Key Points
-
Cortical spreading depression (CSD) is a slowly propagating wave of rapid, near-complete depolarization of brain cells that lasts for about 1 minute and silences brain electrical activity for several minutes; it can be induced in normally metabolizing tissue by depolarizing stimuli that increase extracellular K+ concentration ([K+]e) above a critical value. Longer-lasting spreading depolarizations arise in metabolically compromised brain tissue.
-
CSD initiation depends on the activation of ion channels located in dendrites of pyramidal cells and on the generation of a net self-sustaining inward current that initiates a positive-feedback cycle leading to a regenerative increase in [K+]e and regenerative depolarization. NMDA receptors (NMDARs) and voltage-gated Ca2+ channel (in particular Cav2.1)-dependent release of glutamate have a key role in the positive-feedback cycle that ignites CSD; depending on the method of induction, [Ca2+]e-independent glutamate release may contribute.
-
CSD propagation is probably mediated by interstitial diffusion of K+ released during the depolarization (accompanied by [K+]e-dependent glutamate release), initiating the positive-feedback cycle that ignites CSD in contiguous grey matter.
-
The mechanisms initiating CSD and spreading depolarizations are different. Besides NMDARs, other ion channels and processes (probably including persistent voltage-gated Na+ channels and mitochondrial depolarization) seem to be crucial for the initiation of spreading depolarizations.
-
Propagating depolarizing events in brain have been linked to neurovascular disorders such as migraine and stroke. In migraine, CSD has been linked to migraine aura, trigeminal activation and headache as well as the actions of preventative drugs.
-
It has been increasingly recognized that spreading depolarizations compromise energy metabolism and blood flow, contributing to poor tissue outcome when they erupt in the injured brain. Hence, there is an increasing demand for treatment strategies that selectively block initiation, propagation or enhance recovery to mitigate the impact of chaos and commotion that surrounds CSD and spreading depolarizations.
Abstract
Punctuated episodes of spreading depolarizations erupt in the brain, encumbering tissue structure and function, and raising fascinating unanswered questions concerning their initiation and propagation. Linked to migraine aura and headache, cortical spreading depression contributes to the morbidity in the world's migraine with aura population. Even more ominously, erupting spreading depolarizations accelerate tissue damage during brain injury. The once-held view that spreading depolarizations may not exist in the human brain has changed, largely because of the discovery of migraine genes that confer cortical spreading depression susceptibility, the application of sophisticated imaging tools and efforts to interrogate their impact in the acutely injured human brain.
Similar content being viewed by others
Main
In addition to the targeted and specific electrical activity that occurs in the neural networks of healthy tissue, the brain also exhibits slowly propagating, self-sustaining waves of depolarization of a sizable population of cells that can arise in both apparently normal tissue (for example, in migraine) and metabolically compromised tissue (for example, during ischaemia). Recent evidence suggests that there are several different types of propagating depolarizations, each with their own molecular and electrical signature and characteristic consequences for neuronal function.
Cortical spreading depression (CSD) is a slowly propagating wave of rapid, near-complete depolarization of brain cells that lasts for about 1 minute and silences brain electrical activity for several minutes (hence the name spreading depression)1,2. It is characterized by the collapse of ion homeostasis, profound disruption of transmembrane ionic gradients and the release of neurotransmitters and other molecules from cellular compartments2. Experimental induction of CSD in normally metabolizing brain tissue requires intense depolarizing stimuli that increase the extracellular concentration of K+ ([K+]e) above a critical threshold2.
Metabolic impairment of brain tissue leads to spreading depolarizations that, like CSD, are characterized by abrupt, near-complete sustained depolarization of neurons and massive redistribution of ions, and propagate at a similar rate to CSD2,3. However, in spreading depolarizations, restoration of the ionic gradients, repolarization of the membrane potential and recovery of synaptic transmission and brain function either occur with a prolonged time course compared with CSD or do not occur at all, depending on the degree of local metabolic compromise and impairment of (Na+ + K+)ATPase activity2,3. An example of recurrent spreading depolarizations of longer duration than CSD is the so-called peri-infarct depolarizations, which occur in the ischaemic penumbra after, for example, middle cerebral artery occlusion3. The so-called anoxic depolarization is induced by hypoxia, ischaemia or severe hypoglycaemia in vivo and hypoxia or oxygen-glucose deprivation (OGD) in brain slices2,3. It may reside at one extreme of the spreading depolarization spectrum, as it is characterized by a lack of neuronal membrane repolarization unless there is rapid reperfusion or reoxygenation.
It is important to note that spreading depolarizations do not necessarily begin a spreading depression of brain activity; indeed, in brain tissue exposed to hypoxia or ischaemia, the suppression of action potential-evoked synaptic transmission occurs before the abrupt neuronal depolarization and not as a consequence of it2,3. Furthermore, the mechanisms of initiation of CSD and spreading depolarizations are different. For these reasons, we use the term CSD to refer to the propagating depolarization that is followed by suppression of spontaneous activity and that is evoked in normally metabolizing brain tissue. We use the term spreading depolarization for all propagating depolarizations that are induced within brain tissue with differing degrees of metabolic impairment and/or inhibition of neuronal and glial (Na+ + K+)ATPases, and ranging from mild, CSD-like spreading depolarizations (which occur when the metabolic impairment is small or when only a small fraction of (Na+ + K+)ATPases are inhibited) to anoxic depolarization, which occurs when the metabolic impairment is near-complete.
CSD causes no cell death or long-lasting damage in a normally metabolizing brain4 but imposes a considerable bioenergetic burden on tissue. Clinically, CSD accounts for the spreading visual percept of migraine aura and may trigger headaches5,6,7. However, in the injured brain, there is an eruption of spreading depolarizations, and these exacerbate tissue damage in experimental models of stroke, trauma and subarachnoid haemorrhage3,8. In human brain injury, the evidence for the occurrence of spreading depolarizations is becoming compelling. Hence, there is an undeniable need to identify coherent biophysical and molecular principles that underlie the eruptions and propagations of CSD and spreading depolarizations.
A large part of this Review therefore examines current knowledge of the molecular and cellular mechanisms underlying the initiation and propagation of CSD and spreading depolarizations, and their sustained depolarization phase. Despite important progress in research in this area, these mechanisms remain incompletely understood and controversial owing to the scarcity of direct experimental evidence and the complexity of the phenomena, which involve sequential phases with different mechanisms that make the interpretation of the available data (mainly pharmacological) difficult. The second part summarizes the evidence and emerging importance of CSD and spreading depolarizations in migraine and acute brain injury such as stroke, brain trauma and other conditions. A discussion of short- and long-term effects of CSD on brain integrative function is outside the scope of this Review.
Phenomenology of CSD
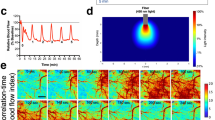
CSD depolarization and associated ionic changes. CSD was first described by Leao1. He reported that brief, repetitive electrical stimulation of the rabbit cerebral cortex induces a spreading depression of spontaneous (and sensory-evoked) electroencephalogram (EEG) activity that lasts for several minutes1 and a large (from −5 to −15 mV) negative variation of the interstitial direct current potential (ΔVo) that lasts for 1 to 2 minutes9. ΔVo is typically characterized by a rapidly attained early peak that is followed by either a less negative plateau or, after a brief decline (called a 'notch'), a slow second negative peak2. This second peak is particularly prominent in the apical dendrite layer (stratum radiatum) of the CA1 hippocampal region, where the Vo waveform has a typical inverted saddle shape (Fig. 1a). Subsequent intracellular recordings from individual cortical neurons revealed near-complete neuronal depolarization (Fig. 1a) and a strong decrease in membrane resistance during the negative ΔVo (Ref. 2). Besides electrical stimulation, topical or intraparenchymal application of high concentrations of KCl are commonly used to induce CSD in the healthy brain.
a | The two top traces show the extracellular potential (Vo) recorded simultaneously in the apical dendrites and pyramidal cell soma regions in the hippocampus during cortical spreading depression (CSD) induced by high K+ ejection in the stratum radiatum. The lower trace shows the membrane potential (Vm) of a pyramidal cell during CSD. During the early phase, the apical dendrites depolarize almost completely, whereas the soma depolarizes only partially. During the main phase, both the soma and dendrites are completely depolarized. During the late phase, only a narrow band in the proximal apical dendrites remains completely depolarized11. b | Changes in the extracellular concentrations of K+ ([K+]e), of Na+ ([Na+]e), of Cl− ([Cl−]e), of Ca2+ ([Ca2+]e) and of glutamate during CSD depolarization are shown: the rapid Vo change during the early phase of CSD is accompanied by a rapid [K+]e increase to 30–60 mM (from a baseline value of 2.7–3.5 mM), a [Na+]e and [Cl−]e decrease to 50–70 mM (from 140–150 mM) and a [Ca2+]e decrease to 0.2–0.8 mM (from 1.0–1.5 mM). The CSD depolarization and associated ionic changes are assumed to propagate across the cerebral cortex from right to left; the red dashed line represents the CSD wavefront. The [K+]e rise is assumed to precede the fast regenerative [K+]e increase associated with the early rapid Vo change, as expected if CSD propagation is mainly mediated by diffusion of K+ in the extracellular space. Part a is adapted with permission from Ref. 11, The American Physiological Society.
CSD is a complex phenomenon that has different phases, some of which are characterized by longitudinal gradients of depolarization within individual neurons10,11. This has been shown in the hippocampus: simultaneous recordings from the dendritic and somatic regions revealed that the ΔVo and the neuronal membrane depolarization start earlier and last longer in the stratum radiatum than in the stratum pyramidale10,11 (Fig. 1a). In particular, there is an early phase, lasting a few seconds, in which the apical dendrites are almost completely depolarized while the soma is only partially depolarized; this is followed by a phase in which the entire somatodendritic membrane is completely depolarized (main phase, lasting 15–20 seconds); the main phase is followed by a late phase that corresponds to the second slow negative ΔVo peak in the stratum radiatum, in which only a narrow band in the proximal apical dendrites remains fully depolarized while the soma is partially repolarized11 (Fig. 1a). These features indicate that the CSD-related depolarization is initiated by the activation of channels located in apical dendrites of pyramidal neurons (early phase) and subsequent activation of other ion channels along most of the somatodendritic membrane (main phase). This is followed by closure of channels in the somatobasal zone, restricting net inward current to a narrow band in the proximal apical dendrites (late phase)11,12. Once initiated, CSD self-propagates in contiguous grey matter at a velocity of 2–5 mm min−1, as if it were a wave. The leading edge of the wave travels in layers containing apical dendrites10,13,14.
The rapid Vo change during the early phase of CSD is accompanied by a rapid increase in [K+]e to 30–60 mM, a rapid decrease in [Na+]e and [Cl−]e to 50–70 mM and in [Ca2+]e to 0.2–0.8 mM (Fig. 1b); it is also associated with a transient increase in extracellular pH (pHe) that is followed by a decrease in pHe during the sustained depolarization2. The reduction in [Na+]e is greater than the increase in [K+]e (whereas Δ[Na+]e and Δ[Cl−]e are similar in magnitude and time course); electroneutrality is probably maintained by efflux of organic anions, including glutamate2. Indeed, several amino acids, including glutamate and aspartate, are released during CSD2 (Fig. 1b). Similar ionic changes occur during hypoxic spreading depolarizations2.
