points along its length with the proximal and distal leg regions that surround the auxilin density in the Fotin et al. map. Much of the auxilin fragment between residue 547 and the J domain is unstructured in solution28,32, but the Fotin et al. map indicates that, when it is bound to clathrin, this part of auxilin is either fully folded or, in some way, condensed to occupy space beneath the clathrin vertex. This raises the possibility that auxilin alters its conformation on binding to clathrin, as has indeed also been shown for the CLCs23. However, biophysical data that support these ideas are not yet available.
Taking a view in the opposite direction — that is, outwards from where the vesicle membrane would be — reveals a 'forest' of TDs (coloured red in Fig. 3b) that project inwards towards the membrane. In native CCVs, this would be the site where the TDs would meet the 'string-like' unfolded regions of clathrin adaptors that extend out from the membrane and contain clathrin-binding motifs. The underside of a clathrin coat therefore functions as a planar multivalent scaffold for the attachment of clathrin to the membrane through numerous clathrin adaptors. The molecular details of the interactions between TDs and the clathrin-box and W-box peptide motifs that are found in the extended regions of clathrin adaptors have been determined by protein crystallography and confirmed by binding studies using structure-directed mutagenesis (Fig. 3c).
Recently, an entirely novel function has been proposed33 for clathrin in chromosome separation during cell division. This was suggested on the basis of an unequal partitioning of chromosomes during mitosis that was caused by the siRNA-mediated depletion of clathrin, as confirmed by immunofluorescence and immunoEM imaging of clathrin that was associated with mitotic spindles. However, as there are no biochemical data to indicate exactly how clathrin functions in this new role, the clathrin structures described here cannot shed light on the molecular mechanisms involved in this process.
Adaptor protein complexes (APs)
The history of the clathrin adaptor complexes can be traced back almost 30 years to the discovery of 'assembly polypeptides' or APs in extracts of clathrin vesicles that promoted the assembly of purified clathrin triskelia into clathrin coats34. Although purified clathrin triskelia could be assembled into clathrin coats in the absence of these assembly polypeptides at low pH, the polypeptides were absolutely required under physiological conditions. Further characterization showed that the assembly polypeptides mainly consisted of two distinct heterotetrameric complexes — AP1 and AP2 — and a third protein that is now generally referred to as AP180 (Ref. 35). The first structural characterization of assembly polypeptides was by deep-etch EM10 and, in agreement with proteolysis studies36,37, indicated that APs are composed of a central brick-like core that is joined to two smaller globular appendages by flexible linkers. The location of the assembly polypeptides between the outer clathrin layer and the inner membrane layer of CCVs was established by observing the differences between EM cage reconstructions that were made in the presence and absence of APs8,9 and through the inspection of clathrin-stripped coats using quick-freeze, deep-etch EM10. Linking clathrin to the membrane — that is, functioning as an adaptor between the two — is the defining function of the APs, so much so that 'AP' has come to mean 'adaptor protein' rather than the original 'assembly polypeptide'.
Organization of adaptor protein complexes (APs). APs are heterotetrameric proteins of which there are four (AP1–4). All APs have two large subunits of 100–130 kDa (α and β2 in AP2; the α-subunit is homologous to the γ-, δ- and ɛ-subunits in AP1, AP3 and AP4, respectively; β2 is homologous to β1, β3 and β4 in AP1, AP3 and AP4, respectively). They also contain a medium-sized subunit of ∼50 kDa (μ1–4 in AP1–4, respectively) and a small subunit of ∼20 kDa (σ1–4 in AP1–4, respectively). In the case of AP1 and AP2, the β-, μ- and σ-subunits share a high degree of homology (∼40–85% sequence identity), whereas α and γ are more diverse (∼25% identity). Many studies have shown that AP2 is the predominant clathrin adaptor at the plasma membrane, whereas, by immunoEM and immunofluorescent microscopy, AP1, -3 and -4 have been shown to localize both to the endosomes and the TGN38. And AP3 might, in fact, be able to function in a clathrin-independent manner in some circumstances39. All four APs bind directly to both YXXΦ motifs (in which X represents any amino acid and Φ represents a large hydrophobic residue) and [D/E]XXXL[L/I] 'acidic dileucine' motifs, at least one of which is contained in many CCV cargo proteins. The heterotetrameric arrangement of the APs is mirrored by that of the β, γ, δ and ζ subcomplex of heptameric coatomer protein-I (COPI). COPI forms a coat around vesicles that mediate both retrograde transport from the Golgi back to the endoplasmic reticulum and intra-Golgi transport (Box 2).