CSD has been associated with large increases in the intracellular concentration of Ca2+ ([Ca2+]i) in both neurons and astrocytes in the cerebral cortex15,16; however, the [Ca2+]i increase in neurons precedes that in astrocytes16, and the CSD-associated neuronal [Ca2+]i wave is unaffected by suppression of the [Ca2+]i increase in astrocytes15,16. Thus, neurons lead, astrocytes follow, and the changes in [Ca2+]e during CSD mainly reflect influx into neurons. In CA1 pyramidal cells, [Ca2+]i increases first in dendrites then in somata17; moreover, the [Ca2+]i increase lasts longer in apical dendrites than in somata and, in the late phase of CSD, a large increase is observed only at the dendrites, in correlation with the main and late phases of the CSD depolarization17.
The transient increase in pHe during CSD onset reflects a transient proton influx into (and/or HCO3− efflux from) neurons, as it is accompanied by a transient decrease in intracellular pH (pHi) in neurons, whereas pHi in astrocytes increases18.
Astrocyte depolarization during CSD seems to be largely passive and is caused by an increase in [K+]e (Ref. 2). Moreover, a fast mitochondrial depolarization coincident with CSD occurs in neurons but not astrocytes18.
Upon the influx of Na+, Cl− and water, the interstitial space shrinks (by 40–70%)2 mainly as a consequence of neuronal swelling in vivo and in vitro18,19. By contrast, astrocytes display only passive swelling in response to CSD-inducing high [K+] solutions in cortical slices18 and do not swell in vivo19.
Within seconds of depolarization, cortical layer 2 dendrites show various morphological changes, including beading and loss of spines19. These changes are reversible within 8–10 minutes, which is about the same time frame for the recovery from suppression of EEG activity.
Cerebral blood flow and metabolic changes. Under most circumstances, cerebral blood flow and brain metabolism are tightly coupled, as brain blood flow rises with an increasing demand for oxygen and glucose. As ATP is used to restore ionic gradients and to repolarize the membrane potential, CSD is associated with a large increase in energy metabolism and therefore a large transient increase in cerebral blood flow20,21,22.
ATP is generated mainly within mitochondria by an oxygen-utilizing process called oxidative phosphorylation. Mitochondrial activity reaches such a high rate during the repolarization phase of CSD that oxygen consumption (cerebral metabolic rate of O2 (CMRO2)) may exceed the ability of blood flow to supply necessary amounts of oxygen. Hence, the tissue partial pressure of O2 (pO2) may decrease to anoxic levels (and haemoglobin desaturates within ischaemic range) for up to 2 minutes after CSD in rodents19,22,23. Further evidence that the brain is deprived of oxygen after CSD comes from the observation that an NADH fluorescent signal increases in small tissue pockets between capillary territories, reflecting decreased NADH utilization owing to reduced oxygen availability19.
Not surprisingly, cerebral blood flow responses to CSD endure for hours. In rodents, CSD-associated blood flow rises are followed by prolonged mild oligaemia5,19,22,23,24 and by a prolonged reduction in tissue pO2 and haemoglobin desaturation22,23. The oligaemia lasts (1–2 hours) much longer than the suppression of spontaneous electrical activity (10–20 minutes) and is partly due to impaired vascular responsivness25,26. The underlying reasons for this oligaemia, however, remain unclear, particularly as it occurs despite an increase in CMRO2 (for about 2 hours)22. Increased synthesis of the vasoconstrictor 20-HETE may explain part of the prolonged oligaemia but not the prolonged uncoupling of blood flow to neuronal activity27. In this regard, a significant second, more prolonged CSD phase that begins after recovery from the initial CSD wave has been reported23. This phase is accompanied by arterial constriction, reduced oxygen delivery and a negative Vo shift. Both phases may be mechanistically distinct.
Mechanisms
Initiation of CSD. Besides neuronal depolarization, the experimental stimuli that have been found to generate CSD in healthy brain tissue cause an increase in local [K+]e and the release of glutamate (and other neurotransmitters) owing to depolarization of presynaptic terminals and activation of voltage-gated Ca2+ (Cav) channels. Both experimental data28,29 and computational models30 support the idea that an increase in [K+]e above a critical value is a key initiating event, as originally proposed by Grafstein31. Modelling has shown that the generation of a net self-sustaining inward current across the membrane is necessary to initiate the positive-feedback cycle that makes the initial gradual neuronal depolarization self-regenerative and confers to CSD its all-or-none characteristics; this is achieved when a sufficient number of voltage-gated and/or [K+]e-dependent channels carrying an inward current are activated by the CSD-inducing depolarizing stimulus. The net inward current leads to membrane depolarization and the release of K+ into the restricted interstitial space, which in turn leads to further activation of voltage-gated and/or [K+]e-dependent channels, further depolarization and an increase in local [K+]e. This may cause complete neuronal depolarization30 if removal of K+ from the interstitium does not keep pace with its release (Fig. 2a).
a | A schematic diagram of the requirements for cortical spreading depression (CSD) initiation as inferred from modelling. As indicated in the green box, the stimuli that can induce CSD in healthy brain tissue produce neuronal depolarization and an increase in local extracellular K+ concentration ([K+]e). Initiation of the positive-feedback cycle that ignites CSD requires the generation of a net inward current as a consequence of the activation of a sufficient number of voltage-gated and/or [K+]e-dependent channels carrying an inward current (blue box). The net inward current leads to membrane depolarization and an increase in [K+]e, which in turn leads to further activation of voltage-gated and/or [K+]e-dependent channels, further depolarization and further increase in local [K+]e. This results in complete neuronal depolarization if the removal of K+ from the interstitium mainly by glial reuptake mechanisms (grey box) does not keep pace with its release. b | A schematic diagram of the initiation mechanism in the case of CSD induced by a brief K+ pulse or by electrical stimulation in which voltage-gated Ca2+ (Cav) Cav2.1 channel-dependent release of glutamate from cortical pyramidal cell synapses and activation of NMDA receptors (NMDARs) have a key role in the positive-feedback cycle that ignites CSD. In this scheme, the glial reuptake mechanisms exert a dampening role by mediating both K+ and glutamate reuptake. Although not illustrated, a relatively minor role of other ion channels (for example, postsynaptic Cav and/or voltage-gated Na+ channels) in the initiation of the positive-feedback cycle cannot be excluded. Vm, membrane potential.
Although there is a general consensus that the regenerative increase in [K+]e is an essential component of the positive-feedback cycle that ignites CSD and that CSD initiation depends on the activation of ion channels located in dendrites of pyramidal cells, the nature of the channels that are crucial for generating the initial net inward current and that mediate local K+ release remains incompletely understood. Moreover, although it is known that local [K+]e increases may modulate synaptic and intrinsic firing properties of individual neurons when in the relevant range for CSD initiation32, the complex feedback loops that intimately link neuronal activity and [K+]e remain incompletely understood.
A mechanistic understanding of the ion channels and mechanisms involved in CSD initiation derives largely from pharmacological studies on experimental CSD. Such studies have been hampered by the fact that CSD involves sequential phases with different pharmacology, and it is often difficult to differentiate between blockade of initiation, propagation or sustained depolarization; and that pharmacological profiles differ depending on the nature of the CSD-inducing stimulus. An insufficient appreciation of these problems underlies some of the confusion and controversy surrounding the initiating mechanisms. Hence, we discuss the methods that contribute to differing CSD pharmacology separately and specify the experimental system. Because the initiating mechanisms in the cerebral cortex and hippocampus are well characterized, they are the primary focus of the discussion.
CSDs induced by electrical stimulation or local application of brief high K+ pulses share a similar pharmacology. NMDA receptor (NMDAR) antagonists but not AMPA–kainate receptor (AMPAKR) antagonists33,34 completely block CSD that is recorded far (≥500 μm) from the electrical or high-K+ stimulation site in the cerebral cortex in vivo33,35,36,37,38 and in vitro in cortical slices14,15,39,40,41. In both cases, NMDAR antagonists completely block CSD even when the intensity of stimulation is several times larger than threshold33,36,41. These studies show that NMDARs are necessary for CSD initiation or propagation (or for both); most studies cannot discriminate between the two. Some pharmacological studies suggest that NMDARs are necessary for CSD propagation and are involved in, but might not be necessary for, CSD initiation, at least in the cerebral cortex. For example, NMDAR antagonists dose-dependently increase the electrical stimulation threshold for CSD induction when applied at subsaturating concentrations in vivo36, and, at high doses, block CSD close to the stimulating electrode when threshold stimulation is used33. However, using suprathreshold stimulation and recording close to the stimulating site, either a non-propagating negative ΔVo of smaller size than a typical CSD33,34,36 or no change in Vo (Ref. 35) was recorded. Moreover, higher doses of a non-competitive NMDAR antagonist are necessary to block CSD initiation compared with those that are needed to block propagation35,40,42.
Complete blockade of a propagating CSD seems to require higher concentrations of competitive NMDAR antagonists in hippocampal than in cortical slices41,43,44, suggesting a role for distinct NMDAR subtypes or region-specific CSD mechanisms. Interestingly, the concentration of competitive NMDAR antagonists required to block CSD is higher than that required to block NMDAR-dependent synaptic transmission17,43, perhaps owing to a higher extracellular concentration of glutamate at the CSD wavefront and/or the involvement of NMDARs with distinct subunit composition and/or subcellular localization43.
The data implicating NMDARs in CSD initiation imply that glutamate has a vital role in this process, as first proposed by van Harreveld45. He showed that topical glutamate application can initiate CSD in the cortex. Pharmacological and functional analyses of mutant mice with an altered CSD threshold (see below) show that the NMDARs implicated in CSD initiation are activated by glutamate released from synaptic terminals after the opening of Cav channels. Indeed, Cav channels seem to be crucial because, in brain slices, the propagating CSD is abolished in Ca2+-free medium15,39,46 or after blocking Cav channels with Cd2+ or Ni2+ (Refs 39,47). Recent evidence suggests that Cav2.1 channels (also known as P/Q-type Ca2+ channels) have a particularly important role because CSD is also abolished after specific blockade of these channels, even when CSD is triggered by largely suprathreshold stimuli41. Other Cav channels such as Cav2.2 (also known as N-type Ca2+ channels) or Cav2.3 (also known as R-type Ca2+ channels) seem to play a less significant part, and Cav1.2 channels (also known as L-type Ca2+ channels) do not seem to be involved41. Further support for the crucial role of Cav2.1 channels is provided by tottering and leaner mice, which carry mutations that produce a partial loss of function of the Cav2.1 channel48. In these mice, the threshold for CSD induction in the cerebral cortex in vivo is greatly increased, and K+-evoked glutamate release (as measured by in vivo microdialysis) is reduced by more than twofold49. Conversely, familial hemiplegic migraine type 1 (FHM1)-knockin mice, which carry gain-of-function mutations in the gene encoding the Cav2.1 channel48,50, show increased action potential-evoked glutamate release at cortical synapses51 and have a lowered threshold for CSD induction in vivo50,52 and in cortical slices51. Restoring evoked glutamate release to the wild-type value (by partial inhibition of Cav2.1 channels) restores the CSD threshold to the wild-type value in FHM1-knockin mice51. This finding supports a causative relationship between increased Cav2.1-dependent glutamate release and facilitation of CSD initiation.
Overall, the data from electrical stimulation or K+ pulse studies support a model for CSD initiation in which Cav (in particular Cav2.1) channel-dependent release of glutamate from cortical pyramidal cell synapses and activation of NMDARs have a key role in the positive-feedback cycle that ignites CSD (Fig. 2b) and possibly point to an additional specific role of postsynaptic Cav2.1 channels.
Astrocytes have also been implicated in CSD initiation. In the adult brain, the α2 (Na+ + K+)ATPase is expressed almost exclusively in astrocytes53,54 and is probably involved in the clearance of K+ (Refs 55,56) and glutamate (through functional coupling with glutamate transporters)54,57,58 during intense neuronal activity. A lowered electrical threshold for CSD in vivo is exhibited by heterozygous FHM2-knockin mice, which carry a loss-function mutation in the gene encoding α2 (Na+ + K+)ATPase that reduces the expression of this protein by 50%59. Furthermore, it has been shown in mouse brain slices that a lower threshold for CSD induction is produced by astrocyte-directed inactivation of the major astrocytic gap junction protein connexin 43 (Ref. 60), probably as a consequence of deficient K+ spatial buffering by astrocytes61. Taken together, these data support a model in which CSD initiation that occurs as a result of positive feedback between a local [K+]e increase, synaptic glutamate release and activation of NMDARs is opposed by the astrocytic α2 (Na+ + K+)ATPase pump, which dampens CSD susceptibility owing to its direct involvement in K+ clearance and/or indirect role in glutamate clearance59,62,63 (Fig. 2).
So far, we have discussed the effect of K+ pulses, but diffuse and/or prolonged application of high [K+] can also induce CSDs in which NMDARs are critically involved both in vivo40,42,64,65 and in brain slices40,66,67,68,69 (although, again, it is controversial whether they are necessary). However, in contrast to the responses following brief K+ pulses, blockade of Cav2.1 channels did not abolish CSDs evoked by continuous perfusion with high [K+] solutions in cortical slices70 or by KCl crystals applied in vivo71. Moreover, CSD induced in cortical slices by minutes perfusion with relatively high K+ was not completely inhibited in Ca2+-free solution18 or after blocking Cav channels with Cd2+ (Ref. 69). This [Ca2+]e-independent CSD is accompanied by a smaller (less than 50%) transient increase in extracellular glutamate levels than control CSD but still requires NMDARs for initiation69. Pharmacological evidence suggests that induction of [Ca2+]e-independent CSD depends on a vicious cycle of glutamate-induced glutamate release involving presynaptic NMDARs and Ca2+ efflux from mitochondria through the Na+/Ca2+ exchanger69.
Tetrodotoxin (TTX), a voltage-gated Na+ (Nav) channel blocker, prevents CSD induced in vivo by repetitive electrical stimulation but not CSD induced by KCl crystal72 or high K+ dialysis10. In cortical slices, the impact of TTX on K+-induced CSD is controversial18,68,69. Overall, CSD caused by high [K+] does not seem to rely on action potential-dependent glutamate release, action potential-dependent K+ efflux or activation of postsynaptic TTX-sensitive Na+ channels, although they may play a part. Perhaps Nav channels have a more important role in CSD induced by pinprick of the cerebral cortex in vivo (Table 1). In general, the pharmacology of CSD induced by pinprick is quite different from that of CSDs induced by electrical stimulation or high levels of KCl (Table 1).
The different CSD pharmacologies summarized in Table 1 underscore the importance of knowing which experimental method of CSD induction is of particular relevance to the specific human condition to be considered below. The differing methods of induction may provide insight into initiating mechanisms that evoke CSD in differing tissue states. For example, sustained high [K+] may better reflect the initiation of events (CSD-like spreading depolarizations) in the ischaemic peri-infarct zone than brief K+ pulses, which may be relevant for migraine and CSD eruption in normal tissue.
Propagation of CSD. The typical slow rate of CSD propagation implies that it is mediated by the diffusion of a chemical substance. Although, as discussed below, most evidence points to diffusion of K+ released in the interstitial space during CSD as the underlying mechanism, the diffusing substance and the diffusion pathway are still debated. Four hypotheses have been proposed: two involve intercellular diffusion and opening of gap junctions in either glial cells or neurons2,73, and the other two are based on interstitial diffusion of a humoral agent, K+ (Ref. 31) or glutamate45.
A role of gap junctions in CSD propagation is challenged by the findings that propagation is not inhibited by carbenoxolone, a gap-junction blocker14,15,74, and that the rate of CSD propagation may even increase after gap-junction blockade75. Conversely, the involvement of humoral mechanisms is strongly supported by the finding that CSD propagation is inhibited in the cerebral cortex by diluting the mediating substance (or substances) using intracerebral microdialysis of a physiological solution76.
As discussed in the previous sections, flooding of K+ and glutamate into the extracellular space during CSD produces a large increase in their extracellular concentrations (Fig. 1b). The local increase in [K+]e above a critical value and [K+]e-induced glutamate release are both key elements in the positive-feedback cycle that ignites CSD (Fig. 2b). Thus, both K+ and glutamate may mediate CSD propagation by diffusing into contiguous grey matter and initiating there the positive-feedback cycle shown in Fig. 2b. Most evidence points to K+ rather than glutamate as the relevant diffusing substance. For example, high levels of K+ but not glutamate can restore CSD propagation after its inhibition by intracerebral dialysis of a physiological solution76,77. Furthermore, the enhancing effects of electric fields on propagation velocity are consistent with a positively (but not negatively) charged diffusing substance31. The higher efficiency of clearance mechanisms for glutamate compared with those of K+ are consistent with this notion78,79.
The failure to detect a [K+]e rise preceding the fast regenerative [K+]e increase associated with CSD has been considered as evidence against K+-mediated propagation2,10,64; however, a rise in [K+]e immediately preceding CSD has been observed in some studies80,81,82.
In brain slices, the propagating K+ wave is accompanied by an almost simultaneous wave of [K+]e-dependent synaptic glutamate release at the CSD wavefront43,83 that is essential for the activation of the NMDARs mediating CSD propagation (see previous section and Figs 1b,2b). Cav (particularly Cav2.1) channel-dependent synaptic glutamate release is required for CSD propagation induced by brief local K+ stimulation15,39,41,46,47. Additional [Ca2+]e-independent mechanisms of glutamate release (involving presynaptic NMDARs and Ca2+ efflux from mitochondria) contribute to CSD propagation induced by more prolonged and diffuse K+ applications18,69.
The importance of Cav channel-dependent release of glutamate and K+ is reinforced by experimental results in mutant mice. Loss-of-function Cav2.1 mutants (or wild-type mice after partial blockade of NMDARs) in vivo show an increased CSD threshold and reduced velocity of CSD propagation36,49. Conversely, the lowered threshold for CSD induction in FHM1 mutants is accompanied by an increased propagation rate50,51,52,84 and facilitated propagation to subcortical areas85. Restoration of glutamate release to wild-type levels (by partial inhibition of the Cav2.1 channel) in FHM1 mutants rescues both the threshold and rate of propagation in cortical slices, supporting a causal relationship between increased Cav2.1 channel-dependent glutamate release and facilitation of both CSD initiation and propagation51. In contrast with glutamate facilitation, GABA limits the rate of propagation in hippocampal slices43.
Overall, the findings are consistent with a model of CSD propagation in which interstitial K+ diffusion initiates the positive-feedback cycle that ignites CSD in contiguous dendritic regions (Fig. 2). The clearance of K+ and glutamate by astrocytes limits the rate and spatial extent of CSD propagation. Indeed, the rate of CSD propagation was increased after selective poisoning of glial metabolism86,87 and was higher in FHM2-knockin and connexin 43-knockout mice in vivo than in wild-type mice59,60. Action potential-dependent glutamate release may contribute to but is not essential for CSD propagation, as TTX slows18 but does not block CSD propagation10,18,69,72 (but see Ref. 68). Although it is now clear that the astrocyte Ca2+ wave is a collateral phenomenon of CSD and not the leading signal in CSD propagation15,16, the available data do not exclude a modulatory role for gliotransmitters88 (particularly glutamate) in CSD propagation.
Initiation of spreading depolarizations. As mentioned earlier, spreading depolarizations are associated with metabolically compromised brain tissue. An understanding of the ion channels and mechanisms involved in the initiation of spreading depolarizations derives largely from studies of the biophysical properties and pharmacology of the specific processes occurring in the few minutes preceding spreading depolarization onset after hypoxia or OGD in acute brain slices (Fig. 3a). The term anoxic depolarization is used below even if, shortly after spreading depolarization initiation, brain slices are reperfused with oxygenated physiological solution, which causes rapid membrane repolarization. The delayed onset (Fig. 3a) reflects the time needed for ATP concentration to fall below the level needed to sustain the activity of (Na+ + K+)ATPase (and other cell pumps) and for subsequent derangement of ion gradients89 and activation of the channels mediating the net self-sustaining inward current and regenerative depolarization.
a | Typical membrane potential (Vm) and extracellular potential (Vo) changes induced in a brain slice by hypoxia or oxygen-glucose deprivation (OGD). After a delay of a few minutes, hypoxia or OGD induce the so-called anoxic depolarization, which is characterized by a rapid, complete neuronal depolarization and a rapid negative ΔVo change. During anoxic depolarization, the extracellular ionic changes are similar to those shown in Fig. 1b for cortical spreading depression (CSD). After anoxic depolarization initiation, the tissue is rapidly reoxygenated to enable membrane repolarization and restoration of ion gradients. The phase preceding anoxic depolarization onset is characterized by a slow membrane depolarization (after a brief hyperpolarization). b | A schematic diagram of an anoxic depolarization initiation mechanism, in which the net self-sustaining inward current in the positive-feedback cycle that ignites anoxic depolarization is mediated by multiple ion channels: besides NMDA receptors (NMDARs) and persistent voltage-gated Na+ (Navp) channels, the diagram includes hypothetical non-specific cation channels (NSCs) whose opening might depend on increased intracellular Ca2+ concentration ([Ca2+]i) (or decreased extracellular Ca2+ concentration ([Ca2+]e)) and hence indirectly on voltage-gated calcium (Cav) channels or on factors (for example, reactive oxygen species) produced by mitochondria depolarization (indicated by ↑ Δψmito) that might depend on increased [Zn2+]i. Both [Ca2+]e-dependent and [Ca2+]e-independent glutamate release contribute to the activation of NMDARs involved in anoxic depolarization initiation. The relative contribution of the different ion channels in the blue box may vary depending on age, brain region and degree of metabolic impairment.
The phase preceding anoxic depolarization onset is characterized by a slow membrane depolarization (after a brief hyperpolarization)90,91,92 (Fig. 3a), a nearly isotonic influx of NaCl and water91, a decrease in pHe (Ref. 93) and a slow gradual increase in [K+]e (Ref. 91), [Ca2+]i (Ref. 94) and [Zn2+]i (Ref. 95). During this phase, mitochondria slowly depolarize95,96 (even in Ca2+-free solution96). Zn2+ chelation reduces mitochondrial depolarization and delays anoxic depolarization onset46,95, supporting a correlation between the two97. After hypoxia, there is a large increase in the frequency of (mostly TTX-insensitive) spontaneous excitatory and inhibitory synaptic potentials98,99,100. By contrast, action potential-evoked synaptic transmission is reduced and may become completely inhibited in the pre-anoxic depolarization phase98,101.
Although pharmacological studies do not provide a consistent picture, they suggest that NMDARs, Cav channels and persistent TTX-sensitive Na+ currents may all be (directly or indirectly) involved in the generation of the net self-sustaining inward current and the initiation of anoxic depolarization a few minutes after hypoxia and/or ischaemia. Their relative contribution remains unclear and varies depending on age and brain region.
NMDAR and AMPAKR antagonists neither block nor delay anoxic depolarization onset in the cerebral cortex in vivo33,35,102,103,104 and in cortical slices105, whereas, in hippocampal slices, they delay anoxic depolarization onset in most studies91,92,94,105; in combination, they prevent anoxic depolarization induction in immature slices92,106.