Core structure. In recent years, the compact molecular architecture that is common to the AP1 and AP2 cores — the N-terminal two thirds (trunk domains) of the large subunits together with a medium and a small subunit — has been described by protein X-ray crystallography40,41 (Box 2). The trunk domains (γ and β1 for AP1, and α and β2 for AP2) share a common superhelical fold that includes a tight ∼90° turn, so that each domain provides two sides of a rectangle. These rectangles correspond to the brick-like structure that is seen by EM (Fig. 4a). Even more striking is the similarity in the folds of the N-terminal domains of μ1 (AP1) and μ2 (AP2) (collectively known as N-μ1/2) and the small subunits σ1 (AP1) and σ2 (AP2) (σ1/2). Both N-μ1/2 and σ1/2 fold into a central β-sheet that is flanked by helices on either side, and each domain fits into the 90° bend in β1/2 and γ/α, respectively (Fig. 4b,c). Intriguingly, this fold is found in other proteins in eukaryotes that also have functions that involve membranes (Box 3).
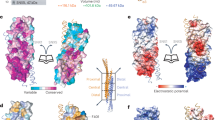
a | An original electron-microscopy image of an adaptor protein complex (AP). Modified with permission from Ref. 10 © (1988) The Rockefeller University Press. b,c | Models of AP1 and AP2 (coloured by subunit as indicated) were made using structures that were determined by X-ray crystallography (Protein Data Bank (PDB) accession files 1W63, 1GYU, 1GW5, 1B9K and 1E42). The linkers and the β1-appendage of AP1 (shaded grey) were modelled, because their structures are unknown. The position of a phosphoinositide mimic that was bound to AP2 is shown in spacefill representation. These AP1 and AP2 structures are in an inactive, closed state, because their μ-subunit YXXΦ-binding sites (X represents any amino acid and Φ represents a large hydrophobic residue) are blocked by their β-subunits. d | The YXXΦ-binding site on μ1 of AP1. A YXXΦ peptide is overlaid in white, and it can be seen that residues from the β1-subunit block the YXXΦ-binding site in the inactive, closed state of AP1. e | The YXXΦ-binding site on μ2 of AP2, again with a YXXΦ peptide overlaid in white and similarly blocked by residues from β2. f | Details of the binding of a YXXΦ peptide to the isolated C-terminal subdomain of μ2. Some of the residues that are important for binding are indicated. Parts d, e and f of this figure were created using coordinates from the PDB accession files 1W63, 1BXX and 1GW5.
Core structure reveals an inactive conformation. The C-terminal domains of the μ-subunits, collectively known as C-μ1/2, have elongated and banana-shaped β-sheet structures that sit on one face of the core structures and complement a shallow depression that is created by the other four subunits in each AP. The contact regions between C-μ1/2 and the β-trunks highlight important implications for function. The YXXΦ-binding sites on the surface of C-μ1/2 comprise hydrophobic pockets for each of the two essential bulky residues of this motif42 and a free β-strand that can form hydrogen bonds with the backbone of the motif. However, in the core structures, these sites are blocked by residues from the corresponding β-subunit. A valine (Val365) sits perfectly in the Φ pocket, while the side chain of Tyr405 blocks the space where the peptide backbone residues of the YX motif would be located (Fig. 4d–f). In other words, these AP1 and AP2 core structures are in an inactive, closed state that necessitates a change in conformation to expose the YXXΦ-binding site in C-μ1/2.
Significantly, the core structure indicates that the linker between N-μ1/2 and C-μ1/2, which contains the main site for phosphorylation (μ1Thr154/μ2Thr156)43 that is essential for the endocytosis of tranferrin44, is surface exposed and flexible. This is consistent with it being a target for protein kinases. In the case of AP2, the kinase responsible is the recently identified α-adaptin-associated kinase-1 (AAK1)45. Several lines of evidence indicate that AAK1 is an important regulator of AP function, possibly by promoting the conformational change in AP2 that is needed for it to bind to endocytic cargo. AAK1 colocalizes to endocytic clathrin-coated pits in vivo45 through its interaction with the α-appendage, and its kinase activity is stimulated by clathrin46,47. Furthermore, phosphorylation of μ2Thr156 enhances the affinity of AP2 for endocytic signals48,49,50, which is consistent with a role for AAK1 phosphorylation in promoting a conformational change in AP2, such that the binding site for endocytic cargo becomes exposed.
AP1 binding to YXXΦ is also accelerated by the phosphorylation of μ1 (Ref. 51) and a candidate for the responsible kinase is GAK (cyclin-G-associated kinase, which is also known as auxilin-2). GAK is a serine/threonine kinase that is related to AAK1 (Refs 52, 53). The precise molecular nature of the conformational change that is required for binding to YXXΦ is unclear, but it requires that the μ-subunits are at least partially dislodged from their binding sites, so that they can 'scan' the nearby membrane for motif-containing cargo.