Both [Ca2+]e-independent and [Ca2+]e-dependent mechanisms of glutamate release contribute to NMDAR activation, which is implicated in anoxic depolarization initiation. Anoxic depolarization onset can be delayed under conditions of low [Ca2+]e (Ref. 94) and is greatly delayed (or totally blocked) after inhibition of Cav channels47,107 but still occurs in Ca2+-free solution46,96,106. Among other mechanisms, glutamate can be released in a [Ca2+]e-independent manner by reversed transport of glutamate transporters. Of note, anoxic depolarization onset is delayed in mice lacking the neuronal glutamate transporter EAAC1 (Ref. 108) and is greatly delayed (or blocked) by pharmacological reduction of glutamate transport in immature hippocampal slices92,106; blockade of the glial glutamate transporter 1 (GLT1; also known as EAAT2) has no effect106,109. However, in adult slices with increased expression of GLT1 (Ref. 110), enhancement of glutamate clearance by GLT1 transporters delays anoxic depolarization onset111 and blockade of glutamate transporters accelerates anoxic depolarization onset108.
The strongest inhibitory effect on anoxic depolarization onset is probably produced by inhibitors of Nav channels. In brain slices, TTX as well as lidocaine or dibucaine strongly delay or block anoxic depolarization onset in most studies105,112,113,114,115,116 (but see Refs 92,98), even at concentrations that only slightly or partially inhibit evoked field potentials or axonal conduction113,116. Moreover, anoxic depolarization onset is delayed when [Na+]e is reduced115. In perfused rat brain, TTX also postpones anoxic depolarization induced by ischaemia and decreases cellular Na+ influx in the pre-anoxic depolarization phase117. Interestingly, hypoxia greatly increases the neuronal persistent Na+ current; this Na+ current is induced at more negative potentials and is more sensitive to TTX and lidocaine than the transient current involved in action potential generation118,119. Being voltage-dependent (and also [K+]e-dependent120), the persistent Na+ current may directly contribute to the generation of the net self-sustaining inward current that initiates the positive-feedback cycle igniting anoxic depolarization30. Moreover, the prolonged Na+ influx during the pre-anoxic depolarization phase may lead to several consequences that could, in principle, contribute to anoxic depolarization initiation: for example, it could increase [Na+]i and enhance metabolic cell stress and thus accelerate the decrease in ATP levels. It could also reduce active reuptake of glutamate and other neurotransmitters as well as indirectly increase [Ca2+]i.
Complete blockade of anoxic depolarization in hippocampal slices can be achieved by a combination of Nav and Cav channel inhibitors and of NMDAR and AMPAKR antagonists121, and in cortical slices by lowering the bath temperature to 22 °C (probably owing to reduced metabolic demand)105.
Ouabain-induced spreading depolarizations are in many respects similar to hypoxia- or OGD-induced spreading depolarizations in terms of physical properties and pharmacology (Box 1).
Although our mechanistic understanding is incomplete, it is clear that the initiating mechanisms of anoxic depolarization and ouabain-induced spreading depolarization differ from those of CSD (Table 1). Some of the specific processes that begin a few minutes preceding spreading depolarization onset (for example, the prolonged Na+ influx, the slow mitochondrial depolarization and Zn2+ influx) seem to be crucial for the initiation of the positive-feedback loops that ignite spreading depolarizations but do not play a part in CSD initiation in healthy brain tissue. Moreover, NMDARs are the key ion channels that mediate most if not all of the net (self-sustaining) inward current that is necessary for initiation of the positive-feedback cycle that ignites CSD (Fig. 2b), whereas other ion channels (that remain unknown but probably include persistent Nav channels), in addition to NMDARs, contribute to the net self-sustaining inward current that initiates spreading depolarizations (Fig. 3b). Their relative contribution remains unclear but probably varies depending on the degree of metabolic (and/or (Na+ + K+)ATPase) impairment. Finally, Ca2+-independent, non-vesicular glutamate release contributes to the activation of NMDARs involved in the initiation of spreading depolarizations, whereas glutamate derived from synaptic Ca2+-dependent vesicular release predominates in CSD initiation.
Sustained depolarization phase during CSD and spreading depolarizations. Although it is clear that the sustained neuronal depolarization during CSD and spreading depolarizations is mainly maintained by Na+ influx through non-selective cationic channels2, the identity of these channels remains incompletely understood.
There is pharmacological evidence that activation of NMDARs contributes to the sustained depolarization phase of CSD10,17,36 and anoxic depolarization106. In hippocampal slices, NMDAR activation is responsible for most of the late CSD phase and for the delayed long-lasting [Ca2+]i increase in the dendrites of CA1 pyramidal cells17. During the last part of the late CSD phase, the increased frequency of spontaneous excitatory postsynaptic currents (EPSCs) and of evoked EPSCs suggests that facilitation of synaptic glutamate release may contribute to NMDAR activation during this phase17.
Furthermore, NMDAR activation accounts for most of the sustained inward current underlying anoxic depolarization in immature hippocampal slices106. Rather than Ca2+-dependent vesicular release, reverse transport of glutamate by neuronal glutamate transporters is the major contributor to NMDAR activation106,109. Reverse transport by glial glutamate transporters may contribute during prolonged ischaemia in vivo in older animals122.
According to a recent study, NMDAR-dependent opening of pannexin 1 (a member of a family of large-pore ion channels with broad expression in the CNS)123 contributes to the inward current underlying anoxic depolarization in hippocampal slices and may actually account for much of its sensitivity to NMDAR antagonists124 (but according to another study, the contribution of pannexins is very small or null125). In vivo studies also report conflicting results regarding the involvement of pannexin channels, as these channels do not appear to open until 30–40 minutes after a relatively brief period (6–8 minutes) of global ischaemia104. These findings notwithstanding, brain infarct volume and neurological deficits after permanent focal cerebral ischaemia were improved in mice lacking both pannexin 1 and pannexin 2 (Ref. 126). Furthermore, there is strong in vivo evidence described below that neuronal pannexin channels open during CSDs induced by pinprick or prolonged topical KCl74.
It will be interesting to investigate whether the cationic, non-selective, Ca2+-permeable acid-sensing ion channel 1 (ASIC1)127, transient receptor potential M2 (TRPM2) channel and TRPM7 channel128 open during CSD and/or spreading depolarizations, as suggested by their properties, including the NMDAR-dependent facilitation of ASIC1 channels via Ca2+/calmodulin-dependent protein kinase II activation129 and the activation of TRPM2 and TRPM7 channels by arachidonic acid, reactive oxygen and nitrogen species and increased [Ca2+]i (Refs 130,131,132), all of which are conditions that occur during ischaemia and to some extent during CSD.
Translational implications
Despite fascinating unanswered questions concerning the underlying mechanisms that initiate and sustain CSD and spreading depolarizations (and the impact of methodological differences), emerging literature implicates CSD, spreading depolarizations and anoxic depolarization in human brain disorders, and these are very briefly reviewed below.
CSD arising in the migrainous brain. Migraine is a headache disorder characterized by throbbing pain, nausea, vomiting and sensitivity to light and sound that lasts for 12–72 hours. The headache is often anticipated by a perceptual disturbance, an aura, the most common being a visual aura characterized by shimmering lights and zig-zag lines beginning in the centre of vision and moving slowly towards the periphery. Accumulating evidence strongly implicates CSD in the genesis of visual aura5,6, and blood-oxygen-level-dependent (BOLD) MRI in humans133 has provided the most technically advanced demonstration of this134. Three additional lines of investigation reinforce the link between aura, CSD and migraine: first, the discovery that certain genetic mutations cause FHM, increase the risk of migraine aura in humans and facilitate CSD in mouse models6,135 (see the section on CSD mechanisms); second, CSD activates the trigeminovascular system in animal models to cause headache; and third, prophylactic drugs raise the electrical and KCl thresholds to evoke CSD as a mechanism of prevention (see below).
Despite strengthening evidence confirming the link between CSD and aura, our understanding of their seemingly unpredictable and spontaneous nature in most patients is only in its nascent stage. Of relevant interest, several mutant proteins expressed in neurons50, astrocytes59, vascular smooth muscle136 or multiple cell types137 have been linked to migraine with aura. When the genes encoding these mutant proteins were knocked into mice, susceptibility to CSD was increased (see Ref. 138 for a review).
A link between headache localization and the neurobiology of aura is provided by CSD-induced activation and sensitization of neuropeptide-containing trigeminal peripheral nociceptors that innervate ipsilateral cranial tissues such as the pia mater, arachnoid and dura mater6,7,139,140,141,142 (Box 2). The brain, unlike the meninges, is insensate. Drastic depolarizations, such as those that occur during CSD, release noxious molecules (for example, K+, protons, arachidonic acid and nitric oxide) into the interstitial space31,81,143. It has been posited that they reach sufficient levels to discharge a network of trigeminal axons140,141, promote conduction to and discharge of second-order neurons142 and cause local neurogenic inflammation and mast cell degranulation within the dura mater layer of the meninges141. The existence of a complex meningoglial network144 may facilitate this mechanism.
In a complementary way, CSD was recently linked to a novel mechanism of trigeminovascular activation, which could provide several therapeutic targets. In this experimental model, intense depolarization and high [K+]e trigger the development of a parenchymal inflammatory response involving the opening of pannexin 1 megachannels and the initiation of an inflammasome cascade involving crosstalk between depolarized neurons, astrocytes and astrocyte foot processes approximating the glial limitans74. In this study, a single pinprick-induced episode of CSD opened neuronal pannexin 1 channels, which in turn promoted caspase 1 activation, HMGB1 (high mobility group protein B1) release into the extracellular space, astrocytic nuclear factor-κB nuclear translocation in cells adjacent to the meninges and, ultimately, trigeminovascular activation (Fig. 4). The pannexin 1 inhibitors carbenoxolone or probenecid blocked the above pro-inflammatory cascade in neurons and glia and inhibited trigeminovascular activation. This finding is of relevance to humans because the intervening and ongoing transcriptional and translational steps provide a plausible mechanism to explain headache persistence plus a delay in headache onset after aura.
Proposed cascade of signalling events is depicted on the cortical surface following cortical spreading depression (CSD) that triggers trigeminovascular activation and possibly headache pain74. CSD evokes transient opening of neuronal pannexin 1 (PANX1) channels, which is followed by release of pro-inflammatory signalling molecules (high mobility group protein B1 (HMGB1) and interleukin-1β (IL-1β)). Among other pro-inflammatory actions, there follows triggering of astrocytic nuclear factor-κB (NF-κB) nuclear translocation and cell expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2; also known as PTGS2) along with sustained cytokine and prostaglandin production. Cytokines and prostaglandins are released from glial cells and cross the glia limitans to reach sensory axons. Following discharge of pial and dural trigeminal axons, impulses are carried centrally (orthodromically; arrows) to the trigeminal ganglion cells and trigeminal nucleus caudalis (TNC). Not shown are the consequences of impulses transmitted antidromically (arrow going to the middle meningeal artery) to promote a CSD-induced neurogenic inflammatory response and mast cell degranulation within the meninges plus reflex dilation of the middle meningeal artery via brainstem connections141. Modified from Karatas, H. et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339, 1092–1095 (2013)74. Reprinted with permission from AAAS.