Phosphoinositide binding and membrane recruitment. The AP core structures also revealed the mechanism of AP recruitment to membranes through the recognition of phosphoinositides. Phosphoinositides are important determinants of membrane identity54 and, as such, are important signals for recruiting APs to different membrane compartments. As a result, the control of specific phosphoinositide levels by a wide range of phosphoinositide-headgroup kinases and phosphatases is an important temporal and spatial switch for dictating the affinity of the different membranes of the endocytic pathway for various APs (and other clathrin adaptors)55,56,57, and therefore for dictating the vesicle-trafficking events that take place at these membranes. For example, AP2 is targeted to the plasma membrane mainly by an α-subunit-mediated interaction with phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) or phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3)58,59, which are phosphoinositides that are enriched in regions of the plasma membrane. Therefore the enzymes that generate them — PtdIns4-position and PtdIns5-position kinases that generate PtdIns(4,5)P2, and the clathrin-activated phosphatidylinositol 3-kinase C2α (Ref. 60) that generates PtdIns(3,4,5)P3 from PtdIns(4,5)P2 — will function to recruit AP2 to nascent plasma-membrane CCV coats and thereby drive CCV formation. Conversely, synaptojanin, which hydrolyses the phosphates on PtdIns(4,5)P2 headgroups, will promote the dissociation of AP2 from the membrane and, consequently, the disassembly or 'death' of a CCV.
The structure of AP2 revealed the exact binding site on the α-subunit for PtdIns(4,5)P2 (actually, for a phosphoinositide-headgroup mimic, D-myo-inositol-1,2,3,4,5,6-hexakisphosphate; InsP6) (Fig. 4c). This site is at the N terminus of the α-subunit and is formed by mainly basic residues. The mode of binding is such that two phosphates of InsP6 are supported by the tips of interacting side chains. Mutation of this site prevents the recruitment of AP2 to PtdIns(4,5)P2-containing membranes, even if these membranes contain YXXΦ-containing cargo50. Unexpectedly, the structure of AP2 revealed a second distinct binding site for InsP6 (Fig. 4c). A basic patch on the surface of C-μ2 was shown to interact with two phosphates of InsP6 through a number of lysine residues. This second site has now also been shown to be important for localizing AP2 to clathrin-coated pits in vivo61 and in helping to drive the conformational change that allows both the PtdIns(4,5)P2-binding and the YXXΦ-binding sites to engage their ligands simultaneously40,41,50.
AP1 recruitment to Golgi membranes also involves phosphoinositides, but, in this case, the supposedly enriched Golgi-membrane marker phosphatidylinositol-4-phosphate (PtdIns4P)54,62 is the probable target, although other data indicate that AP1 binds most strongly to PtdIns(4,5)P2 (Ref. 63). AP1 also binds to the GTP-bound form of ARF1, a member of the ADP-ribosylation factor (ARF) family of small GTPases that are found on many internal membranes throughout the cell64. Data indicate that both interactions are equally important, which indicates a bipartite recruitment mechanism.
A comparison of the structures of AP1 in the absence of phosphoinositides and AP2 in the presence of a phosphoinositide headgroup reveals a potential phosphoinositide-binding site in the γ-subunit of AP1 that is similar to the one in the α-subunit of AP2. Importantly, this site has a lower concentration of basic charge with fewer conserved basic residues than AP2. This is consistent with a preference for PtdIns4P, which has fewer phosphates than PtdIns(4,5)P2 or PtdIns(3,4,5)P3 (Ref. 41). Mutation of a crucial arginine residue in this region, which disturbs the usual perinuclear targeting of AP1, has been proposed to function by reducing the specificity of AP1 for PtdIns4P41. Furthermore, the PtdIns4P pool at the Golgi membranes is dependent on Golgi-resident phosphatidylinositol 4-kinase-IIα. Using siRNA against this kinase reduced the levels of PtdIns4P at the TGN and caused AP1 to become cytosolic, an effect that could be reversed by the addition of PtdIns4P65.
The activation of ARF1 involves the exchange of GDP for GTP, which is catalysed by guanine nucleotide-exchange factors (GEFs). These, in turn, cause the covalently bound myristoyl group of ARF1 to become exposed and able to insert into membranes. By analogy with other small G-protein/effector systems, it is expected that, as a result of conformational changes that are transmitted through the switch-1 and -2 regions of ARF1, the GTP-bound form can probably bind to the large subunits of AP1 (Refs 66, 67), which causes the complex to be recruited onto membranes. Whether this model is correct will only be established when the structure of an AP1–ARF•GTP complex is determined. Inhibition of ARF activation by the GEF inhibitor brefeldin A results in AP1 dissociation from the membrane fraction68,69. It should be noted that the membrane localization of AP2 is not brefeldin-A sensitive, which rules out ARF1–5 as potential direct membrane co-recruitment factors along with PtdIns(4,5)P2 and/or PtdIns(3,4,5)P3. However, ARF6 remains a possible candidate70 because it is mostly localized to the plasma membrane and its GEFs are not sensitive to brefeldin A, although it could also function in an indirect manner through phosphoinositide metabolism71,72.
A final uncertainty in AP1 function is where exactly in the cell AP1 is found (the TGN, endosomes or a TGN–endosome intermediate compartment) and in what pathway(s) it consequently functions (reviewed in Ref. 73). The solution to this problem might come from using structural/biophysical studies to define the true phosphoinositide-binding partner of AP1, because phosphoinositides are mainly localized to a single organelle or organelle subdomain54.