In addition to peripheral activation following aura, there seems to be a substantial role for central modulation of incoming signals that either enhance or inhibit responses in the trigeminal nucleus caudalis145. So, for example, contralaterally projecting corticofugal fibres from the insular cortex directly facilitate responses in laminae I–II neurons within the spinal trigeminal nucleus, whereas primary somatosensory cortical areas provide inhibitory inputs to laminae III–IV. Because these cortical regions are somatotopically specified, it has been suggested that corticofugal projections may contribute to top-down regulation by fine tuning the topographic localization of headache pain145. By contrast, the periaqueductal grey and rostral ventromedial medulla are not somatotopically specified and project widely to the spinal and nucleus caudalis; therefore, their role in pain modulation may not be specific to migraine headache, as proposed on the basis of early functional imaging studies146,147, nor are they likely to serve as brainstem generators for migraine attacks148,149.
Drugs that reduce the frequency and intensity of migraine attacks were discovered mostly by serendipity, and only recently have we begun to understand that CSD provides one target. At least five migraine-preventive drugs blocked CSD when administered chronically150,151. Some may also block activation and sensitization of primary, secondary or tertiary neurons that respond to noxious dural stimulation such as within the ventroposteromedial nucleus of the thalamus152. In humans, treatment with preventive drugs (such as topiramate or valproate) reduces the frequency of aura followed by headache, although this relationship between aura and headache is not without controversy among clinicians because some auras are not followed by headache153.
The experimental model most relevant to 'spontaneous' CSD initiation in migraine is not known, partly because the mechanisms conferring CSD susceptibility are also not known. However, the experimental and clinical evidence points to a disorder of brain excitability characterized by deficient regulation of the cortical excitatory–inhibitory balance6,135. For example, this is supported by recent studies of interictal cortical excitability in migraineurs154,155,156. Moreover, FHM1 mutations lead to a differential effect on synaptic transmission and short-term plasticity at cortical excitatory and inhibitory synapses (note that they have no effect at fast-spiking interneuron synapses) in mouse models51. It has been hypothesized that deficient regulation of the cortical excitatory–inhibitory balance may under certain conditions (for example, in response to migraine triggers) lead to hyperactivity of cortical circuits. This in turn may increase [K+]e above a critical value, thus creating the conditions for CSD ignition6,135. If this hypothesis is correct, electrical or high K+ pulse stimulation seems to be more relevant as a CSD-inducing stimulus than pinprick or ouabain41, although it remains unclear whether a brief high K+ pulse is more physiologically plausible than sustained lower amplitudes changes.
Spreading depolarizations arising in the injured brain. There is little evidence that CSD leads to cell death in the normal brain4, although it is a noxious stimulus that may trigger pain (Box 2). However, when tissue is compromised, as in mild or severe ischaemia, CSD's counterpart, spreading depolarizations, often contribute to lesion development3. Multiple mechanisms that affect blood flow and brain metabolism have been implicated. For example, genetically conferred susceptibility to CSDs causes an exceptional number of spreading depolarizations that accelerate the onset and size of stroke136,157,158. As another example of this link, spreading depolarization waves may erupt in normal brains during very brief periods of ischaemia that do not necessarily cause tissue damage159. Caused by microemboli, these episodes may be of relevance to patients harbouring blood flow connections from veins to arteries that bypass the filtering properties (for example, clot removal) of the lung160; these patients seem to be at risk for both migraine aura and stroke161,162, although not all studies agree on this association163. An intriguing recent report found that responses to microemboli in migraineurs' brains may differ from those in normal brains164. As an example, shortly after microbubbles were injected intravenously into migraineurs with patent foramen ovale (a hole between the right and left atrium, which allows the shunting of blood from the venous to the arterial side, bypassing the lungs), a disturbance in cerebral bioelectrical activity and sometimes headache were evoked, but these effects did not occur in control subjects. Particularly interestingly, migraine with aura has been identified in women as a significant risk factor for stroke165. The mechanism for this remains unknown.
Stroke most often arises from occlusion of a small or large cerebral vessel or from multiple small blood vessels, thereby depriving brain tissue of oxygen and glucose as well as effective waste removal. Stroke is accompanied by a massive release of glutamate as well as by ionic imbalances that contribute to excitotoxic events at the molecular and cellular level that participate in negative tissue outcome166,167 and probably the genesis of spreading depolarizations — so-called killer waves in this context168. As suggested above, anoxic depolarization, spreading depolarization and CSD may exist on a continuum or spectrum. Although it remains to be proven, anoxic depolarization in the ischaemic core may transform into a spreading depolarization at the edges of the ischaemic core; and spreading depolarization may progressively change its features as it travels towards healthy tissue, where it may become CSD (associated with spreading depression of activity). Experimental evidence showing that CSD can be converted into a spreading depolarization by addition of vasoconstrictors is consistent with the notion of a continuum between these events169. Mechanistic differences between CSD and spreading depolarization described above suggest the need for more data to clarify this point.
Within the peri-infarct zone, spreading depolarizations have been observed to cycle around the lesion's outer margin and may provide a potential mechanism to enlarge focal ischaemic brain lesions170 (that is, by decreasing perfusion and delivery of oxygen and glucose171,172). A single spreading depolarization in experimental animals expands the area of severe hypoperfusion by more than 20%173, and the frequency and cumulative duration of spreading depolarizations reportedly correspond to accelerated infarct maturation and core expansion174,175. In clusters, spreading depolarizations portend an even more ominous outcome, potentially leading to anoxic depolarization as a terminal event reflecting tissue deterioration and tissue death176.
Building on the abundant evidence from the preclinical work, Strong, Dreier and the Cooperative Study on Brain Injury Depolarizations (COSBID) initiated studies in patients with brain injury to translate the impact of spreading depolarization and its long-term consequences (for example, 6 months or longer) on patient outcome. Especially notable are their findings in stroke patients (16 in total) who required surgery because of massive brain swelling involving much of one hemisphere (so-called malignant infarction) as a consequence of proximal middle cerebral artery occlusion176. In this group, a total of 127 CSDs and 42 spreading depolarizations were documented over an average of 4.5 days, with many of these appearing in clusters with a delayed recovery phase on an electrocorticogram. Preliminary MRI evidence indicates that the frequency of spreading depolarizations may be associated with tissue deterioration and lesion progression. As in the preclinical scenario, the mechanisms of deterioration may relate to a blood flow versus metabolism mismatch and to the added stress of very unfavourable prolonged propagations and severe hypoperfusion.
Subarachnoid haemorrhage arises when blood escapes from small or large arteries as well as veins and enters the cerebrospinal fluid compartment surrounding the brain. An aneurysm or weakening and ballooning of the arterial wall is most commonly implicated. As a result, intracranial pressure may increase and blood vessels constrict acutely to prevent blood from escaping. The constriction often triggers spreading depolarizations177. During the subacute phase (days after onset), delayed ischaemia sometimes develops, perhaps owing to narrowing of small distal arterioles. It seems that repetitive and spontaneous spreading depolarizations exacerbate ischaemia in this context, especially when electrocorticography suppression is prolonged177,178.
Preclinical findings seem to correlate with the clinical experience in subarachnoid haemorrhage. The COSBID group studied 18 patients with subarachnoid haemorrhage and detected spreading depolarizations in the majority of cases (72%). In nearly every patient, acute ischaemic episodes developed both spatially and temporally in register with the onset of recurrent spreading depolarizations. When clusters were observed179, the reductions in blood flow and tissue oxygen became more acute and severe. Rather than exhibiting transient hyperaemia (as would be expected in healthier brain tissue during spreading depolarization), blood flow dropped along with tissue oxygen availability180. This so-called inverted blood flow response is not invariably present in ischaemia or in other brain injury states but has been reported in various pathological conditions3,8. It may well be driven by the failure to clear extracellular vasoconstrictors such as K+ (>15 mM), a property of the severely hypoxic and ischaemic brain, or the failure to synthesize vasodilators (for example, nitric oxide)181. A greater appreciation of its clinical significance may come from the application of online cerebral blood flow recording techniques in the injured brain.
Spreading depolarizations occur in approximately 50% of patients with severe head trauma, and early evidence from the COSBID group suggests that they may affect overall prognosis182. The risk of evoking spreading depolarization waves increased by 33% during complications such as acute hypotension (<70 mm Hg), raised core temperatures, raised intracranial pressure, reduced plasma glucose levels or the administration of certain drugs183,184. Spreading depolarizations often developed early, singly or in clusters, not infrequently preceded by ischaemic blood flow values. A delayed secondary phase of spreading depolarizations appeared around day 6–7, sometimes associated with raised intracranial pressure or the above-noted physiological and metabolic perturbations. The net impact of these depolarizing events is to provoke even more membrane destabilizations and spreading depolarizations. When steps are taken to rectify the physiological and metabolic perturbations, the frequency of spreading depolarizations is diminished. Nevertheless, the continued eruptions that follow (and especially those that arise on the background of an isoelectric (flat) electrocorticogram) are linked to an increased risk of an unfavourable neurological outcome at 6 months based on a prospective observational study using the extended Glasgow Coma Outcome score182.
Drug treatments to improve outcomes by suppressing spreading depolarizations are at an early but critical stage of development185. The COSBID group reported in a retrospective analysis that the administration of ketamine, an NMDAR antagonist, seemed to reduce spreading depolarizations and spreading depolarization clusters in patients with traumatic brain injury. Patients with malignant stroke or ischaemia after subarachnoid haemorrhage also showed a similar response to ketamine185, suggesting the need for a formalized investigation by a clinical trial. Spreading depolarizations that have been implicated in other brain pathologies such as intraparenchymal and subdural brain haemorrhages are reviewed in Ref. 8.
Conclusions
Accumulating evidence reviewed above strongly supports propagating depolarizations as perpetrators of tissue injury within the compromised brain and in the genesis of migraine aura. Despite the controversial pharmacological profiles reported for CSD, there is an emerging consensus that NMDARs are the key dendritic channels for CSD initiation and that high [K+]e, by acting on contiguous dendritic regions, promotes a self-regenerative feedback cycle, which provides an explanation for wave propagation. These relationships are less clear for spreading depolarizations, as they seem to be relatively insensitive to NMDAR blockers and because their origination and propagation remain unclear. Even greater complexity is introduced when anoxic depolarization erupts after ATP levels drop and inhibitors of Na+ channels delay the inevitability of the prolonged and often terminal depolarization. Hence, there is a compelling need to develop drugs that effectively target CSD and/or spreading depolarization initiation and propagation to prevent and limit migraine aura as well as to mitigate the chaos and commotion that promotes death of the compromised and injured brain.
References
Leao, A. A. P. Spreading depression of activity in the cerebral cortex. J. Neurophysiol. 7, 359–390 (1944). A classic paper containing the first description of a propagating suppression of cortical activity, which was observed in the rabbit cortex, evoked by electrical stimulation and called CSD.
Somjen, G. G. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 81, 1065–1096 (2001).
Dreier, J. P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nature Med. 17, 439–447 (2011).
Nedergaard, M. & Hansen, A. J. Spreading depression is not associated with neuronal injury in the normal brain. Brain Res. 449, 395–398 (1988).
Lauritzen, M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 117, 199–210 (1994).
Pietrobon, D. & Moskowitz, M. A. Pathophysiology of migraine. Annu. Rev. Physiol. 75, 365–391 (2013).
Moskowitz, M. A. The neurobiology of vascular head pain. Ann. Neurol. 16, 157–168 (1984).
Lauritzen, M. et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J. Cereb. Blood Flow Metab. 31, 17–35 (2011).
Leao, A. A. Further observations on the spreading depression of activity in the cerebral cortex. J. Neurophysiol. 10, 409–414 (1947).
Herreras, O. & Somjen, G. G. Propagation of spreading depression among dendrites and somata of the same cell population. Brain Res. 610, 276–282 (1993).
Canals, S. et al. Longitudinal depolarization gradients along the somatodendritic axis of CA1 pyramidal cells: a novel feature of spreading depression. J. Neurophysiol. 94, 943–951 (2005). In this study, simultaneous intracellular recordings from somata and dendrites in hippocampal pyramidal cells (combined with extracellular recordings from the same regions) revealed longitudinal gradients of depolarization within individual neurons during some phases of CSD. The results support the initiation of CSD in apical dendrites, as first reported in reference 10.
Makarova, J., Gomez-Galan, M. & Herreras, O. Variations in tissue resistivity and in the extension of activated neuron domains shape the voltage signal during spreading depression in the CA1 in vivo. Eur. J. Neurosci. 27, 444–456 (2008).
Ochs, S. & Hunt, K. Apical dendrites and propagation of spreading depression in cerebral cortex. J. Neurophysiol. 23, 432–444 (1960).
Vilagi, I., Klapka, N. & Luhmann, H. J. Optical recording of spreading depression in rat neocortical slices. Brain Res. 898, 288–296 (2001).
Peters, O., Schipke, C. G., Hashimoto, Y. & Kettenmann, H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J. Neurosci. 23, 9888–9896 (2003).
Chuquet, J., Hollender, L. & Nimchinsky, E. A. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J. Neurosci. 27, 4036–4044 (2007). This study provides in vivo evidence that the CSD-associated neuronal Ca2+ wave precedes the astrocytic Ca2+ wave and is unaffected by suppression of the intracellular Ca2+ concentration increase in astrocytes, indicating that neurons lead the CSD propagation (see reference 15 for previous similar evidence in vitro).
Aiba, I. & Shuttleworth, C. W. Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. J. Physiol. 590, 5877–5893 (2012).
Zhou, N., Gordon, G. R., Feighan, D. & MacVicar, B. A. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb. Cortex 20, 2614–2624 (2010).
Takano, T. et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nature Neurosci. 10, 754–762 (2007).
Shinohara, M., Dollinger, B., Brown, G., Rapoport, S. & Sokoloff, L. Cerebral glucose utilization: local changes during and after recovery from spreading cortical depression. Science 203, 188–190 (1979).
Busija, D. W., Bari, F., Domoki, F., Horiguchi, T. & Shimizu, K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog. Neurobiol. 86, 379–395 (2008).
Piilgaard, H. & Lauritzen, M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J. Cereb. Blood Flow Metab. 29, 1517–1527 (2009). Polygraph recordings from the rodent cortex showing the consequences of CSD on sustained increases in oxygen utilization and uncoupling of the neurovascular response.
Chang, J. C. et al. Biphasic direct current shift, haemoglobin desaturation and neurovascular uncoupling in cortical spreading depression. Brain 133, 996–1012 (2010).
Fabricius, M., Akgoren, N. & Lauritzen, M. Arginine-nitric oxide pathway and cerebrovascular regulation in cortical spreading depression. Am. J. Physiol. 269, H23–H29 (1995).
Wahl, M., Lauritzen, M. & Schilling, L. Change of cerebrovascular reactivity after cortical spreading depression in cats and rats. Brain Res. 411, 72–80 (1987).
Seitz, I., Dirnagl, U. & Lindauer, U. Impaired vascular reactivity of isolated rat middle cerebral artery after cortical spreading depression in vivo. J. Cereb. Blood Flow Metab. 24, 526–530 (2004).
Fordsmann, J. C. et al. Increased 20-HETE synthesis explains reduced cerebral blood flow but not impaired neurovascular coupling after cortical spreading depression in rat cerebral cortex. J. Neurosci. 33, 2562–2570 (2013).
Reid, K. H., Marrannes, R., De Prins, E. & Wauquier, A. Potassium translocation and spreading depression induced by electrical stimulation of the brain. Exp. Neurol. 97, 345–364 (1987).
Reid, K. H., Marrannes, R. & Wauquier, A. Spreading depression and central nervous system pharmacology. J. Pharmacol. Methods 19, 1–21 (1988).
Kager, H., Wadman, W. J. & Somjen, G. G. Conditions for the triggering of spreading depression studied with computer simulations. J. Neurophysiol. 88, 2700–2712 (2002). A computational model defining the minimal biophysical machinery capable of generating CSD and spreading depolarizations and supporting the idea that a regenerative increase in [K+]e is an essential component of the positive-feedback cycle that ignites CSD (see reference 31 for the original proposal for the key role of an increase in [K+]e in CSD ignition and propagation).
Grafstein, B. Mechanism of spreading cortical depression. J. Neurophysiol. 19, 154–171 (1956).
Frohlich, F., Bazhenov, M., Iragui-Madoz, V. & Sejnowski, T. J. Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist 14, 422–433 (2008).
Lauritzen, M. & Hansen, A. J. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J. Cereb. Blood Flow Metab. 12, 223–229 (1992).
Kruger, H., Heinemann, U. & Luhmann, H. J. Effects of ionotropic glutamate receptor blockade and 5-HT1A receptor activation on spreading depression in rat neocortical slices. Neuroreport 10, 2651–2656 (1999).
Hernandez-Caceres, J., Macias-Gonzalez, R., Brozek, G. & Bures, J. Systemic ketamine blocks cortical spreading depression but does not delay the onset of terminal anoxic depolarization in rats. Brain Res. 437, 360–364 (1987).
Marrannes, R., Willems, R., De Prins, E. & Wauquier, A. Evidence for a role of the N-methyl-D-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain Res. 457, 226–240 (1988). One of the first studies to show that NMDARs have a key role in CSD initiation, are necessary for CSD propagation and contribute to the sustained depolarization phase of CSD that is induced by a local depolarizing stimulus in the normally metabolizing cerebral cortex.
McLachlan, R. S. Suppression of spreading depression of Leao in neocortex by an N-methyl-D-aspartate receptor antagonist. Can. J. Neurol. Sci. 19, 487–491 (1992).
Menniti, F. S. et al. CP-101,606, an NR2B subunit selective NMDA receptor antagonist, inhibits NMDA and injury induced c-fos expression and cortical spreading depression in rodents. Neuropharmacology 39, 1147–1155 (2000).
Footitt, D. R. & Newberry, N. R. Cortical spreading depression induces an LTP-like effect in rat neocortex in vitro. Brain Res. 781, 339–342 (1998).
Petzold, G. C. et al. Increased extracellular K+ concentration reduces the efficacy of N-methyl-D-aspartate receptor antagonists to block spreading depression-like depolarizations and spreading ischemia. Stroke 36, 1270–1277 (2005).
Tottene, A., Urbani, A. & Pietrobon, D. Role of different voltage-gated Ca2+ channels in cortical spreading depression: specific requirement of P/Q-type Ca2+ channels. Channels 5, 110–114 (2011).
Obrenovitch, T. P. & Zilkha, E. Inhibition of cortical spreading depression by L-701,324, a novel antagonist at the glycine site of the N-methyl-D-aspartate receptor complex. Br. J. Pharmacol. 117, 931–937 (1996).
Aiba, I., Carlson, A. P., Sheline, C. T. & Shuttleworth, C. W. Synaptic release and extracellular actions of Zn2+ limit propagation of spreading depression and related events in vitro and in vivo. J. Neurophysiol. 107, 1032–1041 (2012).
Larrosa, B., Pastor, J., Lopez-Aguado, L. & Herreras, O. A role for glutamate and glia in the fast network oscillations preceding spreading depression. Neuroscience 141, 1057–1068 (2006).
van Harreveld, A. Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J. Neurochem. 3, 300–315 (1959).
Dietz, R. M., Weiss, J. H. & Shuttleworth, C. W. Zn2+ influx is critical for some forms of spreading depression in brain slices. J. Neurosci. 28, 8014–8024 (2008). This is the first study to show that Zn2+ influx and associated mitochondrial depolarization can play a crucial part in the generation of spreading depolarizations but are not involved in the initiation of CSD.
Jing, J., Aitken, P. G. & Somjen, G. G. Role of calcium channels in spreading depression in rat hippocampal slices. Brain Res. 604, 251–259 (1993).
Pietrobon, D. CaV2.1 channelopathies. Pflugers Arch. 460, 375–393 (2010).
Ayata, C., Shimizu-Sasamata, M., Lo, E. H., Noebels, J. L. & Moskowitz. M. Impaired neurotransmitter release and elevated threshold for cortical spreading depression in mice with mutations in the α1A subunit of P/Q type calcium channels. Neuroscience 95, 639–645 (2000).
van den Maagdenberg, A. M. et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 41, 701–710 (2004). This is the first study to describe the generation of a knockin mouse model carrying a human mutation that causes FHM; it shows that the migraine mutation produces a gain of function in neuronal Cav2.1 channels and facilitates initiation and propagation of CSD induced by electrical stimulation in vivo . Together with references 49 and 41, this study supports a key role for Cav2.1 channels in CSD initiation and propagation.
Tottene, A. et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Cav2.1 knockin migraine mice. Neuron 61, 762–773 (2009). An analysis of cortical excitatory and inhibitory synaptic transmission in a FHM1-knockin mouse model, which shows increased excitatory neurotransmission owing to increased action potential-evoked glutamate release at pyramidal cell synapses, but unaltered inhibitory neurotransmission at fast-spiking interneuron synapses. Rescue experiments provide evidence of a causative link between increased Cav2.1-dependent glutamate release and facilitation of both initiation and propagation of CSD induced by brief, high [K+] pulses.
van den Maagdenberg, A. M. et al. High cortical spreading depression susceptibility and migraine-associated symptoms in Cav2.1 S218L mice. Ann. Neurol. 67, 85–98 (2010).
McGrail, K. M., Phillips, J. M. & Sweadner, K. J. Immunofluorescent localization of three Na, K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na, K-ATPase. J. Neurosci. 11, 381–391 (1991).
Cholet, N., Pellerin, L., Magistretti, P. J. & Hamel, E. Similar perisynaptic glial localization for the Na+,K+-ATPase α 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb. Cortex 12, 515–525 (2002).
D'Ambrosio, R., Gordon, D. S. & Winn, H. R. Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J. Neurophysiol. 87, 87–102 (2002).
Ransom, C. B., Ransom, B. R. & Sontheimer, H. Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J. Physiol. 522 Pt. 3, 427–442 (2000).
Pellerin, L. & Magistretti, P. J. Glutamate uptake stimulates Na+,K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J. Neurochem. 69, 2132–2137 (1997).
Rose, E. M. et al. Glutamate transporter coupling to Na, K-ATPase. J. Neurosci. 29, 8143–8155 (2009).
Leo, L. et al. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 7, e1002129 (2011). A study describing the generation of a knockin mouse model of FHM2 carrying a loss-of-function mutation in the gene encoding the the α2 (Na+ + K+)ATPase (which is expressed almost exclusively in astrocytes in the adult brain), showing facilitation of both induction and propagation of CSD (as in the FHM1 mouse model).
Theis, M. et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 23, 766–776 (2003).
Kofuji, P. & Newman, E. A. Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056 (2004).
Moskowitz, M. A., Bolay, H. & Dalkara, T. Deciphering migraine mechanisms: clues from familial hemiplegic migraine genotypes. Ann. Neurol. 55, 276–280 (2004).
Pietrobon, D. Familial hemiplegic migraine. Neurotherapeutics 4, 274–284 (2007).
Herreras, O. & Somjen, G. G. Analysis of potential shifts associated with recurrent spreading depression and prolonged unstable SD induced by microdyalisis of elevated K+ in hippocampus of anesthetized rats. Brain Res. 610, 283–294 (1993).