Appendage domains. Structural and biochemical studies have localized the binding sites of a plethora of accessory/regulatory proteins and CLASPs to the appendage domains of APs (see, for example, Refs 3, 5, 74). Appendage-binding partners display different arrangements of short linear binding motifs that are found in different numbers and combinations in the unfolded regions that link their small folded domains32. High-resolution structures of both AP2 appendages (α and β2)74,75,76,77,78 and the γ-appendage of AP179,80 have been determined (Figs 4,5). The β1/2 (β1 and β2 have ∼80% sequence identity) and α-appendages all share a similar bilobal structure — an N-terminal eight- or nine-stranded β-sandwich subdomain and a C-terminal 'platform' subdomain. The structure of the platform subdomain includes a five- or six-stranded β-sheet that is flanked by two helices on one side and a third on the opposite side. By contrast, the γ-appendage has a structure similar only to that of the N-terminal β-sandwich subdomain of the other appendages.
a | A DPF/DPW motif in the Eps15 peptide GSDPFK is shown in complex with the α-appendage of adaptor protein complex-2 (AP2) (Protein Data Bank (PDB) accession file 1KYF). b | An FXDXF motif in the amphiphysin peptide SFFEDNFVPE is shown in complex with the α-appendage of AP2 (PDB accession file 1KY7). c | A WXXF motif in the synaptojanin peptide NPKGWVTFEEEE is shown in complex with the α-appendage of AP2 (PDB accession file 1W80). d | The structure of the β2-appendage of AP2 is shown (PDB accession file 1E42), with a question mark highlighting the place where an ∼16-residue peptide sequence that is found in certain clathrin-associated sorting proteins (CLASPs) is thought to bind. e | For ΨXXΨ motif binding to the AP1 γ-appendage or to the GGA (Golgi-localized, γ-ear-containing, ADP-ribosylation factor (ARF)-binding) appendages, the GGA1 appendage is shown in complex with the p56 peptide DDDDFGGFEAAETFD (PDB accession file 1OM9). The affinities of α-appendage binding to DPF, FXDXF (L. M. Traub, personal communication) and WXXF motifs, β2-appendage binding to a peptide of the CLASP ARH (autosomal recessive hypercholesterolaemia protein), and γ/GGA-appendage binding to ΨXXΨ motifs are shown beneath the structures83. In the motifs, X represents any amino acid and Ψ represents an aromatic amino acid.
Structures of the different appendages in complex with the various peptide motifs they bind are depicted in Fig. 5. The molecular details provided by these structures explain how the specificity of the different sites on the various appendages for different motifs is achieved. Most of the interactions are mediated by mainly hydrophobic residues that are separated by a set number of other residues and that fit into chemically compatible pockets on the appendage surfaces. Further strength/specificity comes from hydrogen-bonding interactions between the peptide backbone of the motif and the appendage surface. The interaction of a single motif is relatively weak (Kd in the 10–100-mM range) and consequently will have a short (<10 s) half-life. The number of copies of a motif for a given site on an adaptor appendage domain will therefore determine the apparent strength of interaction between an accessory/regulatory protein and that appendage site.
The AP2 α-appendage engages DPF/DPW and FXDXF motifs through an overlapping site in the platform subdomain77, whereas WXXF motifs interact through the sandwich subdomain74,78 (Fig. 5a–c). The affinity of a protein for a given appendage must also take into account the fact that some proteins, such as synaptojanin, possess both an FXDXF and a WXXF motif and can therefore bind to both subdomain sites simultaneously. This will increase the apparent strength of binding by an avidity or chelate effect74,81. The β-appendages can probably engage DX[FW] motifs through their platform subdomains, because, in structure, they resemble the platform domain of the α-appendage that binds DX[FW] and FXDXF motifs77,82. However, in β-appendages, this site participates in the relatively tight binding (Kd 1–2 mM) of an ∼16-residue peptide sequence that is found in the CLASPs ARH and the arrestins83 (Fig. 5d). So, if DX[FW] motifs can bind to this site on β-appendages (the expected Kd is 100 μM; Ref. 75), it is probable that they would be competed out by CLASP sequences, which would ensure that CLASPs are preferentially included in forming CCVs.
The AP1 γ-appendage and the highly homologous GGA (Golgi-localized, γ-ear-containing, ARF-binding) appendages bind to ΨXXΨ motifs84 (in which Ψ represents an aromatic amino acid), but only the molecular details of the GGA interaction and its ligand are known at the structural level85,86 (Fig. 5e). A question of interest, therefore, is how do two highly related ΨXXΨ motifs in NECAP (adaptin-ear-binding coat-associated protein) — the sequences WVQF and WGDF — show such remarkably high specificity for opposite faces of the sandwich subdomains of the α- and γ-appendages, respectively78. Acidic residues that lie downstream of WVQF and upstream of WGDF might provide further specificity, so it might be that it is more complicated to predict the affinity of a single motif for a single appendage site than was originally thought.