Peeters, M. et al. Effects of pan-and subtype-selective N-methyl-D-aspartate receptor antagonists on cortical spreading depression in the rat: therapeutic potential for migraine. J. Pharmacol. Exp. Ther. 321, 564–572 (2007).
Anderson, T. R. & Andrew, R. D. Spreading depression: imaging and blockade in the rat neocortical brain slice. J. Neurophysiol. 88, 2713–2725 (2002).
Gniel, H. M. & Martin, R. L. Changes in membrane potential and the intracellular calcium concentration during CSD and OGD in layer V and layer II/III mouse cortical neurons. J. Neurophysiol. 104, 3203–3212 (2010).
Tozzi, A. et al. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc. Natl Acad. Sci. USA 109, 18985–18990 (2012).
Zhou, N. et al. Regenerative glutamate release by presynaptic NMDA receptors contributes to spreading depression. J. Cereb. Blood Flow Metab. 33, 1582–1594 (2013). Pharmacological evidence that [Ca2+]e -independent mechanisms of glutamate release (involving presynaptic NMDARs and Ca2+ efflux from mitochondria) contribute to the initiation and propagation of CSD induced by diffuse and relatively prolonged high [K+] applications.
Petzold, G. C. et al. Nitric oxide modulates spreading depolarization threshold in the human and rodent cortex. Stroke 39, 1292–1299 (2008).
Richter, F., Ebersberger, A. & Schaible, H. G. Blockade of voltage-gated calcium channels in rat inhibits repetitive cortical spreading depression. Neurosci. Lett. 334, 123–126 (2002).
Sugaya, E., Takato, M. & Noda, Y. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J. Neurophysiol. 38, 822–841 (1975).
Herreras, O., Largo, C., Ibarz, J. M., Somjen, G. G. & Martin del Rio, R. Role of neuronal synchronizing mechanisms in the propagation of spreading depression in the in vivo hippocampus. J. Neurosci. 14, 7087–7098 (1994).
Karatas, H. et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339, 1092–1095 (2013). A study detailing a novel mechanism that links CSD and an intracortical signaling mechanism that involves PANX1 channel to release of pro-inflammatory substances that may activate the meningeal trigeminovascular system to cause pain.
Tamura, K., Alessandri, B., Heimann, A. & Kempski, O. The effect of a gap-junction blocker, carbenoxolone, on ischemic brain injury and cortical spreading depression. Neuroscience 194, 262–271 (2011).
Obrenovitch, T. P., Zilkha, E. & Urenjak, J. Intracerebral microdialysis: electrophysiological evidence of a critical pitfall. J. Neurochem. 64, 1884–1887 (1995).
Obrenovitch, T. P. & Zilkha, E. High extracellular potassium, and not extracellular glutamate, is required for the propagation of spreading depression. J. Neurophysiol. 73, 2107–2114 (1995).
Okubo, Y. & Iino, M. Visualization of glutamate as a volume transmitter. J. Physiol. 589, 481–488 (2011).
Meeks, J. P. & Mennerick, S. Astrocyte membrane responses and potassium accumulation during neuronal activity. Hippocampus 17, 1100–1108 (2007).
Kraig, R. P. & Nicholson, C. Extracellular ionic variations during spreading depression. Neuroscience 3, 1045–1059 (1978).
Hansen, A. J. & Zeuthen, T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol. Scand. 113, 437–445 (1981).
Haglund, M. M. & Schwartzkroin, P. A. Role of Na-K pump potassium regulation and IPSPs in seizures and spreading depression in immature rabbit hippocampal slices. J. Neurophysiol. 63, 225–239 (1990).
Carter, R. E., Aiba, I., Dietz, R. M., Sheline, C. T. & Shuttleworth, C. W. Spreading depression and related events are significant sources of neuronal Zn2+ release and accumulation. J. Cereb. Blood Flow Metab. 31, 1073–1084 (2011).
Eikermann-Haerter, K. et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J. Clin. Invest. 119, 99–109 (2009).
Eikermann-Haerter, K. et al. Enhanced subcortical spreading depression in familial hemiplegic migraine type 1 mutant mice. J. Neurosci. 31, 5755–5763 (2011).
Largo, C., Ibarz, J. M. & Herreras, O. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J. Neurophysiol. 78, 295–307 (1997).
Seidel, J. L. & Shuttleworth, C. W. Contribution of astrocyte glycogen stores to progression of spreading depression and related events in hippocampal slices. Neuroscience 192, 295–303 (2011).
Halassa, M. M. & Haydon, P. G. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 72, 335–355 (2010).
Allen, N. J., Karadottir, R. & Attwell, D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J. Neurosci. 25, 848–859 (2005).
Czeh, G., Aitken, P. G. & Somjen, G. G. Membrane currents in CA1 pyramidal cells during spreading depression (SD) and SD-like hypoxic depolarization. Brain Res. 632, 195–208 (1993).
Muller, M. & Somjen, G. G. Na+ and K+ concentrations, extra- and intracellular voltages, and the effect of TTX in hypoxic rat hippocampal slices. J. Neurophysiol. 83, 735–745 (2000).
Allen, N. J., Rossi, D. J. & Attwell, D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J. Neurosci. 24, 3837–3849 (2004).
Martin, R. L., Lloyd, H. G. & Cowan, A. I. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 17, 251–257 (1994).
Tanaka, E., Yamamoto, S., Kudo, Y., Mihara, S. & Higashi, H. Mechanisms underlying the rapid depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J. Neurophysiol. 78, 891–902 (1997).
Medvedeva, Y. V., Lin, B., Shuttleworth, C. W. & Weiss, J. H. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J. Neurosci. 29, 1105–1114 (2009).
Bahar, S., Fayuk, D., Somjen, G. G., Aitken, P. G. & Turner, D. A. Mitochondrial and intrinsic optical signals imaged during hypoxia and spreading depression in rat hippocampal slices. J. Neurophysiol. 84, 311–324 (2000).
Gerich, F. J., Hepp, S., Probst, I. & Muller, M. Mitochondrial inhibition prior to oxygen-withdrawal facilitates the occurrence of hypoxia-induced spreading depression in rat hippocampal slices. J. Neurophysiol. 96, 492–504 (2006).
Hershkowitz, N., Katchman, A. N. & Veregge, S. Site of synaptic depression during hypoxia: a patch-clamp analysis. J. Neurophysiol. 69, 432–441 (1993).
Fleidervish, I. A., Gebhardt, C., Astman, N., Gutnick, M. J. & Heinemann, U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. J. Neurosci. 21, 4600–4608 (2001).
Allen, N. J. & Attwell, D. The effect of simulated ischaemia on spontaneous GABA release in area CA1 of the juvenile rat hippocampus. J. Physiol. 561, 485–498 (2004).
Rosen, A. S. & Morris, M. E. Anoxic depression of excitatory and inhibitory postsynaptic potentials in rat neocortical slices. J. Neurophysiol. 69, 109–117 (1993).
Nellgard, B. & Wieloch, T. NMDA-receptor blockers but not NBQX, an AMPA-receptor antagonist, inhibit spreading depression in the rat brain. Acta Physiol. Scand. 146, 497–503 (1992).
Zhang, E. T., Hansen, A. J., Wieloch, T. & Lauritzen, M. Influence of MK-801 on brain extracellular calcium and potassium activities in severe hypoglycemia. J. Cereb. Blood Flow Metab. 10, 136–139 (1990).
Murphy, T. H., Li, P., Betts, K. & Liu, R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J. Neurosci. 28, 1756–1772 (2008).
Jarvis, C. R., Anderson, T. R. & Andrew, R. D. Anoxic depolarization mediates acute damage independent of glutamate in neocortical brain slices. Cereb. Cortex 11, 249–259 (2001).
Rossi, D. J., Oshima, T. & Attwell, D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403, 316–321 (2000).
Yamamoto, S. et al. Factors that reverse the persistent depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J. Neurophysiol. 78, 903–911 (1997).
Gebhardt, C., Korner, R. & Heinemann, U. Delayed anoxic depolarizations in hippocampal neurons of mice lacking the excitatory amino acid carrier 1. J. Cereb. Blood Flow Metab. 22, 569–575 (2002).
Hamann, M., Rossi, D. J., Marie, H. & Attwell, D. Knocking out the glial glutamate transporter GLT-1 reduces glutamate uptake but does not affect hippocampal glutamate dynamics in early simulated ischaemia. Eur. J. Neurosci. 15, 308–314 (2002).
Furuta, A., Rothstein, J. D. & Martin, L. J. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J. Neurosci. 17, 8363–8375 (1997).
Lipski, J. et al. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience 146, 617–629 (2007).
Aitken, P. G., Jing, J., Young, J. & Somjen, G. G. Ion channel involvement in hypoxia-induced spreading depression in hippocampal slices. Brain Res. 541, 7–11 (1991).
Weber, M. L. & Taylor, C. P. Damage from oxygen and glucose deprivation in hippocampal slices is prevented by tetrodotoxin, lidocaine and phenytoin without blockade of action potentials. Brain Res. 664, 167–177 (1994).
Fung, M. L., Croning, M. D. & Haddad, G. G. Sodium homeostasis in rat hippocampal slices during oxygen and glucose deprivation: role of voltage-sensitive sodium channels. Neurosci. Lett. 275, 41–44 (1999).
Muller, M. & Somjen, G. G. Na+ dependence and the role of glutamate receptors and Na+ channels in ion fluxes during hypoxia of rat hippocampal slices. J. Neurophysiol. 84, 1869–1880 (2000).
Douglas, H. A., Callaway, J. K., Sword, J., Kirov, S. A. & Andrew, R. D. Potent inhibition of anoxic depolarization by the sodium channel blocker dibucaine. J. Neurophysiol. 105, 1482–1494 (2011).
Xie, Y., Dengler, K., Zacharias, E., Wilffert, B. & Tegtmeier, F. Effects of the sodium channel blocker tetrodotoxin (TTX) on cellular ion homeostasis in rat brain subjected to complete ischemia. Brain Res. 652, 216–224 (1994).
Hammarstrom, A. K. & Gage, P. W. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J. Physiol. 510, 735–741 (1998).
Hammarstrom, A. K. & Gage, P. W. Hypoxia and persistent sodium current. Eur. Biophys. J. 31, 323–330 (2002).
Somjen, G. G. & Muller, M. Potassium-induced enhancement of persistent inward current in hippocampal neurons in isolation and in tissue slices. Brain Res. 885, 102–110 (2000).
Muller, M. & Somjen, G. G. Inhibition of major cationic inward currents prevents spreading depression-like hypoxic depolarization in rat hippocampal tissue slices. Brain Res. 812, 1–13 (1998).
Mitani, A. & Tanaka, K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J. Neurosci. 23, 7176–7182 (2003).
MacVicar, B. A. & Thompson, R. J. Non-junction functions of pannexin-1 channels. Trends Neurosci. 33, 93–102 (2010).
Weilinger, N. L., Tang, P. L. & Thompson, R. J. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J. Neurosci. 32, 12579–12588 (2012).
Madry, C., Haglerod, C. & Attwell, D. The role of pannexin hemichannels in the anoxic depolarization of hippocampal pyramidal cells. Brain 133, 3755–3763 (2010).
Bargiotas, P. et al. Pannexins in ischemia-induced neurodegeneration. Proc. Natl Acad. Sci. USA 108, 20772–20777 (2011).
Xiong, Z. G. et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118, 687–698 (2004).
Tymianski, M. Emerging mechanisms of disrupted cellular signaling in brain ischemia. Nature Neurosci. 14, 1369–1373 (2011).