Finally, it should be remembered that many appendage-binding proteins also bind to other components of CCVs, including other accessory/regulatory proteins, clathrin, phosphoinositides, dynamin and cytoskeletal elements, so they need to be considered as part of the dynamic networks of interacting proteins. So, there are many complex factors that govern the steady-state levels and the temporal regulation of accessory/regulatory-protein and CLASP recruitment to the various stages of the life of a CCV. The level of any given accessory/regulatory protein in any given CCV cannot be accurately predicted through motif hunting or 'sequence gazing', and so a systems-biology approach might be required to enable us to understand this problem.
Future directions
The recent high-resolution reconstructions of clathrin cages provide a way to understand clathrin function at an atomic level, and the goal of obtaining higher resolution structures of assembled clathrin bound to many other adaptor proteins now seems more achievable. In order to extend our knowledge of clathrin-coat 'death' to the same level as our understanding of its 'birth' and 'life', it is clear that a sub-15-Å map of HSC70 bound to clathrin cages is urgently required. However, the way is now paved for informed biochemical and cell-biological studies to test the possible mechanisms of CCV uncoating and to continue to investigate CLC function. However, in order to truly understand the mechanisms by which clathrin achieves its dynamic assembly and, in particular, disassembly, it will be necessary to determine the structure of clathrin cages in as many of its different conformational states bound to as many different partners as possible.
The situation with regard to the APs is similar, in that all the evidence that relates to the function of APs as cargo adaptors in CCV formation indicates a molecular mechanism that involves conformational changes. At present, the exact nature of these changes can only be proposed. A concerted effort is needed to determine the structure of APs in a number of different states bound to various ligands, so that we gain a series of snapshots of different stages of AP function. These would, in turn, indicate the biochemical/biophysical experiments that need to be carried out to confirm the functional models that are proposed on the basis of new structures. Will the current structural techniques, with their various technical limitations and the fact that they provide only a series of 'static snapshots', be able to deliver accurate residue-level-resolution functional models? Or will new time-resolved and single-molecule structure-determination techniques be needed? We can only wait and ultimately, we hope, see.
References
Ehrlich, M. et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004).
Merrifield, C. J., Perrais, D. & Zenisek, D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121, 593–606 (2005).
Owen, D. J., Collins, B. M. & Evans, P. R. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 20, 153–191 (2004).
Bonifacino, J. S. & Traub, L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447 (2003).
Traub, L. M. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 163, 203–208 (2003). Introduces the concept of and provides a mechanistic explanation for the function of CLASPs.
Kanaseki, T. & Kadota, K. The 'vesicle in a basket': a morphological study of the coated vesicle isolated from the nerve endings of the guinea pig brain, with special reference to the mechanism of membrane movements. J. Cell Biol. 42, 202–220 (1969).
Ungewickell, E. & Branton, D. Assembly units of clathrin coats. Nature 289, 420–422 (1981).
Vigers, G. P., Crowther, R. A. & Pearse, B. M. Location of the 100 kd–50 kd accessory proteins in clathrin coats. EMBO J. 5, 2079–2085 (1986).
Vigers, G. P., Crowther, R. A. & Pearse, B. M. Three-dimensional structure of clathrin cages in ice. EMBO J. 5, 529–534 (1986). Presents the first 3D reconstruction of a clathrin barrel, on which all subsequent work has been based.
Heuser, J. E. & Keen, J. Deep-etch visualization of proteins involved in clathrin assembly. J. Cell Biol. 107, 877–886 (1988).
Kirchhausen, T. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 15, 705–732 (1999).
Brodsky, F. M., Chen, C. Y., Knuehl, C., Towler, M. C. & Wakeham, D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568 (2001).
Ybe, J. A. et al. Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature 399, 371–375 (1999).
ter Haar, E., Harrison, S. C. & Kirchhausen, T. Peptide-in-groove interactions link target proteins to the β-propeller of clathrin. Proc. Natl Acad. Sci. USA 97, 1096–1100 (2000). Describes the molecular details of peptide binding to clathrin using a clathrin-box motif.
Miele, A. E., Watson, P. J., Evans, P. R., Traub, L. M. & Owen, D. J. Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain β-propeller. Nature Struct. Mol. Biol. 11, 242–248 (2004).
Smith, C. J., Grigorieff, N. & Pearse, B. M. Clathrin coats at 21 Å resolution: a cellular assembly designed to recycle multiple membrane receptors. EMBO J. 17, 4943–4953 (1998).
Fotin, A. et al. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432, 573–579 (2004).
Fotin, A. et al. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature 432, 649–653 (2004). References 17 and 18 describe the molecular details of a clathrin coat at 8-Å resolution and provide a molecular explanation for auxilin/HSC70-catalysed clathrin-coat disassembly.
Heymann, J. B. et al. Visualization of the binding of Hsc70 ATPase to clathrin baskets: implications for an uncoating mechanism. J. Biol. Chem. 280, 7156–7161 (2005).
Crowther, R. A., Finch, J. T. & Pearse, B. M. On the structure of coated vesicles. J. Mol. Biol. 103, 785–798 (1976).