Gao, J. et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 48, 635–646 (2005).
Olah, M. E. et al. Ca2+-dependent induction of TRPM2 currents in hippocampal neurons. J. Physiol. 587, 965–979 (2009).
Aarts, M. et al. A key role for TRPM7 channels in anoxic neuronal death. Cell 115, 863–877 (2003).
Wei, W. L. et al. TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc. Natl Acad. Sci. USA 104, 16323–16328 (2007).
Cao, Y., Welch, K. M., Aurora, S. & Vikingstad, E. M. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch. Neurol. 56, 548–554 (1999).
Hadjikhani, N. et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc. Natl Acad. Sci. USA 98, 4687–4692 (2001). A functional MRI study in a patient showing perturbations in the occipital cortex during visual aura that are consistent with features of CSD in the rodent cortex.
Vecchia, D. & Pietrobon, D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends Neurosci. 35, 507–520 (2012).
Eikermann-Haerter, K. et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome mutations increase susceptibility to spreading depression. Ann. Neurol. 69, 413–418 (2011).
Brennan, K. C. et al. Casein kinase Iδ mutations in familial migraine and advanced sleep phase. Sci. Transl. Med. 5, 183ra56 (2013).
Charles, A. C. & Baca, S. M. Cortical spreading depression and migraine. Nature Rev. Neurol. 9, 637–644 (2013).
Pietrobon, D. & Striessnig, J. Neurobiology of migraine. Nature Rev. Neurosci. 4, 386–398 (2003).
Zhang, X. et al. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J. Neurosci. 30, 8807–8814 (2010).
Bolay, H. et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nature Med. 8, 136–142 (2002).
Zhang, X. et al. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 69, 855–865 (2011).
Lauritzen, M., Hansen, A. J., Kronborg, D. & Wieloch, T. Cortical spreading depression is associated with arachidonic acid accumulation and preservation of energy charge. J. Cereb. Blood Flow Metab. 10, 115–122 (1990).
Mercier, F. & Hatton, G. I. Immunocytochemical basis for a meningeo-glial network. J. Comp. Neurol. 420, 445–465 (2000).
Noseda, R., Constandil, L., Bourgeais, L., Chalus, M. & Villanueva, L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J. Neurosci. 30, 14420–14429 (2010).
Weiller, C. et al. Brain stem activation in spontaneous human migraine attacks. Nature Med. 1, 658–660 (1995).
Goadsby, P. J., Lipton, R. B. & Ferrari, M. D. Migraine—current understanding and treatment. N. Engl. J. Med. 346, 257–270 (2002).
Borsook, D. & Burstein, R. The enigma of the dorsolateral pons as a migraine generator. Cephalalgia 32, 803–812 (2012).
Stankewitz, A., Aderjan, D., Eippert, F. & May, A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J. Neurosci. 31, 1937–1943 (2011).
Ayata, C. Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia 29, 1095–1114 (2009).
Ayata, C., Jin, H., Kudo, C., Dalkara, T. & Moskowitz, M. A. Suppression of cortical spreading depression in migraine prophylaxis. Ann. Neurol. 59, 652–661 (2006). In vivo pharmacological rodent study showing that five preventative drugs for migraine raise the CSD threshold in the cortex, suggesting a common action for these agents.
Andreou, A. P. & Goadsby, P. J. Topiramate in the treatment of migraine: a kainate (glutamate) receptor antagonist within the trigeminothalamic pathway. Cephalalgia 31, 1343–1358 (2011).
Wolthausen, J., Sternberg, S., Gerloff, C. & May, A. Are cortical spreading depression and headache in migraine causally linked? Cephalalgia 29, 244–249 (2009).
Antal, A. et al. Homeostatic metaplasticity of the motor cortex is altered during headache-free intervals in migraine with aura. Cereb. Cortex 18, 2701–2705 (2008).
Conte, A. et al. Differences in short-term primary motor cortex synaptic potentiation as assessed by repetitive transcranial magnetic stimulation in migraine patients with and without aura. Pain 148, 43–48 (2010).
Siniatchkin, M. et al. Abnormal changes of synaptic excitability in migraine with aura. Cereb. Cortex 22, 2207–2216 (2012).
Eikermann-Haerter, K. et al. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation 125, 335–345 (2012).
Arboleda-Velasquez, J. F. et al. Hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proc. Natl Acad. Sci. USA 108, E128–E135 (2011).
Nozari, A. et al. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann. Neurol. 67, 221–229 (2010).
Post, M. C. & Budts, W. The relationship between migraine and right-to-left shunt: fact or fiction? Chest 130, 896–901 (2006).
Schwedt, T. J., Demaerschalk, B. M. & Dodick, D. W. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia 28, 531–540 (2008).
Handke, M., Harloff, A., Olschewski, M., Hetzel, A. & Geibel, A. Patent foramen ovale and cryptogenic stroke in older patients. N. Engl. J. Med. 357, 2262–2268 (2007).
Rundek, T. et al. Patent foramen ovale and migraine: a cross-sectional study from the Northern Manhattan Study (NOMAS). Circulation 118, 1419–1424 (2008).
Sevgi, E. B., Erdener, S. E., Demirci, M., Topcuoglu, M. A. & Dalkara, T. Paradoxical air microembolism induces cerebral bioelectrical abnormalities and occasionally headache in patent foramen ovale patients with migraine. J. Am. Heart Assoc. 1, e001735 (2012).
Kurth, T., Chabriat, H. & Bousser, M. G. Migraine and stroke: a complex association with clinical implications. Lancet Neurol. 11, 92–100 (2012).
Moskowitz, M. A., Lo, E. H. & Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198 (2010).
Hossmann, K. A. Periinfarct depolarizations. Cerebrovasc. Brain Metab. Rev. 8, 195–208 (1996).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nature Med. 17, 796–808 (2011).
Dreier, J. P. et al. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J. Cereb. Blood Flow Metab. 18, 978–990 (1998).
Nakamura, H. et al. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain 133, 1994–2006 (2010).
Yuzawa, I. et al. Cortical spreading depression impairs oxygen delivery and metabolism in mice. J. Cereb. Blood Flow Metab. 32, 376–386 (2012).
Sukhotinsky, I. et al. Perfusion pressure-dependent recovery of cortical spreading depression is independent of tissue oxygenation over a wide physiologic range. J. Cereb. Blood Flow Metab. 30, 1168–1177 (2010).
Shin, H. K. et al. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J. Cereb. Blood Flow Metab. 26, 1018–1030 (2006).
Dijkhuizen, R. M. et al. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 840, 194–205 (1999).
Mies, G., Iijima, T. & Hossmann, K. A. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport 4, 709–711 (1993).
Dohmen, C. et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann. Neurol. 63, 720–728 (2008). A study showing that following middle cerebral artery occlusion, individuals with acute stroke exhibit recurrent spreading depolarizations, which can be detected by recordings from the exposed cortex.
Dreier, J. P. et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 132, 1866–1881 (2009).
Woitzik, J. et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 32, 203–212 (2012).
Dreier, J. P. et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 129, 3224–3237 (2006).
Bosche, B. et al. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann. Neurol. 67, 607–617 (2010).
Dreier, J. P. et al. Nitric oxide modulates the CBF response to increased extracellular potassium. J. Cereb. Blood Flow Metab. 15, 914–919 (1995).
Hartings, J. A. et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 10, 1058–1064 (2011).
Hartings, J. A. et al. Spreading depolarizations and late secondary insults after traumatic brain injury. J. Neurotrauma 26, 1857–1866 (2009).
Sakowitz, O. W. et al. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke 40, e519–522 (2009).
Hertle, D. N. et al. Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain 135, 2390–2398 (2012).
Basarsky, T. A., Feighan, D. & MacVicar, B. A. Glutamate release through volume-activated channels during spreading depression. J. Neurosci. 19, 6439–6445 (1999).
Basarsky, T. A., Duffy, S. N., Andrew, R. D. & MacVicar, B. A. Imaging spreading depression and associated intracellular calcium waves in brain slices. J. Neurosci. 18, 7189–7199 (1998).
Balestrino, M., Young, J. & Aitken, P. Block of (Na+,K+)ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices. Brain Res. 838, 37–44 (1999).
Anderson, T. R., Jarvis, C. R., Biedermann, A. J., Molnar, C. & Andrew, R. D. Blocking the anoxic depolarization protects without functional compromise following simulated stroke in cortical brain slices. J. Neurophysiol. 93, 963–979 (2005).
Moskowitz, M. A., Nozaki, K. I. & Kraig, R. P. Neocortical spreading depression provokes the expression of C-fos protein-like-immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J. Neurosci. 13, 1167–1177 (1993).
Holland, P. R. et al. Acid-sensing ion channel 1: a novel therapeutic target for migraine with aura. Ann. Neurol. 72, 559–563 (2012).
Akerman, S., Holland, P. R. & Goadsby, P. J. Mechanically-induced cortical spreading depression associated regional cerebral blood flow changes are blocked by Na+ ion channel blockade. Brain Res. 1229, 27–36 (2008).
Acknowledgements
The support of the Italian Ministry of University and Research (PRIN2010) and of the University of Padova (Strategic Project 2008 and Progetto Ateneo 2012) to D.P. is gratefully acknowledged. The authors gratefully thank A. Tottene for help with the figures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Ischaemic penumbra
-
The peri-infarct zone of brain tissue that remains vulnerable but viable for several hours after a stroke because of a collateral blood supply.
- Interstitial direct current potential
-
The local mean value of extracellular voltage.
- Stratum radiatum
-
A layer of the hippocampus that contains a portion of the apical dendrites of CA1 pyramidal cells and the axons (Schaffer collaterals) of the CA3 pyramidal cells that form synapses onto the CA1 dendrites.
- Oligaemia
-
A mild or moderate reduction in blood flow to the brain.
- Familial hemiplegic migraine
-
(FHM). An autosomal dominant subtype of classic migraine that is typically associated with prolonged one-sided weakness (motor aura) and is sometimes accompanied by visual, sensory and/or language auras.
- K+ spatial buffering
-
The mechanism by which functionally coupled highly K+-permeable glial cells transfer K+ from regions of increased extracellular K concentration ([K+]e) to regions of lower [K+]e; K+ entry in regions of high [K+]e causes a glial depolarization that spreads through the glial syncytium and generates a net driving force, causing K+ outflow in regions of lower [K+]e.
- Gliotransmitters
-
Transmitter molecules that are released by glial cells and participate in intercellular communication between neurons and glial cells.
- Infarct volume
-
The volume of tissue that dies after the interruption of blood flow in the brain.
- Trigeminovascular system
-
A network of peripheral and central axonal projections from trigeminal ganglion cells that transmit impulses centrally from the meninges and its blood vessels.
Rights and permissions
About this article
Cite this article
Pietrobon, D., Moskowitz, M. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci 15, 379–393 (2014). https://doi.org/10.1038/nrn3770
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3770
This article is cited by
-
The efficacy and safety of metoclopramide in relieving acute migraine attacks compared with other anti-migraine drugs: a systematic review and network meta-analysis of randomized controlled trials
BMC Neurology (2023)
-
Vesicular HMGB1 release from neurons stressed with spreading depolarization enables confined inflammatory signaling to astrocytes
Journal of Neuroinflammation (2023)
-
Future targets for migraine treatment beyond CGRP
The Journal of Headache and Pain (2023)
-
Migraine attacks are of peripheral origin: the debate goes on
The Journal of Headache and Pain (2023)
-
Intravascular laser irradiation of blood as novel migraine treatment: an observational study
European Journal of Medical Research (2023)