Crowther, R. A. & Pearse, B. M. Assembly and packing of clathrin into coats. J. Cell Biol. 91, 790–797 (1981).
Musacchio, A. et al. Functional organization of clathrin in coats: combining electron cryomicroscopy and X-ray crystallography. Mol. Cell 3, 761–770 (1999).
Chen, C. Y. et al. Clathrin light and heavy chain interface: α-helix binding superhelix loops via critical tryptophans. EMBO J. 21, 6072–6082 (2002).
Chen, C. Y. & Brodsky, F. M. Huntingtin-interacting protein 1 (Hip1) and Hip1-related protein (Hip1R) bind the conserved sequence of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J. Biol. Chem. 280, 6109–6117 (2005).
Legendre-Guillemin, V. et al. Huntingtin interacting protein 1 (HIP1) regulates clathrin assembly through direct binding to the regulatory region of the clathrin light chain. J. Biol. Chem. 280, 6101–6108 (2005).
Gruschus, J. M. et al. Structure of the functional fragment of auxilin required for catalytic uncoating of clathrin-coated vesicles. Biochemistry 43, 3111–3119 (2004).
Scheele, U., Kalthoff, C. & Ungewickell, E. Multiple interactions of auxilin 1 with clathrin and the AP-2 adaptor complex. J. Biol. Chem. 276, 36131–36138 (2001).
Scheele, U. et al. Molecular and functional characterization of clathrin- and AP-2-binding determinants within a disordered domain of auxilin. J. Biol. Chem. 278, 25357–25368 (2003).
Takenaka, I. M., Leung, S. M., McAndrew, S. J., Brown, J. P. & Hightower, L. E. Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J. Biol. Chem. 270, 19839–19844 (1995).
Heuser, J. & Steer, C. J. Trimeric binding of the 70-kD uncoating ATPase to the vertices of clathrin triskelia: a candidate intermediate in the vesicle uncoating reaction. J. Cell Biol. 109, 1457–1466 (1989).
Smith, C. J. et al. Location of auxilin within a clathrin cage. J. Mol. Biol. 336, 461–471 (2004).
Dafforn, T. R. & Smith, C. J. Natively unfolded domains in endocytosis: hooks, lines and linkers. EMBO Rep. 5, 1046–1052 (2004).
Royle, S. J., Bright, N. A. & Lagnado, L. Clathrin is required for the function of the mitotic spindle. Nature 434, 1152–1157 (2005).
Keen, J. H., Willingham, M. C. & Pastan, I. H. Clathrin coated vesicles: isolation, dissociation and factor dependent reassociation of clathrin baskets. Cell 16, 303–312 (1979).
Ahle, S. & Ungewickell, E. Purification and properties of a new clathrin assembly protein. EMBO J. 5, 3143–3149 (1986).
Zaremba, S. & Keen, J. H. Limited proteolytic digestion of coated vesicle assembly polypeptides abolishes reassembly activity. J. Cell. Biochem. 28, 47–58 (1985).
Schroder, S. & Ungewickell, E. Subunit interaction and function of clathrin-coated vesicle adaptors from the Golgi and the plasma membrane. J. Biol. Chem. 266, 7910–7918 (1991).
Peden, A. A. et al. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 164, 1065–1076 (2004).
Peden, A. A., Rudge, R. E., Lui, W. W. & Robinson, M. S. Assembly and function of AP-3 complexes in cells expressing mutant subunits. J. Cell Biol. 156, 327–336 (2002).
Collins, B. M., McCoy, A. J., Kent, H. M., Evans, P. R. & Owen, D. J. Molecular architecture and functional model of the endocytic AP2 complex. Cell 109, 523–535 (2002).
Heldwein, E. E. et al. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl Acad. Sci. USA 101, 14108–14113 (2004). Describes the molecular architecture of AP1 and gives insights into the functional mechanisms of AP1.
Owen, D. & Evans, P. A structural explanation for the recognition of tyrosine-based endocytic signals. Science 282, 1327–1332 (1998).
Pauloin, A. & Thurieau, C. The 50 kDa protein subunit of assembly polypeptide (AP) AP-2 adaptor from clathrin-coated vesicles is phosphorylated on threonine-156 by AP-1 and a soluble AP50 kinase which copurifies with the assembly polypeptides. Biochem. J. 296, 409–415 (1993).
Olusanya, O., Andrews, P. D., Swedlow, J. R. & Smythe, E. Phosphorylation of threonine 156 of the μ2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol. 11, 896–900 (2001).
Conner, S. & Schmid, S. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 156, 921–929 (2002).
Conner, S. D., Schroter, T. & Schmid, S. L. AAK1-mediated μ2 phosphorylation is stimulated by assembled clathrin. Traffic 4, 885–890 (2003).
Jackson, A. P. et al. Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor μ2 kinase. J. Cell Biol. 163, 231–236 (2003).
Ricotta, D., Conner, S. D., Schmid, S. L., von Figura, K. & Honing, S. Phosphorylation of the AP2 μ subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J. Cell Biol. 156, 791–795 (2002). Establishes a role for μ2-subunit phosphorylation in AP2 function.
Fingerhut, A., von Figura, K. & Honing, S. Binding of AP2 to sorting signals is modulated by AP2 phosphorylation. J. Biol. Chem. 276, 5476–5482 (2001).
Honing, S. et al. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell 18, 519–531 (2005).
Ghosh, P. & Kornfeld, S. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J. Cell Biol. 160, 699–708 (2003).
Umeda, A., Meyerholz, A. & Ungewickell, E. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur. J. Cell Biol. 79, 336–342 (2000).
Korolchuk, V. & Banting, G. CK2 and GAK/auxilin2 are major protein kinases in clathrin-coated vesicles. Traffic 3, 428–439 (2002).
Simonsen, A., Wurmser, A. E., Emr, S. D. & Stenmark, H. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13, 485–492 (2001).
McPherson, P. et al. A presynaptic inositol-5-phosphatase. Nature 379, 353–357 (1996).
Chung, J. K. et al. Synaptojanin inhibition of phospholipase D activity by hydrolysis of phosphatidylinositol 4,5-biphosphate. J. Biol. Chem. 272, 15980–15985 (1997).
Padron, D., Wang, Y., Yamamoto, M., Yin, H. & Roth, M. Phosphatidylinositol phosphate 5-kinase Iβ recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J. Cell Biol. 162, 693–701 (2003).
Gaidarov, I., Chen, Q., Falck, J., Reddy, K. & Keen, J. A functional phosphatidylinositol 3,4,5-triphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 α subunit. J. Biol. Chem. 271, 20922–20929 (1996).
Gaidarov, I. & Keen, J. H. Phosphoinositide–AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol. 146, 755–764 (1999).
Gaidarov, I., Smith, M. E., Domin, J. & Keen, J. The class II phosphoinositide 3-kinase C2α is activated by clathrin and regulated clathrin-mediated membrane trafficking. Mol. Cell 7, 443–449 (2001).
Rohde, G., Wenzel, D. & Haucke, V. A phosphatidylinositol (4,5)-bisphosphate binding site within μ2-adaptin regulates clathrin mediated endocytosis. J. Cell Biol. 158, 209–214 (2002).
Munro, S. Organelle identity and the targeting of peripheral membrane proteins. Curr. Opin. Cell Biol. 14, 506–514 (2002).
Crottet, P., Meyer, D. M., Rohrer, J. & Spiess, M. ARF1•GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol. Biol. Cell 13, 3672–3682 (2002).
Traub, L. M., Ostrom, J. A. & Kornfeld, S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell Biol. 123, 561–573 (1993).
Wang, Y. J. et al. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299–310 (2003).
Austin, C., Hinners, I. & Tooze, S. Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem. 275, 21862–21869 (2000).
Boehm, M., Aguilar, R. C. & Bonifacino, J. S. Functional and physical interactions of the adaptor protein complex AP-4 with ADP-ribosylation factors (ARFs). EMBO J. 20, 6265–6276 (2001).
Robinson, M. S. & Kreis, T. E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell 69, 129–138 (1992).
Wong, D. H. & Brodsky, F. M. 100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties. J. Cell Biol. 117, 1171–1179 (1992).
Paleotti, O. et al. The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J. Biol. Chem. 280, 21661–21666 (2005).
Krauss, M. et al. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J. Cell Biol. 162, 113–124 (2003).
Tanabe, K. et al. A novel GTPase-activating protein for ARF6 directly interacts with clathrin and regulates clathrin-dependent endocytosis. Mol. Biol. Cell 16, 1617–1628 (2005).
Traub, L. M. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta 1744, 415–437 (2005).
Praefcke, G. et al. Evolving nature of the AP2 α-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 23, 4371–4383 (2004).
Owen, D., Vallis, Y., Noble, M., Hunter, J. & McMahon, H. A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell 97, 805–815 (1999).
Traub, L., Downs, M., Westrich, J. & Fremont, D. Crystal structure of the α appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc. Natl Acad. Sci. USA 96, 8907–8912 (1999).
Brett, T., Traub, L. & Fremont, D. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure 10, 797–809 (2002).
Ritter, B. et al. Two WXXF-based motifs in NECAPs define the specificity of accessory protein binding to AP-1 and AP-2. EMBO J. 23, 3701–3710 (2004).
Nogi, T. et al. Structural basis for the accessory protein recruitment by the γ-adaptin ear domain. Nature Struct. Biol. 9, 527–531 (2002).
Kent, H., McMahon, H., Evans, P., Benmerah, A. & Owen, D. γ-adaptin appendage domain: structure and binding site for eps15 and γ-synergin. Structure 10, 1139–1148 (2002).
Mishra, S. K. et al. Dual engagement regulation of protein interactions with the AP-2 adaptor α appendage. J. Biol. Chem. 279, 46191–46203 (2004).
Owen, D., Vallis, Y., Pearse, B., McMahon, H. & Evans, P. The structure and function of the β2-adaptin appendage domain. EMBO J. 19, 4216–4227 (2000).
Mishra, S. K. et al. Functional dissection of an AP-2 β2 appendage-binding sequence within the autosomal recessive hypercholesterolemia (ARH) protein. J. Biol. Chem. 280, 19270–19280 (2005).
Mills, I. G. et al. EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J. Cell Biol. 160, 213–222 (2003).
Miller, G. J., Mattera, R., Bonifacino, J. S. & Hurley, J. H. Recognition of accessory protein motifs by the γ-adaptin ear domain of GGA3. Nature Struct. Biol. 10, 599–606 (2003).
Collins, B. M., Praefcke, G. J., Robinson, M. S. & Owen, D. J. Structural basis for binding of accessory proteins by the appendage domain of GGAs. Nature Struct. Biol. 10, 607–613 (2003).
Duden, R., Allan, V. & Kreis, T. Involvement of β-COP in membrane traffic through the Golgi complex. Trends Cell Biol. 1, 14–19 (1991).
Schledzewski, K., Brinkmann, H. & Mendel, R. R. Phylogenetic analysis of components of the eukaryotic vesicle transport system reveals a common origin of adaptor protein complexes 1, 2, and 3 and the F subcomplex of the coatomer COPI. J. Mol. Evol. 48, 770–778 (1999).
Hoffman, G. R., Rahl, P. B., Collins, R. N. & Cerione, R. A. Conserved structural motifs in intracellular trafficking pathways: structure of the γCOP appendage domain. Mol. Cell 12, 615–625 (2003).
Watson, P. J., Frigerio, G., Collins, B. M., Duden, R. & Owen, D. J. γ-COP appendage domain — structure and function. Traffic 5, 79–88 (2004).
McMahon, H. T. & Mills, I. G. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr. Opin. Cell Biol. 16, 379–391 (2004).
Eugster, A., Frigerio, G., Dale, M. & Duden, R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 19, 3905–3917 (2000).
Devos, D. et al. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2, e380 (2004).
Gonzalez, L. J., Weis, W. & Scheller, R. A novel SNARE N-terminal domain revealed by the crystal structure of Sec22b. J. Biol. Chem. 276, 24203–24221 (2001).
Tochio, H., Tsui, M., Bandfield, D. & Zhang, M. An autoinhibitory mechanism for non syntaxin SNARE proteins revealed by the structure of Ykt6p. Science 293, 698–702 (2001).
Schwartz, T. & Blobel, G. Structural basis for the function of the β subunit of the eukaryotic signal recognition particle receptor. Cell 112, 793–803 (2003). Provides a possible structural mechanism for the interaction between two widely conserved protein folds — the σ/N-μ2 fold and that of an ARF-family GTPase.
Wilbur, J. D., Hwang, P. K. & Brodsky, F. M. New faces of the familiar clathrin lattice. Traffic 6, 346–350 (2005).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ARH and Dab2
-
Autosomal recessive hypercholesterolaemia protein (ARH) and Disabled-2 (Dab2) are clathrin adaptors that bind to members of the low density lipoprotein (LDL)-receptor family.
- α-helical hairpin
-
A structural motif that comprises a short α-helix joined to another α-helix by a loop. The helices lie side by side, and the motif can be repeated many times with the hairpins stacking on top of one another to form a twisting helical solenoid.
- J domain
-
A conserved domain that stimulates ATP hydrolysis by chaperones of the 70-kDa heat-shock protein (Hsp70) family. It was first identified in the Escherichia coli co-chaperone DnaJ, but it is also found in DnaJ homologues in eukaryotes, as well as in several co-chaperones that recruit Hsp70 proteins for specific cellular processes.
- AP180
-
(adaptor protein of 180 kDa). AP180 is another clathrin adaptor that is found in clathrin coats, but is not related to the APs (adaptor protein complexes). It is a single-chain clathrin adaptor that is composed of an ∼30-kDa helical ANTH (AP180 N-terminal homology) domain at its N terminus followed by a large unstructured region.
Rights and permissions
About this article
Cite this article
Edeling, M., Smith, C. & Owen, D. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol 7, 32–44 (2006). https://doi.org/10.1038/nrm1786
Issue Date:
DOI: https://doi.org/10.1038/nrm1786
This article is cited by
-
NPC1-regulated dynamic of clathrin-coated pits is essential for viral entry
Science China Life Sciences (2022)
-
Mechanism and evolution of the Zn-fingernail required for interaction of VARP with VPS29
Nature Communications (2020)
-
A new role of anterograde motor Kif5b in facilitating large clathrin-coated vesicle mediated endocytosis via regulating clathrin uncoating
Cell Discovery (2018)
-
Kinetic constraints on self-assembly into closed supramolecular structures
Scientific Reports (2017)
-
The long life of an endocytic patch that misses AP-2
Current Genetics (2016)